Abstract
Depletion of extracellular fluids motivates many animals to seek out and ingest water and sodium. Animals with a history of extracellular dehydration display enhanced sodium appetite and in some cases thirst. The progressive increase in sodium intake induced by repeated sodium depletions is known as sensitization of sodium appetite. Administration of the diuretic and natriuretic drug, furosemide, along with a low dose of captopril (furo/cap), elicits thirst and a rapid onset of sodium appetite. In the present studies the furo/cap model was used to explore the physiological mechanisms of sensitization of sodium appetite. However, when thirst and sodium appetite were measured concurrently in the furo/cap model, individual rats exhibited sensitization of either thirst or sodium appetite. In subsequent studies, thirst and sodium appetite were dissociated by offering either water prior to sodium or sodium before water. When water and sodium intake were dissociated in time, the furo/cap model reliably produced sensitization of sodium appetite. It is likely that neuroplasticity mediates this sensitization. Glutamatergic N-methyl-d-aspartate receptor (NMDA-R) activation is critical for the development of most forms of neuroplasticity. Therefore, we hypothesized that integrity of NMDA-R function is necessary for the sensitization of sodium appetite. Pharmacological blockade of NMDA-Rs with systemic administration of MK-801 (0.15mg/kg) prevented the sensitization of fluid intake in general when water and sodium were offered concurrently, and prevented sensitization of sodium intake specifically when water and sodium intake were dissociated. The involvement of NMDA-Rs provides support for the possibility that sensitization of sodium appetite is mediated by neuroplasticity.
Keywords: Sodium appetite, Thirst, Sensitization, Neuroplasticity
Introduction
When depleted of water and sodium many omnivores and herbivores exhibit thirst and sodium appetite. In the laboratory, thirst and sodium appetite are operationally defined as the seeking and ingestion of water and salty substances, respectively (Denton, 1982). When animals are in a state of sodium balance they display aversive behavioral reactions to intraoral delivery of hypertonic saline solutions and eschew ingesting hypertonic saline solutions (Berridge, Flynn, Schulkin, & Grill, 1984). However, in a state of sodium deficiency many animals, including man, display an increased preference for hypertonic saline solutions (Bare, 1949; Berridge, Flynn, Schulkin, & Grill, 1984; Smith & Stricker, 1969; Takamata, Mack, Gillen, & Nadel, 1994). When assessing sodium appetite in laboratory animals, hypertonic saline solution (1.5–3.0% NaCl W/V) intake is commonly used.
Extracellular dehydration is capable of enhancing sodium appetite and in some instances thirst. Rats with a history of sodium depletions exhibit enhanced sodium intake (Falk, 1965; Falk, 1966; Sakai, Fine, Epstein, & Frankmann, 1987; Sakai, Frankmann, Fine, & Epstein, 1989). Although a majority of studies have provided evidence for enhanced sodium intake, by inducing combined sodium and water deficits by intraperitoneal dialysis, Falk found evidence for enhanced water intake when water was offered in a single bottle test (Falk, 1965). When extracellular dehydration is induced repeatedly with the diuretic/natriuretic drug, furosemide, sodium intake gradually increases until it plateaus after 3 or 4 depletions (Sakai, Fine, Epstein, & Frankmann, 1987; Sakai, Frankmann, Fine, & Epstein, 1989). Finally, when sodium depletion is induced repeatedly with the water deprivation-partial rehydration protocol, rats exhibit enhanced sodium appetite (Pereira-Derderian, Vendramini, David, & Menani, 2010). These phenomena are known as sensitization of sodium appetite and thirst, or the increase in sodium or water intake that occurs in response to repeated extracellular dehydration.
Sensitization of fluid intake is an example of a change in behavior due to previous experience. Behavior is a product of the nervous system and, as such, any change in behavior can be associated with neuroplasticity – a change in neuron structure or function. Behavioral and physiological studies of mechanisms of neuroplasticity provide strong support for the idea that NMDA-R activation is necessary for the neuroplasticity that mediates sensitization, habituation, and associative learning and memory (Maren & Quirk, 2004; Trujillo & Akil, 1991; Wolf, 1998). It is likely that neuroplasticity mediates sensitization of sodium appetite. Therefore, we hypothesized that NMDA-R blockade would prevent sensitization of fluid intake.
Sodium appetite can be induced through depletion of extracellular fluids or directly stimulated through pharmacological means (Johnson & Thunhorst, 1997, 2008; Hurley, Thunhorst, & Johnson, in press). Generally these manipulations require a relatively long latency (i.e. on the order of hours to days) between the treatment and expression of sodium appetite. However, the concomitant administration of the diuretic furosemide and the angiotensin converting enzyme inhibitor captopril (furo/cap) induces a rapid sodium appetite and thirst with a latency of approximately an hour (Fitts & Masson, 1989; Masson & Fitts, 1989). The rapid sodium appetite observed in this model is the result of depletion-induced extracellular dehydration concomitant with a small decrease in blood pressure during water and sodium loss (Thunhorst & Johnson, 1994).
The rapid induction of sodium appetite in the furo/cap model allows for the expedient application of pharmacological manipulations prior to the induction and expression of salt appetite. However, the downside to the furo/cap protocol is that it elicits thirst and sodium appetite concurrently (Thunhorst & Johnson, 1994). As such, accurate measurements of sodium and water intake become problematic when both behaviors are measured at once.
In the present work we dissociated thirst from sodium appetite in the furo/cap model by altering the timing of water and sodium access and tested whether sensitization of thirst or sodium appetite would occur in the modified furo/cap paradigms. Additionally, we investigated whether systemic NMDA-R blockade with MK-801 would prevent sensitization of sodium appetite or thirst in two variations of the furo/cap model. The results of these experiments suggest that 1) altering the timing of water and sodium access during extracellular dehydration can have profound effects on the sensitization of fluid intake, and 2) NMDA-R activation is necessary for the sensitization of fluid intake.
2. Materials and methods
Subjects
All experiments were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by The University of Iowa Animal Care and Use Committee. Male Sprague Dawley rats (Harlan Teklad) that weighed between 275–300 grams upon arrival were used. Rats were allowed one week to acclimate to the environment prior to experimentation. They were maintained on a 12/12 light/dark cycle and housed in opaque shoebox (40.5 × 28.5 × 17.5 cm), translucent square (28.5 × 28.5 × 17.5 cm), or wire mesh suspended (24 × 17.2 × 17.0 cm) cages in a temperature and humidity controlled room. Unless noted otherwise, rats had ad libitum access to filtered tap water, 1.8% w/v NaCl solution, and NIH-31, irradiated, modified open-formula mouse/rat diet. Rats had at least 3 days of ad libitum 1.8% saline access before experimentation. In all experiments 1.8% saline intake was used to assess sodium appetite.
Dissociating thirst from sodium appetite in the furo/cap model
Initial experiments using furo/cap to induce thirst and sodium appetite in our laboratory found that when water and saline were offered concurrently, animals displayed sensitization of both thirst and saline intake. This protocol was modified to determine whether sensitization of water or saline intake would occur if the timing of saline and water presentation was altered.
Water and saline were removed and rats received subcutaneous furosemide (10 mg/kg, Hospira Inc, Lake Forest IL) and captopril (5 mg/kg, Sigma-Aldrich) injections. In one group of rats, water access was allowed immediately after furo/cap injections (n=7). Water intake was measured at 30 minutes and 60 minutes post-furo/cap treatment and then at 15-minute intervals until rats exhibited satiation of thirst, defined as the ingestion of <0.4 mls of water within a 15-minute interval. Upon satiation of thirst, rats were allowed access to hypertonic saline. Saline intakes were measured for 90 minutes at 15-minute intervals during the first hour and then at 90 minutes. This fluid presentation order will be referred to as the water-first/saline-second protocol. In addition, a ‘water only’ control group (n=7) received furo/cap with access to water alone over the same time period.
A separate group of rats (n=10) received furo/cap treatment but was not allowed access to water prior to saline presentation. In this experiment, saline was offered 90 minutes after furo/cap treatment to time-lock saline access with the water-first/saline-second protocol. Rats had access to saline alone for a duration of 90 minutes during which saline intake was recorded at 15, 30, and 90 minutes. Water was then provided and the intakes of water and saline were recorded for an additional 90 minutes at 15-minute intervals for the first 30 minutes and then at 90 minutes. This protocol is referred to as the saline-first/water-second protocol.
Sensitization of fluid intake
Rats from the water-first/saline-second protocol were given 3 additional furo/cap treatments for a total of 4 depletions and allowed to recover for 4 days between treatments. To control for observed changes in water intake over successive depletions, a separate group of rats was run in an identical protocol; however they were limited to 4 mls of water intake. An intake of 4 mls was chosen because that was the mean amount of water animals drank during the 4th depletion. To determine whether order of fluid presentation would affect fluid intake sensitization, rats from the saline-first/water second protocol also were repeatedly treated with furo/cap for a total of 4 depletions, each separated by 4 days.
Effect of MK-801 on fluid intake sensitization
All experiments investigating the effect of NMDA-R antagonism on sodium appetite sensitization used MK-801 (0.15 mg/kg, IP) or vehicle (95% saline, 5% DMSO). 0.15 mg/kg MK-801 was chosen based upon studies investigating NMDA-Rs in sensitization and habituation which found that a dose of 0.1 mg/kg MK-801 was sufficient to block those types of learning (Trujillo & Akil, 1991; Wolf, M. E., & Jeziorski, M. 1993). A slightly higher dose was used in the present experiments in order to compensate for increased excretion induced by furosemide. Drug or vehicle was administered 20 minutes prior to furo/cap treatment. In the concurrent fluid presentation protocol rats (n=14 per group) were treated with furo/cap and were offered water and saline concurrently 60 minutes after furo/cap treatment as approximately 60 minutes are required for rats to develop a sodium appetite after furo/cap treatment (Thunhorst & Johnson, 1994). Intakes were measured over a 3-hour time period at 15-minute intervals for the first hour, then at 30-minute intervals for the second hour and then a final recording was taken at 180 minutes. A total of 3 furo/cap treatments, each separated by 1 week, were administered. Urine was collected during the first hour and 20 minutes after MK-801 or vehicle injection. Urine volume, sodium concentration, and potassium concentration were determined with an ion selective electrode electrolyte analyzer (Nova Biomedical, Waltham MA).
To provide further confirmation of the reliability of the effect of MK-801 on sensitization of sodium appetite, the saline-first/water-second protocol was employed. Given that rats rapidly displayed sensitization of sodium appetite in this protocol (see Figure 4A), rats (n=9 in the vehicle group and n=8 in the MK-801 pretreated group) were pretreated with vehicle or MK-801 (0.15 mg/kg) during the first furo/cap treatment only. Rats were allowed to recover for 4 days and then received a second furo/cap treatment. During the second furo/cap treatment all subjects were pretreated with vehicle. Therefore, rats were tested in an MK-801-free state on their second depletion. Based upon the results from the saline-first/water-second protocol where rats exhibited sensitization of sodium appetite during the second depletion with no changes in water intake (see Figure 5), only saline intake was measured during this experiment. Saline intake was measured for a total of 90 minutes at 15-minute intervals for the first 30 minutes and then at 30-minute intervals for the last hour.
Figure 4.
Sensitization of saline intake in the water-first/saline-second protocol is not dependent on a decrease in water intake. Total 1.8% saline intake is displayed on the y-axis. When offered only 4 mls of water to drink during each test, rats displayed a progressive elevation of saline intake (*=p<0.05 vs. depletion 1, #=p<0.01 vs. depletion 1), similar to rats with unlimited water access. All data are expressed as mean ± SEM.
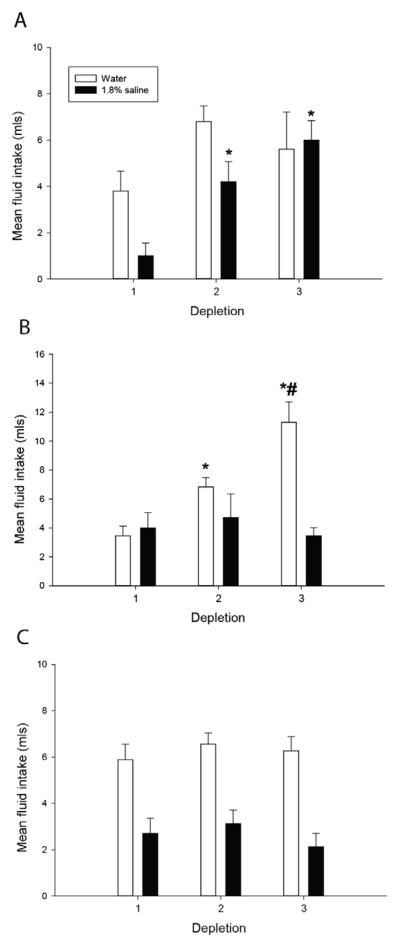
Figure 5.
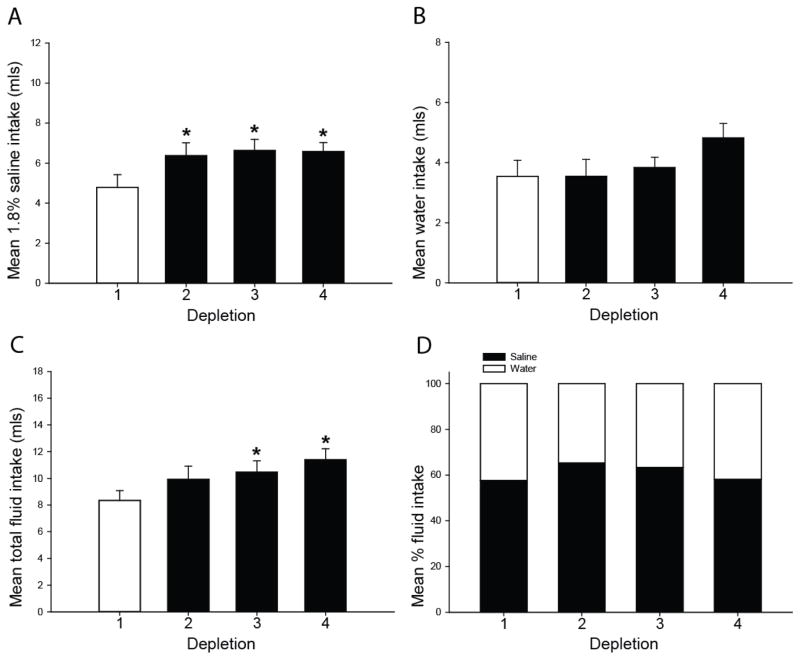
Sensitization of saline intake and total fluid intake in the furo/cap sodium-first/water-second protocol. 1.8% saline intake rapidly sensitizes upon repeated furo/cap treatments (A). Water intake does not significantly change over depletions (B). However, rats exhibit a progressive sensitization of total fluid intake (C). No significant differences in the composition of water or sodium during each depletion were observed (D). *=p<0.05 vs. depletion 1. All data are expressed as mean, and where error bars appear, ± SEM. Please note differences in ordinate across graphs.
Statistics
All results were analyzed using SigmaPlot 12.0 build 12.0.0.182 (Systat Software Inc, San Jose, CA) and α was set at 0.05. Water and saline intake in the studies investigating sensitization in modified furo/cap protocols were analyzed with one-way repeated measures ANOVAs. In studies examining the effect of MK-801 on sensitization, a two-way repeated measures ANOVA was utilized. When a significant main effect or interaction was observed from ANOVA analyses, Newman-Keul’s post tests were applied to determine group and depletion differences.
Results
Dissociation of thirst from sodium appetite
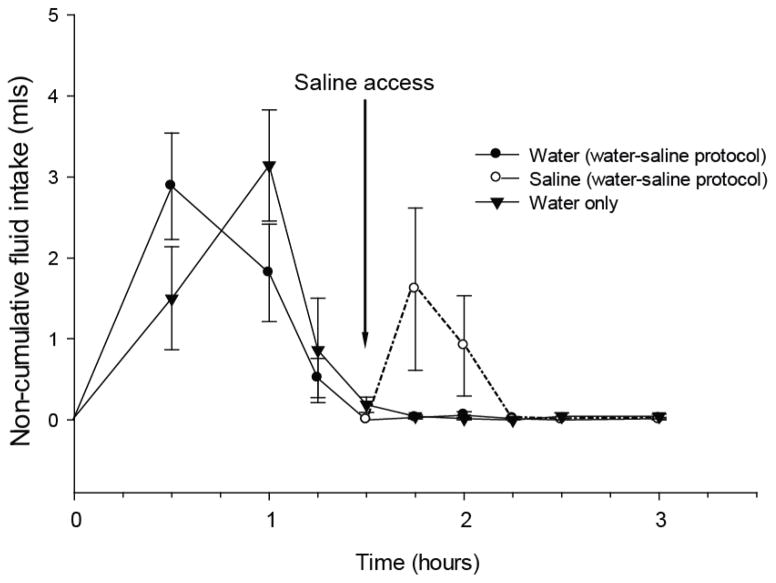
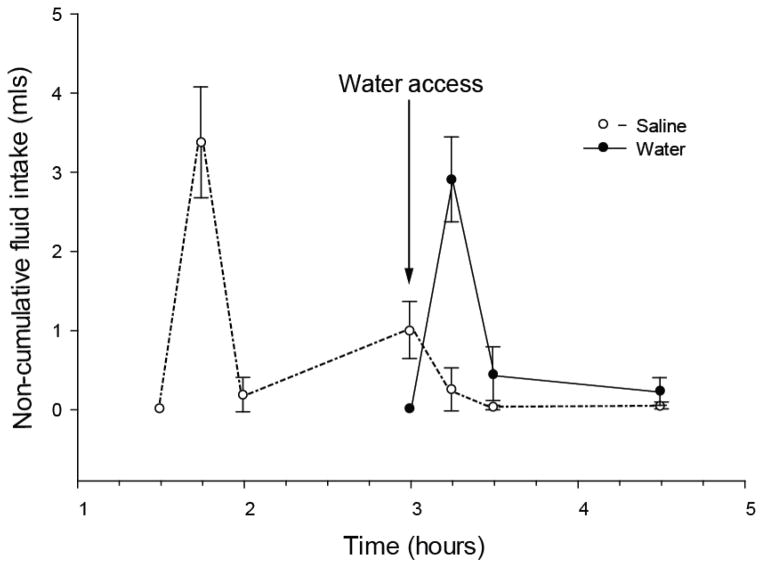
Results as depicted in Figure 1, show that thirst satiated 90 minutes after furo/cap treatment. When saline was offered at the end of this period, saline intake occurred without additional water intake (Figure 1). Rats offered water without saline access displayed similar water intake. In the saline-first/water-second protocol rats rapidly drank saline in the first 15 minutes and then drank only a small amount of saline over the next 75 minutes (Figure 2). After water was presented, rats drank minute amounts of saline; therefore it appears that water intake was also isolated from saline intake in this protocol.
Figure 1.
Dissociation of water and 1.8% saline intake in the water-first/saline-second furo/cap protocol. Non-cumulative intakes are presented on the y-axis and time is on the x-axis. Rats were offered water immediately after furo/cap treatment and allowed to drink until satiation which occurs at approximately 90 minutes. Saline access was then offered in addition to water (arrow). During this time rats drank hypertonic saline and essentially no water. All data are expressed as mean ± SEM.
Figure 2.
Dissociation of water and saline intake in the saline-first/water-second protocol. Non-cumulative intakes are presented on the y-axis and time is on the x-axis. Rats were offered saline 90 minutes after furo/cap treatment. Water was provided after 90 minutes of saline access (arrow). While water was available rats drank water and miniscule amounts of saline. All data are expressed as mean ± SEM.
Sensitization of sodium intake
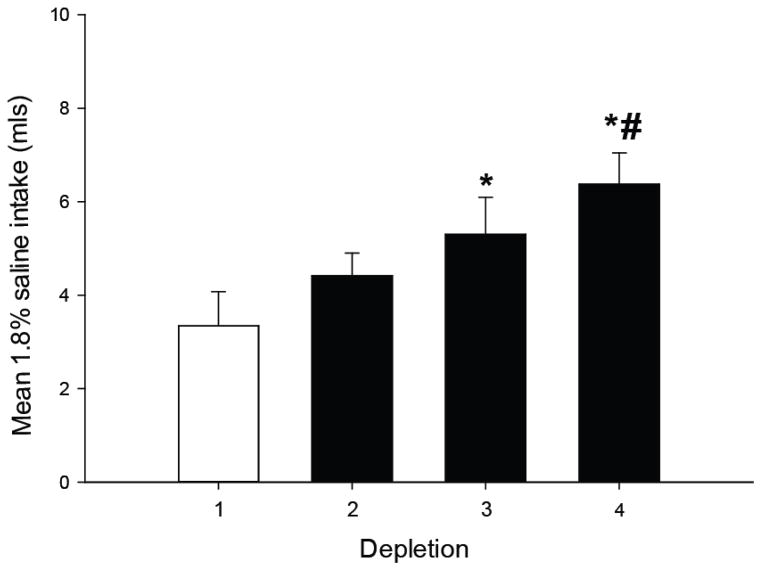
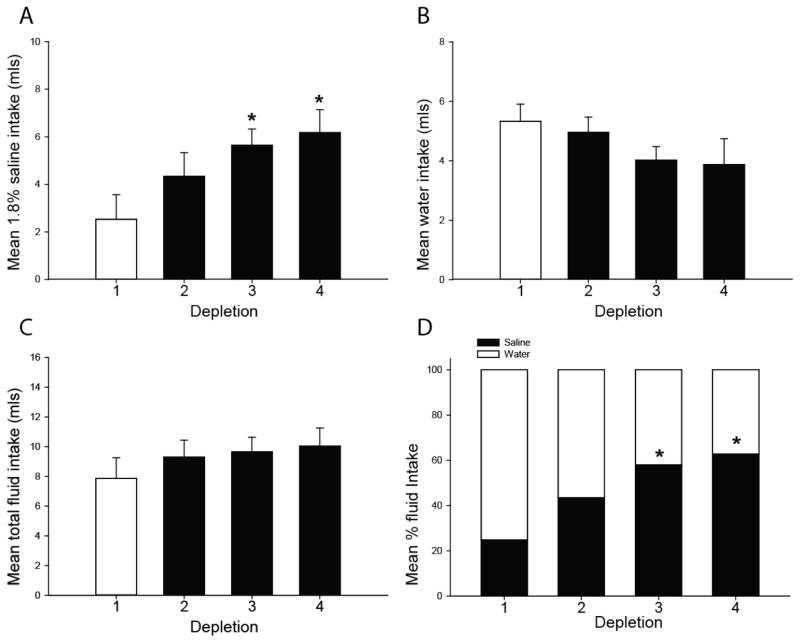
Regardless of the order of access to water or saline, sensitization of saline intake occurs with repeated furo/cap treatments. Specifically, in the water-first/saline-second protocol a main effect of depletion number on saline intake was found, F(3,18)=6.47; p<0.01. Post-hoc tests revealed rats drank significantly more saline on the third and fourth depletion. Therefore, it appeared that a progressive increase in saline intake occurred over repeated furo/cap treatments in this protocol (Figure 3A). Water intake did not significantly change, F(3,18)=2.09; p=0.14 (Figure 3B), nor did total fluid intake, F(3,18)=2.07; p=0.15 (Figure 3C). However, saline intake gradually increased to comprise a greater percentage of total fluid intake, F(3,18)=6.17; p<0.01 (Figure 3D). Enhanced saline intake that occurred in this protocol is not due to a decrease in water intake over successive depletions as rats with limited water access still displayed increased saline intake, F(3,18)=7.03, p<0.01 (Figure 4).
Figure 3.
Sensitization of saline intake in the furo/cap water-first/saline-second protocol. Repeated furo/cap treatments induce a progressive elevation in saline intake (A), but no change in water intake (B) or total fluid intake (C). Over repeated depletions saline intake comprises a greater percentage of fluid intake (D), suggesting a shift in preference from water to saline intake in the water-first/sodium-second protocol (*=p<0.01 vs. depletion 1). All data are expressed as mean, and where error bars appear, ± SEM. Please note differences in ordinate across graphs.
A different profile of sensitization occurred in the saline-first/water-second protocol. Analyses identified a main effect of depletion number on sodium intake, F(3,27)=4.56; p<0.05, and post-hoc tests revealed that rats drank more saline on the second, third, and fourth depletions (Figure 5A). Water intake did not significantly differ over depletions, F(3,27)=2.15; p=0.19 (Figure 5B), however total fluid intake did show a progressive increase such that total fluid intake was greater on the third and fourth depletions compared to the first, F(3,27)=4.61; p<0.05 (Figure 5C). The proportion of water and sodium intake did not change over repeated furo/cap treatments, F(3,27)=0.98; p=0.42 (Figure 5D).
Effect of MK-801 on the sensitization of sodium appetite and thirst
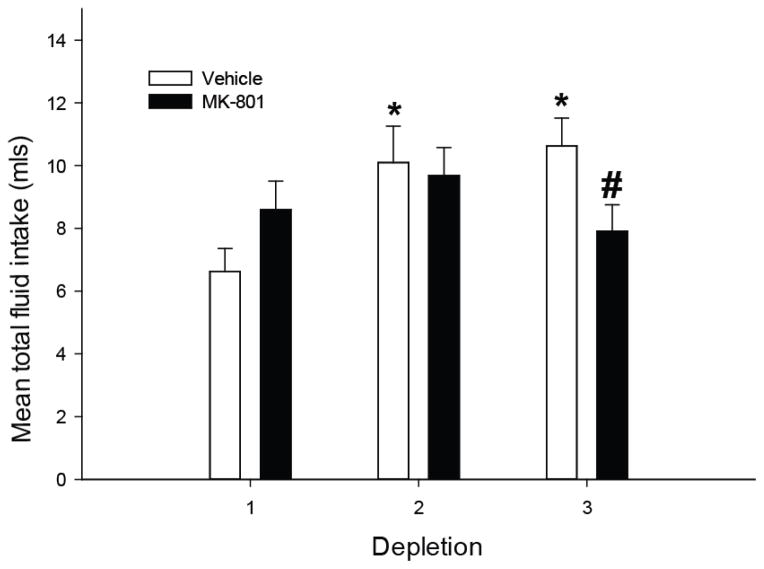
Initial analyses examining combined water and saline intake revealed that there was a significant main effect of depletion, F(2,52)=4.294; p<0.05, and a significant interaction, F(2,52)=4.26; p<0.05. Post-hoc tests showed that vehicle pretreated rats exhibited a sensitized total fluid intake such that combined water and saline intake was significantly greater on depletions 2 and 3 (Figure 6). This effect was blocked by MK-801 pretreatment such that vehicle pretreated rats exhibited greater overall fluid intake on the third depletion compared to MK-801 pretreated rats. MK-801 pretreated rats failed to show a significant elevation of fluid intake during the second and third depletion. Upon closer inspection it appeared that vehicle pretreated rats could be partialed into those that exhibited sensitization of thirst or sodium appetite (Figures 7A and 7B). However, MK-801 pretreatment effectively prevented any sensitization of water or sodium intake (Figure 7C). No significant differences were observed between MK-801 and vehicle pretreated rats in total fluid intake during the first or second furo/cap treatment. No significant differences in urine output were observed (Table 1).
Figure 6.
Effect of MK-801 pretreatment on sensitization of fluid intake when water and sodium were offered concurrently in the furo/cap model. Vehicle pretreated rats exhibited sensitized fluid intake by the second depletion (*=p<0.005 vs vehicle, depletion 1) while MK-801 pretreated rats failed to show any increase in total fluid intake (#=p<0.05 vs vehicle, depletion 3). No significant differences were observed between vehicle and MK-801 pretreated rats during the first or second depletions. No significant differences were observed in total fluid intake during the first depletion. All data are expressed as mean ± SEM.
Figure 7.
Water and saline intakes of vehicle pretreated rats from the concurrent fluid access protocol segregated by whether they exhibited sensitization of saline intake (A) or water intake (B). MK-801 pretreated rats are represented in (C). Rats were categorized as sodium appetite sensitizers if the increase in total fluid intake between the first and third depletion consisted of a 45% or greater increase in saline intake. Two vehicle pretreated rats did not exhibit sensitization of water or saline intake and are not included in this figure. In general, vehicle pretreated rats in the concurrent fluid access protocol displayed either sensitization of water intake (A) or sensitization of saline intake (B), while MK-801 pretreated rats failed to exhibit any changes in fluid intake (C). *=p<0.05 vs depletion 1, #=p<0.01 vs. depletion 2. All data are expressed as mean ± SEM.
Table 1.
Mean urine volume, potassium content, and sodium content in vehicle or MK-801 pretreated rats across depletions. No significant differences were observed.
| Depletion | 1 | 2 | 3 | |
|---|---|---|---|---|
| Urine Volume (mls) | Vehicle | 8.9 | 9.5 | 9.5 |
| MK-801 | 10.3 | 10.4 | 10.4 | |
| Potassium Content (mmol) | Vehicle | .31 | .35 | .35 |
| MK-801 | .31 | .3 | .34 | |
| Sodium Content (mmol) | Vehicle | .95 | 1.1 | 1 |
| MK-801 | .96 | 1 | 1 |
Effect of MK-801 on the sensitization of sodium appetite per se
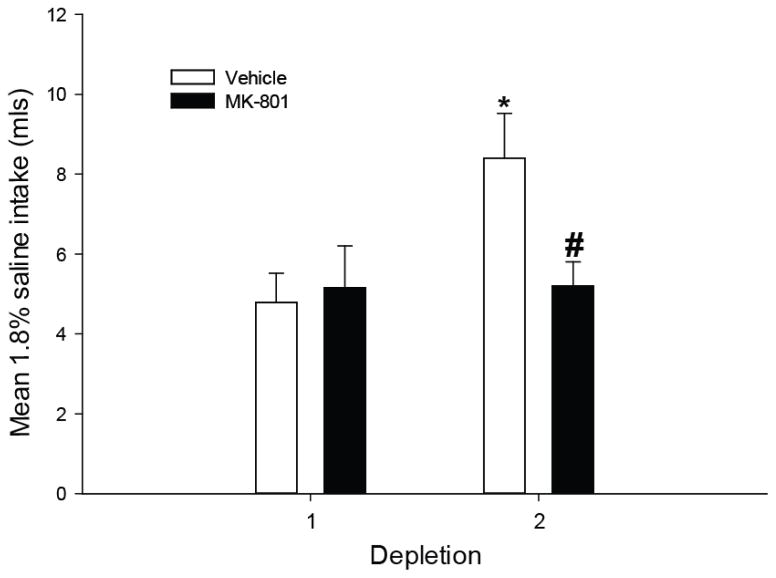
Given that rats developed rapid sodium appetite sensitization in the saline-first/water-second protocol, this protocol was chosen to further examine whether MK-801 would prevent sodium appetite sensitization. Rats were pretreated with MK-801 during the first depletion only, and all rats received a vehicle injection prior to the second depletion. Analyses identified a significant effect of drug pretreatment F(1,15)=7.963; p<0.05 and a significant interaction F(1,15)=7.634, p<0.05. Post-hoc tests revealed that vehicle pretreated rats exhibited greater saline intake during the second depletion and MK-801 pretreated rats failed to show an increase in saline intake (Figure 8). Furthermore, MK-801 pretreated rats drank significantly less saline on the second depletion compared to vehicle pretreated rats. Therefore, a single pretreatment with MK-801 was sufficient to block the sensitization of sodium appetite. Importantly, no significant differences were observed during the first furo/cap treatment which suggests MK-801 pretreatment did not have non-specific effects to disrupt water or sodium intake, but specifically prevented the development of sensitization of sodium appetite.
Figure 8.
Effect of MK-801 pretreatment on sensitization of saline intake in the saline-first/water-second protocol. Vehicle pretreated rats exhibit enhanced saline intake by the second depletion (*=p<0.01 vs. vehicle, depletion 1) while MK-801 pretreated rats fail to display increased intake (#=p<0.05 vs. vehicle, depletion 2). All data are expressed as mean ± SEM.
Discussion
The present findings show that the dynamics of water and sodium intake stimulated by furo/cap treatment are affected by the order in which the fluids are presented. Nonetheless, when the timing of water and saline presentation is controlled by the experimenter, the sensitization of sodium appetite occurs reliably and is independent of whether water or saline access is offered first. Furthermore, NMDA-Rs, which have been heavily implicated in the initiation of neuroplasticity, are critical for sensitization of thirst and sodium appetite.
The order of fluid presentation alters the amount of water and sodium intake seen in the furo/cap model. When water is offered prior to sodium a progressive increase in sodium intake occurs. Interestingly, total fluid intake does not change, but a greater amount of sodium is ingested after each depletion. This suggests that rats exhibit a preference shift from water to sodium after repeated depletions. This preference shift could be an example of incentive relativity (Flaherty, 1996), or a change in reward seeking and consumption produced by prior experience with rewards. Specifically, it may be that the experience of ingesting hypertonic saline during extracellular dehydration results in learning that hypertonic saline provides a greater reward or incentive value than water. This might be explained by physiological evidence demonstrating that repeated sodium depletions alter dendritic morphology (Roitman, Na, Anderson, Jones, & Bernstein, 2002) and c-fos expression (Na, Morris, Johnson, Beltz, & Johnson, 2007) in the nucleus accumbens, a brain area critical in the processes of motivation and reward. It is also possible that the ingestion of water prior to hypertonic saline leads to a hypo-osmotic state. Rats may have learned to shift their preference from water to hypertonic saline to reduce hypo-osmosity. It is interesting to note that one common model used to study salt appetite is extracellular dehydration induced by administration of the diuretic furosemide alone (Frankmann, Dorsa, Sakai, & Simpson, 1986; Sakai, Fine, Epstein, & Frankmann, 1987). In this experimental model of sodium appetite water is given after diuretic treatment but sodium access is delayed for 20–24 hours. Sodium appetite increases in a progressive fashion (i.e. sensitizes) in the furosemide-only model of extracellular dehydration, similar to the water-first/sodium-second protocol employed here (Sakai, Fine, Epstein, & Frankmann, 1987; Sakai, Frankmann, Fine, & Epstein, 1989).
In contrast, when sodium is offered prior to water, rats exhibit a rapid and robust sensitization of sodium appetite that is apparent upon the second depletion. In addition, total fluid intake increases on the third and fourth depletion in this protocol. The increase in total fluid intake observed in this protocol may be due to the ingestion of sufficient sodium on the third and fourth depletion to induce cellular dehydration and a state of osmotic thirst resulting in enhanced water intake to ultimately drive the increase in total fluid intake.
Unfortunately, the present experiments cannot address the circumstances that result in sensitization of thirst. Sensitization of thirst has been reported when water access alone was provided on a second extracellular dehydration (Falk, 1965). Others have found that rats repeatedly treated with furo/cap exhibit sensitized thirst and sodium appetite when water and saline were offered concurrently (Pereira, Menani, & De Luca Jr., 2010). In addition, repeated administration of intracerebroventricular angiotensin II results in enhanced angiotensin II-induced water intake when water is offered in a single bottle test (Moellenhoff et al., 2001). Sensitization of thirst only occurred in a subset of animals in the concurrent fluid access protocol in the present experiments. Our findings, in addition to prior research on sensitization of fluid intake (Bryant, Epstein, Fitzsimons, & Fluharty, 1980; Falk, 1965; Falk, 1966; Sakai, Fine, Epstein, & Frankmann, 1987; Sakai, Frankmann, Fine, & Epstein, 1989), suggest that sensitization of sodium appetite is more reliable, especially when the timing of water and saline access is controlled by the experimenter.
With respect to the physiological mechanisms of sensitization of fluid intake, blockade of NMDA-Rs with systemic MK-801 was sufficient to prevent sensitization of sodium appetite, and when water and sodium were offered together, sensitization of fluid intake in general. These findings implicate glutamatergic NMDA-Rs in experience-dependent changes in fluid intake. Critically, MK-801 was capable of blocking sodium appetite sensitization in the sodium-first/water-second protocol when it was administered during the first depletion only paradigm. This provides strong evidence that NMDA-R blockade prevents the sensitization of sodium appetite without affecting the expression of sodium appetite. An alternate explanation of the present findings is that MK-801 treatment induced nausea or taste aversion. Admittedly, the dose of MK-801 employed in these experiments is higher than the commonly used 0.1 mg/kg dose (Trujillo & Akil, 1991; Wolf & Jeziorski, 1993). A higher dose (0.15 mg/kg) was employed to compensate for enhanced urine excretion induced by furosemide. However, others have found that a dose of 0.3 mg/kg MK-801 is required to produce a conditioned taste aversion to saccharin (Jackson & Sanger, 1989), and a dose of 0.2 mg/kg MK-801 failed to induce a conditioned taste aversion (Traverso, Ruiz, & De la Casa, 2003). This suggests that the 0.15 mg/kg dose employed here probably did not produce a conditioned taste aversion. In addition, our results cannot be explained by differences in urine excretion as we failed to find any significant effect of MK-801 or depletion number on diuretic/natriuretic-induced urine output.
Although our findings support a role for glutamate and NMDA-R activation in the sensitization of fluid intake, the circumstances that lead to glutamate release during extracellular dehydration remain unknown. It may be the case that during hypovolemia-induced thirst and sodium appetite, angiotensin II and aldosterone act on and in the central nervous system (Epstein, 1982; Fluharty & Epstein, 1983) to promote glutamate release and initiate NMDA-R mediated plasticity. In support of a role of angiotensin II and aldosterone in the sensitization of sodium appetite, repeated administration of angiotensin II into the ventricles (Bryant, Epstein, Fitzsimons, & Fluharty, 1980) or administration of ventricular angiotensin II infusions combined with peripheral aldosterone injections induce sensitization of sodium appetite (Sakai, Fine, Epstein, & Frankmann, 1987). Future work investigating the relationship between angiotensin II, aldosterone, glutamate, and NMDA-Rs may provide further insight into the physiological mechanisms governing the sensitization of sodium appetite.
Sensitization of sodium appetite may be mediated by two central nervous system circuits; those involved in body fluid homeostasis and those involved with motivation and reward. Rats with a history of extracellular dehydration display enhanced neuron dendritic arborization and length in the nucleus accumbens shell (Roitman, Na, Anderson, Jones, & Bernstein, 2002). They also exhibit greater sodium depletion-induced expression of the immediate early gene c-fos, a putative marker of neuronal activity, in brain areas involved in body fluid homeostasis and areas involved in motivation and reward compared to rats receiving a sodium depletion for the first time (Na, Morris, Johnson, Beltz, & Johnson, 2007). Specifically, rats with a history of extracellular dehydration express greater fos in limbic areas such as the nucleus accumbens shell and core, medial prefrontal cortex, and basolateral amygdala in addition to brain areas involved in body fluid homeostasis, such as the subfornical organ and paraventricular nucleus of the hypothalamus. Future experiments will aim to delineate what central nervous systemic mechanisms mediate sodium appetite sensitization.
Human Health Implications
Sodium intake in Westernized societies is far in excess of physiological need (Appel et al., 2011; Brown, Tzoulaki, Candeias, & Elliott, 2009; Izzo & Black, 2003). Excess sodium intake has been reported to contribute to cardiovascular disease (Brown, Tzoulaki, Candeias, & Elliott, 2009; Dahl & Love, 1957; Dahl, 1972; Stamler, 1997), but, currently, little is known about the physiological mechanisms that drive excess sodium intake. The progressive increase in sodium intake that occurs over the initial 3 or 4 bouts of sodium depletion provides an animal model of experience-induced excess sodium intake. Research that delineates the physiological mechanisms of excess sodium intake may lead to interventions or treatments that could prevent the development of excess salt consumption or reduce sodium appetite to ultimately improve general health. The findings of this study support the possibility that the experience of extracellular dehydration and activation of NMDA-Rs plays a role in excess sodium intake in Western societies.
References
- Appel LJ, Frohlich ED, Hall JE, Pearson TA, Sacco RL, Seals DR, Sacks FM, Smith SC, Jr, Vafiadis DK, Van Horn LV. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: A call to action from the american heart association. Circulation. 2011;123(10):1138–1143. doi: 10.1161/CIR.0b013e31820d0793. [DOI] [PubMed] [Google Scholar]
- Bare JK. The specific hunger for sodium chloride in normal and adrenalectomized white rats. Journal of Comparative and Physiological Psychology. 1949;42(4):242. doi: 10.1037/h0057987. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Flynn FW, Schulkin J, Grill HJ. Sodium depletion enhances salt palatability in rats. Behavioral Neuroscience. 1984;98(4):652–660. doi: 10.1037//0735-7044.98.4.652. [DOI] [PubMed] [Google Scholar]
- Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: Implications for public health. International Journal of Epidemiology. 2009;38(3):791–813. doi: 10.1093/ije/dyp139. [DOI] [PubMed] [Google Scholar]
- Bryant R, Epstein A, Fitzsimons J, Fluharty S. Arousal of a specific and persistent sodium appetite in the rat with continuous intracerebroventricular infusion of angiotensin II. The Journal of Physiology. 1980;301(1):365. doi: 10.1113/jphysiol.1980.sp013211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl LK. Salt and hypertension. The American Journal of Clinical Nutrition. 1972;25(2):231–244. doi: 10.1093/ajcn/25.2.231. [DOI] [PubMed] [Google Scholar]
- Dahl LK, Love RA. Etiological role of sodium chloride intake in essential hypertension in humans. Journal of the American Medical Association. 1957;164(4):397. doi: 10.1001/jama.1957.02980040037010. [DOI] [PubMed] [Google Scholar]
- Denton DA. The hunger for salt: An anthropological, physiological, and medical analysis. Springer-Verlag; New York: 1982. [Google Scholar]
- De Luca LA, Jr, Pereira-Derderian DT, Vendramini RC, David RB, Menani JV. Water deprivation-induced sodium appetite. Physiology & Behavior. 2010;100(5):535–44. doi: 10.1016/j.physbeh.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Epstein AN. Mineralocorticoids and cerebral angiotensin may act together to produce sodium appetite. Peptides. 1982;3(3):493–494. doi: 10.1016/0196-9781(82)90113-9. [DOI] [PubMed] [Google Scholar]
- Falk JL. Water intake and NaCl appetite in sodium depletion. Psychological Reports. 1965;16:315–25. doi: 10.2466/pr0.1965.16.1.315. [DOI] [PubMed] [Google Scholar]
- Falk JL. Serial sodium depletion and NaCl solution intake. Physiology & Behavior. 1966;1(1):75–77. [Google Scholar]
- Fitts DA, Masson DB. Forebrain sites of action for drinking and salt appetite to angiotensin or captopril. Behavioral Neuroscience. 1989;103(4):865. doi: 10.1037/h0092457. [DOI] [PubMed] [Google Scholar]
- Flaherty CF. Incentive relativity. New York, New York: Cambridge University Press; 1996. [Google Scholar]
- Fluharty SJ, Epstein AN. Sodium appetite elicited by intracerebroventricular infusion of angiotensin II in the rat: II. synergistic interaction with systemic mineralocorticoids. Behavioral Neuroscience. 1983;97(5):746. doi: 10.1037//0735-7044.97.5.746. [DOI] [PubMed] [Google Scholar]
- Frankmann SP, Dorsa DM, Sakai RR, Simpson JB. The Physiology of Thirst and Sodium Appetite. NATO ASI Series. Plenum Press; New York, NY: 1986. A single experience with hyperoncotic colloid dialysis persistently alters water and sodium intake. [Google Scholar]
- Heale V, Harley C. MK-801 and AP5 impair acquisition, but not retention, of the morris milk maze. Pharmacology, Biochemistry, and Behavior. 1990;36(1):145–149. doi: 10.1016/0091-3057(90)90140-d. [DOI] [PubMed] [Google Scholar]
- Hurley SW, Thunhorst RL, Johnson AK. Neural and behavioral sensitization in salt appetite. In: De Luca LA, Johnson AK, Menani JV, editors. Neurobiology of body fluid homeostasis: Transduction and integration. Taylor and Francis; In press. [PubMed] [Google Scholar]
- Izzo JL, Black HR. Hypertension primer: The essentials of high blood pressure. Lippincott Williams & Wilkins; 2003. [Google Scholar]
- Jackson A, Sanger DJ. Conditioned taste aversions induced by phencyclidine and other antagonists of N-methyl-D-aspartate. Neuropharmacology. 1989;28(5):459–464. doi: 10.1016/0028-3908(89)90079-8. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Thunhorst RL. The neuroendocrinology of thirst and salt appetite: visceral sensory signals and mechanisms of central integration. Frontiers in Neuroendocrinology. 18(3):292–353. doi: 10.1006/frne.1997.0153. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Thunhorst RL. The Neuroendocrinology, Neurochemistry and Molecular Biology of Thirst and Salt Appetite. In: Abel L, Blaustein JD, editors. Handbook of Neurochemistry and Molecular Neurobiology: Behavioral Neurochemistry, Neuroendocrinology and Molecular Neurobiology. Plenum Press; New York, NY: [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nature Reviews Neuroscience. 2004;5(11):844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Masson DB, Fitts DA. Subfornical organ connectivity and drinking to captopril or carbachol in rats. Behavioral Neuroscience. 1989;103(4):873. doi: 10.1037/h0092456. [DOI] [PubMed] [Google Scholar]
- Moellenhoff E, Blume A, Culman J, Chatterjee B, Herdegen T, Lebrun CJ, Unger T. Effect of repetitive icv injections of ANG II on c-fos and AT1-receptor expression in the rat brain. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2001;280(4):R1095. doi: 10.1152/ajpregu.2001.280.4.R1095. [DOI] [PubMed] [Google Scholar]
- Na ES, Morris MJ, Johnson RF, Beltz TG, Johnson AK. The neural substrates of enhanced salt appetite after repeated sodium depletions. Brain Research. 2007;1171:104–110. doi: 10.1016/j.brainres.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira DTB, Menani JV, De Luca LA., Jr FURO/CAP: A protocol for sodium intake sensitization. Physiology & Behavior. 2010;99(4):472–481. doi: 10.1016/j.physbeh.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Na E, Anderson G, Jones TA, Bernstein IL. Induction of a salt appetite alters dendritic morphology in nucleus accumbens and sensitizes rats to amphetamine. J Neurosci. 2002;22(11):RC225. doi: 10.1523/JNEUROSCI.22-11-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai RR, Fine WB, Epstein AN, Frankmann SP. Salt appetite is enhanced by one prior episode of sodium depletion in the rat. Behavioral Neuroscience. 1987;101(5):724. doi: 10.1037//0735-7044.101.5.724. [DOI] [PubMed] [Google Scholar]
- Sakai RR, Frankmann SP, Fine WB, Epstein AN. Prior episodes of sodium depletion increase the need-free sodium intake of the rat. Behavioral Neuroscience. 1989;103(1):186. doi: 10.1037//0735-7044.103.1.186. [DOI] [PubMed] [Google Scholar]
- Smith DF, Stricker EM. The influence of need on the rat’s preference for dilute NaCl solutions. Physiology & Behavior. 1969;4(3):407–410. [Google Scholar]
- Stamler J. The INTERSALT study: Background, methods, findings, and implications. The American Journal of Clinical Nutrition. 1997;65(2 Suppl):626S–642S. doi: 10.1093/ajcn/65.2.626S. [DOI] [PubMed] [Google Scholar]
- Takamata A, Mack GW, Gillen CM, Nadel ER. Sodium appetite, thirst, and body fluid regulation in humans during rehydration without sodium replacement. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1994;266(5):R1493–R1502. doi: 10.1152/ajpregu.1994.266.5.R1493. [DOI] [PubMed] [Google Scholar]
- Thunhorst RL, Johnson A. Renin-angiotensin, arterial blood pressure, and salt appetite in rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1994;266(2):R458. doi: 10.1152/ajpregu.1994.266.2.R458. [DOI] [PubMed] [Google Scholar]
- Traverso L, Ruiz G, De la Casa L. Latent inhibition disruption by MK-801 in a conditioned taste-aversion paradigm. Neurobiology of Learning and Memory. 2003;80(2):140–146. doi: 10.1016/s1074-7427(03)00059-5. [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991;251(4989):85–87. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Jeziorski M. Coadministration of MK-801 with amphetamine, cocaine or morphine prevents rather than transiently masks the development of behavioral sensitization. Brain Research. 1993;613(2):291–294. doi: 10.1016/0006-8993(93)90913-8. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Progress in Neurobiology. 1998;54(6):679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]