Abstract
Physical exercise has mood-enhancing and antidepressant properties although the mechanisms underlying these effects are not known. The present experiment investigated the effects of prolonged access to a running wheel on electrical self-stimulation of the lateral hypothalamus (LHSS), a measure of hedonic state, in rats. Rats with continuous voluntary access to a running wheel for either 2 or 5 weeks exhibited dramatic leftward shifts in the effective current 50 (ECu50; current value that supports half of maximum responding) of their LHSS current-response functions compared to their baselines, indicating a decrease in reward threshold, whereas control rats current-response functions after 2 or 5 weeks were not significantly different from baseline. An inverse correlation existed between the change in ECu50 from baseline and the amount an animal had run in the day prior to LHSS testing, indicating that animals that exhibited higher levels of running showed a more robust decrease in LHSS threshold. We conclude that long-term voluntary exercise increases sensitivity to rewarding stimuli, which may contribute to its antidepressant properties.
Keywords: exercise, reward, intracranial self-stimulation, anhedonia, depression
Regular physical exercise has wide-ranging health benefits including positive effects on the cardiovascular and central nervous systems (Cotman & Berchtold, 2002; Laurin, Verreault, Lindsay, MacPherson, & Rockwood, 2001; Linke et al., 2005; Sim et al., 2005; Tillerson, Caudle, Reveron, & Miller, 2003). Along with well-established physiological benefits, exercise affords psychological benefits including anxiolytic and antidepressant effects in patients with major depressive disorder, as well as positive effects on mood in healthy subjects (Brosse, Sheets, Lett, & Blumenthal, 2002; Trivedi, Greer, Grannemann, Chambliss, & Jordan, 2006; Tsatsoulis & Fountoulakis, 2006; Yeung, 1996).
This study was conducted to determine if prolonged voluntary exercise has positive effects on hedonic state in rats by assessing changes in sensitivity to lateral hypothalamic self-stimulation (LHSS) reward following continuous access to a running wheel. The lateral hypothalamus (LH) was chosen for its reliability in supporting intracranial self-stimulation (ICSS) behavior (Morris, Na, Grippo, & Johnson, 2006; Olds, 1962) and because LHSS is in large part rewarding due to activation of the mesolimbic dopamine pathway, a neural pathway also implicated in mediating the rewarding properties of exercise (Brené et al., 2007; Fibiger & Phillips, 1988; Nakajima & McKenzie, 1986; Olds, 1962; Werme et al., 2002; Wise & Rompre, 1989).
In humans, withdrawal from exercise in habitual runners can promote depressive symptoms (Berlin, Kop, & Deuster, 2006; Mondin et al., 1996). Therefore, a second goal of this study was to determine if withdrawal of a running wheel following continuous access can produce anhedonia (i.e., a decrease in sensitivity to reward and a core symptom of major depressive disorder). Finally, we sought to establish the utility of ICSS for investigating the neurobiological adaptations that mediate the antidepressant and rewarding properties of exercise.
Experiment 1: The Effects of 5 Weeks of Voluntary Wheel-Running Exercise on LHSS Reward Thresholds
Materials and Methods
Animals
Adult male Sprague–Dawley rats weighing between 200 and 300 g were used for all experiments and maintained on a 12-h light/dark cycle with ad libitum access to Teklad chow and tap water. All procedures were in accordance with National Institutes of Health Guide to the Care and Use of Laboratory Animals and were approved by the University of Iowa Animal Care and Use Committee.
Surgery
Under Equithesin-like anesthesia (3 ml/kg i.p.; University of Iowa Hospital Pharmacy), rats had bipolar stimulating electrodes (10 mm length; Plastics One, Roanoke, VA) stereotaxically implanted in the LH (3.0 mm posterior to bregma, 1.7 mm lateral to the midline, and 8.5 mm ventral to the skull surface). Stadol (1 ml/kg sc; University of Iowa Hospital Pharmacy) was administered for postoperative analgesia. Rats were allowed a recovery period of at least 1 week following surgery.
Voluntary wheel-running exercise
Following surgery, rats were housed individually with access to a running wheel (circumference 1.065 m). Rats were randomly assigned to “wheel” (n = 12) or “locked-wheel” conditions (n = 5). For all animals, running wheels were locked until after LHSS baseline testing (see below), at which time rats in the wheel group were allowed continuous access to running-wheel exercise for 5 weeks. A duration of 5 weeks was chosen based on previous work suggesting that at least 4 weeks of voluntary wheel running is required for animals to achieve maximum running activity (Burghardt, Fulk, Hand, & Wilson, 2004; Lambert & Jonsdottir, 1998). Furthermore, similar periods of wheel access improve associative learning and prevent or reverse stress-induced depressive-like behaviors (Baruch, Swain, & Helmstetter, 2004; Greenwood et al., 2003; Greenwood, Strong, Dorey, & Fleshner, 2007; van Praag, Christie, Sejnowski, & Gage, 1999; Zheng et al., 2006). The locked-wheel group was maintained in an identical environment except wheels remained in a locked position for the duration of the study. Daily running activity (km/day) was tallied by attaching the running wheel to a cyclocomputer (Protégé 8.0 model, Planet Bike, Madison, WI) that was tested each day to ensure that it was accurately scoring wheel revolutions.
LHSS training and testing
After recovery from surgery, rats were trained in a Plexiglas® operant operant chamber equipped with a lever that delivered a 300-ms train of rectangular pulses with 1-ms duration, similar to previously reported stimulation parameters (Grippo, Moffitt, Beltz, & Johnson, 2006; Morris et al., 2006). Training consisted of 3–4 days of adaptation to the chamber and learning the association between lever pressing and current-pulse delivery. Electrical parameters were set to predetermined values (frequency = 60 Hz; current intensity = 250 μA) and systematically varied with “free” pulses given until the rat began to lever press. During training, current intensities that yielded the best fit to a sigmoidal function when presented as a series of 10 current intensities separated by 25-μA increments were used for each individual rat and were held constant for the duration of the study. Maximum current intensity did not exceed 350 μA for any animal included in the study. Rats that displayed untoward motor effects or did not achieve at least 30 lever presses per minute (LPM) at 250 μA by the second day of training were eliminated from the study (that is, this was a functional assessment of successful electrode placement as described previously; Grippo et al., 2006).
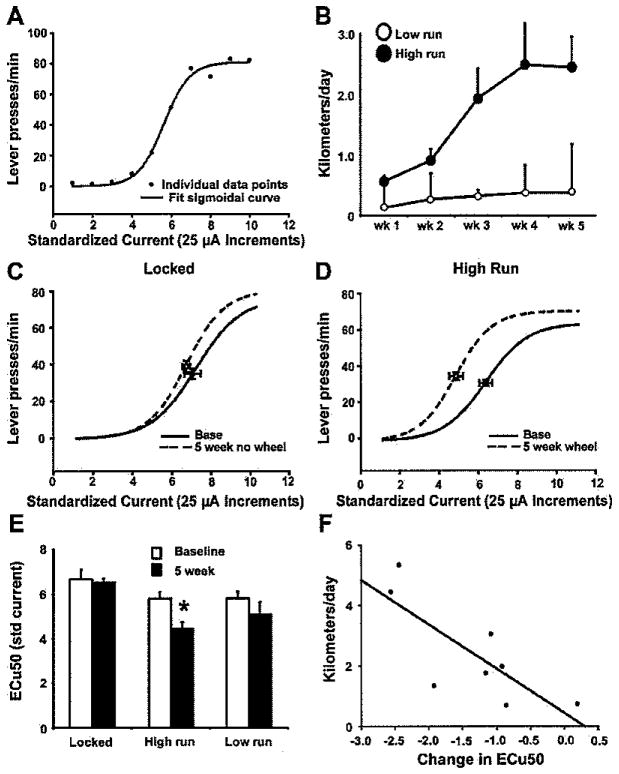
Following LHSS training, baseline LHSS current-response functions were determined similar to methods described previously (Miliaressis, Rompre, Laviolette, Philippe, & Coulombe, 1986; Morris et al., 2006). Current was delivered in a descending series in 10 discrete presentations of 25-μA increments. Rats were allowed to respond for 1 min at each current intensity. On 3 consecutive days, one current-response curve was generated for each rat (LPM at each of the 10 current intensities) and averaged to yield a single baseline curve using the criteria that the response rate was minimal for low levels of current intensity (e.g., 25–75 μA) and increased monotonically, eventually reaching a plateau, so that there was a sigmoid relationship between current intensity and behavioral responses (r2 > .80). Data points were plotted using Sigma Plot (Jandel Scientific, Chicago, IL) and fitted to a three-parameter sigmoidal function from which three parameters were calculated: (1) maximum response rate, (2) current intensity that supports 50% of the maximum response rate (effective current 50 [ECu50]), and (3) minimum response rate. See Figure 1A for a representative LHSS current-response function.
Figure 1.
Five weeks of voluntary wheel running leads to a decrease in lateral hypothalamic self-stimulation reward threshold. (A) A representative current-response curve for one rat. Filled circles illustrate raw data points, and the solid line indicates the fit curve. (B) Average daily running distance for the 5-week high- (>0.5 km/day) and low-run (<0.5 km/day) groups. (C) Mean lateral hypothalamic self-stimulation (LHSS) current-response curves for 5-week locked-wheel control rats. Data are shown as sigmoid curves fit to mean values. Black dots indicate the midpoint effective current 50 (ECu50) of each curve with corresponding vertical and horizontal SEM bars. (D) Mean LHSS current-response curves for animals in the high-run group (>0.5 km/day). (E) Mean ECu50 values + SEM for the locked-wheel control group (n = 5), the high-run (>0.5 km/day; n = 8), and (C) low-run (<0.5 km/day; n = 4) 5-week exercise groups. The high-run group showed a decreased ECu50 postexercise relative to their baseline value, whereas the low-run and locked-wheel groups were not significantly different from baseline. (F) Scatterplot and line of best fit depicting the relationship between change in ECu50 values from baseline plotted against the distance animals ran (km/day) in the day prior to LHSS testing. * p < 0.05 vs. respective baseline value.
Statistical procedures
Mean ECu50 values were compared using repeated-measures ANOVA or paired t tests with post hoc Fischer least significant difference (LSD) tests conducted where necessary. An increased sensitivity to LHSS reward was evidenced by a significant decrease in the ECu50, representing a leftward shift in the manipulation-induced current-response curve relative to the baseline curve. Change in body weight and heart and soleus muscle-to-body weight ratios were analyzed using ANOVA or Student’s t tests. Correlation between the change in ECu50 from baseline and the average distance run in the day previous to LHSS testing was assessed using Pearson’s correlation coefficient (r). A p value of < .05 was required for significance.
Results
Rats that ran more than 0.5 km/day in the 3 weeks prior to LHSS retesting were analyzed as a “high-run” group (n = 8); rats that failed to reach this criterion were placed in a “low-run” group (n = 4) for statistical analyses. Other studies using voluntary running on exercise wheels have found that rats naturally distribute into a high-runners versus low-runners categorization (Burghardt, Pasumarthi, Wilson, & Fadel, 2006); therefore we chose a criterion of 0.5 km/day because there was a clear distinction between animals that would or would not exceed this level of daily running. Rats in the high-run condition ran an average of 1.66 ± 0.35 km/day over the 5 weeks of the study and averaged 2.15 ± 0.51 km/day in the 3 weeks prior to LHSS testing (Figure 1B). Animals in the low-run group averaged 0.34 ± 0.21 km/day over the total 5 weeks and 0.37 ± 0.27 km/day in the 3 weeks prior to LHSS testing (Figure 1B). It is common for rats to exhibit little running when first given access to a wheel and to “ramp up” running over the course of a few weeks (Adlard, Perreau, & Cotman, 2005; Burghardt et al., 2006). Daily running distances between rats exhibit high variability, but an individual rat’s running behavior will often plateau and stabilize at around 3–4 weeks (Adlard et al., 2005; Burghardt et al., 2006).
Five weeks of running-wheel access led to a dramatic leftward shift in LHSS current-response functions compared to baseline in the high-run group (Figure 1D). ECu50 values in the high-run group were significantly decreased compared to baseline following the 5-week exercise period, t(7) = 4.15, p < .01 (Figure 1E). There were no significant differences in ECu50 in the low-run group or the locked-wheel control group relative to baseline (Figure 1C). A one-way ANOVA testing for between groups differences in baseline ECu50 was not significant (p = .267). Maximum number of LPM when comparing baseline to postexercise were not significantly different for any of the groups (mean ± SEM, high-run: baseline = 70.0 ± 4.53, post = 78.34 ± 7.35; locked-wheel group: baseline = 68.24 ± 4.23, post = 77.25 ± 4.23; p = .15, p = .20, respectively). In the high-run group, a strong negative correlation, r = −0.782, p < .05 (Figure 1F), existed between the amount run in the day prior to LHSS retesting and the change in ECu50 from baseline, suggesting that rats that exhibited the greatest amounts of running were most likely to show a more robust increase in sensitivity to LHSS reward.
Body weights at the start of the experiment were not statistically different between groups (locked-wheel group: 240.4 ± 8.3 g; high run: 250 ± 8.9 g; low run: 265 ± 9.5 g); however, a one-way ANOVA showed that the change in body weight from the beginning of the experiment to the end of the running protocol was significant, F(2, 16) = 12.56, p< .01. Post hoc Fischer LSD tests revealed that rats in the high-run group gained significantly less weight than the control (p < .001) and low-run (p < .05) groups (change in body weight, mean ± SEM, locked wheel = 129.4 ± 8.3 g; high run = 96.0 ± 3.6 g; low run = 116.8 ± 2.2 g), in agreement with previous findings (Greenwood et al., 2003, 2007).
At the conclusion of the experiment animals were deeply anesthetized with pentobarbital sodium (Nembutal; 1.0 ml/kg) for the removal of the heart and left soleus muscle. Residual blood was removed from the heart, and the soleus muscle and heart were weighed to assess heart- and soleus muscle-to body weight ratios. Following a significant global F ratio, F(2, 16) = 4.96, p < .05, we found that the left soleus muscle of rats in the high-run group exhibited hypertrophy relative to locked-wheel controls (soleus muscle [mg]: body weight [g], mean ± SEM, high-run = 0.37 ± 0.01; locked wheel = 0.32 ± 0.01; LSD test, p < .05), but were not statistically different from the animals in the low-run group, whereas animals in the low-run group (0.33 ± 0.01) were not different from high-run or control animals. There were no differences in heart-to-body weight ratio between groups (heart [mg]: body weight [g], high run = 3.39 ± 0.09, locked wheel = 3.24 ± 0.04, low run = 3.26 ± 0.06).
Experiment 2: The Effects of 2-Week Voluntary Wheel Running and Subsequent Withdrawal of the Wheel on LHSS Reward Threshold
Materials and Methods
A second experiment was conducted to determine if shorter periods of running-wheel access produced hedonic changes similar to that of 5-week exercise and also to determine the hedonic effects of wheel withdrawal in exercised animals. We chose 2 weeks of exercise based on our results from Experiment 1 in which there was a clear “ramping up” of running activity between 2 and 3 weeks of wheel access (Figure 1B). Following the establishment of baseline, LHSS current-response functions rats were randomly assigned to a wheel (n = 8) or locked-wheel condition (n = 5) and subsequently were allowed voluntary access to a running wheel or a locked wheel for 2 weeks. After 2 weeks animals were retested for LHSS for 3 consecutive days. Following LHSS retesting, all wheels were locked. Rats were then retested for LHSS (“recovery”) on Days 6 and 7 (1-week recovery), and Days 13 and 14 (2-week recovery) following the locking of the wheels.
Results
One rat in the wheel condition did not average >0.5 km/day and was excluded from the analysis. The remaining animals averaged 1.12 ± 0.14 km/day. Body weights were not statistically different at the start of the experiment (no wheel: 234.5 ± 3.08 g; wheel: 239.6 ± 5.7 g) nor was the amount of weight gained between groups during the exercise protocol significantly different (wheel: 37.8 ± 1.38 g; locked wheel: 36.2 ± 1.59 g).
As in Experiment 1, animals allowed to exercise freely on a running wheel exhibited leftward shifts in their LHSS current-response functions relative to baseline with no change observed in animals maintained with a locked wheel (Figure 2A, B). There was a significant overall F ratio, F(3, 21) = 8.75, p = .001, and subsequent LSD post hoc tests revealed that the ECu50 values in the wheel group were significantly lower following 2 weeks of voluntary wheel access relative to baseline (p < .05; Figure 2C) and remained so 1 week after the locking of the wheels (p < 0.05) before returning to baseline 2 weeks after locking the wheels. Unlike Experiment 1, we did not observe a statistically significant correlation between the amount an animal had run in the day prior to LHSS testing and the change in ECu50 (r = −0.289). No significant changes in ECu50 were observed at any time point in the locked-wheel group (not shown). Baseline ECu50 values were not different between groups (p = .191). Importantly, as observed in Experiment 1, maximum number of LPM did not differ from baseline to postexercise or recovery for either group, demonstrating that enhanced motor performance is an unlikely explanation for the shifts in LHSS threshold (maximum LPM, mean ± SEM, locked wheel: baseline = 72.20 ± 8.45, 2-week locked wheel = 75.84 ± 8.51, 1-week recover = 70.60 ± 4.41, 2-week recover = 72.72 ± 6.43; wheel: baseline = 70.69 ± 6.44, 2-week wheel = 74.25 ± 4.17, 1-week recover = 72.50 ± 4.55, 2-week recover = 73.55 ± 4.70).
Figure 2.
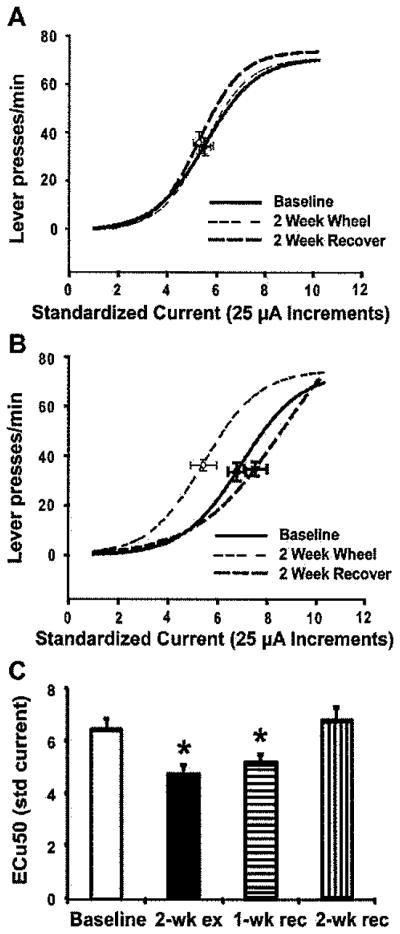
Two weeks of voluntary wheel running exercise decreases the threshold for LHSS that persists 1 week after withdrawal of the wheel. (A) Mean lateral hypothalamic self-stimulation (LHSS) current-response curves for 2-week locked-wheel control rats. (B) Mean lateral hypothalamic self-stimulation (LHSS) current-response curves for 2-week exercised rats showing a robust leftward shift following 2 weeks of wheel running (2 Week Wheel) that returns to baseline following 2 weeks with a locked wheel (2 Week Recover). (C) Mean ECu50 values + SEM animals with access to a running wheel for 2 weeks. ECu50 values were significantly lower than baseline following 2 weeks of wheel access (2-wk ex), remained lower than baseline after 1 week with a locked wheel (1-wk rec) before returning to baseline after 2 weeks with a locked wheel (2-wk rec). * p < 0.05 versus baseline value.
A separate group of rats (n = 14; seven runners and seven locked-wheel controls) were included to determine the effects of 2 weeks of running-wheel access on heart and soleus muscle weights because this could not be determined in the above protocol due to locking of the wheels and subsequent 2-week recovery period. These animals were not tested for LHSS but were euthenized following 17 days of access to the wheels to match the amount of access given to rats in the LHSS experiment. There were no differences in body weights prior to the running protocol or in amount of weight gained over the exercise period (not shown). All animals achieved >0.5 km/day and averaged 1.74 ± 0.78 km/day (not shown). There were no differences in either heart- or soleus muscle-to body weight ratio between the rats with locked wheels versus those that were allowed 2 weeks of free access to a wheel (heart [mg]: body weight [g], mean + SEM, locked wheel = 2.97 + 0.05; wheel = 3.04 + 0.08; soleus muscle [mg]: body weight [g], locked wheel = 0.34 + 0.01; wheel 0.34 + 0.02).
Discussion
The present study, to our knowledge, is the first to report changes in ICSS reward thresholds following long-term voluntary exercise. Either 2 or 5 weeks of access to an exercise wheel led to robust changes in the threshold for LHSS reward in rats. Following 5 weeks of wheel access, sensitivity to LHSS reward was significantly increased in the high-run group (>0.50 km/day), whereas no significant changes in reward threshold were observed in the low-run group (<0.50 km/day). Similar durations of running have beneficial effects on cognition and stress-induced, depressive-like behaviors (Baruch et al., 2004; Greenwood et al., 2003, 2007; van Praag et al., 1999; Zheng et al., 2006). A particularly interesting finding was the significant correlation between the amount of running and the change in ECu50 from baseline in the 5-week high-run animals (Figure 1F), suggesting that greater amounts of exercise may have more robust effects on hedonic state. There were no significant differences in maximum response rate; therefore it is unlikely that enhanced capacity for lever pressing can explain our results.
Although exercise withdrawal can promote depressive-like symptoms in nondepressed humans (Berlin et al., 2006; Mondin et al., 1996), in this study, the hedonic effects of wheel running persisted following withdrawal of an unlocked wheel (Figure 2C). It is possible that anhedonic effects may have been observed had animals been tested for LHSS responding at a shorter interval (e.g., 1–2 days) following wheel withdrawal, although that is difficult to reconcile with the continued decrease in ECu50 observed 1-week after locking the wheels. Withdrawal from drugs of abuse leads to increased thresholds for ICSS (Cryan, Bruijnzeel, Skjei, & Markou, 2003; Cryan, Hoyer, & Markou, 2003; Markou & Koob, 1991), and given the putative stressful nature of wheel withdrawal and the overlap between the neural substrates mediating the rewarding properties of drugs and running, we expected a similar behavioral outcome following the locking of the wheels in exercised rats. Instead we found that decreased LHSS thresholds persisted following 1 week of a locked wheel before returning to baseline levels by 2 weeks. This may be consistent with the long-lasting antidepressant effects of exercise observed up to 10 months after cessation of an exercise regime in patients with major depressive disorder (Babyak et al., 2000). Different durations of prior wheel access may lead to different behavioral outcomes once the wheel is withdrawn.
Based on the lack of cardiac and soleus muscle hypertrophy despite significant decreases in LHSS thresholds following 2 weeks of exercise, it does not appear that robust physical conditioning is necessary for the hedonic effects of exercise to occur. There are, however, alternative measures of physical conditioning than those used in the present study (e.g., change in maximal oxygen uptake, resting bradycardia). The running protocols we used may be sufficiently long to show evidence of conditioning as measured by other methods.
In the present study long-term voluntary running-wheel exercise produced “hyperhedonia,” that is, an increased sensitivity to rewarding stimuli. The ability of physical exercise to reverse hedonic deficits that occur in depressed patients may relate to its antidepressant properties; however, more research is necessary to substantiate this hypothesis. Many central nervous system adaptations are likely relevant for the improvements in mood and affect that result from exercise. For instance salient changes in dopaminergic, serotonergic, and opioidergic signaling, increased production and expression of neurotrophins, and enhanced neurogenesis have been suggested to be relevant to the beneficial effects of physical activity on cognition and affect (Brené et al., 2007; Cotman & Berchtold, 2002; Fordyce & Farrar, 1991; Gobbo & O’Mara, 2005; Neeper, Gomez-Pinilla, Choi, & Cotman, 1995; Sutoo & Akiyama, 1996; van Praag et al., 1999; Werme, Thoren, Olson, & Brene, 2000; Zheng et al., 2006). An important next question to address is which of these neural adaptations that result from running-wheel exercise support the hedonic changes observed in our study. Future work should investigate the neurobiological and molecular mechanisms underlying changes in reward threshold following running-wheel exercise, as well as whether these changes relate to the efficacy of exercise as an antidepressant.
Acknowledgments
We thank Tony Burnes and Terry Beltz for assistance with conducting the experiments, Joanie Rogers and Marilyn Dennis for help with editing and preparing the manuscript, and Ralph Johnson for help with data analysis and presentation. This work was supported in part by National Institutes of Health grants from the National Heart, Lung, and Blood Institute (HL 14388, HL 098207), National Institute of Diabetes and Digestive and Kidney Diseases (DK. 66086), National Institute of Mental Health (MH 80241), and American Heart Association (AHA 0615313Z).
References
- Adlard PA, Perreau VM, Cotman CW. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiology of Aging. 2005;26:511–520. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, Krishnan KR. Exercise treatment for major depression: Maintenance of therapeutic benefit at 10 months. Psychosomatic Medicine. 2000;62:633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Baruch DE, Swain RA, Helmstetter FJ. Effects of exercise on Pavlovian fear conditioning. Behavioral Neuroscience. 2004;118:1123–1127. doi: 10.1037/0735-7044.118.5.1123. [DOI] [PubMed] [Google Scholar]
- Berlin AA, Kop WJ, Deuster PA. Depressive mood symptoms and fatigue after exercise withdrawal: The potential role of decreased fitness. Psychosomatic Medicine. 2006;68:224–230. doi: 10.1097/01.psy.0000204628.73273.23. [DOI] [PubMed] [Google Scholar]
- Brené S, Bjornebekk A, Aberg E, Mathe AA, Olson L, Werme M. Running is rewarding and antidepressive. Physiology & Behavior. 2007;92:136–140. doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: Recent findings and future directions. Sports Medicine. 2002;32:741–760. doi: 10.2165/00007256-200232120-00001. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behavior in rats. Brain Research. 2004;1019:84–96. doi: 10.1016/j.brainres.2004.05.086. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Pasumarthi RK, Wilson MA, Fadel J. Alterations in fear conditioning and amygdalar activation following chronic wheel running in rats. Pharmacology, Biochemistry and Behavior. 2006;84:306–312. doi: 10.1016/j.pbb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences. 2002;25:295–301. doi: 10.1016/S0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology. 2003;168:347–358. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Hoyer D, Markou A. Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biological Psychiatry. 2003;54:49–58. doi: 10.1016/S0006-3223(02)01730-4. [DOI] [PubMed] [Google Scholar]
- Fibiger HC, Phillips AG. Mesocorticolimbic dopamine systems and reward. Annals of the New York Academy of Sciences. 1988;537:206–215. doi: 10.1111/j.1749-6632.1988.tb42107.x. [DOI] [PubMed] [Google Scholar]
- Fordyce DE, Farrar RP. Enhancement of spatial learning in F344 rats by physical activity and related learning-associated alterations in hippocampal and cortical cholinergic functioning. Behavioural Brain Research. 1991;46:123–133. doi: 10.1016/S0166-4328(05)80105-6. [DOI] [PubMed] [Google Scholar]
- Gobbo OL, O’Mara SM. Exercise, but not environmental enrichment, improves learning after kainic acid-induced hippocampal neurodegeneration in association with an increase in brain-derived neurotrophic factor. Behavioural Brain Research. 2005;159:21–26. doi: 10.1016/j.bbr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: Role of dorsal raphe serotonergic neurons. Journal of Neuroscience. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Dorey AA, Fleshner M. Therapeutic effects of exercise: Wheel running reverses stress-induced interference with shuttle box escape. Behavioral Neuroscience. 2007;121:992–1000. doi: 10.1037/0735-7044.121.5.992. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Moffitt JA, Beltz TG, Johnson AK. Reduced hedonic behavior and altered cardiovascular function induced by mild sodium depletion in rats. Behavioral Neuroscience. 2006;120:1133–1143. doi: 10.1037/0735-7044.120.5.1133. [DOI] [PubMed] [Google Scholar]
- Lambert GW, Jonsdottir IH. Influence of voluntary exercise on hypothalamic norepinephrine. Journal of Applied Physiology. 1998;85:962–966. doi: 10.1152/jappl.1998.85.3.962. [DOI] [PubMed] [Google Scholar]
- Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Archives of Neurology. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- Linke A, Adams V, Schulze PC, Erbs S, Gielen S, Fiehn E, Hambrecht R. Antioxidative effects of exercise training in patients with chronic heart failure: Increase in radical scavenger enzyme activity in skeletal muscle. Circulation. 2005;111:1763–1770. doi: 10.1161/01.CIR.0000165503.08661.E5. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Miliaressis E, Rompre PP, Laviolette P, Philippe L, Coulombe D. The curve-shift paradigm in self-stimulation. Physiology & Behavior. 1986;37:85–91. doi: 10.1016/0031-9384(86)90388-4. [DOI] [PubMed] [Google Scholar]
- Mondin GW, Morgan WP, Piering PN, Stegner AJ, Stotesbery CL, Trine MR, Wu MY. Psychological consequences of exercise deprivation in habitual exercisers. Medicine & Science in Sports & Exercise. 1996;28:1199–1203. doi: 10.1097/00005768-199609000-00018. [DOI] [PubMed] [Google Scholar]
- Morris MJ, Na ES, Grippo AJ, Johnson AK. The effects of deoxycorticosterone-induced sodium appetite on hedonic behaviors in the rat. Behavioral Neuroscience. 2006;120:571–579. doi: 10.1037/0735-7044.120.3.571. [DOI] [PubMed] [Google Scholar]
- Nakajima S, McKenzie GM. Reduction of the rewarding effect of brain stimulation by a blockade of dopamine D1 receptor with SCH 23390. Pharmacology, Biochemistry and Behavior. 1986;24:919–923. doi: 10.1016/0091-3057(86)90437-5. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Olds J. Hypothalamic substrates of reward. Physiological Reviews. 1962;42:554–604. doi: 10.1152/physrev.1962.42.4.554. [DOI] [PubMed] [Google Scholar]
- Sim YJ, Kim H, Kim JY, Yoon SJ, Kim SS, Chang HK, Kim CJ. Long-term treadmill exercise overcomes ischemia-induced apoptotic neuronal cell death in gerbils. Physiology & Behavior. 2005;84:733–738. doi: 10.1016/j.physbeh.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Sutoo DE, Akiyama K. The mechanism by which exercise modifies brain function. Physiology & Behavior. 1996;60:177–181. doi: 10.1016/0031-9384(96)00011-X. [DOI] [PubMed] [Google Scholar]
- Tillerson JL, Caudle WM, Reveron ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience. 2003;119:899–911. doi: 10.1016/S0306-4522(03)00096-4. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Greer TL, Grannemann BD, Chambliss HO, Jordan AN. Exercise as an augmentation strategy for treatment of major depression. Journal of Psychiatric Practice. 2006;12:205–213. doi: 10.1097/00131746-200607000-00002. [DOI] [PubMed] [Google Scholar]
- Tsatsoulis A, Fountoulakis S. The protective role of exercise on stress system dysregulation and comorbidities. Annals of the New York Academy of Sciences. 2006;1083:196–213. doi: 10.1196/annals.1367.020. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences, USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werme M, Messer C, Olson L, Gilden L, Thoren P, Nestler EJ, Brene S. Delta FosB regulates wheel running. Journal of Neuroscience. 2002;22:8133–8138. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werme M, Thoren P, Olson L, Brene S. Running and cocaine both upregulate dynorphin mRNA in medial caudate putamen. European Journal of Neuroscience. 2000;12:2967–2974. doi: 10.1046/j.1460-9568.2000.00147.x. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annual Review of Psychology. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Yeung RR. The acute effects of exercise on mood state. Journal of Psychosomatic Research. 1996;40:123–141. doi: 10.1016/0022-3999(95)00554-4. [DOI] [PubMed] [Google Scholar]
- Zheng H, Liu Y, Li W, Yang B, Chen D, Wang X, Halberg F. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behavioural Brain Research. 2006;168:47–55. doi: 10.1016/j.bbr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]