Abstract
Sodium deficiency reliably produces a robust intake of saline in rats, which is associated with an increased preference for sodium solutions at hypertonic concentrations that would normally be avoided. The mechanisms underlying the shift to an increased preference for sodium in the deficient state are not well understood. The current experiments examined the role of opioids on changes of behavioral responses that are modified as a function of body sodium status by studying the intake of 0.3 M saline in a free access drinking test and by characterizing the changes in orofacial-related behaviors in response to intra-orally delivered 0.3 M NaCl. In intake tests, systemic treatment with morphine and naltrexone, respectively enhanced and attenuated intake of 0.3 M saline in sodium depleted rats. In taste reactivity tests systemic treatment with morphine significantly decreased negative responses to 0.3 M saline infusions in both sodium replete and sodium depleted rats. Systemically administered naltrexone significantly decreased positive hedonic responses to 0.3 M saline infusions only in sodium depleted rats. These results indicate that peripheral administration of opioid agonists and antagonists alter both hypertonic saline ingestion in a free access situation and taste reactivity responses to hypertonic saline under sodium replete and deplete conditions. The results indicate that endogenous opioids alter the processing of central information to affect hedonic mechanisms that influence behaviors related to sodium consumption and palatability.
Keywords: Sodium appetite, morphine, naltrexone, taste reactivity test
1. Introduction
Excessive sodium intake is a common practice with an average NaCl intake of approximately 10 g/day in industrialized nations. This is an amount 4 g greater than that recommended by the United States Food and Drug Administration [1–4]. It has been argued that excess dietary sodium intake is associated with an increased incidence of cardiovascular-related illnesses such as hypertension and heart disease [5]. Despite substantial evidence indicating that daily salt intake greatly exceeds physiological need, the cause of this excessive behavior remains elusive. A factor that may contribute to the prevalent use of salt is its ability to enhance the palatability of foods. For example, both hypertensive and heart failure patients are commonly prescribed a low sodium diet as a nonpharmacological treatment [6]. Adherence to a low sodium diet has proved to be remarkably difficult to maintain with many patients reporting a lack of palatability for low sodium foods as one of the important reasons for non-compliance [7, 8].
Sodium replete rats given a two bottle choice test of different concentrations of saline and water typically prefer concentrations close to isotonic, and most strains will drink substantially more 1% saline than water when given 24 h access [9]. Under fluid replete, or near replete, conditions most strains of rat prefer close to isotonic and eschew hypertonic salt solutions. The results of taste reactivity tests are consistent with free access drinking studies with sodium replete rats displaying largely aversive orofacial responses to hypertonic saline solutions [10, 11].
Experimentally-induced sodium deficiency provokes robust ingestion of hypertonic concentrations of saline, with an increase in positive hedonic responses and a decrease in negative affective responses to hypertonic saline [10, 11]. In other words hypertonic concentrations become more palatable when in a state of sodium deficiency which supports the hypothesis that a given taste stimulus can induce pleasant or unpleasant sensations depending upon the homeostatic state of an animal or individual [12]. The neurochemical mechanisms associated with such increases in positive hedonic responses have not been identified; however evidence suggests that opioids may contribute to the enhanced palatability of saline in sodium deficient rats. For example, Lucas, Grillo, and McEwen [13] demonstrated an increase in enkephalin mRNA in the striatum of sodium depleted rats given 2 h access to 2% saline. Moreover, saline intake was attenuated in sodium depleted rats after administration of naltrindole, a delta opioid receptor antagonist.
In intake studies where animals had free access to 3% NaCl, Hubbell and McCutcheon [14] demonstrated that opioid receptor agonists and antagonists modified the intake of saline. Specifically, in sodium deficient rats, systemically administered morphine and naltrexone enhanced or attenuated hypertonic saline intake, respectively. Hubbell and McCutcheon depleted their rats with the diuretic/natriuretic drug furosemide three times and then administered a fourth sodium depletion in association with graded systemic doses of the general opioid receptor antagonist naltrexone. One week later rats were tested again and given 5 doses of the general opioid receptor agonist morphine. Intakes of 3% saline and water were measured after naltrexone or morphine treatment in a 2 h free access sodium appetite test. Morphine increased 3% saline intake two fold at doses ranging from 0.3–3 mg/kg. In contrast, naltrexone decreased 3% saline consumption by 25–40% when given doses ranging from 0.1–10 mg/kg. One caveat to Hubbell and McCutcheon’s experiments is that rats were sodium depleted multiple times, which have been shown to produce sensitization such that the intake of saline incrementally increases following each of the initial 2 to 3 bouts of sodium loss [15–18]. Opioids have been implicated in playing a role in many forms of neuroplasticity [19–22] including sensitization, and it is unknown what effects opioid receptor agonists and antagonists may have in animals with a prior history of sodium deficiency.
The purpose of the present studies was to examine the effects of opioids on both intake and taste reactivity responses to 0.3 M saline. Fluid intake tests and taste reactivity tests were used to determine the intake and palatability of sodium under both sodium replete and deplete conditions in morphine or naltrexone treated rats. In our first experiment the paradigm differed from that used by Hubbell and McCutcheon [14] in that rats were only sodium depleted once during testing. Naïve rats were used because rats depleted of sodium multiple times become sensitized to the effects of sodium deficiency and increase 0.3 M saline intake over the course of multiple sodium depletions [15, 17, 18]. Thus, in order to avoid potentially confounding sensitization effects that might induce changes that alter saline intake, the studies were conducted in rats never previously subjected to experimental sodium deficiency (Experiment 1). It was hypothesized that under conditions of sodium depletion the preferential μ-opioid receptor agonist, morphine, would increase 0.3 M saline intake while the general opioid receptor antagonist, naltrexone, would attenuate intake of 0.3 M saline.
Because the results of Experiment 1 supported our initial hypothesis, we then conducted further experiments to determine if the changes in the palatability of sodium produced as a function of sodium depletion were related to opioid mechanisms. As was mentioned previously, intraoral responses to hypertonic saline change when animals are in a sodium deficient state [10, 11]. However, it is not known if the opioid system is involved in mediating this behavioral response. Thus, Experiments 2 and 3 utilized the taste reactivity test which has been used as a behavioral test of palatability [10, 23] to assess the hedonic value of sodium under sodium replete and deplete conditions. We studied rats under fluid replete and deplete conditions in conjunction with opioid agonist and antagonists treatments. Naltrexone (0.5 or 1 mg/kg; Experiment 2) and morphine (2 or 4 mg/kg; Experiment 3) were systemically administered and taste reactivity responses to intraoral (I–O) infusions of 0.3 M saline were assessed. We predicted that systemic treatment with morphine would enhance positive appetitive taste reactivity responses and/or would decrease negative affective responses to 0.3 M saline in sodium replete rats, and that systemic naltrexone treatment would either decrease positive hedonic responses or enhance negative affective responses to 0.3 M saline in sodium depleted rats.
2. Material and Methods
2.1 Animals
Male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN), weighing 250–350 g at the start of an experiment were used in all studies. All experimental procedures were approved by The University of Iowa Institutional Animal Care and Use Committee. Rats were adapted to the laboratory for one week prior to testing and given ad libitum access to food (#7013, NIH-31 Modified Open Formula Diet, 0.33% sodium, Harlan Laboratories) and tap water. Rats were provided 0.3 M saline and sodium deficient chow (MP Biomedicals, catalogue number 902902) for 3–5 days prior to experimentation. Baseline intakes of 0.3 M saline were recorded daily. The animals were maintained on a 12:12 h light-dark cycle (lights on at 0600 hr) in temperature controlled rooms maintained at 22° C. Testing was conducted at the same time every day from 1300–1600.
2.2 Surgical Procedures
2.2.1 Implantation of intraoral (I–O) cannulas
For experiments 2 and 3, I–O cannulas were implanted into experimentally naïve animals to assess positive hedonic and negative affective responses to 0.3 M saline [10, 11, 23, 24]. I–O cannulas were constructed of polyethylene (PE 50) tubing, flared at one end. A 0.7 cm rubber washer was placed flush against the flared end to anchor the cannula inside the oral cavity. Rats were anesthetized with Ketamine (100 mg/kg, i.p.), and using aseptic techniques, the I–O cannulas were unilaterally implanted by trocar insertion lateral to the first maxillary molar and passed through the cheek beneath the eye. A small incision was made in the scapular area behind the head as an exit for the probe [25]. Ketofen (5 mg/kg, s.c.) was administered for postoperative analgesia. Rats recovered for 3–5 days during which time they were given ad libitum access to moistened powdered chow to facilitate ingestion. The cannulas were flushed with distilled water every other day to prevent occlusion. Rats were habituated to the testing chamber 3 times before experimentation, and during this time I–O cannulas were infused with 1.25 ml of distilled water over the course of 5 min to acclimate animals to the infusion protocol.
2.3 Induction of sodium appetite and sodium appetite test
Rats were given two subcutaneous injections of furosemide (10 mg/kg spaced 1 h apart for a total of 20 mg/kg) to induce water and sodium depletion by natriuresis and diuresis or two comparable isotonic saline injections as a control treatment (i.e., sham depleted) [16]. Over the 3 h period following furosemide administration, body weight changes and volume of urine excreted were recorded to verify the efficacy of the diuretic treatment. A minimum of 15 g of weight loss was specified as the criterion for a verifiable diuresis. Rats were given ad libitum access to distilled water as well as sodium deficient chow. Overnight intakes of distilled water were recorded. Intake and taste reactivity tests were conducted beginning approximately 24 h after furosemide treatments. Graduated cylinders were used for intake tests to determine the amount of fluid consumed.
2.4 Taste reactivity test
Rats were placed in a glass cylinder (i.d.= 20.5 cm) sitting on top of a Plexiglas® stage with a mirror set at a 45° angle underneath the cylinder and stage [26, 27]. 1.25 ml of 0.3 M saline was infused through the I–O cannula over a period of 5 min (0.25 ml/min) using a syringe pump (Harvard Apparatus, Holliston, MA). Responses to 0.3 M saline were recorded using the mirror and a video camera. By viewing the video recordings positive hedonic and negative affective behavioral responses were quantified by an experimenter blind to the treatment. Positive responses included lateral tongue protrusions (2 sec duration), paw licking (2 sec duration), and rhythmic (midline) tongue protrusions (2 sec duration). Negative responses included gapes, headshakes, forelimb flails, face washing (2 sec duration), and chin rubs [11].
2.5.1 Experiment 1. The effects of systemically administered morphine and naltrexone on 0.3 M saline intake after a sodium depletion challenge in experimentally naïve rats
Daily baseline intakes of 0.3 M saline were recorded. A total of 64 rats were used for this experiment. All rats were sodium depleted and randomly assigned to one of seven groups: 1) vehicle treatment (V; i.e., isotonic saline; n=8), 2) morphine (M 0.5, 0.5 mg/kg, n=9), 3) morphine (M1, 1.0 mg/kg, n=10), 4) morphine (M3, 3.0 mg/kg, n=10), 5) naltrexone (N1, 1.0 mg/kg, n=8), 6) naltrexone (N3, 3.0 mg/kg, n=10), and 7) naltrexone (N10, 10.0 mg/kg, n=9). Doses were based on those used by Hubbell and McCutcheon [14]. Thirty minutes prior to the sodium appetite test, rats were given injections of either vehicle, morphine (0.5, 1 or 3 mg/kg, s.c., Sigma Aldrich M8777) or naltrexone (1, 3, or 10 mg/kg i.p., Sigma Aldrich N3136). Rats were given a sodium appetite test and intakes of 0.3 M saline and water were recorded over a 2 h period. Total intakes of 0.3 M saline and water were compared between groups.
2.5.2 Experiment 2. The effects of systemically administered naltrexone on taste reactivity responses to 0.3 M saline in sodium replete and deplete rats
A total of 43 rats were used for this experiment. Following adaptation to 0.3 M saline and sodium deficient chow, rats were randomly assigned to one of 6 groups: 1) sham depleted + vehicle (SV; n=8), 2) sham depleted + 0.5 mg/kg naltrexone (S 0.5, n=7), 3) sham depleted + 1.0 mg/kg naltrexone (S1, n=6), 4) furosemide + vehicle (FV, n=9), 5) furosemide + 0.5 mg/kg naltrexone (F 0.5, n=6), and 6) furosemide + 1.0 mg/kg naltrexone (F1, n=7). Doses used for the current experiment were based on previously published studies using systemic naltrexone on taste reactivity responses [28]. Three days following implantation of I–O cannulas, rats were adapted to the infusion procedure three times. Rats were then sodium or sham depleted using furosemide and isotonic saline, respectively. Twenty-four h after sodium or sham depletion, rats were given naltrexone (0.5 or 1.0 mg/kg i.p.) or vehicle 30 min prior to the taste reactivity test. Ingestive responses to 0.3 M saline were videotaped and scored by an experimenter blind to treatment conditions.
2.5.3 Experiment 3. The effects of systemically administered morphine on taste reactivity responses to 0.3 M saline in sodium replete and deplete rats
A total of 34 rats were used. Following adaptation to 0.3 M saline and sodium deficient chow, rats were fitted with I–O cannulas and randomly assigned to one of 6 groups: 1) sham depleted + vehicle (SV; i.e., isotonic saline; n=6), 2) sham depleted + 2.0 mg/kg morphine (S2, n=6), 3) sham depleted + 4.0 mg/kg morphine (S4, n=6), 4) furosemide + vehicle (FV, n=5), 5) furosemide + 2.0 mg/kg morphine (F2, n=6), and 6) furosemide + 4.0 mg/kg morphine (F4, n=5). Doses in the current experiment were based on previous work examining the effects of systemic morphine on taste reactivity responses to a sucrose-quinine solution delivered by I–O cannula [24]. Three days after implantation of oral cannulas, rats were given experience with I–O infusions of tap water 3 times prior to testing. After this adaptation, rats were sodium or sham depleted using furosemide or isotonic saline, respectively, then subcutaneously injected with morphine (2 or 4 mg/kg) or isotonic saline 24 h after sodium or sham depletion. Thirty minutes after morphine or vehicle treatment, rats were infused with 0.3 M saline for 5 minutes. Ingestive responses to hypertonic saline were videotaped and positive hedonic and negative affective behaviors were scored by an experimenter blind to treatment conditions.
2.6 Statistical Procedures
Mean 0.3 M saline and water intakes in Experiment 1 were analyzed using a one-way Analysis of Variance (ANOVA). Planned comparisons were analyzed using Fisher’s least significant differences (LSD) for post-hoc analyses. A probability value of p<0.05 was considered statistically significant. In Experiments 2 and 3, mean negative affective and positive hedonic behavioral responses were analyzed using a two-way factorial ANOVA to test both main effects of drug and sodium/sham depletion on positive and negative behaviors, as well as to test interaction effects between drug dose and sodium status. If significant interaction effects were seen, Bonferroni t-tests were then used for post-hoc analyses. A p value of less than 0.05 was required for statistical significance. Statistical analysis was conducted using SPSS and SigmaPlot. Individual taste reactivity behaviors do not occur with equal frequency, so positive (e.g., lateral tongue protrusions and paw licking, etc.) and negative behaviors (e.g., gapes and flails, etc.) were summed and those means were used for statistical analyses. Positive and negative behaviors as characteristic individual behaviors (e.g., paw licking, tongue protrusions, etc.) are presented in Tables 1 and 2 for Experiments 2 and 3.
Table 1.
Individual occurrences (mean±SEM) of positive hedonic and negative affective taste reactivity responses in naltrexone or vehicle treated sodium replete and sodium depleted rats.
| Positive Hedonic Behaviors | Negative Affective Behaviors | ||||||
|---|---|---|---|---|---|---|---|
| Groups | LTP | PL | RTP | F | FW | G | HS |
| SV | 0.75±0.62 | 2.38± 1.21 | 10.50±3.17 | 3.00±0.71 | 25.13±6.46 | 2.00±1.43 | 2.57±0.35 |
| S0.5 | 3.00±1.39 | 0.86± 0.37 | 2.57±1.09 | 7.00±3.30 | 8.14±2.22 | 15.71±6.10 | 0.71±0.33 |
| S1 | 1.83±1.17 | 4.50± 3.51 | 5.33±4.74 | 3.50±2.05 | 4.50±2.50 | 14.83±4.38 | 0.67±0.33 |
| FV | 6.89±2.83 | 55.11±16.96 | 25.67±6.62 | 1.33±0.67 | 1.33±1.01 | 5.67±3.45 | 0.56±0.34 |
| F0.5 | 1.17±0.48 | 33.67±15.85 | 2.83±1.28 | 1.33±0.56 | 10.83±3.25 | 6.50±3.63 | 0.33±0.21 |
| F1 | 2.14±0.83 | 18.00± 9.08 | 4.14±0.94 | 1.71±1.23 | 18.43±9.88 | 16.29±6.37 | 0.29±0.29 |
Positive hedonic responses (mean±SEM) were subdivided into lateral tongue protrusions (LTP), paw licks (PL), and rhythmic (midline) tongue protrusions (RTP). Negative affective responses (mean±SEM) were subdivided into flails (F), face wipes (FW), gapes (G), and head shakes (HS). Experimental treatment groups: SV = sham depleted + vehicle; S0.5 = sham depleted + naltrexone (0.5 mg/kg); S1 = sham depleted + naltrexone (1 mg/kg); FV = furosemide depletion + vehicle; F0.5 = furosemide depletion + naltrexone (0.5 mg/kg); F1 = furosemide depletion + naltrexone (1.0 mg/kg).
Table 2.
Individual occurrences (mean±SEM) of positive hedonic and negative affective taste reactivity responses in morphine or vehicle treated rats.
| Positive Hedonic Behaviors | Negative Affective Behaviors | ||||||
|---|---|---|---|---|---|---|---|
| Groups | LTP | PL | RTP | F | FW | G | HS |
| SV | 0.33±0.33 | 12.50± 8.92 | 17.00±4.79 | 2.17±1.22 | 21.00±8.96 | 7.17±3.46 | 2.50±0.85 |
| S2 | 3.83±1.40 | 3.83± 1.42 | 32.00±9.83 | 0.00±0.00 | 3.17±1.76 | 0.83±0.65 | 0.33±0.33 |
| S4 | 0.50±0.34 | 11.83± 8.43 | 33.33±2.78 | 0.00±0.00 | 1.00±1.00 | 1.33±1.33 | 0.00±0.00 |
| FV | 1.20±0.80 | 88.00±19.78 | 18.80±6.21 | 0.80±0.37 | 9.60±3.40 | 1.40±0.98 | 1.00±0.77 |
| F2 | 5.50±2.14 | 21.67±11.22 | 28.83±8.73 | 0.17±0.17 | 0.67±0.33 | 5.00±3.33 | 0.33±0.33 |
| F4 | 0.80±0.58 | 7.40± 2.94 | 43.00±7.31 | 0.00±0.00 | 0.00±0.00 | 0.80±0.49 | 0.60±0.24 |
Positive hedonic responses (mean±SEM) were subdivided into lateral tongue protrusions (LTP), paw licks (PL) and rhythmic (midline) tongue protrusions (RTP). Negative affective responses (mean±SEM) were subdivided into flails (F), face wipes (FW), gapes (G), and head shakes (HS). Experimental treatment groups: SV = sham depleted + vehicle; S2 = sham depleted + morphine (2 mg/kg); S4 = sham depleted + morphine (4 mg/kg); FV = furosemide depletion + vehicle; F2 = furosemide depletion + morphine (2 mg/kg); F4 = furosemide depletion + morphine (4 mg/kg).
3. Results
3.1 Experiment 1. The effects of systemically administered morphine and naltrexone on 0.3 M saline intake after a sodium depletion challenge
3.1.1 0.3 M saline intake in sodium depleted rats given morphine, naltrexone, or vehicle
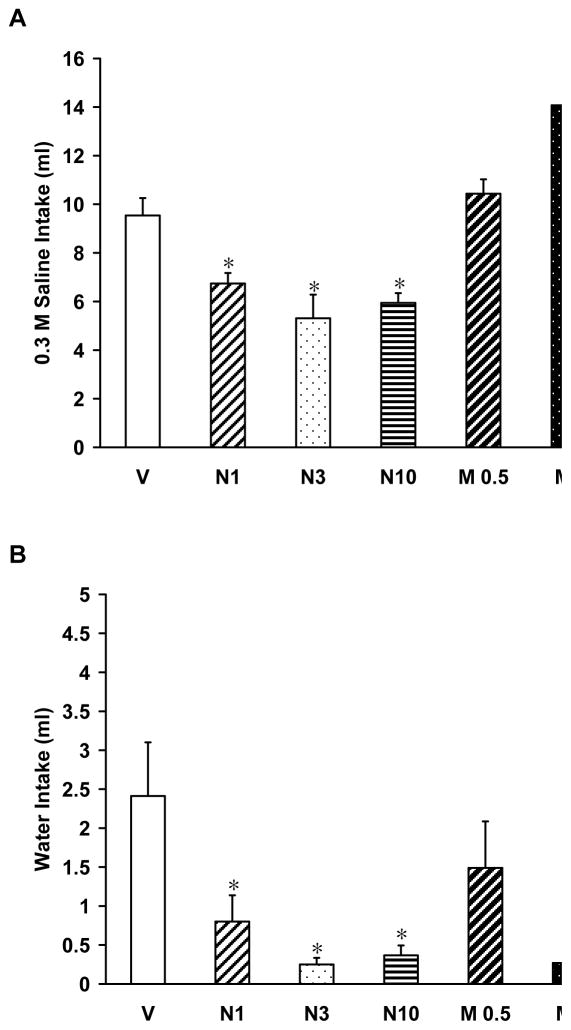
Saline intake of 0.03 M data are presented in Figure 1A. A one-way ANOVA indicated significant differences in 0.3 M saline intake after systemic treatment with morphine, naltrexone, or vehicle (F(6,63)=20.142, p<0.05). All three doses of naltrexone significantly attenuated 0.3 M saline intake as compared to vehicle treated rats (p<0.01 for N1, N3, and N10 groups), while morphine at the two highest doses enhanced ingestion of 0.3 M saline as compared to vehicle treated rats (p<0.01 for M1 and M3). Rats given the two highest doses of morphine also had significantly higher 0.3 M saline intakes than rats given naltrexone (p<0.05).
Figure 1.
Mean (± SEM) water and 0.3 M saline intakes in rats given naltrexone or morphine after furosemide-induced sodium depletion. (A) Rats given naltrexone drank significantly less 0.3 M saline than vehicle and morphine treated rats. Rats given the two highest doses of morphine (M1 and M3) drank significantly more 0.3 M saline than vehicle and naltrexone treated rats. (B) Water intake was significantly affected by acute treatment with naltrexone or morphine. Rats given naltrexone and the two highest doses of morphine decreased water intake during the 2 h test. * p<0.05 compared to vehicle group
3.1.2 Water intake in sodium depleted rats given morphine, naltrexone, or vehicle
Water intake data are presented in Figure 1B. Water intake in rats given systemic vehicle, naltrexone or morphine prior to a sodium appetite test were significantly different [F(6,63)=3.249, p<0.05]. Vehicle treated rats drank significantly more water than rats given naltrexone (p<0.05 for N1, N3, and N10 groups) and rats given morphine at the two highest doses (p<0.05 for M1 and M3).
3.1.3 Sodium depleted induced shift in 0.3 M saline intake after morphine, naltrexone, or vehicle administration
The intake data were analyzed using a 2-way ANOVA to assess if there was a morphine induced shift in 0.3 M saline intake as compared to water intake after systemic administration of morphine, naltrexone, or vehicle. A significant interaction effect was seen [F(6,127)=17.4, p<0.001]. Post-hoc analyses revealed that morphine, naltrexone, and vehicle treated animals drank significantly more 0.3 M saline compared to water (p<0.05).
3.2 Experiment 2. The effects of systemically administered naltrexone on taste reactivity responses to 0.3 M saline in sodium replete and depleted rats
3.2.1 Positive hedonic behaviors in sham or sodium depleted rats given systemic naltrexone or vehicle
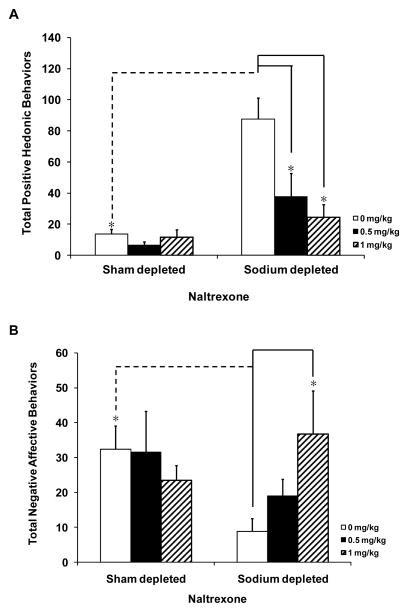
The mean occurrences of individual positive behaviors (e.g., paw licks, lateral and rhythmic tongue protrusions) are presented in Table 1. Positive hedonic taste reactivity responses to 0.3 M saline infusions in sham and in sodium depleted rats given systemic naltrexone are given in Figure 2A. There was a significant interaction effect between sodium status (sodium repleted vs. sodium depleted) and naltrexone dose (F(2,37)=5.742, p<0.05). Post-hoc analyses revealed significant differences in positive hedonic responses to infusions of 0.3 M saline in sodium depleted rats, with both groups of naltrexone treated rats displaying significantly fewer positive hedonic responses than sodium depleted rats given vehicle treatment, (p<0.01 for 0.5 and 1 mg/kg naltrexone groups). There were also significant differences between the 0 mg/kg sham depleted vs, the 0 mg/kg sodium depleted groups with sodium depleted rats showing a significant increase in positive hedonic responses compared to sham depleted rats (p<0.01).
Figure 2.
Mean (± SEM) of total positive hedonic and negative affective responses in sham depleted and furosemide-sodium depleted rats in naltrexone/vehicle treated rats given intraoral (I–O) infusions of 0.3 M saline. Rats were either sham or sodium depleted and then given 0, 0.5, or 1 mg/kg i.p. of systemic naltrexone. (A) Naltrexone significantly attenuated positive hedonic responses in furosemide depleted rats as compared to vehicle treated sodium depleted rats. Positive hedonic responses in sham depleted rats were not affected by systemic naltrexone treatments. (B) Total number of negative affective behaviors was significantly increased in furosemide depleted rats given the highest dose (1 mg/kg) of naltrexone. Negative affective responses in sham depleted rats were not affected by systemic naltrexone treatment. *p<0.05 in vehicle (0 mg/kg) vs. naltrexone (0.5 mg/kg or 1 mg/kg) treatment groups
3.2.2 Total negative affective behaviors in sham or sodium depleted rats given systemic naltrexone or vehicle
Occurrence of individual negative affective behaviors (e.g., flails, face wipes, gapes, and head shakes) is summarized in Table 1. Negative behaviors in sodium and sham depleted rats then given naltrexone prior to a taste reactivity test are presented in Figure 2B. There was no significant interaction and no main effect in negative behaviors after naltrexone treatment, however, using a Bonferroni t-test a significant decrease in negative affective responses in the 0 mg/kg sodium depleted condition compared to the 0 mg/kg sham depleted group (p<0.01) was found.
3.3 Experiment 3. The effects of systemically administered morphine on taste reactivity responses to 0.3 M saline in sodium replete and depleted rats
3.3.1 Positive hedonic behaviors in sham or sodium depleted rats given systemic morphine or vehicle
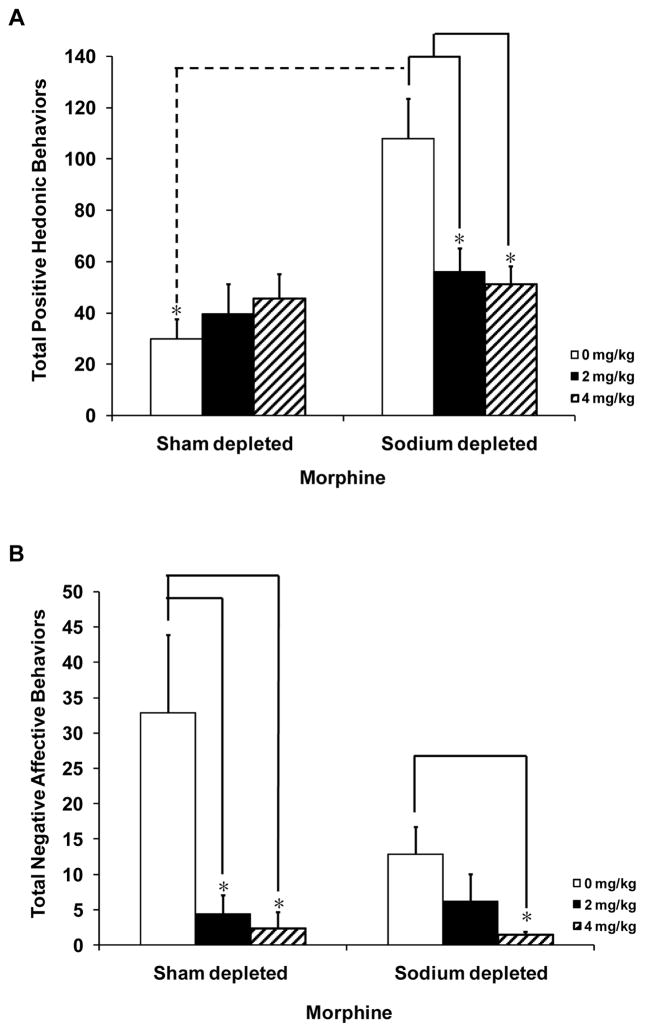
The occurrences of individual positive appetitive behaviors in rats given systemic morphine or vehicle are presented in Table 2. The total number of positive hedonic behaviors in sham or sodium depleted rats given morphine or vehicle can be found in Figure 3A. A two-way ANOVA showed a significant interaction effect between sodium status (sodium replete and sodium depleted) and morphine dose [F(2,28)=6.968, p<0.05]. There is a significant main effect of sodium depletion on appetitive responses [F(1,28)=15.46, p<0.05]. Post-hoc analyses revealed significant differences in positive hedonic responses between sodium depleted rats given vehicle versus sodium depleted rats given morphine at both doses (p<0.001). Moreover, an increase in positive hedonic I–O responses in sodium depleted rats versus sham depleted rats in the vehicle condition (p<0.001) was found.
Figure 3.
Mean (± SEM) of total positive hedonic and negative affective responses in sham depleted and furosemide-sodium depleted rats pretreated with morphine or vehicle treated rats given I–O infusions of 0.3 M saline. (A) Positive hedonic responses (lateral tongue protrusions, rhythmic tongue protrusions, and paw licks) were quantified during I–O infusions of 0.3 M saline. Furosemide treated rats given vehicle (0 mg/kg) showed significantly more positive hedonic behaviors than sodium depleted rats given the two highest doses of morphine (2, 4 mg/kg), * p<0.05 compared to 0 mg/kg morphine in the furosemide treated group. (B) Negative affective responses (gapes, flails, head shakes, and face wipes) were quantified during I–O infusions of 0.3 M saline. Sham depleted rats given morphine (2 or 4 mg/kg) showed significantly fewer negative affective responses to I–O infusions of 0.3 M saline. Sodium depleted rats given the highest dose of morphine (4 mg/kg) displayed significantly fewer negative affective responses than sodium depleted rats given vehicle treatment. * p<0.05 as compared to 0 mg/kg group for both sham depleted and sodium depleted groups
3.3.2 Negative affective behaviors in sham or sodium depleted rats given systemic morphine or vehicle
Individual negative affective behaviors can be found in Table 2. The interaction effect of sodium depletion and morphine treatment was not significantly different. However, the total number of negative affective behaviors in sham or sodium depleted rats given systemic treatment with morphine was significantly different as there was a significant main effect for morphine dose on negative responses to 0.3 M saline [F(2,28)=8.037, p<0.05, Figure 3B]. An independent t-test with a Bonferroni correction showed significant differences between rats given the highest dose of morphine (4 mg/kg) and vehicle treated rats, indicating that morphine treated rats displayed significantly fewer negative affective responses than vehicle control rats (p<0.01). A follow-up comparison between the 0 mg/kg sham depleted vs. the 0 mg/kg sodium depleted groups did not show significant differences in negative affective responses.
4. Discussion
The major objective of the present experiments was to combine the methods of sodium depletion-induced salt appetite with measures to assess the preference and palatability of 0.3 M saline to determine the effects of opioid receptor agonist and antagonist in sodium replete and deplete states. Specifically, 0.3 M sodium solution intake and taste reactivity to the same saline solution were studied in the sodium replete and deplete states while morphine or naltrexone was administered. The primary findings of the present studies indicated that: 1) systemically administered morphine enhanced and naltrexone attenuated 0.3 M NaCl intake, respectively, in experimentally naïve, sodium depleted rats, 2) systemically administered naltrexone reduced positive hedonic responses and enhanced negative affective responses in sodium depleted rats, and 3) systemic morphine administration attenuated both positive hedonic and negative affective responses in sodium depleted rats.
In a previous report investigating the role of opioids on salt appetite [14], rats were depleted three times to establish a stable baseline before they were administered naltrexone in conjunction with a fourth sodium depletion. In this same study the animals were then given morphine prior to a sodium appetite test the following day. Because past studies have demonstrated sensitization effects after repeated sodium depletions [15, 17], it is possible that exposure to multiple sodium depletions may have produced a ceiling effect thereby obscuring the full pharmacological effects of morphine and naltrexone. Thus, it was important to replicate these findings using naïve animals. In Experiment 1 rats received their initial sodium depletion in conjunction with vehicle, naltrexone, or morphine administered 30 min prior to a first sodium appetite test. In the current experiments, rats were naïve to both drug treatment and to sodium depletion. The current studies are consistent with previous findings [14] showing that sodium depleted rats given systemic morphine and naltrexone increased and decreased 0.3 M saline intake during a sodium appetite test, respectively, compared to sodium replete rats given vehicle treatment.
The fact that the intake of hypertonic saline was decreased in sodium depleted rats given naltrexone (Experiment 1) suggests that the acceptability of saline decreased significantly following naltrexone treatment in sodium depleted rats. However, this conclusion is based on intake tests and therefore the relative palatability/aversive nature of NaCl in naltrexone treated sodium depleted rats cannot be determined. Thus, in Experiment 2, naltrexone was used to test taste reactivity responses to 0.3 M saline in sodium replete and deplete rats. The taste reactivity test is a behavioral test that directly examines species typical responses to infused solutions, and has been validated in numerous studies [10, 23, 28]. Positive hedonic behaviors were decreased by peripheral treatment of naltrexone in rats depleted of sodium (Experiments 2), while negative affective behaviors were increased in sodium depleted rats given the highest dose of systemic naltrexone. Morphine enhanced intake of hypertonic saline, as demonstrated by intake tests (Experiment 1), suggests that morphine tends to enhance the palatability of 0.3 M saline. Taste reactivity tests demonstrated that morphine decreased negative affective responses to saline in sodium replete rats when administered peripherally (Experiment 3).
Systemically administered naltrexone influenced taste reactivity responses in sodium depleted rats with positive hedonic responses being significantly decreased in both naltrexone groups (0.5 and 1 mg/kg), and negative affective responses being significantly increased in rats given the highest dose of naltrexone (1 mg/kg). These results suggest that naltrexone at these doses was sufficient to block endogenous opioid mechanisms that are important for mediating palatability. Supporting this hypothesis, other studies have shown that 0.5 and 1 mg/kg doses of naltrexone reduces both palatability and the consumption of 10% alcohol [29], as well as saccharin and sucrose solutions [28].
The attenuation of both negative affective and positive hedonic taste reactivity responses in sodium depleted rats given morphine suggests that in sodium depleted rats, positive responding may have reached a ceiling that could not be overcome by morphine administration. Investigators have found that morphine did not produce enhancement of positive hedonic responses to highly palatable substances. Parker, Maier, Rennie, and Crebolder [28] found that morpine did not change the taste reactivity responses (i.e., palatability) of a 20% sucrose solution. In contrast, morphine enhances the palatability of substances that produce a mixture of positive and negative ingestive responses. Lynch and Libby [30] found that morphine does not alter preferences for low concentrations of saccharin but that it does increase preference for a 10% saccharin solution, which when given I–O to control rats, elicits both positive hedonic and negative affective responses. In the current experiments, morphine decreased negative affective responses to 0.3 M saline in sodium replete rats. This concentration of saline is typically aversive to rats when sodium replete. These data suggest that in sodium replete rats, 0.3 M saline becomes less aversive under the influence of morphine. Thus, the effects of morphine on taste reactivity may be specific for negative responses rather than positive ones.
Systemic naltrexone effectively decreased positive appetitive responses in sodium depleted rats (Experiment 2) which implies that endogenous opioids are involved in modulating sodium palatability. However, somewhat unexpectedly, systemically administered morphine also decreased positive appetitive responses in sodium depleted rats (Experiment 3). It was anticipated that morphine would either enhance or at least not change positive hedonic responses in sodium depleted rats compared to sodium depleted rats given vehicle. One potential explanation for this unanticipated finding may be related to the pharmacological specificity of morphine. Morphine, while primarily a μ-opioid receptor agonist, is also a weak κ receptor agonist [31]. Systemic administration of κ agonists have been shown to reduce feeding in lean Zucker rats compared to saline vehicle controls, both of which were maintained on a 6 h feeding schedule [32]. Perhaps systemically administered morphine may have been acting at other opioid receptor subtypes in addition to the μ receptor to produce the decrease in positive appetitive responses. Naltrexone is a competitive antagonist for κ and δ opioid receptors as well as μ-opioid receptors. Thus, some of the behavioral effects seen in the current study may not be a purely μ-opioid receptor mediated response.
A role of opioids in regulating the palatability of foods is well-established. It is widely believed that opioids function to enhance palatability, thereby increasing consumption [33–37]. Systemic administration of naloxone or naltrexone, opioid receptor antagonists, to humans negatively affects palatability ratings of food [38–40]. Naloxone reduces pleasantness ratings of highly palatable foods (i.e., those high in sugar and fat content) in obese, bulimic, and normal weight controls and also significantly decreases ingestion of sweet high fat foods such as cookies and chocolate in binge eaters [41]. Naloxone has also been found to block ANG II-induced 1.5% saline intake in mice [42]. Morphine or DAMGO, μ-opioid receptor agonists, elicit hedonic positive appetitive responses to sucrose and quinine [24, 28, 43]. Mu opioid receptor agonists also induce conditioned taste preferences to a dilute mixture of monosodium glutamate and sodium chloride and increase preference for a typically negative affective concentration of 3% saline in mice [44, 45]. Alternatively, opioid receptor antagonists increase negative affective responses to palatable substances such as sucrose solutions and hypotonic saline solutions [28] and also decrease consumption of sweet pellets under ad libitum and food-deprived conditions [34, 46].
Several researchers contend that the endogenous opioid system promotes overeating and may be responsible in advancing the onset and incidence of obesity, thereby increasing the prevalence of diseases associated with obesity (e.g., diabetes, cardiovascular disease and some forms of cancer) [34, 47, 48]. To date, most studies have targeted the role of opioids in modulating fat and sugar intake, neglecting the effect opioids may have on salt intake. Not only is it possible that increased intake of salty foods could contribute to the etiology of obesity, but it is also recognized to have a negative impact on cardiovascular health, with high salt intake being associated with hypertension and cardiovascular disease. These data implicate opioids in mediating the palatability of sodium not only through intake tests but also through the taste reactivity tests, suggesting that opioids play a pervasive role in their effects on ingestive behavior and hedonic responses.
Acknowledgments
This work was supported in part by grants from the National Heart, Lung, and Blood Institute HL 14388 and HL 098207, the National Institute of Diabetes and Digestive and Kidney Diseases DK 66086 and the National Institute of Mental Health MH 80241 to AKJ, and the American Heart Association (0625661Z) to MJA.
Contributor Information
Elisa S. Na, Email: elisa-na@uiowa.edu.
Michael J. Morris, Email: michael-j-morris@uiowa.edu.
Alan Kim Johnson, Email: alan-johnson@uiowa.edu.
References
- 1.de Wardener HE, He FJ, MacGregor GA. Plasma sodium and hypertension. Kidney Int. 2004;66:2454–66. doi: 10.1111/j.1523-1755.2004.66018.x. [DOI] [PubMed] [Google Scholar]
- 2.MacGregor G, De Wardener HE. Salt, Diet and Health. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- 3.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 4.Michell AR. The Clinical Biology of Sodium: The Physiology and Pathophysiology of Sodium in Mammals. New York: Elsevier Science; 1995. [Google Scholar]
- 5.Haddy FJ. Role of dietary salt in hypertension. Life Sci. 2006;79:1585–92. doi: 10.1016/j.lfs.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Evaluation and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 7.Hollenberg NK. The influence of dietary sodium on blood pressure. J Am Coll Nutr. 2006;25:240S–6S. doi: 10.1080/07315724.2006.10719573. [DOI] [PubMed] [Google Scholar]
- 8.Horvathova H, Kimlikova K, Balazovjech I, Kyselovic I. Compliance and the therapeutic effect in patients with arterial hypertension. Bratisl Lek Listy. 2003;104:149–54. [PubMed] [Google Scholar]
- 9.Khavari KA. Some parameters of sucrose and saline ingestion. Physiol Behav. 1970;5:663–6. doi: 10.1016/0031-9384(70)90227-1. [DOI] [PubMed] [Google Scholar]
- 10.Berridge KC, Flynn FW, Schulkin J, Grill HJ. Sodium depletion enhances salt palatability in rats. Behav Neurosci. 1984;98:652–60. doi: 10.1037//0735-7044.98.4.652. [DOI] [PubMed] [Google Scholar]
- 11.Berridge KC, Schulkin J. Palatability shift of a salt-associated incentive during sodium depletion. Q J Exp Psychol B. 1989;41:121–38. [PubMed] [Google Scholar]
- 12.Cabanac M. Physiological role of pleasure. Science. 1971;173:1103–7. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- 13.Lucas LR, Grillo CA, McEwen BS. Involvement of mesolimbic structures in short-term sodium depletion: in situ hybridization and ligand-binding analyses. Neuroendocrinology. 2003;77:406–15. doi: 10.1159/000071312. [DOI] [PubMed] [Google Scholar]
- 14.Hubbell CL, McCutcheon NB. Opioidergic manipulations affect intake of 3% NaCl in sodium-deficient rats. Pharmacol Biochem Behav. 1993;46:473–6. doi: 10.1016/0091-3057(93)90382-4. [DOI] [PubMed] [Google Scholar]
- 15.Sakai RR, Fine WB, Epstein AN, Frankmann SP. Salt appetite is enhanced by one prior episode of sodium depletion in the rat. Behav Neurosci. 1987;101:724–31. doi: 10.1037//0735-7044.101.5.724. [DOI] [PubMed] [Google Scholar]
- 16.Sakai RR, Frankmann SP, Fine WB, Epstein AN. Prior episodes of sodium depletion increase the need-free sodium intake of the rat. Behav Neurosci. 1989;103:186–92. doi: 10.1037//0735-7044.103.1.186. [DOI] [PubMed] [Google Scholar]
- 17.Na ES, Morris MJ, Johnson RF, Beltz TG, Johnson AK. The neural substrates of enhanced salt appetite after repeated sodium depletions. Brain Res. 2007;1171:104–10. doi: 10.1016/j.brainres.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falk JL. Serial sodium depletion and NaCl solution. Physiol Behav. 1966;1:75–7. [Google Scholar]
- 19.Cao JL, Vialou VF, Lobo MK, Robison AJ, Neve RL, Cooper DC, et al. Essential role of the cAMP-cAMP response-element binding protein pathway in opiate-induced homeostatic adaptations of locus coeruleus neurons. Proc Natl Acad Sci U S A. 2010;107:17011–6. doi: 10.1073/pnas.1010077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mickiewicz AL, Napier TC. Repeated exposure to morphine alters surface expression of AMPA receptors in the rat medial prefrontal cortex. Eur J Neurosci. 2011;33:259–65. doi: 10.1111/j.1460-9568.2010.07502.x. [DOI] [PubMed] [Google Scholar]
- 21.Robinson TE, Gorny G, Savage VR, Kolb B. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse. 2002;46:271–9. doi: 10.1002/syn.10146. [DOI] [PubMed] [Google Scholar]
- 22.Robinson TE, Kolb B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse. 1999;33:160–2. doi: 10.1002/(SICI)1098-2396(199908)33:2<160::AID-SYN6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 23.Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–79. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 24.Doyle TG, Berridge KC, Gosnell BA. Morphine enhances hedonic taste palatability in rats. Pharmacol Biochem Behav. 1993;46:745–9. doi: 10.1016/0091-3057(93)90572-b. [DOI] [PubMed] [Google Scholar]
- 25.Na ES, Fitts DA. Conditioned taste aversion and c-Fos expression in cholestatic rats. Brain Res. 2001;918:187–90. doi: 10.1016/s0006-8993(01)02982-1. [DOI] [PubMed] [Google Scholar]
- 26.Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain Res. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- 27.Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci. 2000;20:8122–30. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker LA, Maier S, Rennie M, Crebolder J. Morphine- and naltrexone-induced modification of palatability: analysis by the taste reactivity test. Behav Neurosci. 1992;106:999–1010. doi: 10.1037//0735-7044.106.6.999. [DOI] [PubMed] [Google Scholar]
- 29.Ferraro FM, 3rd, Hill KG, Kaczmarek HJ, Coonfield DL, Kiefer SW. Naltrexone modifies the palatability of basic tastes and alcohol in outbred male rats. Alcohol. 2002;27:107–14. doi: 10.1016/s0741-8329(02)00220-3. [DOI] [PubMed] [Google Scholar]
- 30.Lynch WC, Libby L. Naloxone suppresses intake of highly preferred saccharin solutions in food deprived and sated rats. Life Sci. 1983;33:1909–14. doi: 10.1016/0024-3205(83)90675-6. [DOI] [PubMed] [Google Scholar]
- 31.Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician. 2008;11:S133–53. [PubMed] [Google Scholar]
- 32.Leighton GE, Hill RG, Hughes J. The effects of the kappa agonist PD-117302 on feeding behaviour in obese and lean Zucker rats. Pharmacol Biochem Behav. 1988;31:425–9. doi: 10.1016/0091-3057(88)90369-3. [DOI] [PubMed] [Google Scholar]
- 33.Arbisi PA, Billington CJ, Levine AS. The effect of naltrexone on taste detection and recognition threshold. Appetite. 1999;32:241–9. doi: 10.1006/appe.1998.0217. [DOI] [PubMed] [Google Scholar]
- 34.Glass MJ, Billington CJ, Levine AS. Opioids and food intake: distributed functional neural pathways? Neuropeptides. 1999;33:360–8. doi: 10.1054/npep.1999.0050. [DOI] [PubMed] [Google Scholar]
- 35.Hanlon EC, Baldo BA, Sadeghian K, Kelley AE. Increases in food intake or food-seeking behavior induced by GABAergic, opioid, or dopaminergic stimulation of the nucleus accumbens: is it hunger? Psychopharmacology (Berl) 2004;172:241–7. doi: 10.1007/s00213-003-1654-0. [DOI] [PubMed] [Google Scholar]
- 36.Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–77. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- 37.Levine AS, Grace MK, Cleary JP, Billington CJ. Naltrexone infusion inhibits the development of preference for a high-sucrose diet. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1149–54. doi: 10.1152/ajpregu.00040.2002. [DOI] [PubMed] [Google Scholar]
- 38.Fantino M, Hosotte J, Apfelbaum M. An opioid antagonist, naltrexone, reduces preference for sucrose in humans. Am J Physiol. 1986;251:R91–6. doi: 10.1152/ajpregu.1986.251.1.R91. [DOI] [PubMed] [Google Scholar]
- 39.Yeomans MR, Gray RW. Selective effects of naltrexone on food pleasantness and intake. Physiol Behav. 1996;60:439–46. doi: 10.1016/s0031-9384(96)80017-5. [DOI] [PubMed] [Google Scholar]
- 40.Yeomans MR, Gray RW. Effects of naltrexone on food intake and changes in subjective appetite during eating: evidence for opioid involvement in the appetizer effect. Physiol Behav. 1997;62:15–21. doi: 10.1016/s0031-9384(97)00101-7. [DOI] [PubMed] [Google Scholar]
- 41.Drewnowski A, Krahn DD, Demitrack MA, Nairn K, Gosnell BA. Taste responses and preferences for sweet high-fat foods: evidence for opioid involvement. Physiol Behav. 1992;51:371–9. doi: 10.1016/0031-9384(92)90155-u. [DOI] [PubMed] [Google Scholar]
- 42.Kuta CC, Bryant HU, Zabik JE, Yim GK. Stress, endogenous opioids and salt intake. Appetite. 1984;5:53–60. doi: 10.1016/s0195-6663(84)80050-1. [DOI] [PubMed] [Google Scholar]
- 43.Clarke SN, Parker LA. Morphine-induced modification of quinine palatability: effects of multiple morphine-quinine trials. Pharmacol Biochem Behav. 1995;51:505–8. doi: 10.1016/0091-3057(95)00042-u. [DOI] [PubMed] [Google Scholar]
- 44.Lett BT, Grant VL, Koh MT. Naloxone attenuates the conditioned place preference induced by wheel running in rats. Physiol Behav. 2001;72:355–8. doi: 10.1016/s0031-9384(00)00427-3. [DOI] [PubMed] [Google Scholar]
- 45.Mucha RF, Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 1985;86:274–80. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- 46.Levine AS, Weldon DT, Grace M, Cleary JP, Billington CJ. Naloxone blocks that portion of feeding driven by sweet taste in food-restricted rats. Am J Physiol. 1995;268:R248–52. doi: 10.1152/ajpregu.1995.268.1.R248. [DOI] [PubMed] [Google Scholar]
- 47.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–95. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]