Abstract
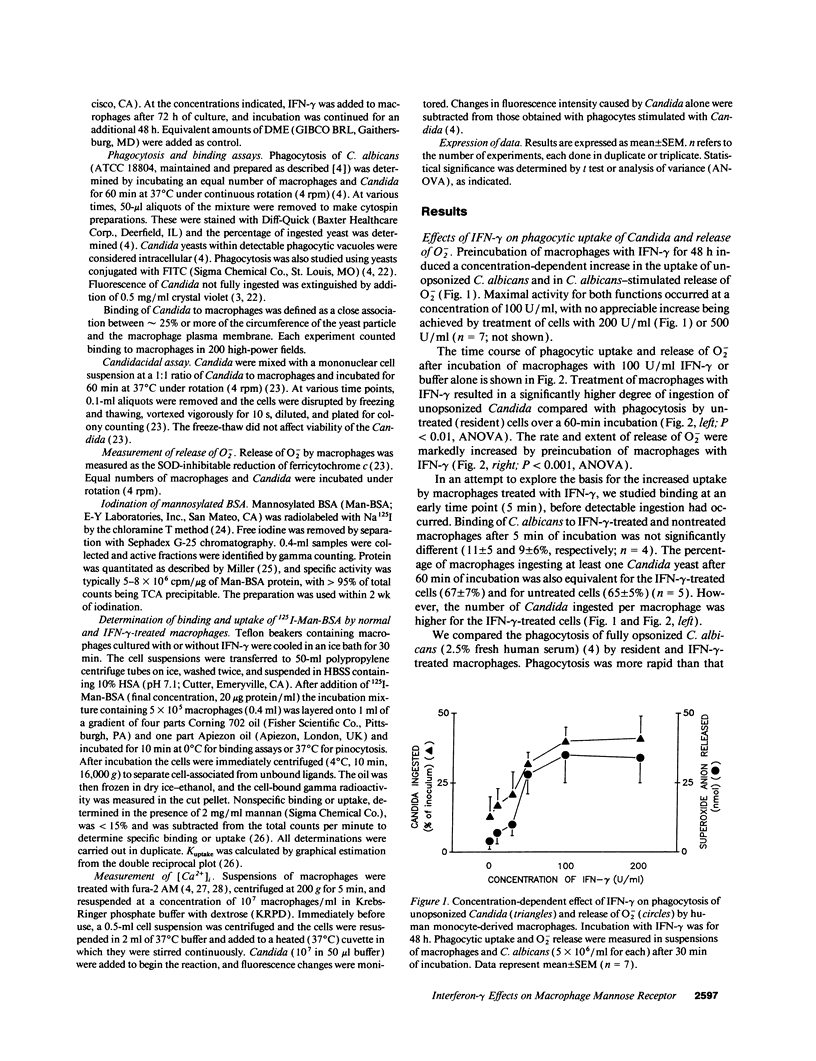
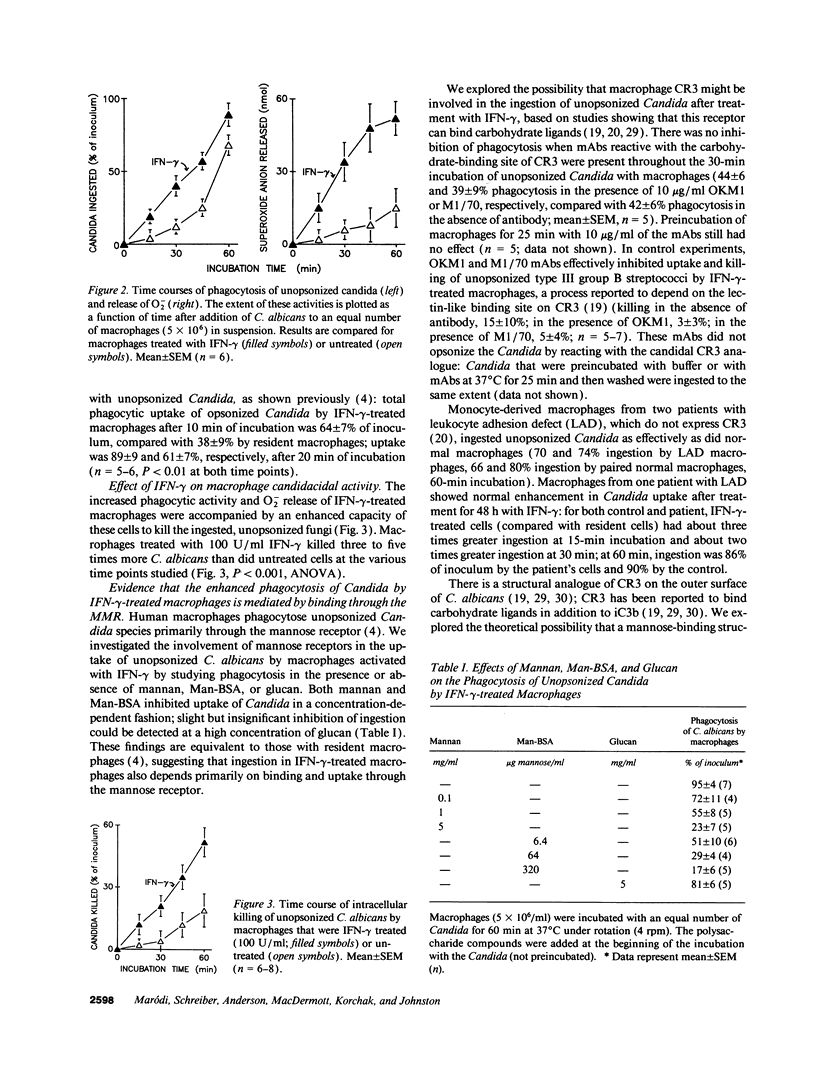
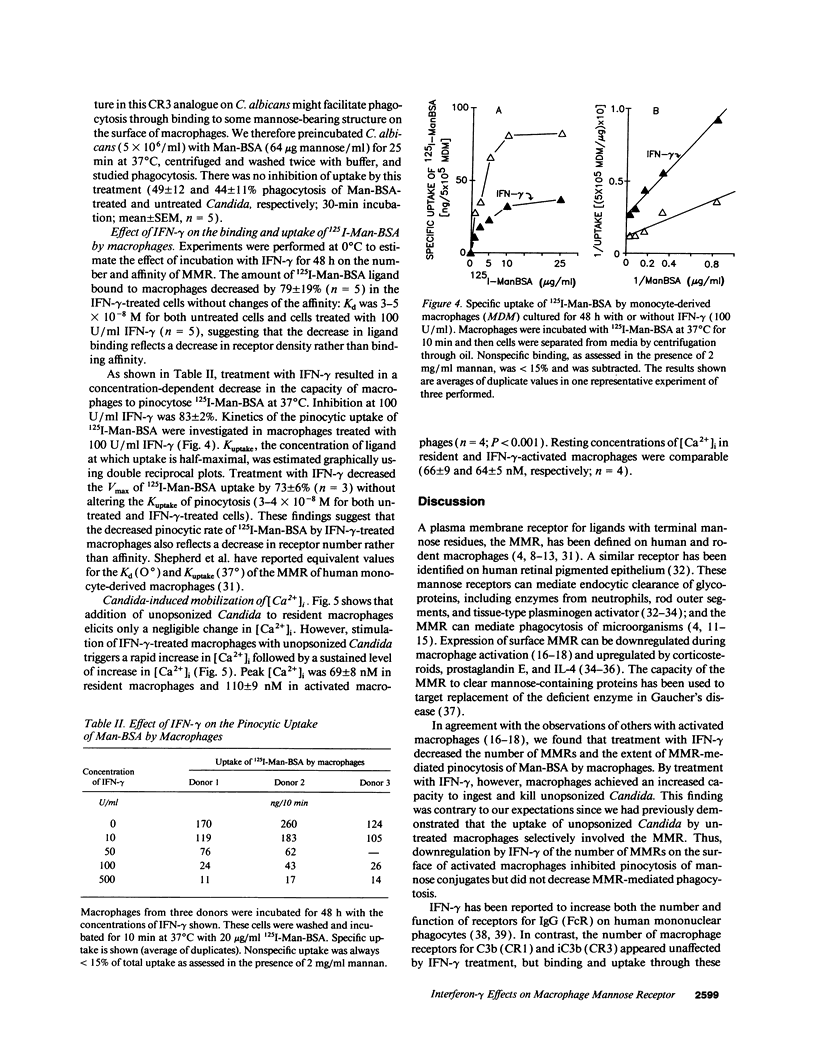
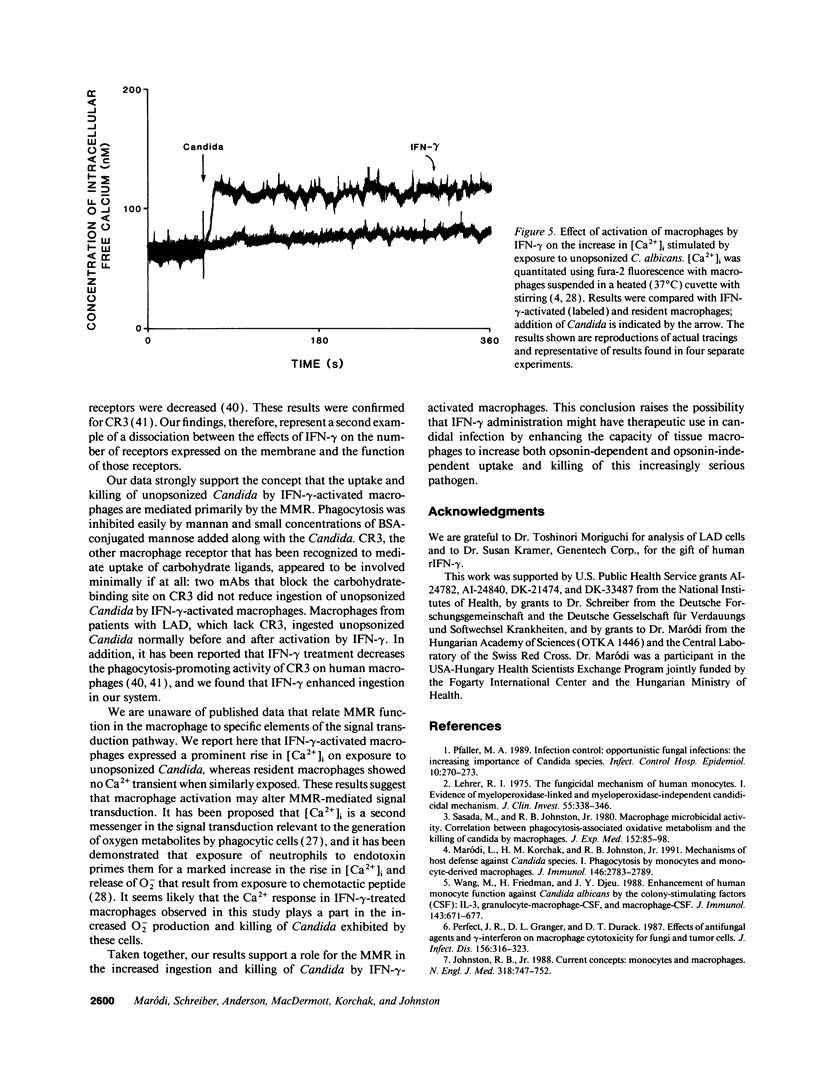
In contrast to its macrophage-activating capacity, IFN-gamma downregulates expression of the macrophage mannose receptor (MMR), which mediates uptake of Candida and other microorganisms. We found that IFN-gamma induced a concentration-dependent increase in the capacity of human monocyte-derived macrophages to ingest and kill both opsonized and unopsonized Candida albicans and to release superoxide anion upon stimulation with Candida. Mannan or mannosylated albumin inhibited this activated uptake of unopsonized Candida, but glucan did not. Addition of mAb to complement receptor (CR) 3 did not inhibit ingestion; macrophages that lacked CR3 (leukocyte adhesion defect) showed normal upregulation of ingestion by IFN-gamma. The increased candidacidal activity of IFN-gamma-activated macrophages was associated with reduced expression of MMR by a mean of 79% and decreased pinocytic uptake of 125I-mannosylated BSA by 73%; K(uptake) of pinocytosis was not changed. Exposure of resident macrophages to unopsonized Candida did not elicit a transient increase in intracellular free Ca2+ ([Ca2+]i); macrophages activated by IFN-gamma expressed a brisk increase in [Ca2+]i on exposure to Candida. These data suggest that macrophage activation by IFN-gamma can enhance resistance to C. albicans infection in spite of downregulation of the MMR, perhaps through enhanced coupling of the MMR to microbicidal functions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Schmalsteig F. C., Finegold M. J., Hughes B. J., Rothlein R., Miller L. J., Kohl S., Tosi M. F., Jacobs R. L., Waldrop T. C. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J Infect Dis. 1985 Oct;152(4):668–689. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- Barton N. W., Brady R. O., Dambrosia J. M., Di Bisceglie A. M., Doppelt S. H., Hill S. C., Mankin H. J., Murray G. J., Parker R. I., Argoff C. E. Replacement therapy for inherited enzyme deficiency--macrophage-targeted glucocerebrosidase for Gaucher's disease. N Engl J Med. 1991 May 23;324(21):1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S., Enkel H. Interaction of Mycobacterium avium complex with human macrophages: roles of membrane receptors and serum proteins. Infect Immun. 1991 May;59(5):1697–1702. doi: 10.1128/iai.59.5.1697-1702.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Gordon S. Down-regulation of mannosyl receptor-mediated endocytosis and antigen F4/80 in bacillus Calmette-Guérin-activated mouse macrophages. Role of T lymphocytes and lymphokines. J Exp Med. 1982 Jun 1;155(6):1623–1637. doi: 10.1084/jem.155.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Sastry K., Bailly P., Warner A. Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J Exp Med. 1990 Dec 1;172(6):1785–1794. doi: 10.1084/jem.172.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Williams D. J., Koziel H., Armstrong M. Y., Warner A., Richards F. F., Rose R. M. Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature. 1991 May 9;351(6322):155–158. doi: 10.1038/351155a0. [DOI] [PubMed] [Google Scholar]
- Forehand J. R., Pabst M. J., Phillips W. A., Johnston R. B., Jr Lipopolysaccharide priming of human neutrophils for an enhanced respiratory burst. Role of intracellular free calcium. J Clin Invest. 1989 Jan;83(1):74–83. doi: 10.1172/JCI113887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson K. S., Vercellotti G. M., Bendel C. M., Hostetter M. K. Molecular mimicry in Candida albicans. Role of an integrin analogue in adhesion of the yeast to human endothelium. J Clin Invest. 1991 Jun;87(6):1896–1902. doi: 10.1172/JCI115214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyre P. M., Morganelli P. M., Miller R. Recombinant immune interferon increases immunoglobulin G Fc receptors on cultured human mononuclear phagocytes. J Clin Invest. 1983 Jul;72(1):393–397. doi: 10.1172/JCI110980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich F., Dierich M. P. Candida albicans and Candida stellatoidea, in contrast to other Candida species, bind iC3b and C3d but not C3b. Infect Immun. 1985 Nov;50(2):598–600. doi: 10.1128/iai.50.2.598-600.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imber M. J., Pizzo S. V., Johnson W. J., Adams D. O. Selective diminution of the binding of mannose by murine macrophages in the late stages of activation. J Biol Chem. 1982 May 10;257(9):5129–5135. [PubMed] [Google Scholar]
- Johnston R. B., Jr Current concepts: immunology. Monocytes and macrophages. N Engl J Med. 1988 Mar 24;318(12):747–752. doi: 10.1056/NEJM198803243181205. [DOI] [PubMed] [Google Scholar]
- Jungi T. W., Lerch P. G., Brcic M. The effect of recombinant interferon-gamma on human monocyte-derived macrophages. Eur J Immunol. 1987 May;17(5):735–738. doi: 10.1002/eji.1830170526. [DOI] [PubMed] [Google Scholar]
- Konish M., Shepherd V., Holt G., Stahl P. Uptake of glycoproteins and glycoconjugates by macrophages. Methods Enzymol. 1983;98:301–304. doi: 10.1016/0076-6879(83)98157-0. [DOI] [PubMed] [Google Scholar]
- Korchak H. M., Vosshall L. B., Zagon G., Ljubich P., Rich A. M., Weissmann G. Activation of the neutrophil by calcium-mobilizing ligands. I. A chemotactic peptide and the lectin concanavalin A stimulate superoxide anion generation but elicit different calcium movements and phosphoinositide remodeling. J Biol Chem. 1988 Aug 15;263(23):11090–11097. [PubMed] [Google Scholar]
- Lehrer R. I. The fungicidal mechanisms of human monocytes. I. Evidence for myeloperoxidase-linked and myeloperoxidase-independent candidacidal mechanisms. J Clin Invest. 1975 Feb;55(2):338–346. doi: 10.1172/JCI107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maródi L., Forehand J. R., Johnston R. B., Jr Mechanisms of host defense against Candida species. II. Biochemical basis for the killing of Candida by mononuclear phagocytes. J Immunol. 1991 Apr 15;146(8):2790–2794. [PubMed] [Google Scholar]
- Maródi L., Korchak H. M., Johnston R. B., Jr Mechanisms of host defense against Candida species. I. Phagocytosis by monocytes and monocyte-derived macrophages. J Immunol. 1991 Apr 15;146(8):2783–2789. [PubMed] [Google Scholar]
- Otter M., Barrett-Bergshoeff M. M., Rijken D. C. Binding of tissue-type plasminogen activator by the mannose receptor. J Biol Chem. 1991 Jul 25;266(21):13931–13935. [PubMed] [Google Scholar]
- Pawlowski N. A., Kaplan G., Abraham E., Cohn Z. A. The selective binding and transmigration of monocytes through the junctional complexes of human endothelium. J Exp Med. 1988 Nov 1;168(5):1865–1882. doi: 10.1084/jem.168.5.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect J. R., Granger D. L., Durack D. T. Effects of antifungal agents and gamma interferon on macrophage cytotoxicity for fungi and tumor cells. J Infect Dis. 1987 Aug;156(2):316–323. doi: 10.1093/infdis/156.2.316. [DOI] [PubMed] [Google Scholar]
- Pfaller M. A. Infection control: opportunistic fungal infections--the increasing importance of Candida species. Infect Control Hosp Epidemiol. 1989 Jun;10(6):270–273. doi: 10.1086/646021. [DOI] [PubMed] [Google Scholar]
- Roecklein J. A., Swartz R. P., Yeager H., Jr Nonopsonic uptake of Mycobacterium avium complex by human monocytes and alveolar macrophages. J Lab Clin Med. 1992 Jun;119(6):772–781. [PubMed] [Google Scholar]
- Sasada M., Johnston R. B., Jr Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med. 1980 Jul 1;152(1):85–98. doi: 10.1084/jem.152.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger L. S., Horwitz M. A. Phagocytosis of Mycobacterium leprae by human monocyte-derived macrophages is mediated by complement receptors CR1 (CD35), CR3 (CD11b/CD18), and CR4 (CD11c/CD18) and IFN-gamma activation inhibits complement receptor function and phagocytosis of this bacterium. J Immunol. 1991 Sep 15;147(6):1983–1994. [PubMed] [Google Scholar]
- Schreiber S., Blum J. S., Chappel J. C., Stenson W. F., Stahl P. D., Teitelbaum S. L., Perkins S. L. Prostaglandin E specifically upregulates the expression of the mannose-receptor on mouse bone marrow-derived macrophages. Cell Regul. 1990 Apr;1(5):403–413. doi: 10.1091/mbc.1.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd V. L., Abdolrasulnia R., Garrett M., Cowan H. B. Down-regulation of mannose receptor activity in macrophages after treatment with lipopolysaccharide and phorbol esters. J Immunol. 1990 Sep 1;145(5):1530–1536. [PubMed] [Google Scholar]
- Shepherd V. L., Campbell E. J., Senior R. M., Stahl P. D. Characterization of the mannose/fucose receptor on human mononuclear phagocytes. J Reticuloendothel Soc. 1982 Dec;32(6):423–431. [PubMed] [Google Scholar]
- Shepherd V. L., Tarnowski B. I., McLaughlin B. J. Isolation and characterization of a mannose receptor from human pigment epithelium. Invest Ophthalmol Vis Sci. 1991 May;32(6):1779–1784. [PubMed] [Google Scholar]
- Smith C. L., Baker C. J., Anderson D. C., Edwards M. S. Role of complement receptors in opsonophagocytosis of group B streptococci by adult and neonatal neutrophils. J Infect Dis. 1990 Aug;162(2):489–495. doi: 10.1093/infdis/162.2.489. [DOI] [PubMed] [Google Scholar]
- Speert D. P., Wright S. D., Silverstein S. C., Mah B. Functional characterization of macrophage receptors for in vitro phagocytosis of unopsonized Pseudomonas aeruginosa. J Clin Invest. 1988 Sep;82(3):872–879. doi: 10.1172/JCI113692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P. D. The macrophage mannose receptor: current status. Am J Respir Cell Mol Biol. 1990 Apr;2(4):317–318. doi: 10.1165/ajrcmb/2.4.317. [DOI] [PubMed] [Google Scholar]
- Stein M., Keshav S., Harris N., Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992 Jul 1;176(1):287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. E., Bezouska K., Drickamer K. Contribution to ligand binding by multiple carbohydrate-recognition domains in the macrophage mannose receptor. J Biol Chem. 1992 Jan 25;267(3):1719–1726. [PubMed] [Google Scholar]
- Wang M., Friedman H., Djeu J. Y. Enhancement of human monocyte function against Candida albicans by the colony-stimulating factors (CSF): IL-3, granulocyte-macrophage-CSF, and macrophage-CSF. J Immunol. 1989 Jul 15;143(2):671–677. [PubMed] [Google Scholar]
- Wilson M. E., Pearson R. D. Evidence that Leishmania donovani utilizes a mannose receptor on human mononuclear phagocytes to establish intracellular parasitism. J Immunol. 1986 Jun 15;136(12):4681–4688. [PubMed] [Google Scholar]
- Wright S. D., Detmers P. A., Jong M. T., Meyer B. C. Interferon-gamma depresses binding of ligand by C3b and C3bi receptors on cultured human monocytes, an effect reversed by fibronectin. J Exp Med. 1986 May 1;163(5):1245–1259. doi: 10.1084/jem.163.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]



