Abstract
Purpose
To examine the expression of pro-interleukin-1β (pro-IL-1β) and interleukin-1β (IL-1β) in the vitreous body of patients with neovascular age-related macular degeneration(nAMD), polypoidal choroidal vasculopathy (PCV), proliferative diabetic retinopathy (PDR), retinal vein occlusion (RVO) or Eales’ disease to further elucidate the role of IL-1β and inflammation in the pathogenesis of neovascular retinal disease.
Design
Prospective clinical laboratory investigation study.
Methods
All patients enrolled had vitreous hemorrhage due to nAMD, PCV, PDR, RVO or Eales’ disease that required vitrectomy. Patients were excluded for any history of active intraocular inflammation, or other ophthalmic surgery besides vitrectomy. Control samples were obtained from patients with idiopathic macular epiretinal membrane. A total of fifty vitreous samples were collected from patient during vitrectomy. Pro-IL-1β and IL-1β expression were measured by enzyme-linked immunosorbent assay (ELISA). Results were analyzed statistically using nonparametric tests.
Results
Expression of pro-IL-1β protein was increased by 2.83-fold and 9.19-fold in PCV and nAMD vitreous samples relative to control, respectively. Expression of IL-β protein was increased by 10-fold and 4.83-fold in PCV and nAMD vitreous samples relative to control, respectively.
Conclusions
Our results demonstrate that expression of pro-IL-1β and IL-1β proteins is higher in PCV and nAMD. The roles of pro-IL-1β and IL-1β as inflammatory mediators in the development of PCV and nAMD may be associated with photoreceptor degeneration and neovascularization which necessitates further study.
Introduction
As the leading cause of irreversible blindness in the elderly, age-related macular degeneration (AMD) is associated with many risk factors, including genetic and environmental factors such as oxidative stress and inflammation [1–3]. Polypoidal choroidal vasculopathy (PCV) is described as a special form of choroidal vasculopathy distinct from neovascular AMD (nAMD) with choroidal neovascularization (CNV). Idiopathic PCV is distinguishable from other choroidal neovascular disorders by its several unique clinical features and angiographic findings. However, there remains some controversy over whether or not PCV represents a subtype of AMD.
Inflammation has been shown to be associated with a variety of retinal diseases including AMD, PCV and proliferative diabetic retinopathy (PDR) [4]. Both the innate and adaptive immune systems are activated in these diseases. Various cytokines associated with protection and innate immunity are recruited in inflammatory eye diseases, including pattern recognition receptors (PRRs)[5].
Interleukin-1β (IL-1β) is a key pro-inflammatory cytokine which can initiate innate immune processes associated with inflammation, infection, and autoimmunity [6, 7]. IL-1β is secreted in an inactive pro-interleukin-1β (pro-IL-1β) form and requires proteolytic cleavage by caspase-1 to be released in its active form [8]. Caspase-1, also known as IL-1 converting enzyme (ICE), is a cysteine protease that specifically cleaves the 31kD pro-IL-1β precursor to produce the mature, 17kD active IL-1β [8, 9]. The caspase-1 activation platform, referred to as the inflammasome, links the sensing of pathogen and danger signals to pro-IL-1β activation[10]. The NLRP3 inflammasome has been previously reported to be involved in AMD [11–13]. Previous studies showed that caspase-1 activity increased in galactosemic mice and diabetic patients. C-reactive protein (CRP), a nonspecific marker of acute and chronic inflammation, was shown to be elevated in both PCV and nAMD [14, 15]. Furthermore, evidence of local inflammatory responses has been found both in macular drusen deposits [16] and in arterioles near drusen-like deposits [17–19].
IL-1β binds to the interleukin-1 receptor (IL-1R), which activates the myeloid differentiation factor 88 (MyD88) adapter protein to initiate a downstream signaling pathway [20]. IL-1β is associated with chronic inflammatory disease and its expression is maintained at a relatively constant level [14, 21, 22]. Although IL-1β was shown to be the major product of inflammasome cascade activation, levels of pro-IL-1β and IL-1β in the vitreous body from eyes with nAMD, PCV and other retinal vascular disease have not been previously studied.The present study attempted to address this issue by by simultaneously analyzing the expression of pro-IL-1β and IL-1β in the vitreous obtained during vitrectomy using an enzyme-linked immunosorbent assay (ELISA). Expression in vitreous from diseased eyes was compared to that in vitreous from eyes with idiopathic macular epiretinal membrane only.
Methods
Patients
Our prospective study was performed with the approval of the Ethical Committee of Peking University People’s Hospital and was conducted in accordance with the Declaration of Helsinki.
All participants gave written informed consent and were subsequently enrolled between April 2012 and January 2013. This prospective series included vitreous samples from fifty patients with vitreous hemorrhage due to nAMD with CNV, PCV, PDR, RVO or Eales’ disease who had received vitreous aspiration during vitrectomy. Ten patients with nAMD, ten with PCV, eight with PDR, eight with Eales’ disease and eight with RVO were enrolled. Six samples from patients with idiopathic macular epiretinal membrane were used as the control group (Table 1).
Table 1. The concentration of IL-1β protein level.
| IL-1β | Mean concentration(pg/ml) | SEM | N | P value |
|---|---|---|---|---|
| control | 0.41 | 0.19 | 6 | |
| PDR | 0.67 | 0.17 | 8 | - |
| nAMD | 1.98 | 0.49 | 10 | * |
| PCV | 4.20 | 0.5 | 10 | ** |
| Eales’ | 1.55 | 0.43 | 8 | * |
| RVO | 1.60 | 0.09 | 8 | * |
*<0.05 compared with control group
**P<0.01 compared with control group
- no significantly change compared with control group
All patients received a standard ophthalmic examination by a retinal specialist (Dr. Xuan Shi and Dr. Xiaoxin Li). The diagnoses of nAMD and PCV were made according to the International Classification System for AMD and the classification system for PCV [23–26]. Each patient also received fluorescein angiography (FA), optic coherence tomography (OCT), and indocyanine green angiogram (ICGA) testing.
All patients underwent vitrectomy, performed by Dr. Xuan Shi and Dr. Xiaoxin Li at the Peking University People’s Hospital, Beijing, China, after approval by the hospital’s institutional review board.
Patients were enrolled that had vitreous hemorrhage due to nAMD, PCV, PDR, RVO or Eales’ disease that required vitrectomy, and that had not had any other ophthalmic surgery previously. Prior to enrollment, all patients gave written informed consent to have vitreous aspiration sampling performed during vitrectomy. Any patients who were receiving intravitreal injection treatment during the period of enrollment, who had active intraocular inflammation, recent cerebral vascular accident or myocardial infarction, or had other systemic diseases which precluded vitrectomy were excluded from the study. Patients with idiopathic macular epiretinal membrane and underwent vitrectomy were selected as the control group.
Sample Collection
The sampling procedure was undergone during the 3-port lens-sparing vitrectomy with manual suction by sterile syringes. Approximately 100μl undiluted vitrectomy samples were obtained from the midvitreous of patients. The intraocular irrigating solution (Alcon) was not opened until the procedure completed.
The undiluted vitreous samples were aspirated by sterile syringes and centrifuged at 3000 rpm for 10 minutes at 4°C to remove cells and debris, then immediately transferred to store at -80°C until the time of assay. The technician and doctor involved in the study were masked to all the samples.
ELISA
Pro-IL-1β and IL-1β expression of all the samples were measured by enzyme-linked immunosorbent assay (ELISA) using a kit for human anti- pro-IL-1β (DLBP00; R&D Systems) and a kit for human anti- IL-1β (QLB00B; R&D Systems). Each assay was performed according to the manufacturer’s instruction. The optical density was determined at 450 nm using an absorption spectrophotometer. The optical density mean values were reading for three times for quantitative analysis.
A standard curve for this assay was built with recombinant human pro-IL-1β and IL-1β (R&D Systems).
Statistical Analysis
Results were analyzed statistically using nonparametric tests because of the skewed distribution (Kruskal-Wallis variance analysis and Bonferroni corrected Mann–Whitney U test) and were expressed as the median and range. A P value less than 0.05 was considered statistically significant. Statistical analysis was performed using SPSS software version 11.5 (SPSS, Inc., Chicago, IL).
Results
The concentrations of IL-1β Levels in the Vitreous Samples
The concentrations of IL-1β in nAMD, PCV, RVO, PDR, Eales' disease and idiopathic macular epiretinal membrane patients’ vitreous samples were measured by ELISAs. Our results showed that the concentration of IL-1β was 0.41±0.19 pg/ml (mean±SEM) in control group. However, the concentration of IL-1β was 4.20±0.5 pg/ml in PCV vitreous samples and 1.98±0.49 pg/ml in nAMD vitreous samples. At the same time, the concentration of IL-1β was 1.60±0.09 pg/ml in RVO vitreous samples and 1.55±0.43 pg/ml in Eales’ disease vitreous samples. The concentration of IL-1β was 0.67±0.17 pg/ml in PDR vitreous samples (Table 1).
The concentrations of pro-IL-1β Levels in the Vitreous Samples
The concentrations of pro-IL-1β in nAMD, PCV, RVO, PDR, Eales' disease and idiopathic macular epiretinal membrane patients’ vitreous samples were measured by ELISAs. Our results showed that the concentration of pro-IL-1β was 21.63±5.15 pg/ml (mean±SEM) in control group. However, the concentration of IL-1β was 198.91±73.79pg/ml in nAMD vitreous samples and 119.19±73.86 pg/ml in RVO vitreous samples. At the same time, the concentration of pro-IL-1β was 61.23±5 pg/ml in PCV vitreous samples and 72.03±17.71 pg/ml in Eales’ disease vitreous samples. The concentration of IL-1β was 36.09±14.03pg/ml in PDR vitreous samples (Table 2).
Table 2. The concentration of Pro-IL-1β protein level.
| Pro-IL-1β | Mean Concentration(pg/ml) | SEM | N | P value |
|---|---|---|---|---|
| control | 21.63 | 5.15 | 6 | |
| PDR | 36.09 | 14.03 | 8 | - |
| nAMD | 198.91 | 73.79 | 10 | * |
| PCV | 61.23 | 5.0 | 10 | * |
| Eales’ | 72.03 | 17.71 | 8 | * |
| RVO | 119.19 | 73.86 | 8 | - |
*<0.05 compared with control group
**P<0.01 compared with control group
- no significantly change compared with control group
IL-1β Levels were markedly elevated in the PCV, nAMD, Eales’ disease and RVO Vitreous Samples
The concentrations of IL-1β levels in the vitreous samples were listed in Table 1. The concentration of IL-1β in PCV, nAMD, Eales’ disease and RVO vitreous samples were significantly elevated when compared with control group (Table 1, Fig 1). A 10-fold and 4.83-fold increase of IL-1β protein expression was detected in PCV (P<0.01) and nAMD (P<0.05) vitreous body respectively compared to control group.
Fig 1. The concentration of IL-1β protein in vitreous samples.
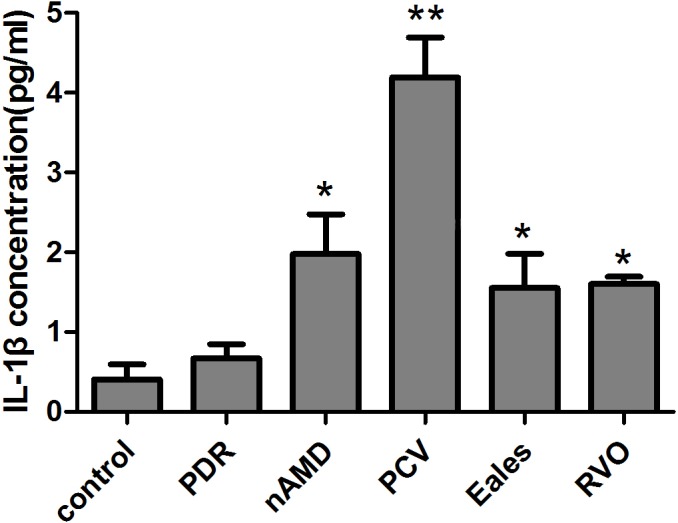
The concentrations of IL-1β in nAMD, PCV, RVO, PDR, Eales' disease and idiopathic macular epiretinal membrane patients vitreous samples were measured by ELISAs. The concentration of IL-1β was 4.20±0.5 pg/ml in PCV vitreous samples and 1.98±0.49 pg/ml in nAMD vitreous samples and 1.55±0.43 pg/ml in Eales’ disease vitreous samples and 1.60±0.09 pg/ml in RVO vitreous samples, which were markedly elevated when compared with control group (0.41±0.19 pg/ml; p<0.05) (mean±SEM).
Pro-IL-1β Levels were markedly elevated in the PCV, nAMD and Eales’ disease Vitreous Samples
The concentrations of pro-IL-1β levels in the vitreous samples were listed in Table 2. The concentration of pro-IL-1β in nAMD, PCV and Eales’ disease vitreous samples were significantly elevated when compared with control group (Table 2, Fig 2). A 2.83-fold and 9.19-fold increase of pro-IL-1β protein expression was detected in PCV (P<0.05) and nAMD (P<0.05) vitreous body respectively compared to control group.
Fig 2. The concentration of Pro-IL-1β protein in vitreous samples.
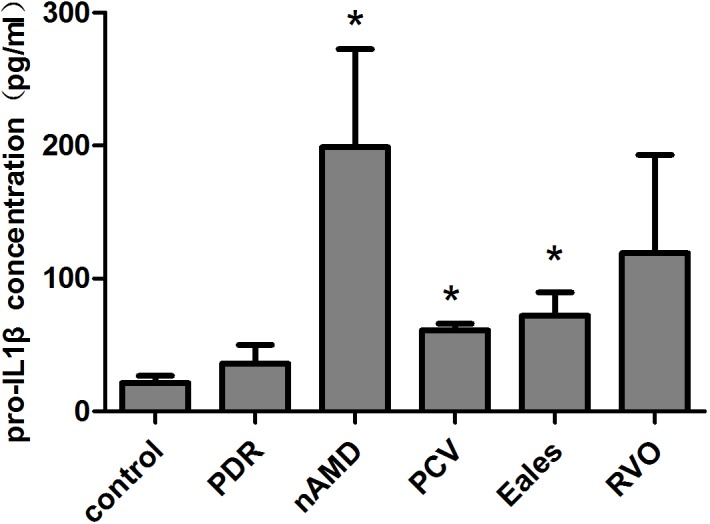
The concentrations of pro-IL-1β in nAMD, PCV, RVO, PDR, Eales' disease and idiopathic macular epiretinal membrane patients vitreous samples were measured by ELISAs. The concentration of pro-IL-1β was 198.91±73.79 pg/ml in nAMD vitreous samples and 61.23±5 pg/ml in PCV vitreous samples and 72.03±17.71 pg/ml in Eales’ disease vitreous samples, which were markedly elevated when compared with control group (21.63±5.15 pg/ml; p<0.05) (mean±SEM).
Discussion
There are more and more evidence for local inflammatory responses in the pathophysiology of AMD, PDR, RVO, Eales’ disease and PCV [16, 27, 28]. IL-1β, a key pro-inflammatory cytokine, can promote inflammation cascades and play an important role in these diseases [14, 21, 22]. Inflammasome links the sensing of pathogen and danger signals to pro-IL-1β activation. NLRP3 inflammasome is also reported to be involved in AMD which plays a role on detecting components of bacteria [11].
IL-1β is the major factor to active inflammasome cascades. However, there is few data on inflammatory cytokines expression in AMD and PCV vitreous samples. We therefore analyzed the protein level of pro-IL-1β and IL-1β in vitreous samples from patients with nAMD, PCV and other retinal vascular diseases. Our result showed that pro-IL-1β and IL-1β levels were markedly elevated in the PCV, nAMD vitreous samples compared to control group. This indicated that the IL-1β related inflammatory pathway may be associated with nAMD and PCV. However, we were unable to distinguish nAMD and PCV from vitreous samples on the basis of the expression of IL-1β or pro-IL-1β proteins, though PCV samples contained higher levels of IL-1β while nAMD samples contained higher levels of pro-IL-1β. The reason remains unclear as the number of subjects enrolled in our present study was limited. We assumed that IL-1β might be involved in both diseases although nAMD and PCV have different etiology, pathology and mechanisms.
Previous studies showed that the level of IL-1β increased in retinas of diabetic rats. The IL-1β expression was also reported to be elevated in the vitreous of PDR and Eales’ disease patients[27, 29–32]. Another study showed controversial result that the IL-1β was detected in only 10.3% vitreous samples from PDR patients at very low levels[33]. Our result showed that pro-IL-1β and IL-1β levels were elevated in the Eales’ disease vitreous samples compared to control group. These results indicated that IL-1β maybe involved in the Eales’ disease which was consistent with previous studies[27].
However, interestingly, from our results, pro-IL-1β and IL-1β levels were not markedly elevated in the PDR vitreous samples which differed from most of previous studies[27, 32, 34]. Currently, it is believed that the vascular barrier breakdown in the diabetic retinopathy and inflammation may play a role in the diabetic retinopathy[35]. It is a very long process that non proliferative diabetic retinopathy develops into proliferative diabetic retinopathy. Thus, the intraocular cytokines levels should be a dynamic process too. One study showed that the IL-1β level in DR patients’ aqueous humor differed with different DR severities and increased as the DR progressed[34]. We assumed that the level of pro-IL-1β and IL-1β levels were not markedly elevated in the PDR vitreous samples in our study maybe related to the DR severities. Patients enrolled in our study were diagnosed with vitreous hemorrhage due to PDR which was the milder stage of PDR. The other reason maybe because that the number of patients diagnosed with PDR in our study was limited.
nAMD, PCV, RVO, PDR and Eales’ disease have different etiology, pathology and demonstrate individual features. The symptoms and signs of these diseases are with great difference. Immunoinflammatory mechanism may be involved in those diseases but may not have a similar inflammation cytokine expression pattern in the vitreous. The capillary circulation is disturbed, the capillary endothelial cells is injured in DR. Then, minute NPAs is formed and become more and more extensive as the disease progresses, causing neovascularization and the formation of fibrovascular membrane in the interface between the retina and vitreous body[36]. Conversely, there is a pigmental epithelial (RPE) cell dysfunction and the choroidal vessels are dilated in AMD. Then new vessels sprout from the choroidal vessels, penetrate the Bruch’s membrane and grow into the subretinal space. Indeed, the RPE cell is one of the most important systems that regulate the immune response in the eye. Thus, the pathway between RPE damage and ocular inflammation is likely a two way street whereby RPE damage potentiates inflammation, which further worsens RPE degeneration [1]. Such a relatively RPE cell dysfunction may be one reason that the inflammatory reaction in nAMD is more severe than in DR.
RPE cells were the key immune cells in the macula immune defence. The dysfunction of RPE cells could trigger the inflammatory response, which could further trigger the photoreceptors degeneration and neovascularization development [11, 37–39]. This might be one of the mechanisms of PCV and nAMD development.
IL-1β was involved in the abnormal angiogenesis process. However, the reason that IL-1β plays different role in the development of PCV and nAMD remains unknown. One possibility was that IL-1β was upregulated in both PCV and nAMD conditions but with different level and time window which may further trigger different cell signaling pathways to activate different abnormal angiogenesis process. The increased expression of IL-1β in PCV and nAMD patients raises a possibility that the inflammasome could be involved in these diseases. Previous studies concluded controversial conclusion on the role of inflammasome in AMD [40]. Some concluded that inflammasome was harmful and the other concluded that it is beneficial [13, 41]. Inflammasome may play a dual role in AMD. The role of inflammasome and this hypothesis needs to be verified in our further studies.
In addition, an analysis of the IL-1β levels in the serum have been studied and listed in the supplemental data (S1 Text and S1 Fig) to demonstrate whether the higher concentrations of IL-1β in the vitreous of nAMD and PCV patients are due to the increased concentration in the serum. Our results in the supplemental data showed that there was a significant decrease in the concentration of IL-1β in PCV (P<0.05) and nAMD(P<0.01) serum samples compared with control group. Thus, we assumed that the decrease in the concentration of IL-1β in the blood of nAMD and PCV patients may not interference the result on the increase in the concentration of IL-1β in the vitreous samples of nAMD and PCV patients.
Taken together, our results suggested that IL-1β related inflammatory mechanism may be associated with nAMD and PCV. This association could be a result of the activation of inflammation, which further stimulates RPE cells to trigger the photoreceptors degeneration and neovascularization. The different role of IL-1β in the development of PCV and nAMD needs to be verified in further studies.
Supporting Information
The concentrations of IL-1β in nAMD, PCV and idiopathic macular epiretinal membrane patients’ serum samples were measured by ELISAs. The concentration of IL-1β was 2.28±0.17 pg/ml (mean±SEM) in control group. The concentration of IL-1β was 0.53±0.14 pg/ml in PCV serum samples and 0.47±0.12 pg/ml in nAMD serum samples. There was a significant decrease in the concentration of IL-1β in PCV (P<0.05) and nAMD (P<0.01) serum samples compared with control group.
(TIF)
The concentrations of IL-1β in nAMD, PCV and idiopathic macular epiretinal membrane patients’ serum samples were measured by ELISAs to determine the intereference of blood on the result. The results showed that the decrease in the concentration of IL-1β in the blood of nAMD and PCV patients may not interference the result on the increase in the concentration of IL-1β in the vitreous samples of nAMD and PCV patients.
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Xiaoxin Li was supported by National Basic Research Program of China (973 Program, 2011CB510200). Min Zhao was supported by Peking University People’s Hospital Research and Development Funds (RDB2013-21, 2118000540). Yujing Bai was supported by Beijing Nova Program (Z131102000413004). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Stefánsson E, Geirsdóttir Á, Sigurdsson H. Metabolic physiology in age related macular degeneration. Prog Retin Eye Res. 2011; 30(1): 72–80. 10.1016/j.preteyeres.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 2. Jager RD, Mieler WF, Miller JW. Age-Related Macular Degeneration. N Engl J Med. 2008; 358(24): 2606–2617. 10.1056/NEJMra0801537 [DOI] [PubMed] [Google Scholar]
- 3. Wankun X, Wenzhen Y, Min Z, Weiyan Z, Huan C, Wei D, et al. Protective effect of paeoniflorin against oxidative stress in human retinal pigment epithelium in vitro. Mol Vis. 2011; 17: 3512–3522. [PMC free article] [PubMed] [Google Scholar]
- 4. Klein R, Myers CE, Cruickshanks KJ, Gangnon RE, Danforth LG, Sivakumaran TA, et al. Markers of inflammation, oxidative stress, and endothelial dysfunction and the 20-year cumulative incidence of early age-related macular degeneration: the Beaver Dam Eye Study. JAMA Ophthalmol. 2014; 132(4): 446–455. 10.1001/jamaophthalmol.2013.7671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leal SM Jr., Pearlman E. The role of cytokines and pathogen recognition molecules in fungal keratitis—Insights from human disease and animal models. Cytokine. 2012; 58(1): 107–111. 10.1016/j.cyto.2011.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rock KL, Lai JJ, Kono H. Innate and adaptive immune responses to cell death. Immunological reviews. 2011; 243(1): 191–205. 10.1111/j.1600-065X.2011.01040.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu J, Yin Z, Cao S, Gao W, Liu L, Yin Y, et al. Systematic review and meta-analysis on the association between IL-1B polymorphisms and cancer risk. PLoS One. 2013; 8(5): e63654 10.1371/journal.pone.0063654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunological reviews. 2011; 243(1): 206–214. 10.1111/j.1600-065X.2011.01044.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kostura MJ, Tocci MJ, Limjuco G, Chin J, Cameron P, Hillman AG, et al. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci U S A. 1989; 86(14): 5227–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Okumura C, Haraikawa K, Suehiro S, Ito Y, Masumoto J. [The inflammasome]. Nihon rinsho Japanese journal of clinical medicine. 2013; 71(8): 1497–1504. [PubMed] [Google Scholar]
- 11. Doyle SL, Campbell M, Ozaki E, Salomon RG, Mori A, Kenna PF, et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med. 2012; 18(5): 791–798. 10.1038/nm.2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anderson OA, Finkelstein A, Shima DT. A2E induces IL-1ss production in retinal pigment epithelial cells via the NLRP3 inflammasome. PLoS One. 2013; 8(6): e67263 10.1371/journal.pone.0067263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stanton CM, Wright AF. Inflammatory biomarkers for AMD. Adv Exp Med Biol. 2014; 801: 251–257. 10.1007/978-1-4614-3209-8_32 [DOI] [PubMed] [Google Scholar]
- 14. Kikuchi M, Nakamura M, Ishikawa K, Suzuki T, Nishihara H, Yamakoshi T, et al. Elevated C-reactive protein levels in patients with polypoidal choroidal vasculopathy and patients with neovascular age-related macular degeneration. Ophthalmology. 2007; 114(9): 1722–1727. [DOI] [PubMed] [Google Scholar]
- 15. Colak E, Majkic-Singh N, Zoric L, Radosavljevic A, Kosanovic-Jakovic N. The role of CRP and inflammation in the pathogenesis of age-related macular degeneration. Biochemia medica: casopis Hrvatskoga drustva medicinskih biokemicara / HDMB. 2012; 22(1): 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson LV, Leitner WP, Staples MK, Anderson DH. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res. 2001; 73(6): 887–896. [DOI] [PubMed] [Google Scholar]
- 17. Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002; 134(3): 411–431. [DOI] [PubMed] [Google Scholar]
- 18. McGeer PL, McGeer EG. Inflammation and the degenerative diseases of aging. Annals of the New York Academy of Sciences. 2004; 1035: 104–116. [DOI] [PubMed] [Google Scholar]
- 19. Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2006; 51(2): 137–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nature reviews Immunology. 2013; 13(6): 397–411. 10.1038/nri3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annual review of immunology. 2009; 27: 519–550. 10.1146/annurev.immunol.021908.132612 [DOI] [PubMed] [Google Scholar]
- 22. Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annual review of immunology. 2011; 29: 707–735. 10.1146/annurev-immunol-031210-101405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995; 39(5): 367–374. [DOI] [PubMed] [Google Scholar]
- 24. Hou J, Tao Y, Li XX, Zhao MW. Clinical characteristics of polypoidal choroidal vasculopathy in Chinese patients. Graefes Arch Clin Exp Ophthalmol. 2011; 249(7): 975–979. 10.1007/s00417-010-1575-7 [DOI] [PubMed] [Google Scholar]
- 25. Koh AH, Chen LJ, Chen SJ, Chen Y, Giridhar A, Iida T, et al. Polypoidal choroidal vasculopathy: evidence-based guidelines for clinical diagnosis and treatment. Retina. 2013; 33(4): 686–716. 10.1097/IAE.0b013e3182852446 [DOI] [PubMed] [Google Scholar]
- 26. Cackett P, Wong D, Yeo I. A classification system for polypoidal choroidal vasculopathy. Retina. 2009; 29(2): 187–191. 10.1097/IAE.0b013e318188c839 [DOI] [PubMed] [Google Scholar]
- 27. Murugeswari P, Shukla D, Kim R, Namperumalsamy P, Stitt AW, Muthukkaruppan V. Angiogenic potential of vitreous from Proliferative Diabetic Retinopathy and Eales' Disease patients. PLoS One. 2014; 9(10): e107551 10.1371/journal.pone.0107551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feng J, Zhao T, Zhang Y, Ma Y, Jiang Y. Differences in aqueous concentrations of cytokines in macular edema secondary to branch and central retinal vein occlusion. PLoS One. 2013; 8(7): e68149 10.1371/journal.pone.0068149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vincent JA, Mohr S. Inhibition of caspase-1/interleukin-1beta signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes. 2007; 56(1): 224–230. [DOI] [PubMed] [Google Scholar]
- 30. Kowluru RA, Odenbach S. Role of interleukin-1beta in the development of retinopathy in rats: effect of antioxidants. Invest Ophthalmol Vis Sci. 2004; 45(11): 4161–4166. [DOI] [PubMed] [Google Scholar]
- 31. Krady JK, Basu A, Allen CM, Xu Y, LaNoue KF, Gardner TW, et al. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005; 54(5): 1559–1565. [DOI] [PubMed] [Google Scholar]
- 32. Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (Lond). 2006; 20(12): 1366–1369. [DOI] [PubMed] [Google Scholar]
- 33. El-Asrar AM, Nawaz MI, Kangave D, Geboes K, Ola MS, Ahmad S, et al. High-mobility group box-1 and biomarkers of inflammation in the vitreous from patients with proliferative diabetic retinopathy. Mol Vis. 2011; 17: 1829–1838. [PMC free article] [PubMed] [Google Scholar]
- 34. Dong N, Xu B, Wang B, Chu L. Study of 27 aqueous humor cytokines in patients with type 2 diabetes with or without retinopathy. Mol Vis. 2013; 19: 1734–1746. [PMC free article] [PubMed] [Google Scholar]
- 35. Funatsu H, Yamashita H, Noma H, Mimura T, Nakamura S, Sakata K, et al. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2005; 243(1): 3–8. [DOI] [PubMed] [Google Scholar]
- 36. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA: the journal of the American Medical Association. 2007; 298(8): 902–916. [DOI] [PubMed] [Google Scholar]
- 37. Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000; 14(7): 835–846. [PubMed] [Google Scholar]
- 38. Edwards AO, Ritter R 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005; 308(5720): 421–424. [DOI] [PubMed] [Google Scholar]
- 39. Kaarniranta K, Salminen A. Age-related macular degeneration: activation of innate immunity system via pattern recognition receptors. J Mol Med (Berl). 2009; 87(2): 117–123. 10.1007/s00109-008-0418-z [DOI] [PubMed] [Google Scholar]
- 40. Rosenbaum JT. Eyeing macular degeneration—few inflammatory remarks. N Engl J Med. 2012; 367(8): 768–770. 10.1056/NEJMcibr1204973 [DOI] [PubMed] [Google Scholar]
- 41. Campbell M, Doyle SL. An eye on the future of inflammasomes and drug development in AMD. J Mol Med (Berl). 2013; 91(9): 1059–1070. 10.1007/s00109-013-1050-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The concentrations of IL-1β in nAMD, PCV and idiopathic macular epiretinal membrane patients’ serum samples were measured by ELISAs. The concentration of IL-1β was 2.28±0.17 pg/ml (mean±SEM) in control group. The concentration of IL-1β was 0.53±0.14 pg/ml in PCV serum samples and 0.47±0.12 pg/ml in nAMD serum samples. There was a significant decrease in the concentration of IL-1β in PCV (P<0.05) and nAMD (P<0.01) serum samples compared with control group.
(TIF)
The concentrations of IL-1β in nAMD, PCV and idiopathic macular epiretinal membrane patients’ serum samples were measured by ELISAs to determine the intereference of blood on the result. The results showed that the decrease in the concentration of IL-1β in the blood of nAMD and PCV patients may not interference the result on the increase in the concentration of IL-1β in the vitreous samples of nAMD and PCV patients.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


