Abstract
Gliotransmission, a process involving active vesicular release of glutamate and other neurotransmitters by astrocytes, is thought to play a critical role in many brain functions. A new paper by Nedergaard et al. (2014) identifies an experimental flaw in these previous studies suggesting that astrocytes may not perform active vesicular release after all.
Over the past two decades, the role of glia in the brain has undergone a renaissance of sorts. These cells, once relegated as mere passive bystanders of nervous system development and function, have garnered new respect as active contributors to brain physiology. Astrocytes, for example, had long been thought to exist for primarily homeostatic roles of clearing excess synaptic neurotransmitters, maintaining metabolic balance, and sustaining the blood brain barrier. A large body of work, however, soon identified several predominant roles for astrocytes in active neurodevelopmental and functional processes. These come in multiple facets; astrocytes are critically required for synapse formation and function, neuronal migration, synapse phagocytosis, and even active waste clearance. But these aspects of astrocytes function in brain physiology have shared the spotlight with another tantalizing theory, that astrocytes could actively modify synaptic activity by the release of “gliotransmitters.”
In the early 1990s, fundamental observations demonstrated that glutamate could evoke rises in the intracellular calcium (Ca2+) concentration in cultured astrocytes and that the increase in Ca2+ concentration in astrocytes could evoke a Ca2+ response in adjacent neurons (Cornell-Bell et al., 1990; Nedergaard, 1994). This was an alluring finding, because it indicated that astrocytes not only receive information from neurons but also that they could potentially feed signals back to neuronal networks. This idea quickly gained traction and gave rise to the novel theory of the “tripartite” synapse. This new model proposed that signal integration and transduction at synapses should be considered in terms of not only presynaptic and postsynaptic terminals but also adjacent perisynaptic astrocytic processes. Since the coining of this term nearly two decades ago, over 100 studies have been published on the role of gliotransmission in normal brain function. But over time, significant dissent in the field has questioned the paradigm of astroglial transmitter release and modulation of synaptic transmission. This topic has been reviewed extensively from perspectives both in favor of astrocytic transmitter release (Araque et al., 2014; Halassa and Haydon, 2010) as well as those to the contrary (Agulhon et al., 2008; Nedergaard and Verkhratsky, 2012).
The main criticism against astrocytic transmitter release has been concern about the nonphysiological nature of many of the experiments in support of gliotransmition. Most of these studies have been performed on cultured astrocytes, raising the question of whether gliotransmitter release actually occurs in vivo. Perhaps the strongest in vivo evidence in support of gliotransmission was the development of a transgenic mouse line in which vesicular release could be specifically inhibited in astrocytes. In these mice, the formation of the SNARE complex between vesicles and the plasma membrane is inhibited by the expression of a dominant-negative domain of the vesicle-associated membrane protein 2 (VAMP2) protein, which interferes with endogenous VAMP2 expression and thus prevents VAMP2-mediated membrane fusion (Pascual et al., 2005). Most importantly, the glial-fibrillary acidic protein (GFAP) promoter is used to drive dominant negative SNARE (dnSNARE) expression selectively in astrocytes, therefore restricting the inhibition of vesicular release to the glial cohort of interest. Exciting observations from these mice support many of the in vitro findings of gliotransmission. In fact, the elimination of astrocytic transmitter exocytosis implicated glia in processes that were traditionally considered strictly neuronal, including hippocampal LTP (Pascual et al., 2005), sleep-wake cycles (Halassa et al., 2009), and pain (Foley et al., 2011). Crucially, however, all of these observations rely on the premise that the specificity of the dnSNARE manipulation is restricted to astrocytes. This presumption is challenged in a fundamental study published recently in The Journal of Neuroscience, where authors Fujita et al. (2014) propose that many of these observations in dnSNARE mice should be reexamined due to leaky neuronal transgene expression. The implications of this work raise critical questions about a number of studies that use the GFAP promoter to drive transgene expression, extending beyond the dnSNARE mice. Furthermore, hesitation about the validity of the dnSNARE findings in astrocytes suggests that it may be necessary to reconsider a large bulk of evidence in favor of gliotransmission.
There were two compelling reasons for the authors to assess the cellular specificity of the dnSNARE mice. First, ever since the inception of using the GFAP promoter for targeting transgene expression to astrocytes, suspicions soon arose about the fidelity of the driver (Su et al., 2004). These qualms were oftentimes overlooked, and despite quiet apprehension, over 200 studies have since been published using the GFAP promoter as an astrocyte-specific driver. Second, considering how integral VAMP2-mediated exocytosis is to neuronal transmission, the authors worried that even a minimal amount of neuronal transgene expression could have significant consequences. Off-target expression of dnSNARE in neurons could majorly impact neural network activity, because glutamatergic transmission critically depends upon the formation of the SNARE complex between synaptic vesicles and the presynaptic membrane. In fact, deletion of endogenous VAMP2 in neurons reduces synaptic fusion events by over 100-fold and is lethal immediately after birth, suggesting that even a minor disruption of neuronal vesicular release has the potential to suppress glutamatergic transmission.
To achieve inducible and reversible temporal control of dnSNARE transgene expression, the dnSNARE mice utilize a tetracycline-controlled transcriptional activation system. In these mice, the GFAP promoter drives expression of the tetracycline transactivator (tTA), which binds to the tetracycline operator (tetO) directly upstream of a gene of interest (in this case, dnSNARE). In the presence of the tetracycline analog doxycycline (Dox), tTA is inhibited, and tetO-mediated transcription is suppressed. Conversely, when Dox is removed, the tTA in GFAP-expressing cells is free to activate tetO-mediated dnSNARE expression. In summary, the “On-Dox” condition behaves like wild-type, and “Off-Dox” triggers transgene expression.
The authors first examined how inhibition of vesicular fusion in GFAP-expressing cells could alter overall cortical neural activity. Surprisingly, they observed a dramatic reduction in EEG power 2 weeks after discontinuing Dox treatment (thus turning on dnSNARE transgene expression). This effect was reversible, as the EEG power recovered completely after the mice were again exposed to Dox. This striking magnitude of EEG suppression (several fold) seemed too dramatic to arise solely from the inhibition of gliotransmission, which is purported to affect synaptic transmission by 20%–30%. Therefore, Fujita et al. (2014) next asked if leaky neuronal transgene expression might contribute to the observed phenotype. They harvested cortical tissue from dnSNARE mice and utilized fluorescence-activated cell sorting (FACS) to separate neurons from other brain cell types and then looked for transgene expression in the neuron versus non-neuronal pools. Surprisingly, the authors discovered significant dnSNARE transcripts in the neuronal population. In addition to dnSNARE, these mice express two reporter genes, LacZ and EGFP, under tetO control. Both LacZ and EGFP transcripts were also abundant in FACS sorted neurons.
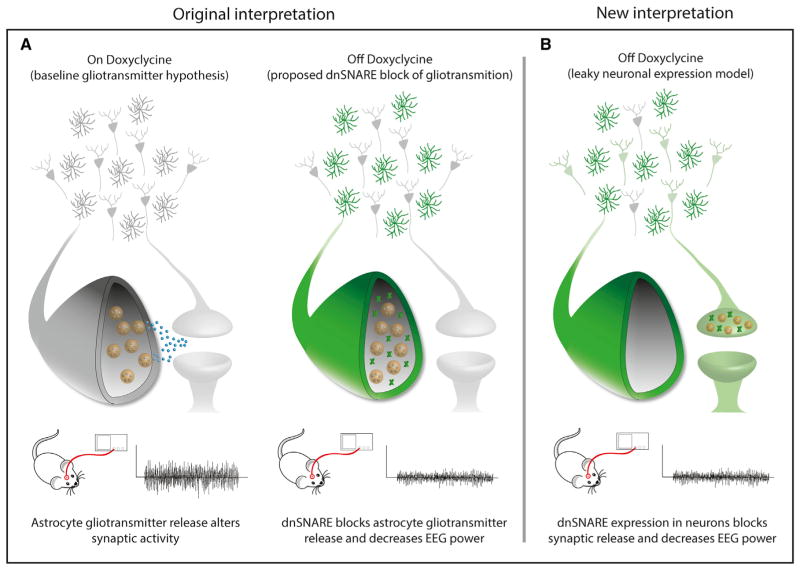
To validate their transcriptome findings of leaky neuronal transgene expression, the authors used immunohistochemical analysis to evaluate protein expression in situ. Since EGFP is also under tetO control in dnSNARE mice, the authors utilized EGFP expression as a proxy for the presence of dnSNARE in the adult cortex. As expected, strong EGFP expression colocalized with GFAP-positive astrocytes, but the authors also noted a low to moderate level of EGFP expression ubiquitously across most NeuN-positive neurons. To ensure that these results were not merely an artifact of EGFP expression, Fujita et al. (2014) also examined expression of lacZ, a second tetO reporter in dnSNARE mice. Identically to EGFP, lacZ expression was detectable in NeuN-positive neurons in both cortex and hippocampus. These findings demonstrate that the GFAP promoter drives transgene expression in neurons in dnSNARE mice and that direct suppression of neuronal vesicular release may be responsible for the suppression of EEG power in these mice (summarized in Figure 1).
Figure 1. Original and New Interpretations of dnSNARE Mice Phenotypes.
(A) Schematic (A) depicts the interpretation of dnSNARE mice with the presumption that transgene expression is restricted to astrocytes. In the presence of Dox, tetO-mediated dnSNARE expression is repressed in all cells. In the Off-Dox condition, however, astrocytic vesicular transmitter release is inhibited, and a subsequent reduction in EEG power is observed. These observations suggest that astrocyte gliotransmitter release contributes to baseline neural activity. However, the EEG power suppression in absence of Dox is more potent than it would be expected based on prior reports on gliotransmission.
(B) In scenario (B), new data demonstrate that suppression of EEG power is the result of direct inhibition of exocytosis of synaptic vesicles as a consequence of leaky dnSNARE expression in neurons.
Is neuronal transgene expression via the GFAP promoter a specific artifact of dnSNARE mice? Considering the significant implications of their findings, the authors examined a second mouse line where transgene expression is supposedly restricted to astrocytes under the GFAP driver. As in the dnSNARE mice, this second independent strain contained identical extensive neuronal expression of the transgene in cortex and hippocampus. Of note, however, both this and the dnSNARE mice utilize the same inducible tetracycline system. The authors report that tetO-dnSNARE mice without GFAP-tTA (these mice should have absolutely no transgene expression) exhibit a basal leakiness of expression throughout the brain. Thus, it is possible to interpret that neuronal transgene expression could be a result of leaky tetO activity as opposed to nonspecific GFAP promoter activation.
Previous studies of dnSNARE mice have implicated adenosine as an integral player by which astrocytes modulate synaptic transmission (via the proposed release of ATP that is immediately degraded to adenosine) (Halassa et al., 2009; Hines and Haydon, 2013; Nadjar et al., 2013; Pascual et al., 2005). The indications for adenosine as a causative agent, however, have been largely indirect. Here, Fujita et al. (2014) directly quantified extracellular concentrations of adenosine in vivo using microdialysis to examine whether glial (or neuronal) adenosine release was indeed affected by the presence of dnSNARE. As expected, adenosine levels were consistently higher in dark phases when mice are awake and active, in contrast to lower adenosine levels in the light phase when mice (nocturnal animals) are sleeping. These diurnal fluctuations were present in both wild-type and dnSNARE animals during the “On-Dox” phase when transgene expression should be off. Interestingly, once Dox was removed, and astrocyte vesicular fusion subsequently inhibited, extracellular adenosine levels remained almost identical to wild-type mice. This was also true following episodes of sleep deprivation, where previous reports from dnSNARE mice had reported that SNARE-dependent adenosine release from astrocytes contributed to accumulation of sleep pressure. Of note, a number of studies have utilized adenosine biosensors to measure extracellular adenosine in acute slices from dnSNARE mice and have reached conclusions contrary to those found by Fujita et al. (2014). The authors point out that these biosensor measurements detect adenosine concentrations in slice preparation studies that are 6–200 times higher than reported here in vivo. Fujita et al. (2014) suggest that discrepancies between the ex vivo biosensor recordings and in vivo micro-dialysis technique may arise from the ischemia and traumatic injury that occurs during slice preparations.
Implications for Gliotransmission
Since its inception as a model by which astrocytes modulate neuronal activity, the theory of gliotransmission has received a proportionate amount of acceptance and criticism. Advocators of the theory argue that repeated experimental paradigms consistently converge on the presence of active Ca2+-dependent vesicular transmitter release from astrocytes. Others, however, have concerns about the experimental evidence in support of the exocytic release of gliotransmitters. In particular, they argue that the majority of studies in favor of gliotransmission have been performed in vitro and raise concerns about the physiological nature of the observations. For example, although the Ca2+-dependent release of glutamate from cultured astrocytes has been demonstrated via a number of methods, the mechanism of such release is unclear. Any disruptions of membrane permeability or anion channel opening can result in the efflux of cytosolic glutamate. In fact, when cultured astrocytes are made to swell, they release glutamate through volume-regulated anion channels (Kimelberg et al., 1990), and simple depolarization techniques of astrocytes are sufficient to open hemi-channels that cause cytoplasmic contents (including ATP and glutamate) to spill out of the cell. Further arguments claim that glutamate concentrations inside astrocytes are insufficiently low due to the high concentration of the enzyme glutamine synthetase, which converts glutamate into glutamine—although computational models argue that functionally relevant levels of astrocyte glutamate may still be present (Hamilton and Attwell, 2010). Finally, a myriad of conflicting data exists regarding the question of whether astrocytes contain the necessary mechanical machinery for Ca2+-dependent gliotransmitter release. For example, transcriptome-level data sets suggest that astrocytes express little to no synaptotagmin proteins, which are required for Ca2+-mediated vesicle fusion (Zhang et al., 2014). The one exception is the presence of synaptotagmin 11, although it is still unclear whether this protein is Ca2+ dependent and whether it mediates kiss-and-run exocytosis as opposed to full membrane fusion. Finally, although several electron microscopy studies demonstrate that astrocytes may contain vesicular compartments that could be used for exocytic release, astrocytes lack structurally organized release zones apposed to neuronal postsynaptic densities.
A number of studies on cultured astrocytes provide provocative data in support of exocytic transmitter release. For example, capacitance measurements indicate that release of glutamate from cultured astrocytes is accompanied by an increase in membrane area, as would be expected for exocytic events. But one might argue that manipulation to cultured astrocytes is distinct from what occurs in vivo. It is possible that either cultured astrocytes express different proteins than those in situ that endow them with the ability to release transmitters or, conversely, that the manipulations to cultured astrocytes required to induce transmitter release are nonphysiological. Alternatively, like the observations made by Fujita et al. (2014) in vivo, one might question whether measurements of glutamate release by astrocytes in vitro is largely an artifact of small numbers of contaminating neurons. Typical methods for purifying astrocytes by proliferating young cells in serum-containing media generate cultures that include a significant population of stem cells. Thus, although neuronal contamination may initially be absent in these cultures, a small number of neurons may be present several days later when performing experimental assays. Interestingly, when Foo et al. (2011) established a method for directly purifying astrocytes from rodent brains to >99% purity (without expanding the population in culture), no evoked glutamate release could be induced.
The creation of the dnSNARE mice provided perhaps the most significant response to criticisms about in vitro and ex vivo experiments on astrocyte gliotransmission. A number of groups quickly utilized the dnSNARE mice to implicate astrocyte-secreted molecules in various brain functions, although interestingly, almost all of these involved astrocyte ATP release and not glutamate. In light of the findings presented by Fujita et al. (2014), however, it is with caution that such observations implicating in vivo gliotransmission should be endorsed. It is possible that many of these findings may in some part be attributable to nonspecific inhibition of synaptic transmission in neurons. And it is with this major caveat that the evidence for gliotransmission should be critically re-evaluated. It is important to note that the term gliotransmission, itself, may be a largely semantic source of debate. Most would agree in the validity of gliotransmission when the definition is limited to the astrocytic secretion of neuroactive molecules. It is the specific Ca2+-dependent vesicular release mechanism commonly affiliated with gliotransmission that has garnered such heightened scrutiny.
The findings by Fujita et al. (2014) emphasize the necessity for careful analysis of cell specificity in a number of conditional mouse models. It is likely that neuronal expression of target genes may be present in other adult transgenic lines that rely on the GFAP promoter for astrocyte-specific expression. Of course, each founder line will vary in leakiness depending upon the site of integration of the GFAP promoter, and thus, cell specificity may be more or less of an issue depending upon the particular transgenic line. In any case, careful scrutiny should be taken to ensure cell type specificity. In conclusion, this work provides a valuable reminder about the caveats of mouse genetics and reinvigorates the debate about gliotransmission. Above all else, it is an exemplar of the scientific method—continually challenging and attempting to rebut our most exciting hypotheses.
References
- Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD. Neuron. 2008;59:932–946. doi: 10.1016/j.neuron.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SHR, Robitaille R, Volterra A. Neuron. 2014;81:728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Foley JC, McIver SR, Haydon PG. Mol Pain. 2011;7:93. doi: 10.1186/1744-8069-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo LC, Allen NJ, Bushong EA, Ventura PB, Chung WS, Zhou L, Cahoy JD, Daneman R, Zong H, Ellisman MH, Barres BA. Neuron. 2011;71:799–811. doi: 10.1016/j.neuron.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Chen MJ, Li B, Smith NA, Peng W, Sun W, Toner MJ, Kress BT, Wang L, Benraiss A, et al. J Neurosci Published online December. 2014;10:2014. doi: 10.1523/JNEUROSCI.2585-14.2014. http://dx.doi.org/10.1523/JNEUROSCI.2585-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. Neuropharmacology. 2009;57:343–346. doi: 10.1016/j.neuropharm.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D. Nat Rev Neurosci. 2010;11:227–238. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- Hines DJ, Haydon PG. J Neurosci. 2013;33:4234–4240. doi: 10.1523/JNEUROSCI.5495-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK, Anderson E, Kettenmann H. Brain Res. 1990;529:262–268. doi: 10.1016/0006-8993(90)90836-z. [DOI] [PubMed] [Google Scholar]
- Nadjar A, Blutstein T, Aubert A, Laye S, Haydon PG. Glia. 2013;61:724–731. doi: 10.1002/glia.22465. [DOI] [PubMed] [Google Scholar]
- Nedergaard M. Science. 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Verkhratsky A. Glia. 2012;60:1013–1023. doi: 10.1002/glia.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Su M, Hu H, Lee Y, d’Azzo A, Messing A, Brenner M. Neurochem Res. 2004;29:2075–2093. doi: 10.1007/s11064-004-6881-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, et al. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]