Abstract
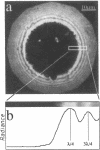
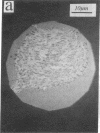
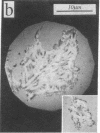
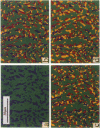
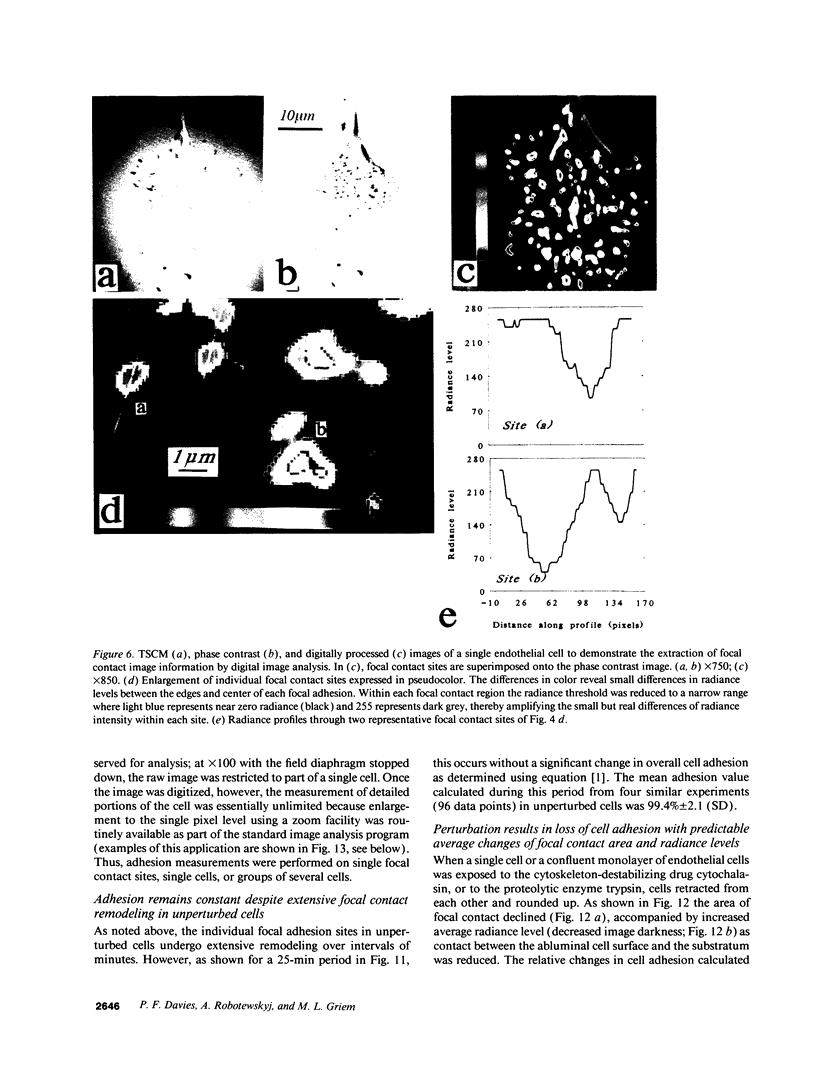
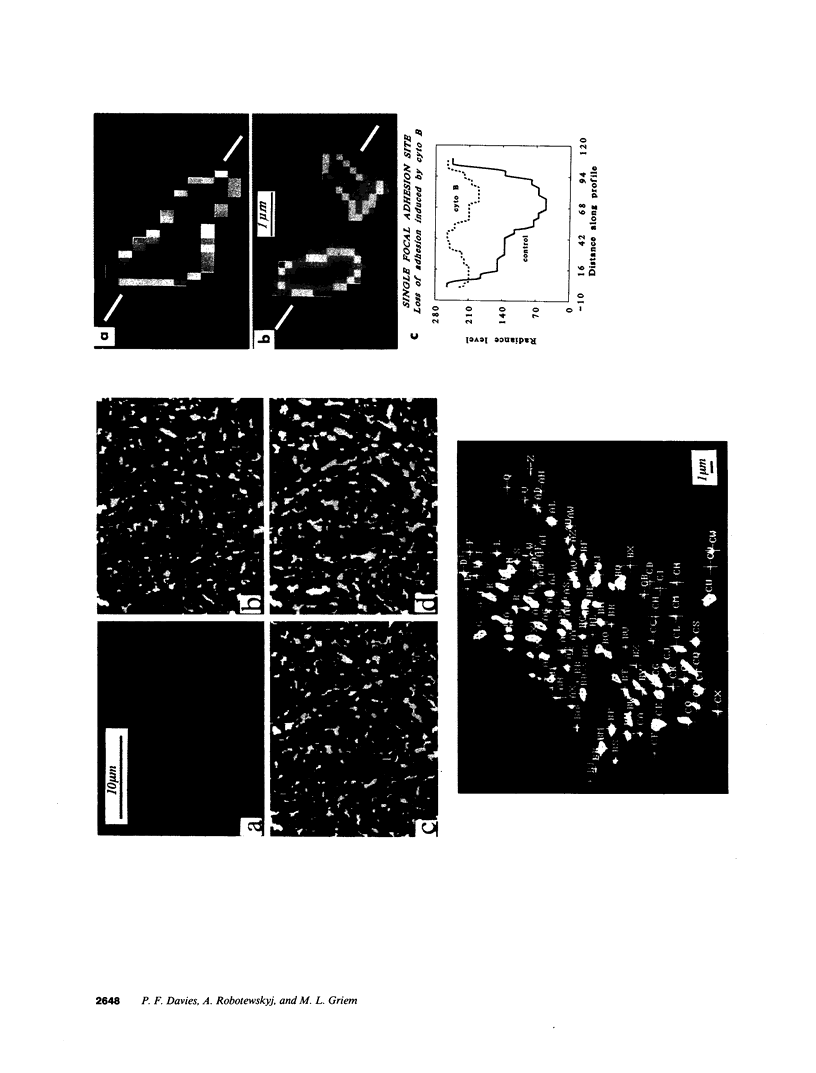
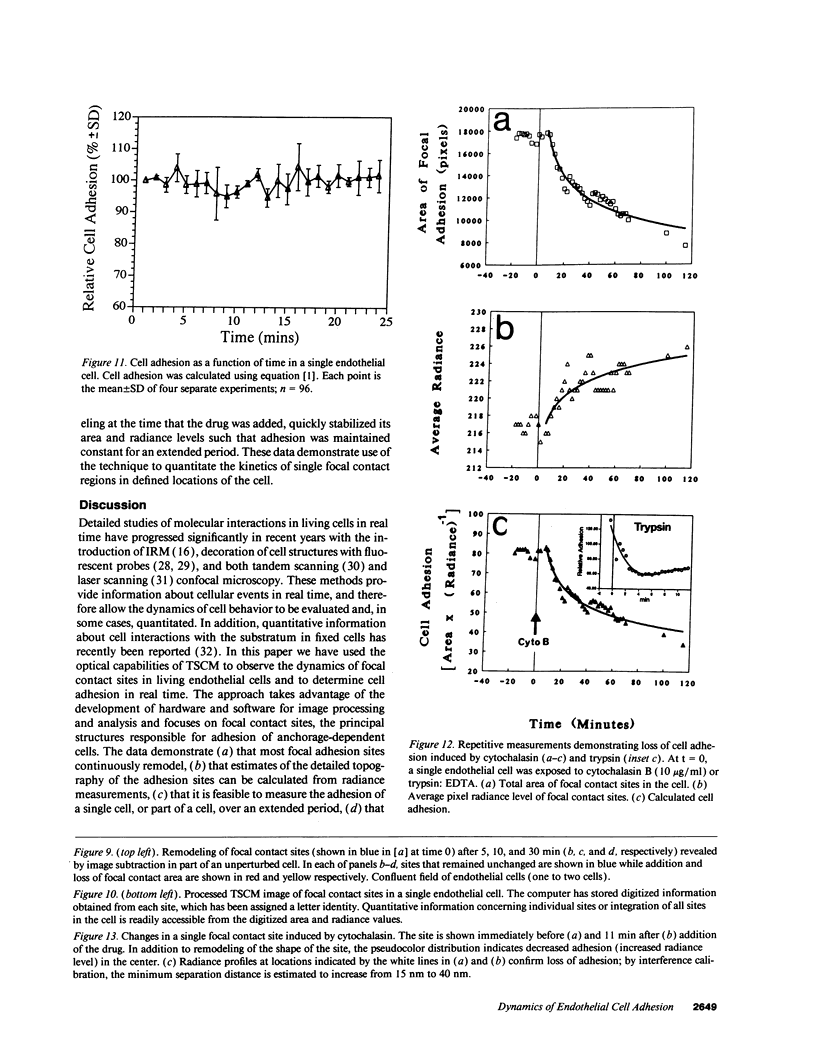
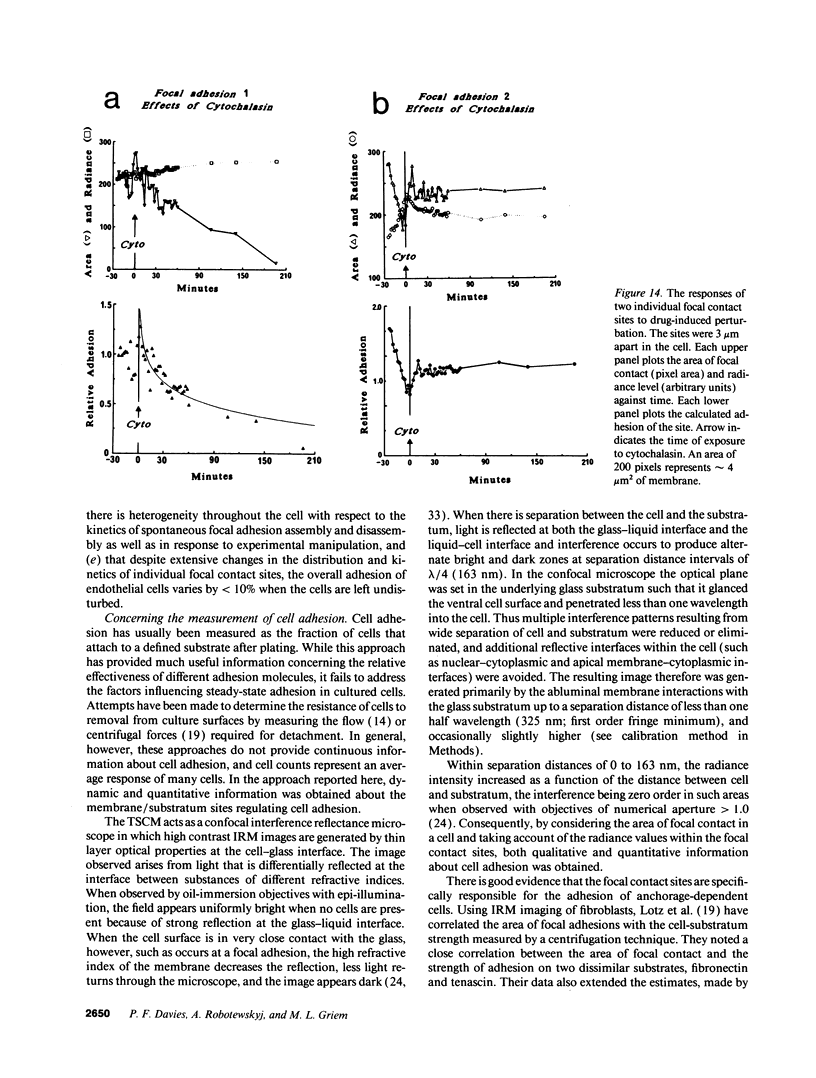
Real time measurements of cell-substratum adhesion in endothelial cells were obtained by tandem scanning confocal microscopy of sites of focal contact (focal adhesions) at the abluminal cell surface. Focal contact sites were sharply defined (low radiance levels) in the living cell such that the images could be enhanced, digitized, and isolated from other cellular detail. Sites of focal contact are the principal determinant of cell-substratum adhesion. Measurements of (a) the focal contact area and (b) the closeness of contact (inverse radiance) were used to nominally define the adhesion of a single cell or field of cells, and to record spontaneous and induced changes of cell adhesion in real time. The topography of focal contacts was estimated by calculating separation distances from radiance values using a calibration technique based on interference ring optics. While slightly closer contact was noted between the cell membrane and substratum at or near the center of each focal contact, separation distances throughout the adhesion regions were always < 50 nm. Subtraction of consecutive images revealed continuous spontaneous remodeling of individual focal adhesions in unperturbed cells during periods of < 1 min. Despite extensive remodeling of focal contact sites, however, cell adhesion calculated for an entire cell over extended periods varied by < 10%. When cytoskeletal stability was impaired by exposure to cytochalasin or when cells were exposed to proteolytic enzyme, endothelial adhesion declined rapidly. Such changes were recorded at the level of single cells, groups of cells, and at single focal adhesions. In both unperturbed and manipulated cells, the dynamics of remodeling and cell adhesion characteristics varied greatly between individual sites within the same cell; disappearance of existing sites and appearance of new ones often occurred within minutes while adjacent sites underwent minimal remodelling. Tandem scanning confocal microscopy image analysis of living cells in real time provides repetitive spatial, temporal, and quantitative information about cell adhesion. Such an approach should allow more precise quantitative analyses to be made of the interactions between extracellular matrix, adhesion proteins, integrins, and the cytoskeleton in the living cell.
Full text
PDF


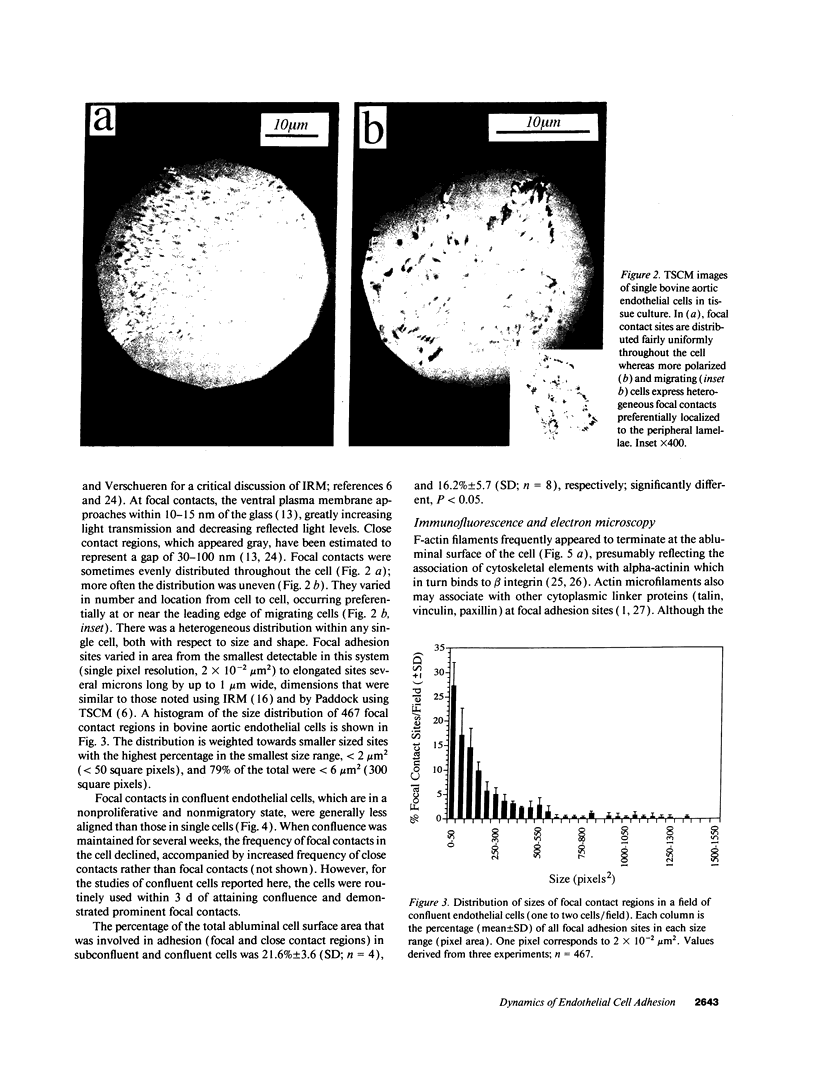



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
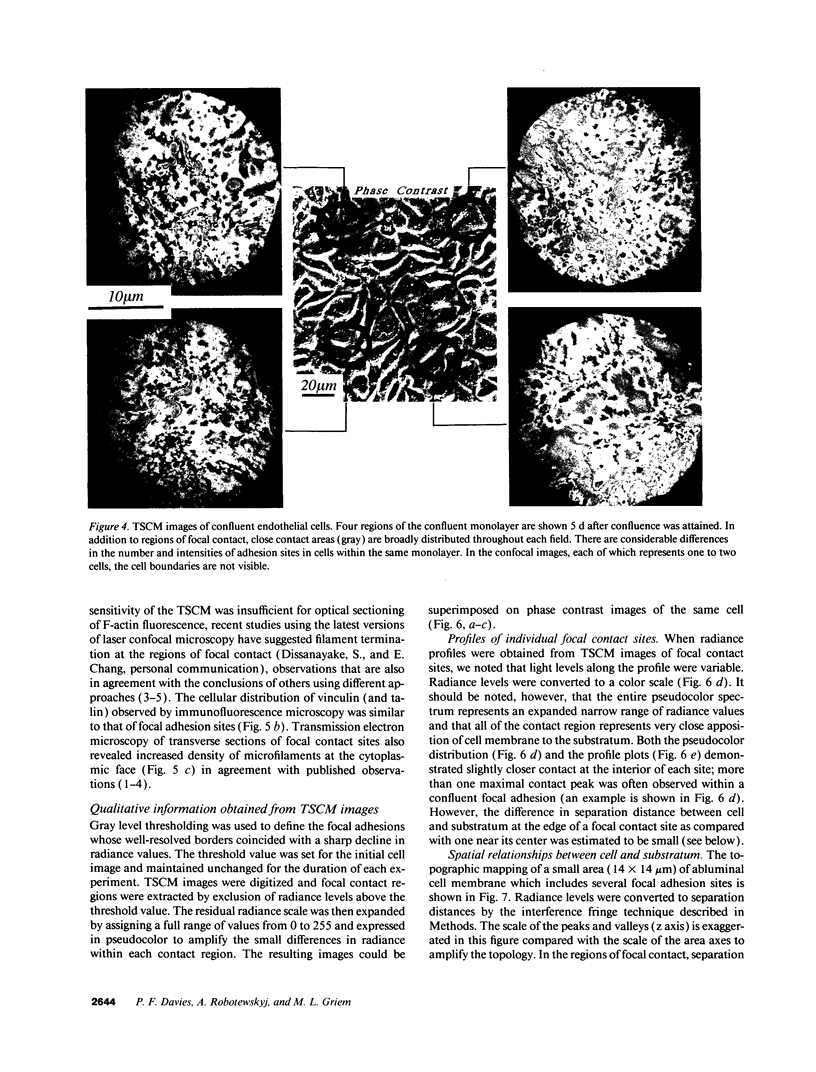
- Abercrombie M., Heaysman J. E., Pegrum S. M. The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Exp Cell Res. 1971 Aug;67(2):359–367. doi: 10.1016/0014-4827(71)90420-4. [DOI] [PubMed] [Google Scholar]
- Bell G. I. Models for the specific adhesion of cells to cells. Science. 1978 May 12;200(4342):618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Bereiter-Hahn J., Fox C. H., Thorell B. Quantitative reflection contrast microscopy of living cells. J Cell Biol. 1979 Sep;82(3):767–779. doi: 10.1083/jcb.82.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyde A., Dillon C. E., Jones S. J. Measurement of osteoclastic resorption pits with a tandem scanning microscope. J Microsc. 1990 May;158(Pt 2):261–265. doi: 10.1111/j.1365-2818.1990.tb02999.x. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Horwitz A. F. Integrin, a transmembrane glycoprotein complex mediating cell-substratum adhesion. J Cell Sci Suppl. 1987;8:231–250. doi: 10.1242/jcs.1987.supplement_8.13. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Davies P. F., Rennke H. G., Cotran R. S. Influence of molecular charge upon the endocytosis and intracellular fate of peroxidase activity in cultured arterial endothelium. J Cell Sci. 1981 Jun;49:69–86. doi: 10.1242/jcs.49.1.69. [DOI] [PubMed] [Google Scholar]
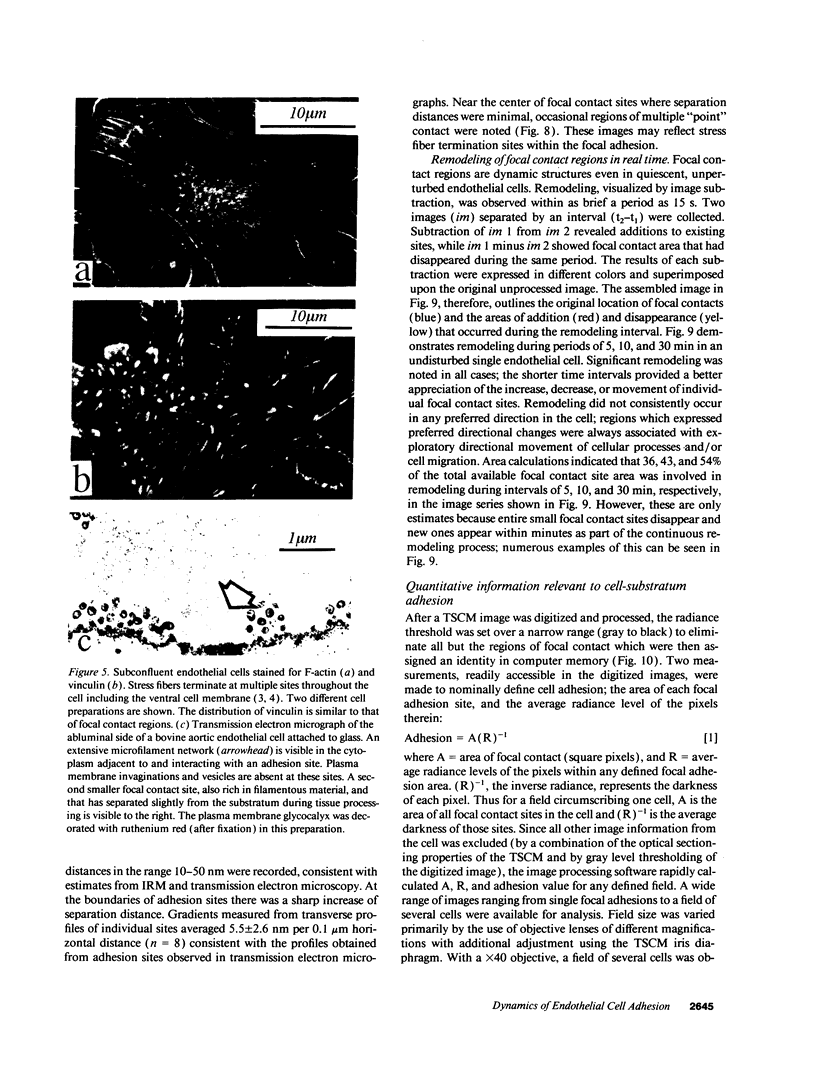
- Davies P. F., Tripathi S. C. Mechanical stress mechanisms and the cell. An endothelial paradigm. Circ Res. 1993 Feb;72(2):239–245. doi: 10.1161/01.res.72.2.239. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Geiger B., Avnur Z., Kreis T. E., Schlessinger J. The dynamics of cytoskeletal organization in areas of cell contact. Cell Muscle Motil. 1984;5:195–234. doi: 10.1007/978-1-4684-4592-3_5. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Birdwell C. R. Determination of cellular shape by the extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 1978 Nov;38(11 Pt 2):4155–4171. [PubMed] [Google Scholar]
- Hay E. D., Meier S. Stimulation of corneal differentiation by interaction between cell surface and extracellular matrix. II. Further studies on the nature and site of transfilter "induction". Dev Biol. 1976 Aug;52(1):141–157. doi: 10.1016/0012-1606(76)90014-2. [DOI] [PubMed] [Google Scholar]
- Heath J. P., Dunn G. A. Cell to substratum contacts of chick fibroblasts and their relation to the microfilament system. A correlated interference-reflexion and high-voltage electron-microscope study. J Cell Sci. 1978 Feb;29:197–212. doi: 10.1242/jcs.29.1.197. [DOI] [PubMed] [Google Scholar]
- Hyatt S. L., Klauck T., Jaken S. Protein kinase C is localized in focal contacts of normal but not transformed fibroblasts. Mol Carcinog. 1990;3(2):45–53. doi: 10.1002/mc.2940030202. [DOI] [PubMed] [Google Scholar]
- Ingber D. E., Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol. 1989 Jul;109(1):317–330. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. Integrins as mechanochemical transducers. Curr Opin Cell Biol. 1991 Oct;3(5):841–848. doi: 10.1016/0955-0674(91)90058-7. [DOI] [PubMed] [Google Scholar]
- Izzard C. S., Lochner L. R. Formation of cell-to-substrate contacts during fibroblast motility: an interference-reflexion study. J Cell Sci. 1980 Apr;42:81–116. doi: 10.1242/jcs.42.1.81. [DOI] [PubMed] [Google Scholar]
- Lotz M. M., Burdsal C. A., Erickson H. P., McClay D. R. Cell adhesion to fibronectin and tenascin: quantitative measurements of initial binding and subsequent strengthening response. J Cell Biol. 1989 Oct;109(4 Pt 1):1795–1805. doi: 10.1083/jcb.109.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P. A., Pasquale E. B., Wang J. Y., Singer S. J. Phosphotyrosine-containing proteins are concentrated in focal adhesions and intercellular junctions in normal cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6576–6580. doi: 10.1073/pnas.82.19.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal B., Sanger J. M., Sanger J. W. Visualization of myosin in living cells. J Cell Biol. 1987 Oct;105(4):1753–1760. doi: 10.1083/jcb.105.4.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Hök M. Thrombospondin modulates focal adhesions in endothelial cells. J Cell Biol. 1989 Sep;109(3):1309–1319. doi: 10.1083/jcb.109.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuckolls G. H., Turner C. E., Burridge K. Functional studies of the domains of talin. J Cell Biol. 1990 May;110(5):1635–1644. doi: 10.1083/jcb.110.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole T. E., Mandelman D., Forsyth J., Shattil S. J., Plow E. F., Ginsberg M. H. Modulation of the affinity of integrin alpha IIb beta 3 (GPIIb-IIIa) by the cytoplasmic domain of alpha IIb. Science. 1991 Nov 8;254(5033):845–847. doi: 10.1126/science.1948065. [DOI] [PubMed] [Google Scholar]
- Olesen S. P., Clapham D. E., Davies P. F. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988 Jan 14;331(6152):168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- Otey C. A., Pavalko F. M., Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J Cell Biol. 1990 Aug;111(2):721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock S. W. Tandem scanning reflected-light microscopy of cell-substratum adhesions and stress fibres in Swiss 3T3 cells. J Cell Sci. 1989 May;93(Pt 1):143–146. doi: 10.1242/jcs.93.1.143. [DOI] [PubMed] [Google Scholar]
- Pratt K. J., Jarrell B. E., Williams S. K., Carabasi R. A., Rupnick M. A., Hubbard F. A. Kinetics of endothelial cell-surface attachment forces. J Vasc Surg. 1988 Apr;7(4):591–599. [PubMed] [Google Scholar]
- Qwarnström E. E., MacFarlane S. A., Page R. C., Dower S. K. Interleukin 1 beta induces rapid phosphorylation and redistribution of talin: a possible mechanism for modulation of fibroblast focal adhesion. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1232–1236. doi: 10.1073/pnas.88.4.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammak P. J., Borisy G. G. Direct observation of microtubule dynamics in living cells. Nature. 1988 Apr 21;332(6166):724–726. doi: 10.1038/332724a0. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M. Selection and characterization of bovine aortic endothelial cells. In Vitro. 1978 Dec;14(12):966–980. doi: 10.1007/BF02616210. [DOI] [PubMed] [Google Scholar]
- Semich R., Robenek H. Organization of the cytoskeleton and the focal contacts of bovine aortic endothelial cells cultured on type I and III collagen. J Histochem Cytochem. 1990 Jan;38(1):59–67. doi: 10.1177/38.1.1688450. [DOI] [PubMed] [Google Scholar]
- Shuman H., Murray J. M., DiLullo C. Confocal microscopy: an overview. Biotechniques. 1989 Feb;7(2):154–163. [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Truskey G. A., Burmeister J. S., Grapa E., Reichert W. M. Total internal reflection fluorescence microscopy (TIRFM). II. Topographical mapping of relative cell/substratum separation distances. J Cell Sci. 1992 Oct;103(Pt 2):491–499. doi: 10.1242/jcs.103.2.491. [DOI] [PubMed] [Google Scholar]
- Ts'ao C. H., Glagov S. Basal endothelial attachment. Tenacity at cytoplasmic dense zones in the rabbit aorta. Lab Invest. 1970 Nov;23(5):510–516. [PubMed] [Google Scholar]
- Turner C. E., Glenney J. R., Jr, Burridge K. Paxillin: a new vinculin-binding protein present in focal adhesions. J Cell Biol. 1990 Sep;111(3):1059–1068. doi: 10.1083/jcb.111.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschueren H. Interference reflection microscopy in cell biology: methodology and applications. J Cell Sci. 1985 Apr;75:279–301. doi: 10.1242/jcs.75.1.279. [DOI] [PubMed] [Google Scholar]
- Wechezak A. R., Wight T. N., Viggers R. F., Sauvage L. R. Endothelial adherence under shear stress is dependent upon microfilament reorganization. J Cell Physiol. 1989 Apr;139(1):136–146. doi: 10.1002/jcp.1041390120. [DOI] [PubMed] [Google Scholar]