Abstract
Objective
The SH2 domain-containing protein-tyrosine phosphatase Shp2 has been implicated in a variety of growth factor signaling pathways, but its metabolic role in some peripheral insulin-responsive tissues remains unknown.
Materials/Methods
To address the metabolic function of Shp2 in adipose tissue, we generated mice with adipose-specific Shp2 deletion using Adiponectin (Adipoq)-Cre transgenic mice. We then analyzed insulin sensitivity, glucose tolerance and body mass in adipose-specific Shp2 deficient and control mice on regular chow and high fat diet (HFD).
Results
Control mice on a HFD exhibited increased Shp2 expression in various adipose depots compared with those on regular chow. Adipoq-Cre mice enabled efficient and specific deletion of Shp2 in adipose tissue. However, adipose Shp2 deletion did not significantly alter body mass in mice on chow or HFD. In addition, mice with adipose Shp2 deletion exhibited comparable insulin sensitivity and glucose tolerance compared with controls. Consistent with this, basal and insulin-stimulated Erk and Akt phosphorylation were comparable in adipose tissue of Shp2-deficient and control mice.
Conclusions
Our findings indicate that adipose-specific Shp2 deletion does not significantly alter systemic insulin sensitivity and glucose homeostasis.
Keywords: Protein-tyrosine phosphatases, diabetes, glucose homeostasis, body weight, adiposity
INTRODUCTION
Metabolic syndrome and type 2 diabetes are complex disorders that are associated with obesity and sedentary life style [1, 2]. The increasing incidence of obesity worldwide has focused attention on adipose tissue function and contribution to whole body metabolic homeostasis. White adipose tissue (WAT) is specialized in lipid storage and adipokine secretion and is a regulator of energy balance and systemic insulin sensitivity [3].
Tyrosyl phosphorylation is a major regulator of insulin signaling and is tightly controlled by the opposing actions of protein-tyrosine kinases (PTKs) and protein-tyrosine phosphatases (PTPs) [4]. Src homology phosphatase 2 (Shp2) is a ubiquitously expressed non-transmembrane protein-tyrosine phosphatase that contains two SH2 domains, a tyrosine phosphatase domain, a C-terminal region with phosphorylation sites and a proline-rich domain [5]. Multiple studies indicate that Shp2 plays an essential role in most receptor tyrosine kinase signaling pathways [6, 7]. However, its function in regulating glucose homeostasis and energy balance in vivo requires additional investigation.
In vivo studies have not completely resolved the physiological role of Shp2 in insulin signaling and glucose homeostasis. Targeted mutation of Shp2 exon 3 in mice leads to embryonic lethality [8], precluding detailed studies of the effects of global Shp2 deletion. Hemizygous mice are viable but do not manifest any apparent defects in insulin action [9]. On the other hand, transgenic mice that express a presumptive dominant negative mutant of Shp2 in skeletal muscle, liver and adipose tissue exhibit insulin resistance and impaired insulin-stimulated glucose uptake [10]. Shp2 deletion in striated and cardiac muscle results in insulin resistance, impaired glucose uptake in muscle cells, and glucose intolerance [11], although these mice also exhibit marked dilated cardiomyopathy [11, 12]. In addition, Shp2 deletion in the pancreas causes defective glucose-stimulated insulin secretion and impaired glucose tolerance [13]. Moreover, we recently reported that mice lacking Shp2 in the liver exhibit increased hepatic insulin action and enhanced systemic insulin sensitivity [14]. However, the role of adipose Shp2 in regulating insulin sensitivity and glucose homeostasis in vivo remains unknown.
Shp2 also is implicated in regulating adiposity, body mass and leptin signaling (reviewed in [15, 16]). In vitro biochemical studies identify Shp2 as a positive mediator of leptin signaling through regulating tyrosine 985 site of leptin receptor [17-19]. These findings are supported by in vivo deletion of Shp2 in postmitotic forebrain neurons with the mice developing early onset obesity and leptin resistance [20] In addition, mice with proopiomelanocortin (POMC) neuron-specific Shp2 deletion exhibit elevated adiposity, decreased leptin sensitivity and reduced energy expenditure [21]. Together, these studies demonstrate a role for Shp2 in regulating energy balance, at least in part, through modulating leptin signaling.
In this study we assessed the physiological effects of Shp2 in adipose tissue using tissue-specific knockout approach. We determined the metabolic effects of adipose Shp2 deletion on body mass, systemic insulin sensitivity and glucose homeostasis in chow and high fat diet-fed mice.
METHODS
Mouse studies
Shp2-floxed (Shp2fl/fl) mice were generated previously [22]. Adiponectin (Adipoq)-Cre mice were generated and kindly provided by Dr. E. Rosen (BIDMC/Harvard University). Shp2fl/fl mice were on a mixed 129Sv/J x C57Bl/6J background and Adipoq-Cre mice were on a mixed FVB x C57Bl/6J background. All mice studied were age-matched and were maintained on a 12-hour light-dark cycle with free access to water and food. Mice were placed on standard lab chow (Purina lab chow, # 5001), and in some experiments, switched to a high fat diet (HFD; 60% kcal from fat, # D12492, Research Diets) at weaning. Genotyping for the Shp2 floxed allele and for the presence of Cre was performed by polymerase chain reaction (PCR), using DNA extracted from tails [14]. Mouse studies were conducted in line with federal regulations and were approved by the Institutional Animal Care and Use Committee at University of California Davis.
Metabolic measurements
Glucose was measured in blood collected from the tail using a glucometer (Home Aide Diagnostics). Serum insulin was determined by enzyme linked immunosorbent assay (ELISA) using mouse insulin as a standard (Crystal Chem). Serum leptin was assayed by ELISA using rat leptin standard (Crystal Chem). Free fatty acid (FFA) and triglyceride (TG) concentrations were measured by an enzymatic colorimetric method (Wako). Fed glucose measurements were taken between 7-9 am and, where indicated, from mice fasted for 12 hrs. For insulin tolerance tests (ITTs), mice were fasted for 4 hrs and injected intraperitoneally (i.p.) with 1 mU/g body weight human insulin (HumulinR; Eli Lilly). Blood glucose values were measured before and at 15, 30, 45, 60, 90 and 120 min post-injection. For glucose tolerance tests (GTTs), overnight-fasted mice were injected with 20% D-glucose at 2 mg/g body weight, and glucose was measured before and at 30, 60, 90 and 120 min following injection.
Isolation of adipocytes
Three grams of adipose tissue were incubated at 37°C for 60 minutes in siliconized tubes containing 20 ml isolation buffer {0.1 M HEPES, pH 7.4, 0.12 M NaCl, 0.05 M KCl, 1.2 mM CaCl2, 0.6 mM, MgSO4.7H2O and 1.5% (w/v) bovine serum albumin Fraction V (Fisher)} containing 0.002% (w/v) collagenase (Worthington). Tissue remnants were removed by filtration through a nylon screen (pore size 250 μm) (Tetko) into a siliconized tube. Adipocytes were allowed to float to the surface for 5 min whereafter the infranatant was aspirated through a siliconized injection needle. Further purification of adipocytes was performed by adding 5 ml isolation buffer and 2 ml dinonylphthalate oil (Fluka). Cells were allowed to float to the surface by centrifugation at 1,000 × g for 5 min. The supernatant was transferred to Eppendorf tubes and cells were pelleted and lysed in radio-immunoprecipitation assay (RIPA) buffer (150 mM NaCl, 1.0% IGEPAL® CA-630, 0.5% sodium deoxycholate, 0.1% SDS, and 50 mM Tris, pH 7.4 and I add 5 mM EDTA, 1 mM NaF, 1 mM sodium orthovanadate and protease inhibitors).
Biochemical analyses
For insulin signaling experiments, 30 week-old male mice were fasted overnight, injected i.p. with insulin (10 mU/g body weight), and sacrificed 10 minutes after injection. Tissues were ground in liquid nitrogen and lysed using RIPA buffer. Lysates were clarified by centrifugation at 13,000 rpm for 10 min and protein concentrations were determined using bicinchoninic acid protein assay kit (Pierce Chemical). Proteins were resolved by SDS-PAGE and transferred to PVDF membranes. Immunoblotting of lysates was performed with antibodies for Shp2 (Santa Cruz; 1/10,000), PTP1B (Millipore; 1/5,000), TCPTP (Mediamab; 1/2,000), pAkt (1/5,000), Akt (1/5,000), pErk (1/10,000), Erk (1/10,000) (all from Cell Signaling) and Tubulin (Santa Cruz; 1/5,000). Proteins were visualized using enhanced chemiluminescence (ECL, Amersham Biosciences) and pixel intensities of immuno-reactive bands were quantified using FluorChem 8900 (Alpha Innotech).
Statistical analyses
Data are expressed as means ± standard error of the mean (SEM). Statistical analyses were performed using the JMP program (SAS Institute). ITTs, GTTs, body weight and adiposity data were analyzed by analysis of variance (ANOVA). Post-hoc analysis was performed using Tukey-Kramer honestly significant difference test. For biochemistry studies, comparisons between groups were performed using unpaired two-tailed Student’s t test.
RESULTS
Generation of adipose-specific Shp2 knockout mice
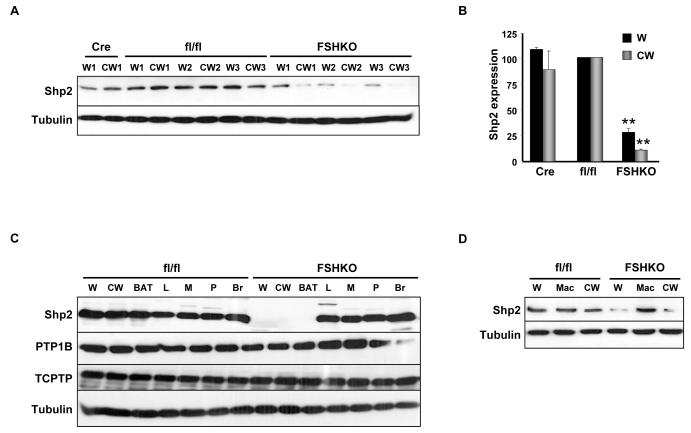
To investigate the role of adipose Shp2 in regulating body mass and glucose homeostasis, we assessed the physiological effects of its deletion in adipose tissue using Cre-LoxP approach. Mice with adipose-specific Shp2 deletion were generated by crossing Shp2fl/fl (fl/fl) mice to BAC transgenic mice expressing Cre recombinase under the control of the Adiponectin locus (Adipoq-Cre) to generate Adipoq-Shp2fl/+ mice. These mice were crossed to Shp2fl/fl, yielding Adipoq-Shp2fl/fl (hereafter termed fat-specific Shp2 KO; FSHKO). FSHKO mice survived to adulthood, and were fertile. Efficiency of Shp2 deletion was determined using immunoblot analysis of lysates from whole white adipose tissue (W) and purified adipocytes from collagenase-treated white adipose tissue (CW) (Fig. 1A, B). Shp2 protein expression was comparable between Cre and fl/fl mice in white adipose tissue and purified adipocytes. On the other hand, FSHKO mice exhibited decreased Shp2 expression by ~70% in white adipose (W) and ~85% in collagenase-treated white adipose (CW) compared with controls (Fig. 1B). These findings are consistent with complete deletion of Shp2 in adipocytes; the residual Shp2 in FSHKO white adipose (W) lysates likely reflects Shp2 expression in other cell types in the adipose tissue, such as vascular endothelial cells and macrophages. Indeed, immuno-staining of Shp2 in WAT sections of FSHKO and control mice supports this notion (data not shown). Shp2 levels were unchanged in other peripheral insulin-responsive tissues (liver and muscle), pancreas, brain and macrophages confirming the specificity of deletion (Fig. 1C, D). The expression of other PTPs known to regulate glucose homeostasis, protein-tyrosine phosphatase 1B (PTP1B) [23, 24] and its closely related T cell protein-tyrosine phosphatase (TCPTP) [25, 26] was unaltered in FSHKO mice (Fig. 1C). In addition, Shp2 deletion also was observed in adipose tissue of old (60 weeks) FSHKO mice on regular chow (Fig. 1D). Therefore, this approach enables efficient and specific deletion of Shp2 in adipose tissue.
Figure 1. Adipose-specific Shp2 deletion.
(A) Immunoblots of Shp2 expression in lysates of white adipose tissue (W) and purified adipocytes from collagenase-treated white adipose tissue (CW) from Adipoq-Cre (Cre), Shp2flx/flx (fl/fl) and Adipoq-Shp2flx/flx (FSHKO) mice on a HFD for 12 weeks. Blots were probed with anti-Tubulin antibodies (bottom panel) as a loading control. Numbers reflect samples from different mice (W1 and CW1 are from the same mouse; W2 and CW2 are from a different mouse). (B) Quantitative determination of Shp2 protein expression (normalized to Tubulin) from six mice per genotype. Note that compared with control mice, adipose Shp2 protein expression was decreased by ~70% and 85% in W and CW, respectively. (C) Shp2 protein expression in lysates from white adipose tissue, collagenase-treated white adipose tissue, brown adipose tissue (BAT), liver (L), muscle (M), pancreas (P) and brain (Br). Blots were probed for PTP1B, TCPTP and Tubulin. (D) Shp2 expression in W, CW and bone marrow-derived macrophages (Mac) from mice on regular chow for 60 weeks. ** Indicates statistically significant difference (P < 0.01) between FSHKO and fl/fl mice.
Adipose-specific Shp2 deletion does not significantly alter body mass or adiposity
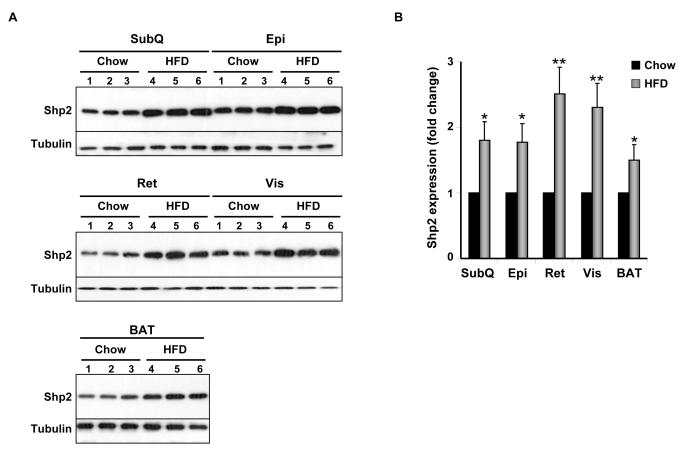
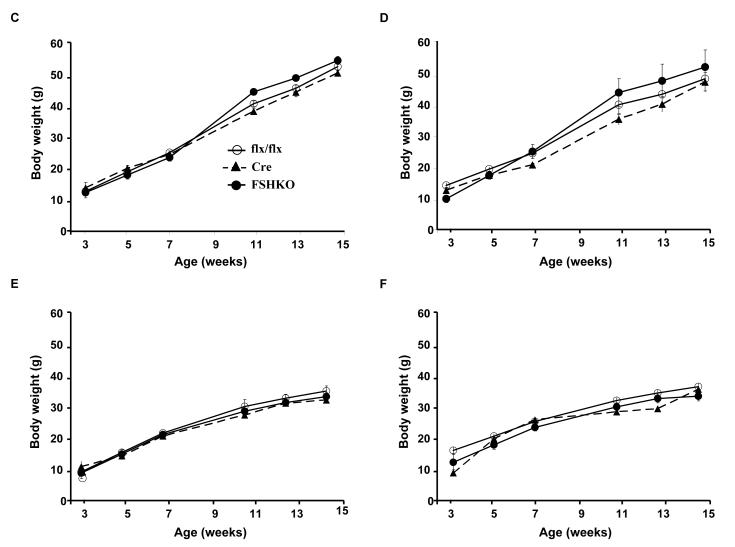
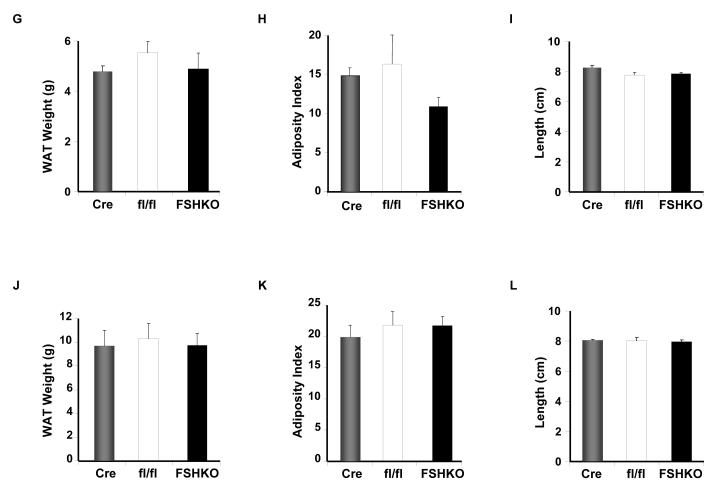
Shp2 protein expression was determined in various adipose depots of control mice fed regular chow or HFD (for 12 weeks). Immunoblot analysis of lysates revealed that Shp2 was expressed in subcutaneous (SubQ), epididymal (Epi), retroperitoneal (Ret), visceral (Vis) and brown adipose tissue (BAT) depots of mice fed regular chow (Fig. 2A). Notably, mice fed a HFD exhibited significantly increased Shp2 expression in all examined adipose depots compared with those fed regular chow (Fig. 2A, B). Next, we evaluated the effect of adipose Shp2 deletion on body mass and adiposity in mice fed regular chow or challenged with a HFD. As expected, on HFD mice gained more weight than their counterparts on regular chow, but comparable body weights (females and males) were detected between genotypes on either diet (Fig. 2C-F). Similar data were obtained in another independent cohort of mice on a HFD for 24 weeks (data not shown). In line with this observation, white adipose tissue weight was similar in FSHKO mice compared with controls on a HFD in both genders (Fig. 2G, J). In addition, adiposity index (total adipose depot weight (g) ÷ body weight (g) × 100), which correlates strongly with body fat percentage [27], was comparable between genotypes (Fig. 2H, K). Similar head-rump length was also observed in mice of different genotypes (Fig. 2I, L). Moreover, we assayed several parameters of whole-body lipid homeostasis. Leptin is a cytokine that is produced by adipocytes and its levels typically reflect body fat content with lean animals normally having low serum leptin [28, 29]. Consistent with their comparable adiposity and body weight, FSHKO mice exhibited similar fasted serum leptin concentrations compared with controls (Table 1). Furthermore, fasted serum triglyceride and free fatty acid concentrations were comparable between FSHKO and controls. Together, our data indicate that adipose Shp2 protein expression increases after high fat feeding but its deletion does not significantly alter adiposity and body weight under the tested conditions.
Figure 2. Effects of adipose-specific Shp2 deletion on body weight and adiposity.
(A) Shp2 protein expression in different adipose depots (SubQ: subcutaneous; Epi: epididymal; Ret: retroperitoneal; Vis: visceral; BAT: brown adipose tissue) of wild type male mice on regular chow or a HFD (for 12 weeks). Each lane represents sample from a different mouse. (B) Quantitative determination of Shp2 protein expression, normalized to Tubulin, from 3 mice per genotype. Body weight of male (C, E) and female (D, F) Cre (n= 9), flx/flx (n= 9), and FSHKO (n= 9) mice on a HFD (C, D) and chow (E, F). Total white adipose tissue weight (G, J), adiposity index (H, K), and head-rump length (cm) (I, L) of male (G-I) and female (J-L) mice on HFD for 12 weeks. Adiposity index (H) in FSHKO and fl/fl mice (H) has a P value of 0.052. * Indicates statistically significant difference in Shp2 expression between chow and HFD fed mice (*, P < 0.05; **, P < 0.01).
Table 1. Metabolic variables in mice with adipose-specific Shp2 deletion.
Male flx/flx, FSHKO and Cre mice were fed a HFD upon weaning. Serum was collected from fed or fasted mice at 10 weeks of age (6 weeks on HFD) and the indicated metabolic parameters were measured. Values are expressed as the mean ± SEM of measurements obtained for 6-8 animals per genotype.
| Genotype | flx/flx | FSHKO | Cre |
|---|---|---|---|
| Metabolic parameters | |||
|
Glucose (mg/dl)
Fed Fasted |
178 ± 14 | 197 ± 19 | 175 ± 13 |
| 128 ± 10 | 139 ± 5 | 129 ± 9 | |
|
Insulin (ng/ml)
Fed Fasted |
10.5 ± 1.6 | 11.2 ± 1.2 | 10.7 ± 1.7 |
| 3.2 ± 0.6 | 2.6 ± 0.5 | 2.9 ± 0.5 | |
|
Insulin/Glucose ratio
Fed Fasted |
0.05 ± 0.01 | 0.06 ± 0.02 | 0.06 ± 0.01 |
| 0.025 ± 0.01 | 0.018 ± 0.01 | 0.022 ± 0.02 | |
|
Leptin (ng/ml)
Fasted |
8.16 ± 0.62 | 8.96 ± 1.90 | 9.71 ± 1.10 |
|
TG (mg/dl)
Fasted |
15.9 ± 1.6 | 13.4 ± 0.8 | 18.3 ± 4.6* |
|
FFA (mM)
Fasted |
1.86 ± 0.3 | 1.41 ± 0.2 | 1.45 ± 0.2 |
indicates statistically significant difference between Cre and FSHKO.
Adipose-specific Shp2 deletion does not significantly alter systemic glucose homeostasis
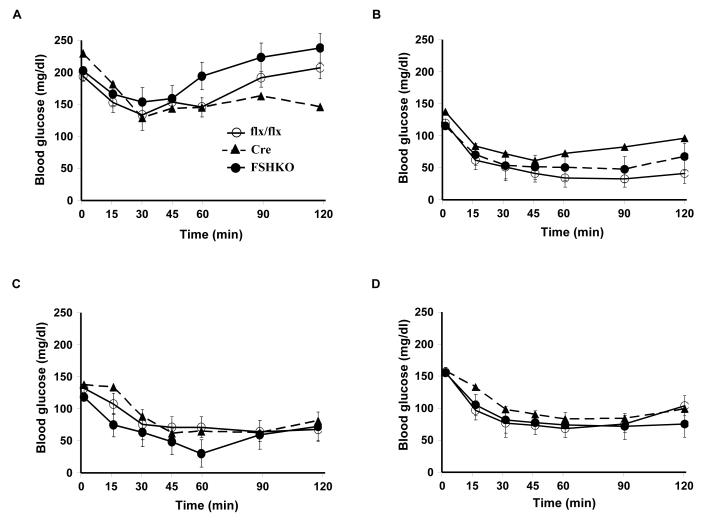
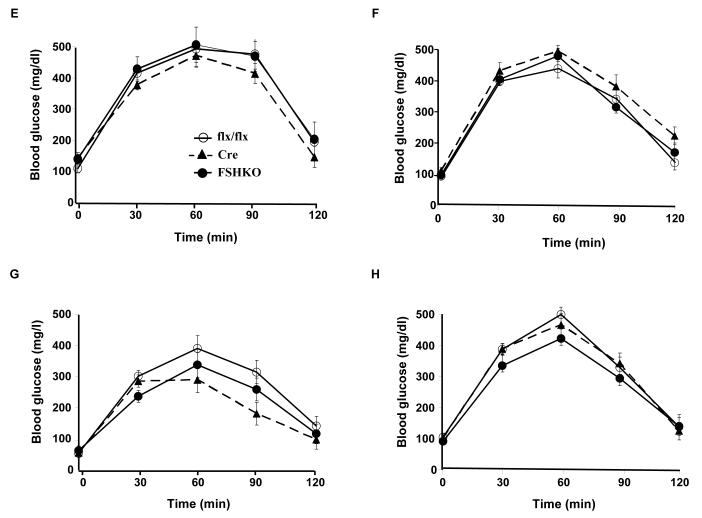
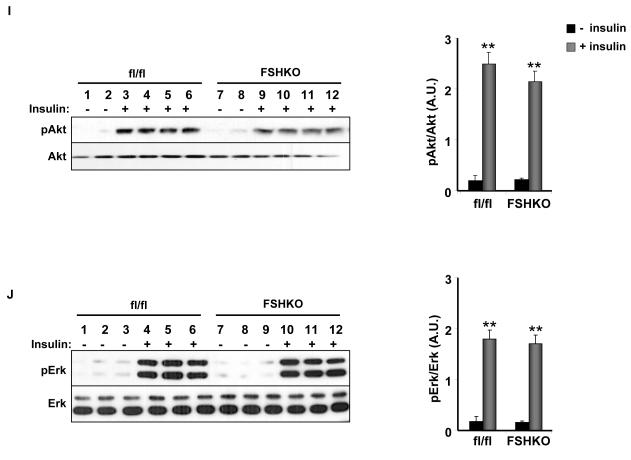
Body weights of control and FSHKO mice on regular chow and HFD were comparable suggesting that any potential differences in glucose homeostasis are primary and not caused by body weight alterations. We assayed several metabolic parameters in control and FSHKO mice on a HFD (Table 1). FSHKO mice exhibited comparable fed and fasted glucose and insulin concentrations compared with controls. In addition, insulin/glucose ratio was comparable between genotypes. To directly evaluate insulin sensitivity in vivo, male and female mice on regular chow and a HFD were subjected to ITTs at 14 weeks of age (Fig. 3A-D). On either diet, FSHKO mice exhibited comparable insulin sensitivity to controls. In addition, we tested the ability of mice to clear glucose from the peripheral circulation during intraperitoneal GTTs (Fig. 3E-H). Similarly, FSHKO mice exhibited comparable glucose tolerance to controls on either diet. Additional ITTs and GTTs were performed in another independent cohort of mice on a HFD (for 24 weeks) revealing comparable results (data not show). Next, we evaluated phosphorylation of Akt (Ser473) and Erk in control and FSHKO mice on a HFD at basal and insulin-stimulated (10 min) conditions (Fig. 3I, J). As expected, insulin induced significant Akt (Ser473) and Erk phosphorylation in adipose tissue of FSHKO and control mice. FSHKO mice exhibited a trend for decreased insulin-induced Akt phosphorylation, but it did not reach statistical significance (P=0.4). In addition, no significant differences were observed in insulin-induced Erk phosphorylation in FSHKO and control mice (P=0.5) (Fig. 3J). Collectively, our data indicate that adipose Shp2 deletion does not significantly alter systemic insulin sensitivity and glucose tolerance.
Figure 3. Insulin sensitivity and glucose tolerance in mice with adipose-specific Shp2 deletion.
(A-D) Insulin tolerance tests (ITTs) in male (A, C) and female (B, D) Cre (n= 9), flx/flx (n= 9), and FSHKO (n= 9) mice on a HFD (A, B) and chow (C, D) at 14 weeks of age (insulin 1 mU/g B.W.). (E-H) Glucose tolerance tests (GTTs) in male (E, G) and females (F, H) Cre (n= 9), flx/flx (n= 9), and FSHKO (n= 9) mice on a HFD (E, F) and chow (G, H) at 15 weeks of age (glucose dose, 2 mg/g B.W.). (I, J) Male mice (30 weeks old) were injected intraperitoneally with saline or insulin (10 mU/g B.W.) and sacrificed after 10 minutes. Total adipose tissue lysates were immunoblotted for pAkt (S473) (I) and pErk (J) and the corresponding total proteins. Bar graph indicates quantitation of Akt and Erk phosphorylation (adjusted to protein level) from at least 4 mice per group. All blots were scanned and quantified using FluorChem 8900 and statistical analysis was performed using two-tailed Student’s t-test. * Indicates statistically significant difference between basal and insulin stimulated conditions for each genotype (*, P < 0.05; **, P < 0.01).
DISCUSSION
The role of Shp2 in regulating glucose homeostasis, as well as its specific functions in adipose tissue, has heretofore remained largely unresolved. To begin to address these issues, we generated mice with adipose-specific Shp2 deletion using the novel Adipoq-Cre transgenic mice. These mice might provide some advantages compared with the commonly-used fatty acid binding protein 2 (aP2)-Cre mice that have been utilized for adipose-specific deletion. Although aP2 is predominantly expressed in adipocytes postnatally [30], it is also expressed in non-adipose tissues (such as trigeminal ganglia, dorsal root ganglia and vertebrae) during development [31]. In addition, aP2 is expressed in activated macrophages [32], which are implicated in the regulation of adipose tissue inflammation and function [33, 34]. On the other hand, Adipoq-Cre mice do not express Cre in bone marrow-derived macrophages (Fig. 1). Although additional studies are required to fully evaluate the utility of Adipoq-Cre mice; they enabled efficient and specific deletion of Shp2 in various adipose depots.
Our studies demonstrated that Shp2 expression was dynamically regulated in adipose tissue depots of mice on a HFD. The regulatory point(s) for adipose Shp2 abundance in response to high fat feeding remains to be determined, and could be attributed to increased expression and/or pretranslational alterations (involving mRNA stability or gene transcription). Number of factors can contribute to increased adipose Shp2 expression including, but not limited to, insulin resistance. Of note, Shp2 (and PTP1B) expression and activity are increased in liver and muscle of diabetic rats [35]. In addition, improved insulin sensitivity in obese subjects following weight loss is accompanied by decreased PTP1B expression and activity in adipose tissue [36]. Thus, in subjects with insulin resistance, reduction of the elevated PTP activity (in one or more tissue) could potentially reduce the risk of developing diabetes and may have beneficial metabolic effects. Additional factors can contribute to increased PTP expression in vivo. Zabolotny et al. report that inflammation underlies PTP1B over-expression in diabetes and obesity [37]. Given that Shp2 plays a role in TNF receptor and interleukin 6 signaling [38, 39], inflammatory responses might contribute, at least in part, to the regulation of adipose Shp2 expression. Preliminary studies revealed increased inflammatory response in adipose tissue of FSHKO mice on a HFD compared with controls (Bettaieb and Haj, unpublished observations). Since adipose inflammation accompanies obesity in humans [33], additional studies are warranted to address the potential role of adipose Shp2 in inflammation and the metabolic implications of such regulation.
Body weights of control and FSHKO mice on regular chow and HFD were comparable indicating that adipose Shp2 deletion did not significantly alter body mass under these experimental conditions. In line with this, FSHKO and control mice exhibited comparable leptin concentrations. Our findings and previous reports on Shp2 deletion in muscle [11, 12], suggest that Shp2 deletion in these peripheral insulin-responsive tissues does not significantly alter body mass. On the other hand, neuronal Shp2 has been identified as a regulator of energy balance and adiposity in vivo, at least in part, through modulating leptin signaling [20, 21]. Additional studies are required to fully assess the effects of adipose Shp2 deficiency on energy balance and adipokine secretion under different experimental conditions such as various diets and/or prolonged high fat feeding.
Shp2 plays diverse roles in peripheral tissues to modulate systemic insulin sensitivity and glucose homeostasis. Transgenic mice that express a presumptive dominant negative mutant of Shp2 to varying levels in liver, skeletal muscle and adipose tissue exhibit insulin resistance, impaired insulin-stimulated glucose uptake and decreased IRS1 phosphorylation in skeletal muscle and liver [10]. In addition, Shp2 deletion in muscle leads to insulin resistance and glucose intolerance [11]. The dilated cardiomyopathy that also develops in these mice [11, 12] could lead to secondary changes in muscle cells that affect insulin sensitivity. However, the similarity between the muscle-specific KO and transgenic mutant mice suggest that Shp2 is a positive modulator of insulin signaling in muscle. On the other hand, we recently reported that mice lacking Shp2 in the liver exhibit increased hepatic insulin action and enhanced systemic insulin sensitivity, indicating that Shp2 is a negative regulator of insulin signaling in the liver. Our current findings indicate that adipose-specific Shp2 deletion does not significantly alter systemic insulin sensitivity and glucose homeostasis. Consistent with their insulin sensitivity, FSHKO and control mice exhibited comparable Akt and Erk phosphorylation. However, we cannot exclude alterations in Akt and/or Erk signaling at later times post stimulation. The mechanism(s) through which Shp2 plays distinct roles in individual peripheral insulin-responsive tissues remains unclear. Conceivably, Shp2 has distinct substrates in different tissues. Alternatively, Shp2 may affect the same pathways in different tissues, but the effects of those pathways and/or the feedback regulatory pathways may differ in a tissue-specific manner. At any rate, these studies highlight the need to dissect the tissue-specific roles of Shp2.
In summary, our studies indicated that adipose Shp2 deletion did not significantly alter body mass and systemic glucose homeostasis. It would be of interest to examine if enhanced/prolonged metabolic challenge(s) will lead to the manifestation of metabolic alterations in FSHKO mice. Finally, we do not exclude other biologically relevant effects of adipose Shp2 deletion such as nonshivering thermogenesis, endoplasmic reticulum stress, adipokine secretion and inflammation. Indeed, Shp2 plays a role in TNF receptor and interleukin 6 signaling, thus it will be important to explore these and other signaling systems, in FSHKO mice.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Benjamin Neel (Ontario Cancer Institute) and Evan Rosen (BIDMC/Harvard University) for Shp2fl/fl and Adipoq-Cre mice, respectively. We thank members of the laboratory for critical comments on the manuscript.
FUNDING This work was supported in part by grant from Center for Health and Nutrition Research, Junior Faculty Award from the American Diabetes Association (7-06-JF-28), Research Grant from the Juvenile Diabetes Research Foundation (1-2009-337) and NIH grant R56DK084317 to F.G.H.
Abbreviations
- Shp2
Src homology phosphatase 2
- PTP
protein-tyrosine phosphatase
- ITT
insulin tolerance test
- GTT
glucose tolerance test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest
DISCLOSURE STATEMENT The authors declare no conflict of interest
AUTHOR CONTRIBUTIONS A. B., performed research, collected and analyzed data and co-wrote manuscript, K. M., performed research, I.M., performed research, N.N., performed research, S.C., performed research, S.L., performed research, F.G.H., designed study, data interpretation and manuscript writing.
REFERENCES
- 1.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 2.Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annual review of physiology. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- 3.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 5.Sugimoto S, Lechleider RJ, Shoelson SE, Neel BG, Walsh CT. Expression, purification, and characterization of SH2-containing protein tyrosine phosphatase, SH-PTP2. The Journal of biological chemistry. 1993;268:22771–22776. [PubMed] [Google Scholar]
- 6.Feng GS. Shp2-mediated molecular signaling in control of embryonic stem cell self-renewal and differentiation. Cell research. 2007;17:37–41. doi: 10.1038/sj.cr.7310140. [DOI] [PubMed] [Google Scholar]
- 7.Chan G, Kalaitzidis D, Neel BG. The tyrosine phosphatase Shp2 (PTPN11) in cancer. Cancer metastasis reviews. 2008;27:179–192. doi: 10.1007/s10555-008-9126-y. [DOI] [PubMed] [Google Scholar]
- 8.Saxton TM, Henkemeyer M, Gasca S, Shen R, Rossi DJ, Shalaby F, Feng GS, Pawson T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. The EMBO journal. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arrandale JM, Gore-Willse A, Rocks S, Ren JM, Zhu J, Davis A, Livingston JN, Rabin DU. Insulin signaling in mice expressing reduced levels of Syp. The Journal of biological chemistry. 1996;271:21353–21358. doi: 10.1074/jbc.271.35.21353. [DOI] [PubMed] [Google Scholar]
- 10.Maegawa H, Hasegawa M, Sugai S, Obata T, Ugi S, Morino K, Egawa K, Fujita T, Sakamoto T, Nishio Y, et al. Expression of a dominant negative SHP-2 in transgenic mice induces insulin resistance. The Journal of biological chemistry. 1999;274:30236–30243. doi: 10.1074/jbc.274.42.30236. [DOI] [PubMed] [Google Scholar]
- 11.Princen F, Bard E, Sheikh F, Zhang SS, Wang J, Zago WM, Wu D, Trelles RD, Bailly-Maitre B, Kahn CR, et al. Deletion of Shp2 tyrosine phosphatase in muscle leads to dilated cardiomyopathy, insulin resistance, and premature death. Molecular and cellular biology. 2009;29:378–388. doi: 10.1128/MCB.01661-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kontaridis MI, Yang W, Bence KK, Cullen D, Wang B, Bodyak N, Ke Q, Hinek A, Kang PM, Liao R, et al. Deletion of Ptpn11 (Shp2) in cardiomyocytes causes dilated cardiomyopathy via effects on the extracellular signal-regulated kinase/mitogen-activated protein kinase and RhoA signaling pathways. Circulation. 2008;117:1423–1435. doi: 10.1161/CIRCULATIONAHA.107.728865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang SS, Hao E, Yu J, Liu W, Wang J, Levine F, Feng GS. Coordinated regulation by Shp2 tyrosine phosphatase of signaling events controlling insulin biosynthesis in pancreatic beta-cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7531–7536. doi: 10.1073/pnas.0811715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuo K, Delibegovic M, Matsuo I, Nagata N, Liu S, Bettaieb A, Xi Y, Araki K, Yang W, Kahn BB, et al. Altered glucose homeostasis in mice with liver-specific deletion of Src homology phosphatase 2. The Journal of biological chemistry. 2010;285:39750–39758. doi: 10.1074/jbc.M110.153734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends in biochemical sciences. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 16.Feng GS. Shp2 as a therapeutic target for leptin resistance and obesity. Expert opinion on therapeutic targets. 2006;10:135–142. doi: 10.1517/14728222.10.1.135. [DOI] [PubMed] [Google Scholar]
- 17.Carpenter LR, Farruggella TJ, Symes A, Karow ML, Yancopoulos GD, Stahl N. Enhancing leptin response by preventing SH2-containing phosphatase 2 interaction with Ob receptor. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6061–6066. doi: 10.1073/pnas.95.11.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Friedman JM. Leptin receptor activation of SH2 domain containing protein tyrosine phosphatase 2 modulates Ob receptor signal transduction. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:9677–9682. doi: 10.1073/pnas.96.17.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjorbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, Neel BG, Myers MG, Jr., Flier JS. Divergent roles of SHP-2 in ERK activation by leptin receptors. The Journal of biological chemistry. 2001;276:4747–4755. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- 20.Zhang EE, Chapeau E, Hagihara K, Feng GS. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16064–16069. doi: 10.1073/pnas.0405041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banno R, Zimmer D, De Jonghe BC, Atienza M, Rak K, Yang W, Bence KK. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. The Journal of clinical investigation. 120:720–734. doi: 10.1172/JCI39620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang SQ, Yang W, Kontaridis MI, Bivona TG, Wen G, Araki T, Luo J, Thompson JA, Schraven BL, Philips MR, et al. Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Molecular cell. 2004;13:341–355. doi: 10.1016/s1097-2765(04)00050-4. [DOI] [PubMed] [Google Scholar]
- 23.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 24.Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Molecular and cellular biology. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galic S, Klingler-Hoffmann M, Fodero-Tavoletti MT, Puryer MA, Meng TC, Tonks NK, Tiganis T. Regulation of insulin receptor signaling by the protein tyrosine phosphatase TCPTP. Molecular and cellular biology. 2003;23:2096–2108. doi: 10.1128/MCB.23.6.2096-2108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukushima A, Loh K, Galic S, Fam B, Shields B, Wiede F, Tremblay ML, Watt MJ, Andrikopoulos S, Tiganis T. T-cell protein tyrosine phosphatase attenuates STAT3 and insulin signaling in the liver to regulate gluconeogenesis. Diabetes. 59:1906–1914. doi: 10.2337/db09-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu S, Kim K, Haus KA, Espinal GM, Millon LV, Warden CH. Identification of positional candidate genes for body weight and adiposity in subcongenic mice. Physiol Genomics. 2007;31:75–85. doi: 10.1152/physiolgenomics.00267.2006. [DOI] [PubMed] [Google Scholar]
- 28.Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004;53(Suppl 1):S143–151. doi: 10.2337/diabetes.53.2007.s143. [DOI] [PubMed] [Google Scholar]
- 29.Ahima RS. Revisiting leptin’s role in obesity and weight loss. The Journal of clinical investigation. 2008;118:2380–2383. doi: 10.1172/JCI36284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross SR, Graves RA, Greenstein A, Platt KA, Shyu HL, Mellovitz B, Spiegelman BM. A fat-specific enhancer is the primary determinant of gene expression for adipocyte P2 in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:9590–9594. doi: 10.1073/pnas.87.24.9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urs S, Harrington A, Liaw L, Small D. Selective expression of an aP2/Fatty Acid Binding Protein 4-Cre transgene in non-adipogenic tissues during embryonic development. Transgenic Res. 2006;15:647–653. doi: 10.1007/s11248-006-9000-z. [DOI] [PubMed] [Google Scholar]
- 32.Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, Parker RA, Suttles J, Fazio S, Hotamisligil GS, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nature medicine. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neels JG, Olefsky JM. Inflamed fat: what starts the fire? The Journal of clinical investigation. 2006;116:33–35. doi: 10.1172/JCI27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad F, Goldstein BJ. Alterations in specific protein-tyrosine phosphatases accompany insulin resistance of streptozotocin diabetes. The American journal of physiology. 1995;268:E932–940. doi: 10.1152/ajpendo.1995.268.5.E932. [DOI] [PubMed] [Google Scholar]
- 36.Cheung A, Kusari J, Jansen D, Bandyopadhyay D, Kusari A, Bryer-Ash M. Marked impairment of protein tyrosine phosphatase 1B activity in adipose tissue of obese subjects with and without type 2 diabetes mellitus. The Journal of laboratory and clinical medicine. 1999;134:115–123. doi: 10.1016/s0022-2143(99)90115-4. [DOI] [PubMed] [Google Scholar]
- 37.Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. The Journal of biological chemistry. 2008;283:14230–14241. doi: 10.1074/jbc.M800061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.You M, Flick LM, Yu D, Feng GS. Modulation of the nuclear factor kappa B pathway by Shp-2 tyrosine phosphatase in mediating the induction of interleukin (IL)-6 by IL-1 or tumor necrosis factor. The Journal of experimental medicine. 2001;193:101–110. doi: 10.1084/jem.193.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Podar K, Mostoslavsky G, Sattler M, Tai YT, Hayashi T, Catley LP, Hideshima T, Mulligan RC, Chauhan D, Anderson KC. Critical role for hematopoietic cell kinase (Hck)-mediated phosphorylation of Gab1 and Gab2 docking proteins in interleukin 6-induced proliferation and survival of multiple myeloma cells. The Journal of biological chemistry. 2004;279:21658–21665. doi: 10.1074/jbc.M305783200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.