Abstract
Background
It is well-known that there is a close relationship between metabolic syndrome (MetS) and microalbuminuria. However, some recent studies have found that even normal range albuminuria was associated with MetS and cardiometabolic risk factors. The purpose of this study is to analyze the relationship between MetS and normal range albuminuria and to calculate the cutoff value for albuminuria that correlates with MetS in the representative fraction of Korean population.
Methods
Data were obtained from the 2011–2012 Korea National Health and Nutrition Examination Survey and included 9,650 subjects aged ≥19 years. We measured metabolic parameters: fasting blood glucose, waist circumference, blood pressure, and lipids, and albumin-to-creatinine ratio (ACR). The optimal ACR cutoff points for MetS were examined by the receiver operating characteristic curve. Multivariate logistic regression was used to obtain the prevalence of MetS and its components according to the ACR levels.
Results
The first cutoff value of ACR were 4.8 mg/g for subjects with ≥3 components of MetS. There was a graded association between ACR and prevalence of MetS and its components. If ACR was <4 mg/g, there was no significant increase in the prevalence of MetS or its components. From the ACR level of 4–5 mg/g, the prevalence of MetS significantly increased after adjusting for age, sex, body mass index, smoking, alcohol intake, exercise, and medications for diabetes mellitus and hypertension (odds ratio; 95% confidence intervals = 1.416; 1.041–1.926).
Conclusions
Albuminuria within the normal range (around 5 mg/g) was associated with prevalence of MetS in the Korean population.
Introduction
Microalbuminuria, which is generally defined as a urinary albumin-to-creatinine ratio (ACR) of 30–300 mg/g [1], was associated with an elevated risk for cardiovascular disease and death in general population as well as in patients with diabetes mellitus (DM) and hypertension (HTN) [2–5]. Metabolic syndrome (MetS) is the most useful and widely accepted description of the cluster of metabolic abnormalities related to cardiovascular risk factors [6]. Several studies have reported a close relationship between MetS and microalbuminuria [7–10]. However, some recent studies have found that even normal range albuminuria was associated with MetS and cardiometabolic risk factors [11–15]. Jassen et al. found that low-grade albuminuria, which is far below the microalbuminuric range, was associated with cardiovascular risk factors in a sample of 40,619 Caucasians [13]. Vyssoulis et al. found that low-grade albuminuria was significantly associated with the prevalence of MetS in 6,650 hypertensive patients [11]. Two Asian studies also showed low-grade albuminuria to be positively associated with MetS in middle aged/elderly Chinese and middle-aged Korean men [14, 15]. In addition, other studies have showed that even normal range, very low-grade albuminuria is associated with elevated incidence of DM, HTN, central obesity [16–18], and cardiovascular disease [19–21]. Several studies already examined Korean population, but these studies only included middle-aged men or subjects undergoing a medical check-up. In addition, there was no study that investigated the cutoff value of albuminuria predictive of MetS in the representative fraction of Korean population.
Therefore, we investigated the relationship between normal range albuminuria and MetS and calculated a novel cutoff value for albuminuria predictive of MetS in the Korean whole population.
Materials and Methods
Subjects
We analyzed the data from the 2011–2012 Korea National Health and Nutrition Examination Survey (KNHANES). KNHANES has been performed since 1998 by the Division of Chronic Disease Surveillance at the Korean Center for Disease Control and Prevention and is a nationwide survey assessing national health and nutritional states. The survey consists of 3 parts: a health interview survey, a health examination survey, and a nutrition survey [22]. A total of 12,859 subjects aged ≥19 years were included in the survey. We excluded subjects who had malignancy, liver cirrhosis, chronic hepatitis B or C, thyroid disease, renal disease, or pulmonary or extrapulmonary tuberculosis, and those who were pregnant. We also excluded participants with missing data or who had not fasted for the required 8 hours before blood sample collection. A total of 9,650 subjects were included in the present study. All subjects provided written informed consent, and the study protocol was approved by the institutional review board of the Korean Center for Disease Control and Prevention.
Anthropometric and biochemical measurements
The heights (cm) and weights (kg) of the subjects were measured to the nearest 0.1 cm and 0.1 kg, respectively, with light clothing on and shoes removed. Waist circumference (WC) was measured to the nearest 0.1 cm on a horizontal plane at the midpoint level between the iliac crest and the costal margin at the end of normal expiration. Body mass index (BMI) was calculated by dividing the weight (kg) by the square of height (m2). Blood pressure (BP) was measured thrice using a mercury sphygmomanometer (Baumanometer; W. A. Baum Co., Inc., Copiague, NY, USA). Each subject was seated and rested for at least 5 minutes before BP was measured. The final resting BP value was calculated by taking the average of the second and third measurements. We used this calculated average BP value for statistical analysis. Blood samples were obtained after at least 8 hours of fasting and were analyzed for serum levels of fasting blood glucose (FBG), total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), triglycerides (TG), and low-density lipoprotein-cholesterol (LDL-C). We used the first morning urine samples of subjects. We checked urine and serum creatinine levels and the urine albumin level by kinetic colorimetry and turbidimetric assays by using the Hitachi Automatic Analyzer 7600. Albuminuria was calculated by a urine albumin-to creatinine ratio. We also calculated the estimated glomerular filtration rate (eGFR; mL/min/1.73 m2) using the following equation from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI): eGFR (mL/min/1.73 m2) = 141 × min(serum creatinine/κ, 1)α × max(serum creatinine/κ, 1)−1.209 × 0.993age × 1.018 [if female] × 1.159 [if African American], where κ is 0.7 for women and 0.9 for men, α is -0.411 for men and -0.329 for women, min indicates minimum serum creatinine/κ or 1, and max indicates maximum serum creatinine/κ or 1 [23].
Lifestyle variables
Alcohol consumption, smoking status, and physical activity were measured using the self-report questionnaire. Alcohol use was determined based on the amount of alcohol consumed per day during the 1-month period before the baseline interview; heavy drinkers were defined as those with alcohol consumption of ≥30 g/day. Current smokers were defined as those who were smoking currently and had smoked ≥100 cigarettes over their lifetime. The amount of physical activity performed was assessed using the International Physical Activity Questionnaire short form modified for the Korean population [24]. Subjects were divided into 2 groups: exercise and non-exercise. Regular physical exercise was defined as moderate intensity exercise for more than 5 times per week for more than 30 minutes or vigorous intensity exercise for more than 3 times per week for more than 20 minutes.
Definition of MetS
MetS was defined according to the American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement (AHA/NHLBI) criteria for Asians [25]. Participants with 3 or more of the following 5 criteria were defined as having MetS: high WC (≥90 cm for men, ≥80 cm for women), high TG (≥ 150 mg/dL or use of anti-dyslipidemic medication), low HDL-C (men < 40 mg/dL, and women < 50 mg/dL or use of anti-dyslipidemic medication), high BP (≥130/85 mmHg or use of antihypertensive drug treatment), and high BG (≥100 mg/dL or on use of drug treatment for elevated glucose levels).
Statistical analyses
Data are presented as either means ± standard errors (SEs) for continuous variables or as percentage (SE) for categorical variables. The chi-squared test was used for categorical variables and t-test was used for continuous variables.We used logarithmic transformation for the variables with skewed distributions such as TG and ACR to achieve a normal distribution. In order to analyze the baseline characteristics of study participants, we divided participants according to the presence/absence of MetS and compared the mean values of cardiometabolic risk factors using a Chi-squared test or t-test. To determine the optimal ACR cutoff points for metabolic syndrome and its components, we computed the receiver operating characteristic (ROC) curve. The optimal cutoff values were obtained from the maximal Youden’s index, calculated as (sensitivity + specificity—1) and the optimal combination of sensitivity and specificity. Multivariate logistic regression analyses were applied to examine the odds ratios (ORs) and 95% confidence intervals (CI) of MetS prevalence according to the ACR level. All statistical tests were 2-tailed and a p-value of <0.05 was considered to be statistically significant. Statistical analyses were performed using SAS software package version (9.2) for Windows (SAS Institute, Cary, NC, USA).
Results
Characteristics of participants
Table 1 shows the baseline characteristics of participants with and without MetS. Age, WC, SBP, DBP, FBG, and TG were higher in the MetS group than that in the non-MetS group (all p values < 0.001). ACR was higher in the MetS group than those in the non-MetS group (ACR: 8.1 ± 0.5 vs. 4.1 ± 0.1 mg/g, respectively; all p values< 0.001).
Table 1. General characteristics of subjects with and without MetS.
| MetS | |||
|---|---|---|---|
| Variable | No | Yes | p value* |
| Unweighted (n) | 6622 | 3028 | |
| Age (years) | 42.1 ± 0.3 | 54.4 ± 0.4 | <0.001 |
| Sex (male, %) | 53.1 (0.7) | 50.8 (1.1) | 0.106 |
| BMI (kg/m2) | 23.0 ± 0.1 | 26.2 ± 0.1 | <0.001 |
| WC (cm) | 78.6 ± 0.2 | 89.4 ± 0.2 | <0.001 |
| SBP (mmHg) | 114.2 ± 0.3 | 128.3 ± 0.4 | <0.001 |
| DBP (mmHg) | 74.7 ± 0.2 | 81.2 ± 0.3 | <0.001 |
| FBG (mg/dL) | 92.0 ± 0.2 | 110.3 ± 0.7 | <0.001 |
| TC (mg/dL) | 186.3 ± 0.6 | 198 ± 1.0 | <0.001 |
| TG** (mg/dL) | 93.0 ± 1.5 | 180.5 ± 4.9 | <0.001 |
| HDL-C (mg/dL) | 54.8 ± 0.2 | 45.1 ± 0.2 | <0.001 |
| LDL-C (mg/dL) | 110.4 ± 0.5 | 113.7 ± 0.9 | 0.002 |
| eGFR (mL/min/1.73 m2) | 96.7 ± 0.3 | 88.6 ± 0.4 | <0.001 |
| ACR** (mg/g) | 4.1 ± 0.1 | 8.1 ± 0.5 | <0.001 |
| Heavy drinker (yes, %) | 9.8 (0.5) | 13.0 (0.8) | <0.001 |
| Current smoker (yes, %) | 24.6 (0.8) | 25.4 (1.1) | 0.317 |
| Regular exercise (yes, %) | 20.0 (0.7) | 16.0 (1) | 0.001 |
| Rural area (yes, %) | 18.0 (2.0) | 24.8 (2.6) | <0.001 |
| Education level (yes, %) | |||
| high school or more ≥10 | 79.0 (0.8) | 52.3 (1.4) | <0.001 |
| Monthly income (yes, %) | |||
| lowest quartile | 11.8 (0.6) | 21.9 (1.1) | <0.001 |
| DM medication (yes, %) | 3.7 (0.3) | 27.2 (1.1) | <0.001 |
| HTN medication (yes, %) | 14.0 (0.6) | 60.5 (1.3) | <0.001 |
* p values were obtained using the Chi-squared test and Student’s t-test.
** Log transformation used to normalize data
Data are presented as mean ± SE or percentages (SE).
MetS, metabolic syndrome; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; eGFR, estimated glomerular filtration rate; ACR, albumin-to-creatinine ratio
ACR cutoff values according to the MetS components
ACR cutoff values analyzed by the ROC curve according to the number of MetS components and the individual MetS components (Table 2). The ACR cutoff value was 5.3 mg/g for subjects with ≥1 MetS component; 5.3 mg/g, for subjects with ≥2 components; 4.8 mg/g, for subjects with ≥3 components; 4.9 mg/g, for subjects with ≥4 components; and 5.8 mg/g, for subjects with all 5 MetS components. The ACR cutoff points for high BP, high BG, low HDL-C, high TG, and high WC were 5.3, 5.3, 5.2, 5.2, and 4.8 mg/g, respectively.
Table 2. The first ACR cutoff according to the number of and individual components of MetS.
| Variables | Cutoff (mg/g) | Sensitivity (%) | Specificity (%) | AUC (95% CI) |
|---|---|---|---|---|
| Number of MetS components | ||||
| ≥1 | 5.3 | 40.2 | 79.8 | 0.617 (0.608–0.627) |
| ≥2 | 5.3 | 46.1 | 76.7 | 0.641 (0.632–0.651) |
| ≥3 | 4.8 | 54.1 | 70.2 | 0.653 (0.644–0.663) |
| ≥4 | 4.9 | 59.4 | 66.9 | 0.672 (0.663–0.682) |
| 5 | 5.8 | 60.3 | 69.2 | 0.680 (0.671–0.689) |
| MetS components | ||||
| High WC | 4.8 | 47.1 | 68.2 | 0.591 (0.581–0.600) |
| High BP | 5.3 | 49.8 | 75.9 | 0.659 (0.650–0.669) |
| High BG | 5.3 | 49.5 | 71.4 | 0.633 (0.624–0.643) |
| Low HDL-C | 5.2 | 44.0 | 69.0 | 0.579 (0.569–0.589) |
| High TG | 5.2 | 42.9 | 68.6 | 0.573 (0.563–0.583) |
ACR, albumin-to-creatinine ratio; MetS, metabolic syndrome; AUC, area under the ROC curve; CI, confidential interval; WC, waist circumference; BP, blood pressure; BG, blood glucose; HDL-C, high-density lipoprotein-cholesterol; TG, triglyceride
The mean ACR values and the percentage of subjects with ACR values over 5 mg/g
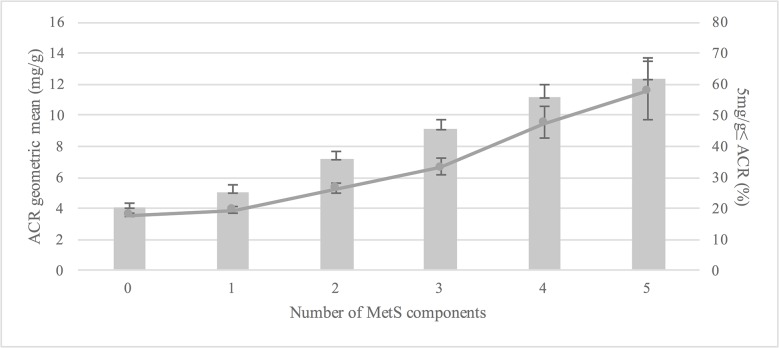
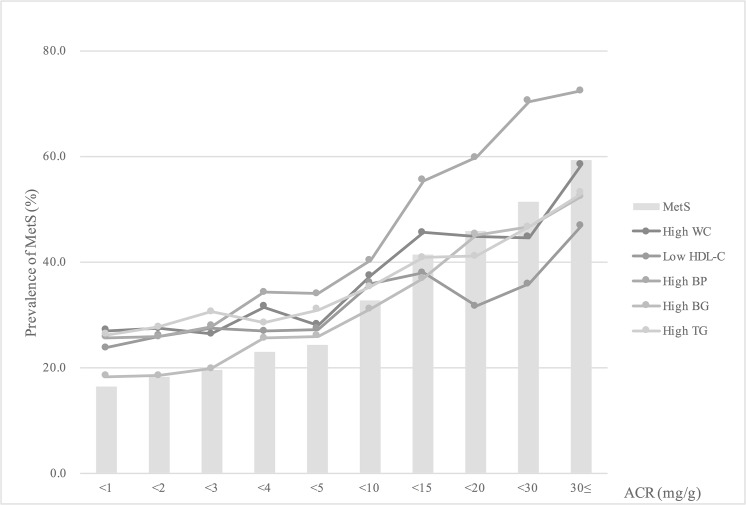
Geometric mean values of ACR increased according to the number of MetS components (presented as a line). Percentages of subjects whose ACR values were over 5 mg/g (presented as a bar graph) also increased according to the number of MetS components (p for trend < 0.001) (Fig 1). Fig 2 shows a graded association between ACR increase and the prevalence of MetS and its components. ACR <5mg/g did not show a significant increase in the prevalence of MetS or its components, but ACR levels ≥5 mg/g showed a remarkable increase.
Fig 1. The geometric mean ACR values and the percentage of subjects with ACR values over 5 mg/g with an increase in the number of MetS components.
All p for trends are <0.001. The geometric mean values of ACR were presented as a line, and the percentage of subjects with ACR values over 5 mg/g was presented as a bar graph. ACR, albumin-to-creatinine ratio; MetS, metabolic syndrome.
Fig 2. Graded association between ACR increase and the prevalence of MetS and its components.
MetS, metabolic syndrome; ACR, albumin-to-creatinine ratio; WC, waist circumference; HDL-C, high-density lipoprotein-cholesterol; BP, blood pressure; BG, blood glucose; TG, triglyceride.
MetS prevalence according to the different ACR level
The adjusted ORs and 95% CIs for MetS prevalence were analyzed according to the different ACR level (Table 3). In model 1, which was adjusted for age and sex, the prevalence of MetS increased significantly even among people with very low ACR levels (≥1 mg/g). However, in model 4, which was adjusted for age, sex, BMI, smoking, alcohol consumption, exercise, and DM and HTN medication use, the prevalence of MetS significantly increased from the ACR levels of 4–5 mg/g (OR; 95% CI = 1.416; 1.041–1.926).
Table 3. Adjusted odds ratios of MetS prevalence according to the ACR level.
| ORs (95% CI) for MetS prevalence | ||||
|---|---|---|---|---|
| ACR (mg/g) | Model 1 | Model 2 | Model 3 | Model 4 |
| <1.0 | 1 | 1 | 1 | 1 |
| 1.0–1.9 | 1.303 (1.035–1.641) | 1.146 (0.882–1.489) | 1.129 (0.863–1.478) | 1.066 (0.807–1.407) |
| 2.0–2.9 | 1.415 (1.125–1.779) | 1.419 (1.114–1.808) | 1.382 (1.078–1.772) | 1.271 (0.981–1.647) |
| 3.0–3.9 | 1.557 (1.231–1.969) | 1.410 (1.078–1.845) | 1.388 (1.060–1.816) | 1.318 (0.999–1.740) |
| 4.0–4.9 | 1.707 (1.313–2.219) | 1.610 (1.202–2.157) | 1.583 (1.182–2.119) | 1.416 (1.041–1.926) |
| 5.0–9.9 | 2.140 (1.773–2.584) | 1.935 (1.568–2.387) | 1.893 (1.529–2.344) | 1.722 (1.378–2.152) |
| 10.0–14.9 | 2.831 (2.169–3.695) | 2.423 (1.787–3.287) | 2.408 (1.766–3.283) | 2.146 (1.531–3.009) |
| 15.0–19.9 | 3.386 (2.388–4.801) | 2.665 (1.732–4.099) | 2.359 (1.511–3.681) | 1.930 (1.202–3.097) |
| 20.0–29.9 | 3.850 (2.676–5.540) | 3.464 (2.420–4.959) | 3.225 (2.245–4.633) | 2.635 (1.790–3.878) |
| ≥30.0 | 4.742 (3.719–6.046) | 3.370 (2.563–4.432) | 3.349 (2.540–4.416) | 2.428 (1.813–3.251) |
Odds ratios and 95% confidence intervals were obtained by multivariable logistic regression analyses.
Model 1 was adjusted for age and sex.
Model 2 was adjusted for covariates of model 1 plus body mass index.
Model 3 was adjusted for covariates of model 2 plus smoking, alcohol intake, and regular exercise.
Model 4 was adjusted for covariates of model 3 plus use of medication for HTN and DM, and eGFR.
MetS, metabolic syndrome; ACR, albumin-to-creatinine ratio; OR, odd ratio; CI, confidential interval; eGFR, estimated glomerular filtration rate
Comparison of the prevalence of MetS using different combination of MetS components
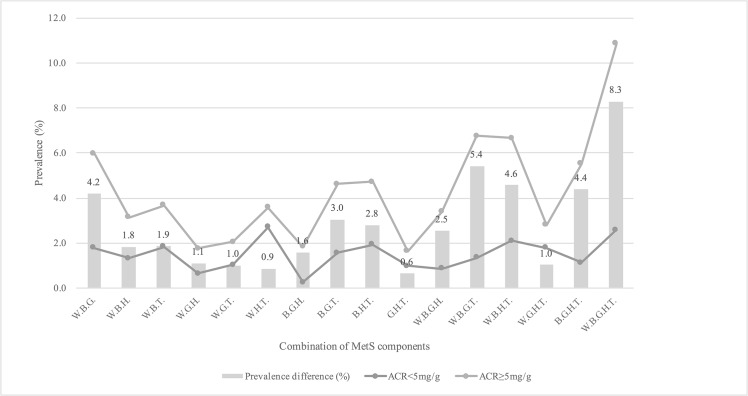
We compared the prevalence of MetS using different combination of MetS components using an ACR cutoff value of 5 mg/g (Fig 3). Except for 2 combinations of high WC + low HDL-C + high TG and high BG + low HDL-C + high TG, a cutoff point of 5 mg/g associated with all other combinations (all p < 0.05). In subjects with ACR ≥5 mg/g, the most frequently reported pattern was a combination of high WC + high BP + high BG + low HDL-C + high TG. In subjects with ACR <5 mg/g the most frequently reported pattern was a combination of high WC + low HDL-C + high TG. Among combinations of 3 MetS components, the ACR level was most predictive of high WC + high BP + high BG and least predictive of high WC + high BG + high TG. Among combinations of 4 MetS components, the ACR level was most predictive of high WC + high BP + high BG + high TG and least predictive of high WC + high BG + low HDL-C + high TG (5.4% and 1.0%, respectively). The maximum difference predicted by the cutoff was in the combination of all MetS components (8.3%).
Fig 3. Comparison of the prevalence of MetS using different combination of MetS components with an ACR cutoff value of 5 mg/g.
W, high WC; B, high BP; G, high BG; H, low HDL-C; T, high TG; MetS, metabolic syndrome; ACR, albumin-to-creatinine ratio.
Discussion
In this study, elevated ACR within the normal range was associated with a higher prevalence of MetS and its components. We identified a cutoff value for albuminuria of 4.8 mg/g, which significantly associated with the prevalence of MetS and its components in a representative sample of the South Korean population.
Many studies in different countries, including Korea, have shown an association between microalbuminuria and MetS [7–10]. Those studies use the standard definition of microalbuminuria. However, several recent studies suggested that individuals with high-normal albuminuria also have an increased risk of cardiometabolic risk factors including MetS [11–15]. Very low levels of microalbuminuria are also associated with coronary heart disease and cardiovascular mortality. The Third Copenhagen City Heart Study showed that microalbuminuria, defined as urinary albumin excretion >4.8 μg/min, was a strong and independent predictor of coronary heart disease and death in hypertensive patients and in participants with no history of coronary heart disease [19, 20]. Moreover, low levels of microalbuminuria (>5 μg/min) were associated with increased risk of MetS [4]. The Framingham Heart Study also reported that low-grade albuminuria (ACR ≥3.9 mg/g in men, ≥7.5 mg/g in women) was associated with a significant increase in the risk of the first cardiovascular event and mortality in non-hypertensive and non-diabetic subjects [21]. Ruggenenti et al. also found that even very low-grade albuminuria (ACR of 1–2 μg/min) was significantly associated with increased cardiovascular risk in type 2 DM patients [26]. In South Korea, Oh et al. reported that high-normal albuminuria (>4.87 μg/mg) was associated with significantly increased incidence of MetS in 4,338 middle-aged men participated in the medical check-up programs by themselves [14]. These findings show that the previous definition of microalbuminuria does not adequately reflect cardiometabolic abnormalities such as MetS
Some mechanisms may explain the association between normal range albuminuria and MetS and its components. One mechanism is an elevated vascular resistance. Vasodilatation in response to nitric oxide has been notably decreased at slightly elevated albuminuria in healthy subjects [27], and early carotid atherosclerotic lesions was independently associated with normal range albuminuria in type 2 DM patients [12]. Increased vascular renin-angiotensin system activity is also observed in the subjects with normal range albuminuria [28]. Another mechanism is based on the insulin resistance which also plays a role in the development of MetS. Insulin resistance could be involved in the development of microalbuminuria by increasing glomerular hydrostatic pressure, renal vascular permeability, renal sodium reabsorption, and aggregating glomerular hyperfiltration [29]. Endothelial dysfunction is also an important factor associated with hypertension and microalbuminuria [30].
There are some limitations to this study. First, this study is cross-sectional, making it difficult to explain causal relationships or describe clear mechanisms relating low-normal albuminuria to MetS and its components. Second, we used only one morning urine sample for evaluation of albuminuria. 24-h urine collection is generally recommended to assess albuminuria. However, a single morning urine sample is known to be correlated with 24-h urine albumin excretion rates [31].
Despite these limitations, this study has several strengths. First, it is a large epidemiologic study found the association between normal range albuminuria and MetS, based on the representative fraction of Korean population. Second, to the best of our knowledge, this is the first study to suggest a cutoff value of ACR associated with the prevalence of MetS and its components in the Korean population.
In conclusion, the prevalence of MetS and its components are increased in normal range albuminuria around 5 mg/g. Clinicians should carefully evaluate the risk of MetS in the patients with normal range albuminuria. Further prospective studies are needed to demonstrate a causal relationship between MetS and normal range albuminuria.
Acknowledgments
The authors thank the Korean Center for Disease Control and Prevention, which performed the KNHANES and the subjects who participated in the KNHANES.
Data Availability
Data are available at https://knhanes.cdc.go.kr/knhanes/eng/index.do.
Funding Statement
The authors have no support or funding to report.
References
- 1. Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33(5):1004–10. Epub 1999/04/23. doi: S0272638699001870 [pii]. . [DOI] [PubMed] [Google Scholar]
- 2. Gerstein HC, Mann JF, Pogue J, Dinneen SF, Halle JP, Hoogwerf B, et al. Prevalence and determinants of microalbuminuria in high-risk diabetic and nondiabetic patients in the Heart Outcomes Prevention Evaluation Study. The HOPE Study Investigators. Diabetes Care. 2000;23 Suppl 2:B35–9. Epub 2000/06/22. . [PubMed] [Google Scholar]
- 3. Wachtell K, Ibsen H, Olsen MH, Borch-Johnsen K, Lindholm LH, Mogensen CE, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann Intern Med. 2003;139(11):901–6. Epub 2003/12/04. doi: 139/11/901 [pii]. . [DOI] [PubMed] [Google Scholar]
- 4. Klausen KP, Parving HH, Scharling H, Jensen JS. The association between metabolic syndrome, microalbuminuria and impaired renal function in the general population: impact on cardiovascular disease and mortality. J Intern Med. 2007;262(4):470–8. Epub 2007/09/19. doi: JIM1839 [pii] 10.1111/j.1365-2796.2007.01839.x . [DOI] [PubMed] [Google Scholar]
- 5. Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106(14):1777–82. . [DOI] [PubMed] [Google Scholar]
- 6. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–80. 10.1111/j.1464-5491.2006.01858.x . [DOI] [PubMed] [Google Scholar]
- 7. Choi HS, Ryu SH, Lee KB. The relationship of microalbuminuria with metabolic syndrome. Nephron Clin Pract. 2006;104(2):c85–93. 10.1159/000093995 . [DOI] [PubMed] [Google Scholar]
- 8. Palaniappan L, Carnethon M, Fortmann SP. Association between microalbuminuria and the metabolic syndrome: NHANES III. Am J Hypertens. 2003;16(11 Pt 1):952–8. . [DOI] [PubMed] [Google Scholar]
- 9. Sheng CS, Hu BC, Fan WX, Zou J, Li Y, Wang JG. Microalbuminuria in relation to the metabolic syndrome and its components in a Chinese population. Diabetol Metab Syndr. 2011;3(1):6 10.1186/1758-5996-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Konta T, Hao Z, Abiko H, Ishikawa M, Takahashi T, Ikeda A, et al. Prevalence and risk factor analysis of microalbuminuria in Japanese general population: the Takahata study. Kidney Int. 2006;70(4):751–6. 10.1038/sj.ki.5001504 . [DOI] [PubMed] [Google Scholar]
- 11. Vyssoulis G, Karpanou E, Spanos P, Kyvelou SM, Adamopoulos D, Stefanadis C. Urine albumin excretion, within normal range, reflects increasing prevalence of metabolic syndrome in patients with essential hypertension. J Clin Hypertens (Greenwich). 12(8):597–602. Epub 2010/08/11. doi: JCH306 [pii] 10.1111/j.1751-7176.2010.00306.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li MF, Tu YF, Li LX, Lu JX, Dong XH, Yu LB, et al. Low-grade albuminuria is associated with early but not late carotid atherosclerotic lesions in community-based patients with type 2 diabetes. Cardiovascular Diabetology. 2013;12(1):110. doi: Artn 110 10.1186/1475-2840-12-110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Janssen WM, Hillege H, Pinto-Sietsma SJ, Bak AA, De Zeeuw D, de Jong PE, et al. Low levels of urinary albumin excretion are associated with cardiovascular risk factors in the general population. Clinical chemistry and laboratory medicine: CCLM / FESCC. 2000;38(11):1107–10. 10.1515/CCLM.2000.165 . [DOI] [PubMed] [Google Scholar]
- 14. Oh CM, Park SK, Kim HS, Kim YH, Kim O, Ryoo JH. High-normal albuminuria predicts metabolic syndrome in middle-aged Korean men: A prospective cohort study. Maturitas. 77(2):149–54. Epub 2013/11/30. doi: S0378-5122(13)00324-1 [pii] 10.1016/j.maturitas.2013.10.013 . [DOI] [PubMed] [Google Scholar]
- 15. Zhang J, Chen Y, Xu Y, Li M, Wang T, Xu B, et al. Low-Grade Albuminuria Is Associated with Metabolic Syndrome and Its Components in Middle-Aged and Elderly Chinese Population. PLoS One. 2013;8(6):e65597 10.1371/journal.pone.0065597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ritz E, Viberti GC, Ruilope LM, Rabelink AJ, Izzo JL, Katayama S, et al. Determinants of urinary albumin excretion within the normal range in patients with type 2 diabetes: the Randomised Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) study. Diabetologia. 2010;53(1):49–57. 10.1007/s00125-009-1577-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park SK, Moon SY, Oh CM, Ryoo JH, Park MS. High Normal Urine Albumin-to-Creatinine Ratio Predicts Development of Hypertension in Korean Men. Circ J. 78(3):656–61. Epub 2013/12/18. doi: DN/JST.JSTAGE/circj/CJ-13-0745 [pii]. . [DOI] [PubMed] [Google Scholar]
- 18. Cubeddu LX, Hoffmann IS, Aponte LM, Nunez-Bogesits R, Medina-Suniaga H, Roa M, et al. Role of salt sensitivity, blood pressure, and hyperinsulinemia in determining high upper normal levels of urinary albumin excretion in a healthy adult population. American Journal of Hypertension. 2003;16(5):343–9. 10.1016/S0895-7061(03)00057-8 . [DOI] [PubMed] [Google Scholar]
- 19. Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, Jensen G, Clausen P, Scharling H, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110(1):32–5. Epub 2004/06/24. 10.1161/01.CIR.0000133312.96477.4801.CIR.0000133312.96477.48[pii]. . [DOI] [PubMed] [Google Scholar]
- 20. Klausen KP, Scharling H, Jensen G, Jensen JS. New definition of microalbuminuria in hypertensive subjects: association with incident coronary heart disease and death. Hypertension. 2005;46(1):33–7. Epub 2005/06/02. doi: 01.HYP.0000169153.78459.50 [pii] 10.1161/01.HYP.0000169153.78459.50 . [DOI] [PubMed] [Google Scholar]
- 21. Arnlov J, Evans JC, Meigs JB, Wang TJ, Fox CS, Levy D, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112(7):969–75. Epub 2005/08/10. doi: CIRCULATIONAHA.105.538132 [pii] 10.1161/CIRCULATIONAHA.105.538132 . [DOI] [PubMed] [Google Scholar]
- 22. Park HA. The Korea national health and nutrition examination survey as a primary data source. Korean journal of family medicine. 2013;34(2):79 10.4082/kjfm.2013.34.2.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67(6):2089–100. 10.1111/j.1523-1755.2005.00365.x . [DOI] [PubMed] [Google Scholar]
- 24. Chun MY. Validity and reliability of korean version of international physical activity questionnaire short form in the elderly. Korean J Fam Med. 2012;33(3):144–51. 10.4082/kjfm.2012.33.3.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–52. 10.1161/CIRCULATIONAHA.105.169404 . [DOI] [PubMed] [Google Scholar]
- 26. Ruggenenti P, Porrini E, Motterlini N, Perna A, Ilieva AP, Iliev IP, et al. Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with type 2 diabetes. J Am Soc Nephrol. 23(10):1717–24. Epub 2012/09/01. doi: ASN.2012030252 [pii] 10.1681/ASN.2012030252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clausen P, Jensen JS, Jensen G, Borch-Johnsen K, Feldt-Rasmussen B. Elevated urinary albumin excretion is associated with impaired arterial dilatory capacity in clinically healthy subjects. Circulation. 2001;103(14):1869–74. . [DOI] [PubMed] [Google Scholar]
- 28. Nicholl DD, Hemmelgarn BR, Turin TC, MacRae JM, Muruve DA, Sola DY, et al. Increased urinary protein excretion in the "normal" range is associated with increased renin-angiotensin system activity. Am J Physiol Renal Physiol. 2012;302(5):F526–32. 10.1152/ajprenal.00458.2011 . [DOI] [PubMed] [Google Scholar]
- 29. Tucker BJ, Anderson CM, Thies RS, Collins RC, Blantz RC. Glomerular hemodynamic alterations during acute hyperinsulinemia in normal and diabetic rats. Kidney Int. 1992;42(5):1160–8. . [DOI] [PubMed] [Google Scholar]
- 30. Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32(4):219–26. Epub 1989/04/01. . [DOI] [PubMed] [Google Scholar]
- 31. Hutchison AS, O'Reilly DS, MacCuish AC. Albumin excretion rate, albumin concentration, and albumin/creatinine ratio compared for screening diabetics for slight albuminuria. Clinical chemistry. 1988;34(10):2019–21. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available at https://knhanes.cdc.go.kr/knhanes/eng/index.do.