Abstract
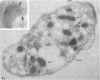
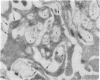
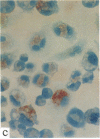
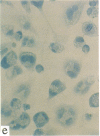
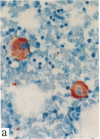
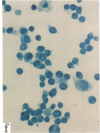
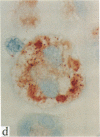
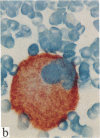
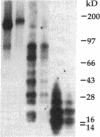
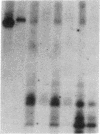
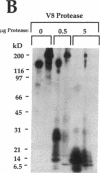
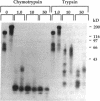
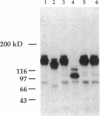
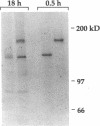
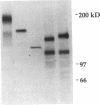
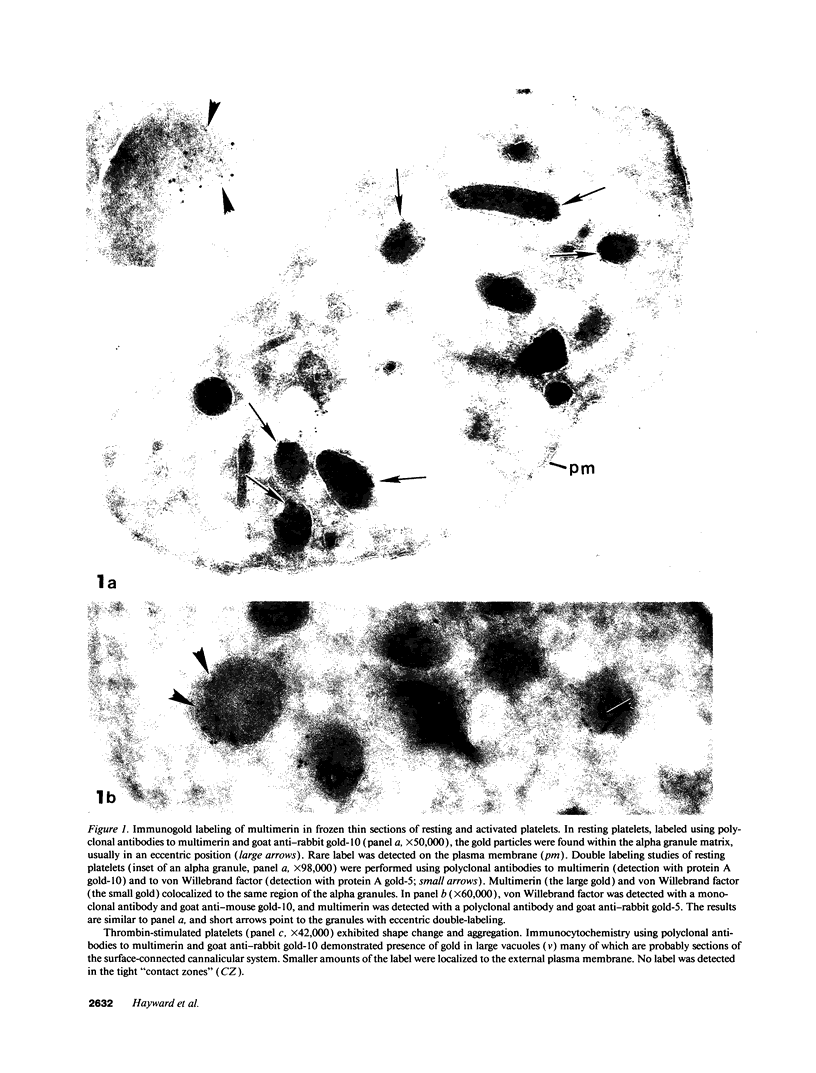
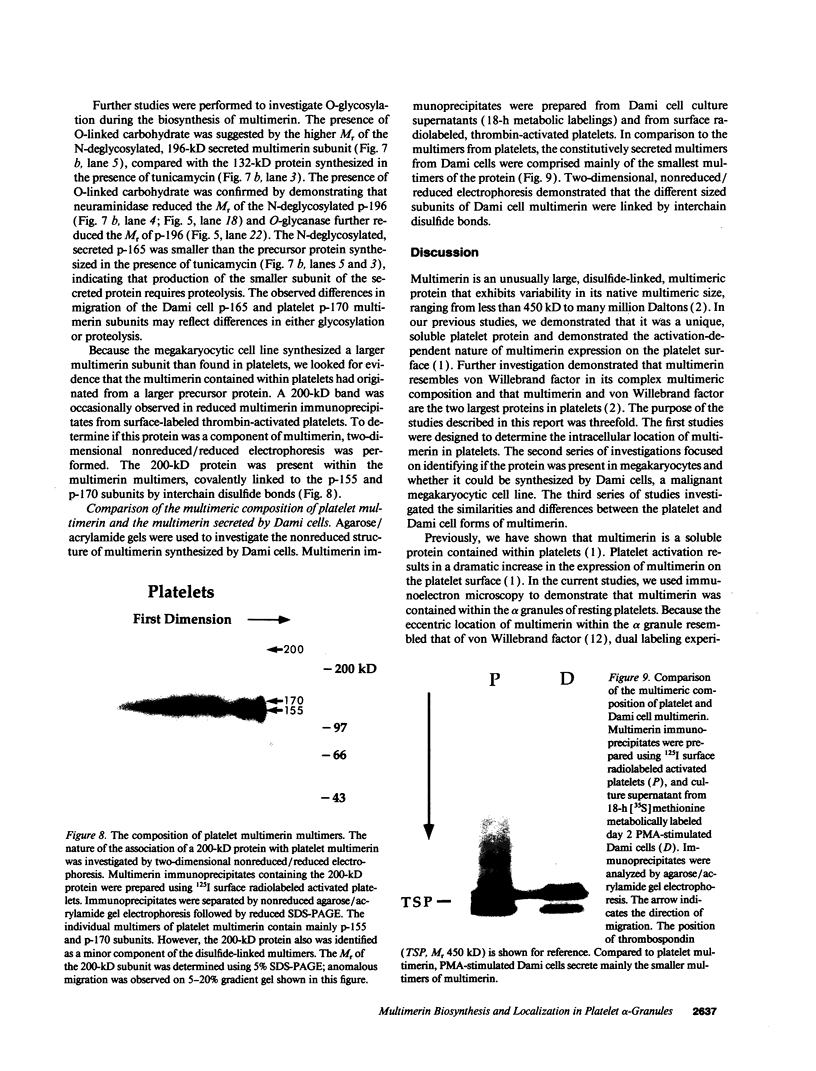
In this report, we describe the intracellular localization of multimerin in platelets and its biosynthesis by Dami cells, a megakaryocytic cell line. Immunoelectron microscopy was used to examine frozen thin sections of resting and activated platelets. Multimerin was localized within the platelet alpha-granule in an eccentric position. Within activated platelets, multimerin was found in the surface-connected open cannalicular system and on the external plasma membrane. Light microscopic immunocytochemistry demonstrated multimerin in normal megakaryocytes and in Dami cells after stimulation with PMA. Confirmation of multimerin biosynthesis by Dami cells was obtained by metabolic labeling studies. Both platelet and Dami cell multimerin demonstrated several subunit sizes on reduced SDS-PAGE. However, peptide mapping confirmed structural homology between the different multimerin subunits. Glycosidase digestion demonstrated that multimerin is heavily glycosylated with mainly complex, N-linked carbohydrate. In contrast to the multimerin isolated from platelets, cultured Dami cells secreted mainly smaller multimers of the protein. Biosynthesis of multimerin by a megakaryocytic cell line supports endogenous biosynthesis by megakaryocytes as the origin of this platelet alpha-granule protein.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton D. F., Miller L. J., Kishimoto T. K., Springer T. A. Leukocyte adhesion receptors are stored in peroxidase-negative granules of human neutrophils. J Exp Med. 1987 Dec 1;166(6):1641–1653. doi: 10.1084/jem.166.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cramer E. M., Meyer D., le Menn R., Breton-Gorius J. Eccentric localization of von Willebrand factor in an internal structure of platelet alpha-granule resembling that of Weibel-Palade bodies. Blood. 1985 Sep;66(3):710–713. [PubMed] [Google Scholar]
- George J. N. Platelet immunoglobulin G: its significance for the evaluation of thrombocytopenia and for understanding the origin of alpha-granule proteins. Blood. 1990 Sep 1;76(5):859–870. [PubMed] [Google Scholar]
- Greenberg S. M., Rosenthal D. S., Greeley T. A., Tantravahi R., Handin R. I. Characterization of a new megakaryocytic cell line: the Dami cell. Blood. 1988 Dec;72(6):1968–1977. [PubMed] [Google Scholar]
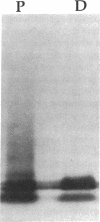
- Hayward C. P., Smith J. W., Horsewood P., Warkentin T. E., Kelton J. G. p-155, a multimeric platelet protein that is expressed on activated platelets. J Biol Chem. 1991 Apr 15;266(11):7114–7120. [PubMed] [Google Scholar]
- Johnston G. I., Kurosky A., McEver R. P. Structural and biosynthetic studies of the granule membrane protein, GMP-140, from human platelets and endothelial cells. J Biol Chem. 1989 Jan 25;264(3):1816–1823. [PubMed] [Google Scholar]
- Kelton J. G., Smith J. W., Horsewood P., Humbert J. R., Hayward C. P., Warkentin T. E. Gova/b alloantigen system on human platelets. Blood. 1990 Jun 1;75(11):2172–2176. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- McEver R. P., Beckstead J. H., Moore K. L., Marshall-Carlson L., Bainton D. F. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989 Jul;84(1):92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Marder V. J. Biosynthesis of von Willebrand protein by human endothelial cells. Identification of a large precursor polypeptide chain. J Biol Chem. 1983 Feb 25;258(4):2065–2067. [PubMed] [Google Scholar]