Abstract
Glucokinase activators (GKAs) are being developed and clinically tested for potential antidiabetic therapy. The potential benefits and limitations of this approach continue to be intensively debated. To contribute to the understanding of experimental pharmacology and therapeutics of GKAs, we have tested the efficacy of one of these agents (Piragliatin) in isolated islets from humans with type 2 diabetes mellitus (T2DM), from mice with glucokinase (GK) mutations induced by ethyl-nitroso-urea (ENU) as models of Maturity Onset Diabetes of the Young linked to GK and Permanent Neonatal Diabetes Mellitus linked to GK (PNDM-GK) and finally of islets rendered glucose insensitive by treatment with the sulphonyl urea compound glyburide in organ culture. We found that the GKA repaired the defect in all three instances as manifest in increased glucose-induced insulin release and elevated intracellular calcium responses. The results show the remarkable fact that acute pharmacological activation of GK reverses secretion defects of β-cells caused by molecular mechanism that differ vastly in nature, including the little understood multifactorial lesion of β-cells in T2DM of man, the complex GK mutations in mice resembling GK disease and acute sulphonylurea failure of mouse β-cells in tissue culture. The implications of these results are to be discussed on the theoretical basis underpinning the strategy of developing these drugs and in light of recent results of clinical trials with GKAs that failed for little understood reasons.
Keywords: β-cells, diabetes therapy, glucokinase, glucokinase activators
Introduction
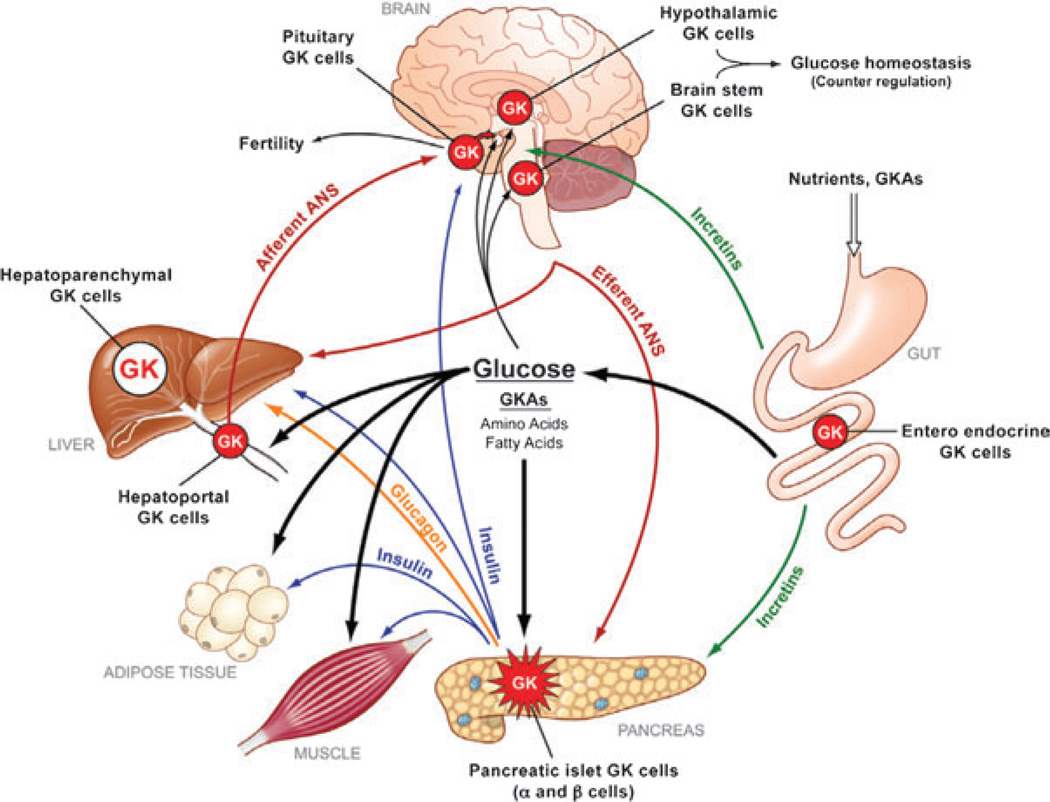
Glucokinase (GK)-expressing cells are essential components of the glucose homeostatic system of man and common laboratory animals (figure 1) [1]. This includes prominently the insulin producing β-cells of the pancreas – where the enzyme serves as glucose sensor – and equally significant the parenchymal cells of the liver – where it serves as general regulator of intermediary metabolism controlling glycogen synthesis, gluconeogenesis, lipid synthesis and urea production [1,2]. It is not surprising then that mutations of the GK gene were found to cause diabetes or hypoglycemia in humans when the mutation inhibits or activates GK, respectively [3–11]. Because of its central role in glucose homeostasis, GK was targeted for drug development with the goal to augment its activity and thereby reduce hyperglycaemia in patients with diabetes mellitus. This effort was successful and resulted in the discovery of small molecular weight allosteric glucokinase activators (GKAs) which were shown to enhance glucose-stimulated insulin release and reduce hepatic glucose production in normal and diabetic laboratory animals and also in patients with T2DM [2,12–15]. However, in a recent trial of 54 weeks with 587 T2DM patients, the GKA MK-0941 lost efficacy over time and resulted in increased serum triglycerides and systolic blood pressure which lead to the termination of this particular trial but also raises questions about the soundness of the approach to use GKAs as antidiabetic medication [16]. In this report, we briefly discuss published and new information which validates and strengthens the rationale and continued efforts to develop suitable GKAs for treating diabetes mellitus, but we also emphasize that our understanding of the central role of GK in glucose homeostasis and in the pathogenesis of T2DM remains woefully fragmentary, which greatly limits future work and potential progress in the exploration of this pharmacologically very powerful and promising new class of antidiabetic oral agents.
Figure 1.
The network of glucokinase (GK)-containing tissues throughout the body and their connections. Expression of GK in the pancreatic islet α and β-cells, other endocrine cells (including pituitary and gut) and cells in the portal vascular tree and central nervous system (including the hypothalamus and brain stem) makes up about 0.1% of the body’s total GK complement. The liver contains the rest (about 99.9%). The pancreatic islet cells and the liver constitute the basic GK-dependent regulatory system maintaining glucose homeostasis. ANS, autonomic nervous system; GKA, glucokinase activator. With permission from reference [1].
Enzyme Kinetics and Molecular Biophysics of the Pancreatic Islet Cell GK Glucose Sensor
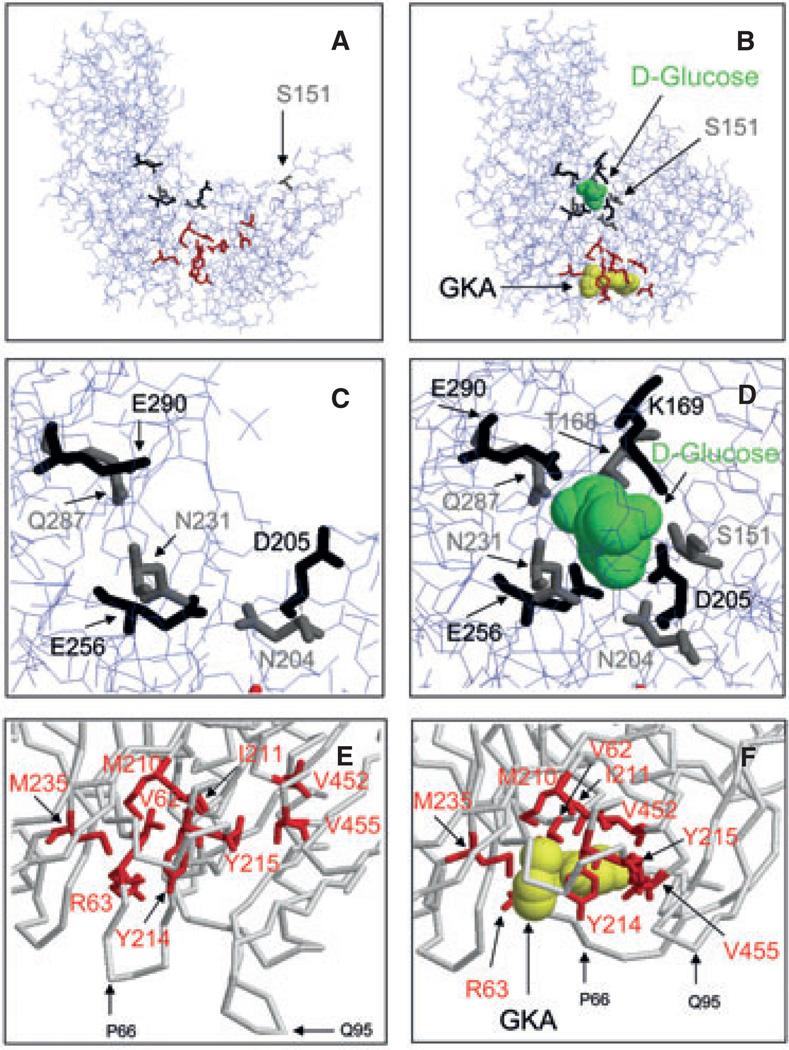
Any meaningful discussion of GKAs in pharmacological diabetes therapy has to be grounded in a basic comprehension of the enzyme kinetics and the molecular biophysics of GK, which explains its role as a glucose sensor and drug receptor. The glucose-phosphorylating enzyme GK operates as a monomer with half saturation at about 7 mM glucose and shows cooperative kinetics with regard to its substrate glucose as indicated by a Hill coefficient of about 1.7 [1,14,17–19]. This co-operativity is usually explained by the ‘ligand induced slow transition’ model (the LIST model) which proposes that the enzyme exists in two interconvertible conformations with low and high affinity for glucose and that the catalytic cycle is relatively fast compared to the rate of the structural transitions [19–22]. As shown in figure 2, glucose induces a large structural change resulting in the closure of the substrate site (panels A–D). During the course of R&D for GKAs and the parallel biochemical genetic work on activating mutations of GK causing hyperinsulinaemia in children, it was discovered that the enzyme has an allosteric activator site which is occluded in the open conformation but is rendered accessible to GKAs when glucose is bound as illustrated most clearly by panels E and F of figure 2. The locations of the contact amino acids comprising the glucose-binding site and of those amino acids that form the drug receptor are shown in the open, ligand free state of GK and also in the closed form of the ternary complex (figure 2, panels A–F). It should be realized, however, that more advanced molecular biophysics models of GK co-operativity propose that as many as six distinct conformations exist in equilibrium that is governed by the glucose concentration [23]. It is known that ligand binding stabilizes the GK protein [23], which is highly thermolabile as indicated by its Delta G of denaturation as low as 1.5 Kcal/mole (at 20 °C). This mechanism of protein stabilization is of physiologically considerable significance as shown by induction of the enzyme by culturing isolated pancreatic islets in the presence of mannoheptulose, which competes with glucose for the glucose-binding site of GK, but is not phosphorylated, an effect powerfully enhanced by GKAs [20,24]. It is not surprising then that functional and structural instability as a result of genetic mutation can cause loss of enzyme resulting in Maturity Onset Diabetes of the Young linked to GK (MODY-2) or Permanent Neonatal Diabetes mellitus linked to GK (PNDM-GK) in humans and also in animals [3–9] (see example below). The molecular features of GK are the basis of the enzyme’s glucose sensor function in pancreatic β-cells. Playing this role in pancreatic β-cells, it operates together with the KATP channel and the voltage sensitive calcium channel by widely accepted mechanisms [25]. Figure 1 indicates that GK serves also as glucose sensor of the pancreatic α-cell mediating direct glucose suppression of glucagon secretion. However, the glucose sensor role of GK remains speculative in that case as in most others depicted in figure 1. Some investigators favour the view that glucose suppression of glucagon secretion is entirely indirect mediated by paracrine factors including insulin, somatostatin or zinc [26,27], whereas others including our laboratory have provided strong evidence that glucose exerts a direct inhibitory action on α-cells which implicates GK as the sensor [28–30]. The hypothetical role of GK as glucose sensor of α-cells is of high relevance in the context of this discussion because it extrapolates to the possibility that GKAs might affect glucagon release directly.
Figure 2.
Effects of glucokinase activator (GKA) binding on glucokinase (GK) structure. Panels A and B: Synergistic binding of glucose and GKA to GK induces a large conformational change of the protein structure which allows the full visualization of the glucose-binding site in the crystal structure and causes a major rearrangement of the allosteric drug receptor site. Panels C and D provide a detailed view of the glucose-binding site in the open and closed conformation. Panels E and F illustrate how glucose binding provides access of the GKA to the allosteric site, which is occluded in the open conformation. The orientation of the cutouts in panels C and D is the same as in panels A and B but it is slightly changed in panels E and F to better visualize the critical opening of the V62–G72 loop when ligands bind. Figure design based on Kamata et al. [22].
GKAs Restore Defective Function of β-cells of Severely Diabetic Mice with Ethyl-Nitroso-Urea-Induced GK Mutations
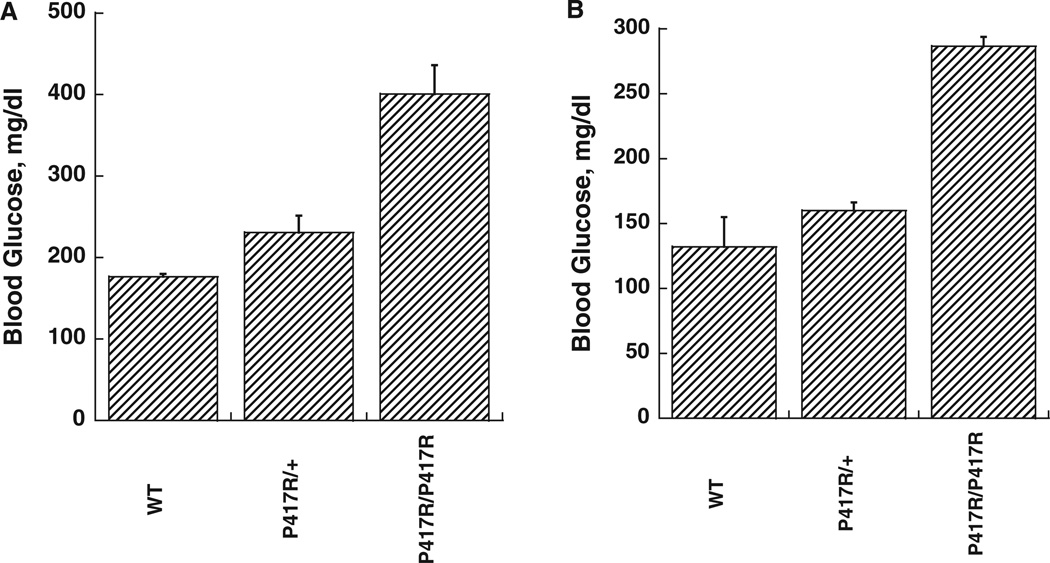
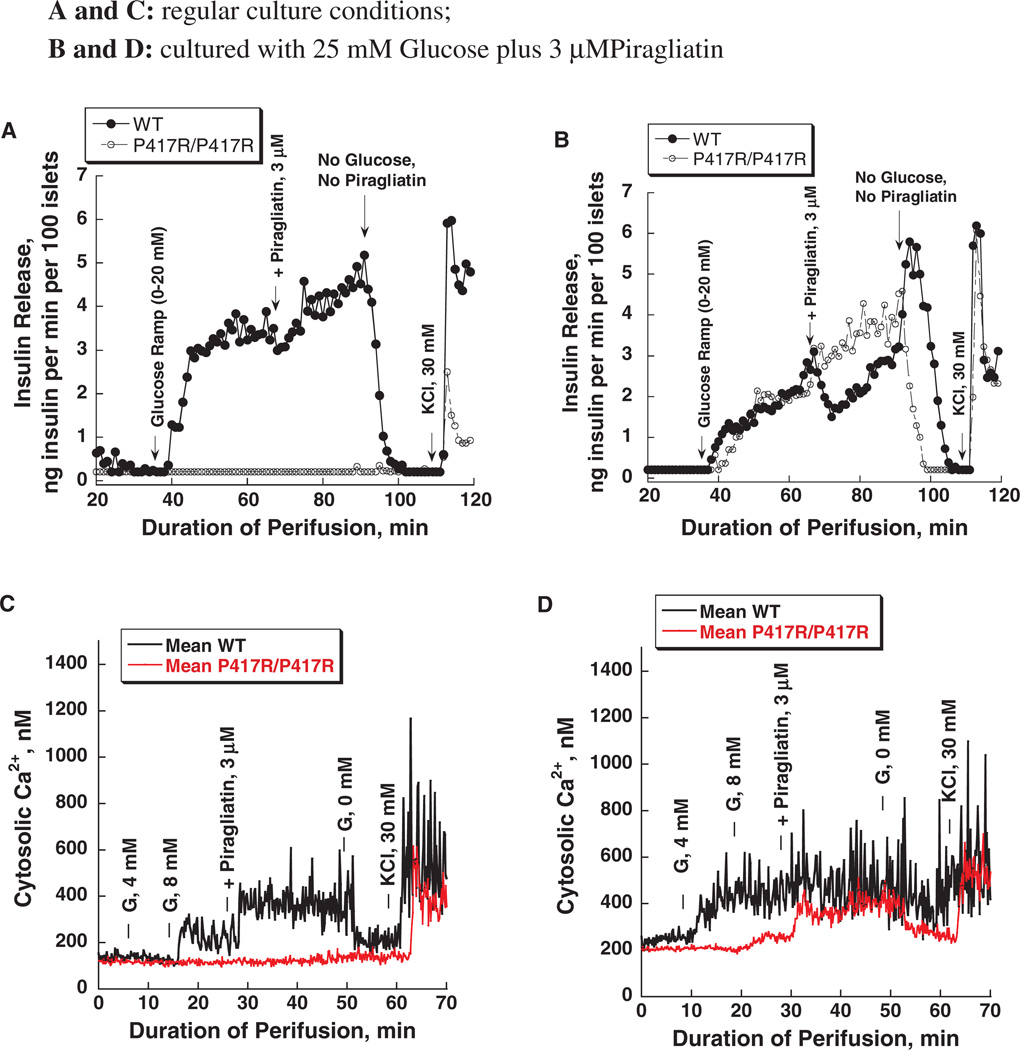
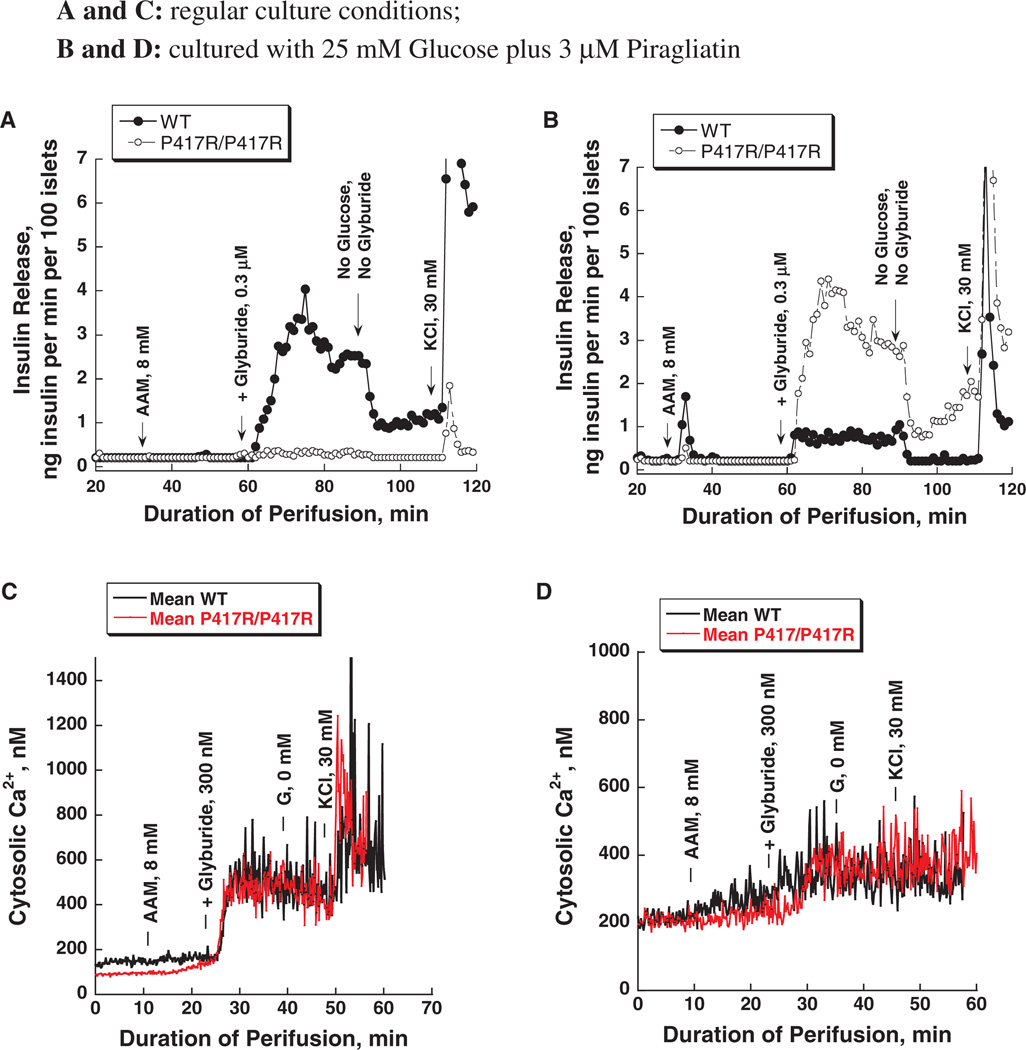
We have recently reported on a mouse model of GK linked MODY (one allele affected) and PNDM (both alleles affected), which were generated in the course of ethyl-nitroso-urea (ENU) mutagenesis studies [31]. Diabetes in two different mouse lines was found to be caused by inactivating mutations of the GK gene: K140E and P417R. These mutations reduce the catalytic capacity of the enzyme only moderately but also increase thermal lability of the protein. This results in very mild forms of MODY-2 in heterozygous and severely diabetic forms of PNDM in homozygous mice, which still survive to adulthood [figure 3 shows the blood sugars in the fed state of the GK-P417R line measured before shipping the animals from Chicago to Philadelphia (panel A) and before sacrificing the animals for islet isolation at the University of Pennsylvania (panel B)]. Acute treatment with GKA lowered the blood glucose in the P417R line but not in the K140E line [31]. This difference in responsiveness was unexpected in view of the similarity of the molecular defect and the clinical phenotype. We speculated that culturing isolated islets from these mice for several days in the presence of high glucose plus a GKA should normalize β-cell function through ligand induced protein stabilization which can be expected to require days for developing a therapeutic effect. This turned out to be so as shown by the results with the P417R/P417R line presented in figures 4 and 5. When islets from either homozygous mouse line are cultured for 3–4 days in RPMI with 10 mM glucose present β-cells are functionally severely impaired as indicated by a total lack of glucose-stimulated insulin release and the abnormal intracellular calcium tracings (figure 4A, C). Culturing in high glucose plus 3 µM of the GKA Piragliatin repairs the defects in both diabetic lines. Note that the transient inhibitory effect of Piragliatin on the insulin release profile in perifusion experiments with control islets, most pronounced following culture in high glucose plus the GKA (panel 4B) and also the high calcium baselines in controls and experimentals (panel 4D) are most likely an indication that the treatment resulted in marked induction of GK and overstimulation of the cells. Islets from mutant mice lacked an insulin response to glyburide but exhibited a normal calcium rise (figure 5, panels A and C) illustrating the involvement of critical, glucose-dependent factors in addition to elevated calcium in stimulus secretion coupling. It is also possible that insulin stores are critically reduced in the P417R/P417R islets when untreated. Exposing islets to high glucose plus the activator changes glyburide responsiveness of controls and experimentals. The insulin response of islets with mutant GK was normalized but that of the control islets was reduced after exposing islets to high glucose plus the activator for several days (panel 5B). The calcium baseline is greatly elevated in controls and experimentals, the glyburide effects are muted and, strikingly, high potassium has no effect (panel 5D) showing that the aggressive treatment applied here fully depolarizes the β-cell and that this effect is plausibly more pronounced in controls than in the islets with mutant GK.
Figure 3.
Blood glucose levels of mice glucokinase (GK)P417R/P417R and GKP417R/+ mice. Panel A shows the blood sugars in the fed state of the GK-P417R line measured before shipping the animals from Chicago to Philadelphia; Panel B: blood glucose levels before sacrificing the animals for islet isolation at the University of Pennsylvania. The small difference in blood sugar levels of the fed animals is more likely due to reduced food intake as a result of shipping the animals which were used 1 day after arrival.
Figure 4.
Effects of glucose and Piragliatin on insulin secretion and intracellular calcium concentrations of islets isolated from control mice and from mice with a GKP417R/P417R mutation induced by ethyl-nitroso-urea (ENU). Panel A: insulin release profile. Islets were cultured for 3–4 days in RPMI 1640 medium with 10 mM glucose present before they were used for the perifusion experiment. A physiological amino acid mixture was present throughout the entire run at 4.0 mM. Initially, islets were perfused in the absence of glucose for 30 min and then a glucose ramp from 0 to 20 mM was applied during the next 25 min. The highest 20 mM concentration was maintained for another 15 min and then 3 µM of Piragliatin was added to the perfusate for the next 25 min. Afterwards, all stimuli were removed for 20 min and islets were then depolarized with 30 mM KCl. Panel B: islets were cultured for 3–4 days with 25 mM glucose and 3 µM Piragliatin. The same experimental protocol as describe in panel A was employed to study insulin secretion. Panels C and D show Ca2+ profiles for the same islets preparations and conditions comparable to those presented in panels A and B.
Figure 5.
Amino acid- and glyburide-stimulated insulin secretion and intracellular Ca2+ in islets isolated from control and glucokinase (GK)P417R/P417R mice. Panels A and C: insulin release and Ca2+ profiles of islets cultured for 3–4 days in regular RPMI 1640 culture medium with 10 mM glucose. Panels B and D: insulin release and Ca2+ profiles of islets cultured for 3–4 days with 25 mM glucose and 3 µM Piragliatin. Note that in contrast to experiments presented in figure 4, the amino acid stimulus was introduced later during the course of the perifusion.
We interpret these results as follows: extended exposure of islet cells to the glucose/drug mixture is sufficient to induce the cells’ enzyme level primarily by stabilizing the labile mutant GK protein and thereby augmenting glucose metabolism which then results in repair of the secretory defect. In order to fully test this minimal working, hypothesis will require quantification of GK protein levels and activity, of GK mRNA as well as establishing a time course and the glucose and drug dependencies of the repair process.
GKAs Partly Repair Glyburide-Induced β-cell Dysfunction in an Islet Culture Model
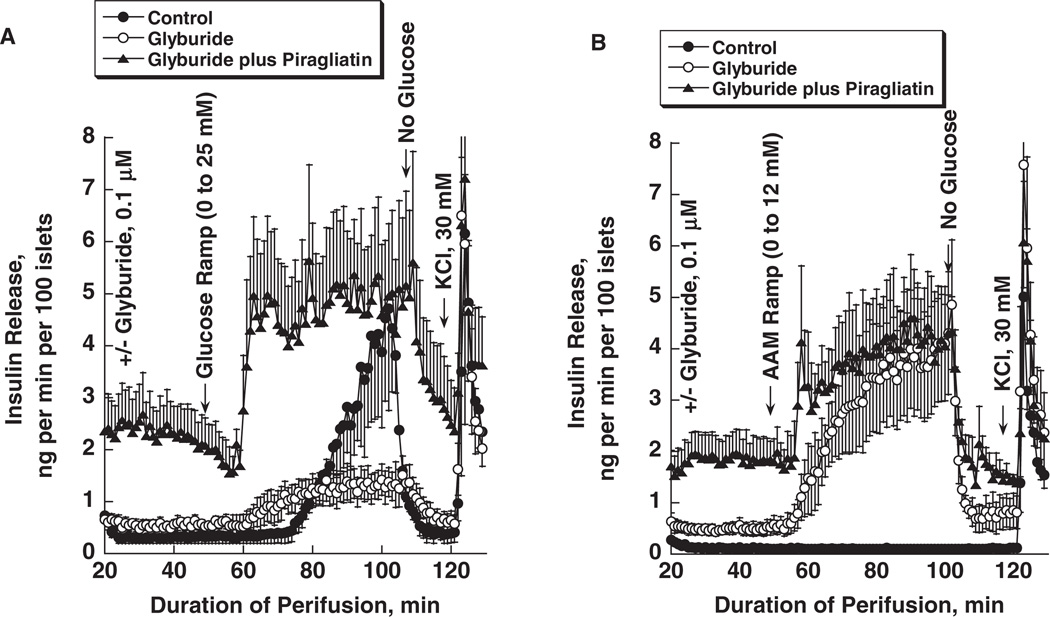
Sulphonylurea failure is known to develop with prolonged duration of using this class of antidiabetic agents [32,33]. There is no ready explanation for this phenomenon. It has been shown, however, that GKAs restore defective β-cell function in model experiments of acute sulphonylurea failure in normal rats treated with glimepiride [34]. In order to understand the underlying biochemical pharmacology of this restoration process, we have begun to explore this phenomenon with an islet culture model of acute sulphonylurea failure. Mouse islets were cultured for 3–4 days in RPMI with 10 mM glucose with or without 0.1 µM glyburide present and were then tested in a perifusion system with the same drug concentration to assess glucose and amino acid induced insulin secretion using nutrient ramps to establish concentration dependencies and define thresholds for stimulation of secretion. As shown in figure 6A, inhibition of the KATP channel for several days in culture and continued during the perifusion step doubles basal insulin secretion, shifts the glucose threshold to the left but decreases maximal glucose stimulated insulin release by about 75%. Furthermore, glyburide treatment renders the β-cell highly sensitive to a physiological mixture of amino acids with a threshold of about 2 mM and a maximal response of about 10 times basal at 12 mM of the mixture (figure 6B). The result is comparable to that seen in isolated islets of sulfonylurea type 1 receptor (SUR-1) KO mice which also lose glucose responsiveness but are sensitized to amino acids [35]. The GKA Piragliatin at 3 µM, superimposed on glyburide during culture and perifusion, raises basal insulin release more than fivefold and greatly sensitizes the β-cells to glucose as indicated by a sharply defined threshold of about 5 mM. It also seems that low glucose causes a transient inhibition of release before the threshold is reached at about 5 mM (figure 6A). This treatment also increases the sensitivity of β-cells to amino acids as indicated by the biphasic release profile elicited by the amino acid ramp with a sharply defined threshold of about 2 mM (figure 6B).
Figure 6.
Piragliatin restores glucose sensitivity and enhances amino acid induced insulin release in glyburide-treated mouse islets. Three experimental conditions were used during islet culturing (for 3 days) and perifusion: (i) control islets were cultured in RPMI 1640 media containing 10 mM glucose without further additions; (ii) 0.1 µM glyburide was added to the culture medium described in condition 1; (iii) 0.1 µM glyburide plus 3 µM Piragliatin were added to the culture medium described under 1. During perifusion, the drugs were present at the same concentrations as during culture. Panel A: insulin secretion was stimulated by a glucose ramp from 0 to 25 mM extending over a period of 50 min. Panel B: insulin release was stimulated by a physiological amino acid mixture ramp from 0 to 12 mM extending over a period of 50 min. Results are presented as mean ± s.e. of three to four experiments.
On the basis of current understanding of stimulus secretion coupling and the limited information available at this stage of the study, it is difficult to explain the profound effect of prolonged GKA treatment on insulin secretion of glyburide depolarized islets stimulated by glucose or by amino acids. The extrapolation to the in vivo model of sulphonylurea failure [34] is also tenuous. Still, glyburide treatment of islets in culture results in the expected phenotype of fuel-stimulated insulin release: β-cells are depolarized, intracellular calcium is probably elevated causing enhanced basal insulin release and glucose augments basal release by providing metabolic coupling factors that are postulated to amplify the effect of calcium by an unknown mechanism [25]. It can also be expected that at 10 mM glucose 3 µM Piragliatin stimulates glucose metabolism and energy production maximally during the culture period resulting in the striking amplification of the glyburide effect. As apparent from figures 6A, B, insulin secretion of the islets primed with glyburide and Piragliatin in the presence of 10 mM glucose is maintained at five to seven times the rate of controls even in the absence of glucose suggesting that calcium, second messengers and metabolic coupling factors remain elevated for a considerable length of time after islets have been removed from the stimulatory culture medium. Such persistent activation would also explain the prompt biphasic response to the glucose and amino acid ramps (see below for further comments). A widely accepted view distinguishes between triggering and amplification mechanisms in fuel-stimulated insulin release [25]. It appears then according to this concept that GKAs greatly potentiate triggering as well as amplification processes. Hyperstimulation of glucose metabolism is usually considered detrimental to β-cell function [36,37]. Yet even under the extreme conditions of this study, there is little evidence for that, at least for the 4-day duration of the experiment. The hyper responsiveness of the glyburide-treated islets to an amino acid mixture requires special comments. It is difficult to reason that it is because of enhanced intermediary metabolism and generation of metabolic coupling factors in view of the results with glucose stimulation in this situation, showing greatly reduced efficacy. In view of our earlier observation that inhibition of the SUR-1 receptor greatly sensitizes the β-cell to acetylcholine, glucagon-like peptide-1 (GLP-1) or IBMX [38], one should consider the intriguing possibility that amino acid stimulation in this experimental condition might be due to activation of G-protein-coupled receptors including the heterodimeric T1R1/T1R3 (‘Umami’ receptor) or the homodimeric CaR (calcium receptor) which are involved in taste sensation of amino acids and are being discovered to be present in mammalian endocrine cells [39] including nutrient sensing cells of the gut. In further support of this view, it was observed that amino acids increase cAMP and insulin secretion in islets of SUR-1 KO mice and that this effect is inhibited by exendin-9–39 an antagonist of the GLP-1 receptor of the β-cell [40]. The presence of such nutrient receptors (NRs) in β-cells could also explain the diagnostic protein intolerance and the high acute insulin release due to an intravenous calcium challenge of children with hyperinsulinism caused by inhibitory KATP channel mutation [41]. Similar speculations offer themselves logically to explain the astonishing sensitivity of glyburide plus Piragliatin treated islets to glucose stimulation in view of the discovery of G-protein-coupled sweet taste receptors in pancreatic β-cells which could be unmasked when cells are sensitized by GK activation coupled with membrane depolarization [42,43]. The present experimental design promises to be useful for characterizing and identifying such mechanisms possibly involved in stimulus secretion coupling of the islet β-cell, both in the triggering and the amplification processes. It would be informative to test whether islets isolated from rats with sulphonylurea failure and of those that were repaired with GKA treatment [34] show functional phenotypes resembling those observed here in the organ culture model.
GKAs Repair Defective Bioenergetics, Calcium Metabolism and Insulin Secretion of Isolated Islets from Human Subjects with T2DM
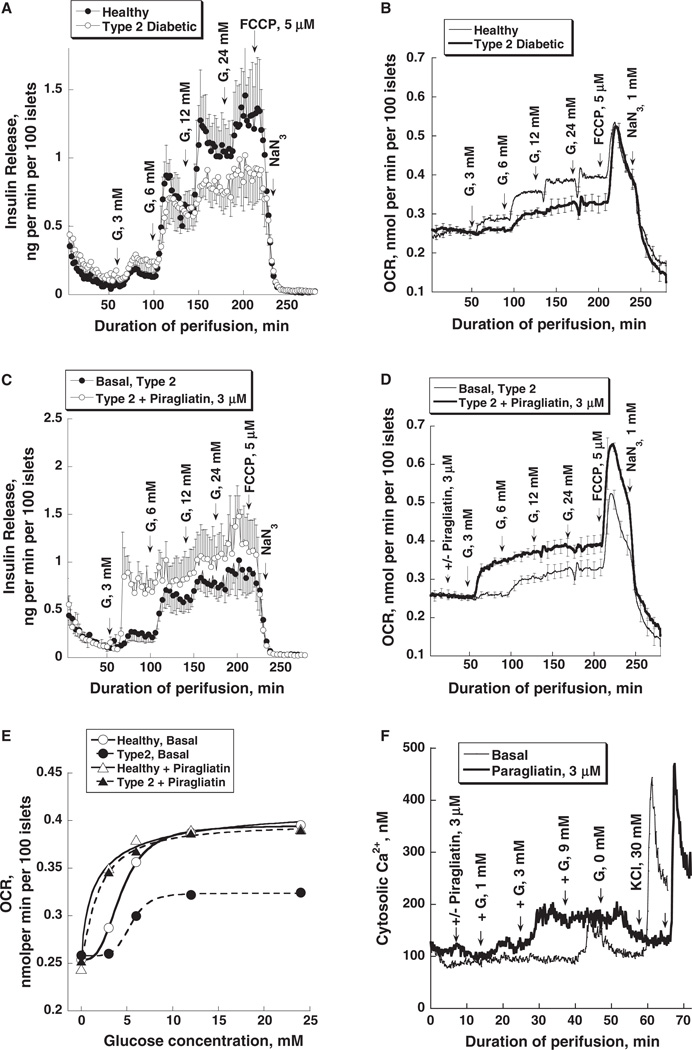
In two clinical trials, it was reported that GKAs are effective blood glucose lowering agents in T2DM [13,16]. In a 7-day trial, it was shown that Piragliatin-stimulated insulin release and reduced hepatic glucose production in a group of subjects with T2DM. Normoglycemia was achieved with the highest dose [13]. However, the trial was discontinued for undisclosed reasons. In a 54-week trial, MK-0941 significantly reduced blood glucose and HBA1c at the 14-week time point but lost effectiveness as the trial proceeded and also caused hypertriglyceridemia and an increase of systolic blood pressure resulting in termination of the project [16]. Additional trials with chemically different GKAs are underway and promise to settle some of the questions raised by previous failed attempts. Their outcome will be critical for the future of GKAs as antidiabetic drugs. To contribute to the understanding of the biochemical pharmacology of GKAs in T2DM, we have investigated the effects of Piragliatin on glucose-stimulated insulin release, respiration and calcium metabolism of isolated islets isolated from the pancreas of individuals with T2DM. We made the following observations [44]: islets from diabetics showed reduced glucose-induced insulin release, a marked reduction of glucose-induced respiration and lower intracellular calcium responses when exposed to glucose (figure 7). The defect of glucose-stimulated insulin release observed here with islets from diabetics is moderate and seen only at glucose concentrations at 6 mM and above (panel 7A). It is important to realize that these islets were cultured at 23 °C for periods exceeding 7 days with 5 mM glucose present before they were studied at the physiological temperature. We speculate that this extended conditioning improves β-cell function, in part perhaps by eliminating the diabetic environment and deleting critically involved toxic macrophages [45]. The defects of bioenergetics and calcium metabolism are far more severe (panels 7B, E and F). These defects were largely repaired by 3 µM Piragliatin except that maximal insulin release remained lower than in the controls.
Figure 7.
Impaired insulin release, oxygen consumption and intracellular Ca2+ concentration of isolated islets from type 2 diabetic organ donors and the restoration of insulin secretion and respiration by a glucokinase activator (GKA). Panel A shows the insulin release patterns of healthy and type diabetic islets with glucose stimulation using stepwise increases of glucose from 0 to 3, 6, 12 and 24 mM. Panel B shows the oxygen consumption profiles for the same experiments during stepwise increase of glucose concentration followed by treatment with 5 µM of the uncoupler of Ox/Phos FCCP and 1 mM Na-azide. O2 consumption was determined with a method based on phosphorescence quenching of metalloporphyrins by oxygen [63]. Panels C and D show the insulin release and oxygen consumption patterns of type 2 diabetic islets in the absence and presence of Piragliatin (3 µM). Panel E: comparison of OCR glucose dependencies of healthy islets and those of diabetics and of the effect of Piragliatin on these two sets of islets. Panel F: GKA effect on intracellular calcium concentrations in islets from type 2 diabetic organ donors. HbA1c levels for the pancreas donors with T2DM were 9.3, 11.0 and 7.4% as compared to an average of 5.6 % for controls.
There is obviously a giant gap between these acute studies with isolated islets from diabetics and the long-term clinical trials referred to above which makes extrapolations difficult. However, it seems reasonable to conclude that the ability of GKAs to repair a profound defect of the bioenergetics of the pancreatic islet tissue in T2DM as shown in our experiments is the critical downstream effect of the drug and that it is most probably operative and beneficial in subjects receiving the drug. Panel 7E shows indeed that the GKA normalizes the greatly reduced oxygen consumption rate (OCR) of T2DM islets in a way not possible even by extreme glucose concentration of 25 mM which indicates that the drug repairs dysregulation and not simply restores capacity of the enzyme. This data underscore the need for intensive studies of GK regulation in islet cells from subjects with T2DM and suitable animal models of the disease.
Discussion
It is now about 45 years since GK was identified in the β-cell islets of ob/ob mice [46], about 25 years since GK was shown in human islets [47] and about 10 years since the discovery of GKAs [12]. Knowledge related to the basic biochemistry and the role of GK in glucose homeostasis in health and disease has since expanded tremendously as any literature survey would convincingly demonstrate. However, as illustrated by the three presently discussed examples dealing with the biochemical pharmacology of GKAs, there remain large gaps in our understanding of this prominent molecule as glucose sensor, regulator of intermediary metabolism and drug receptor.
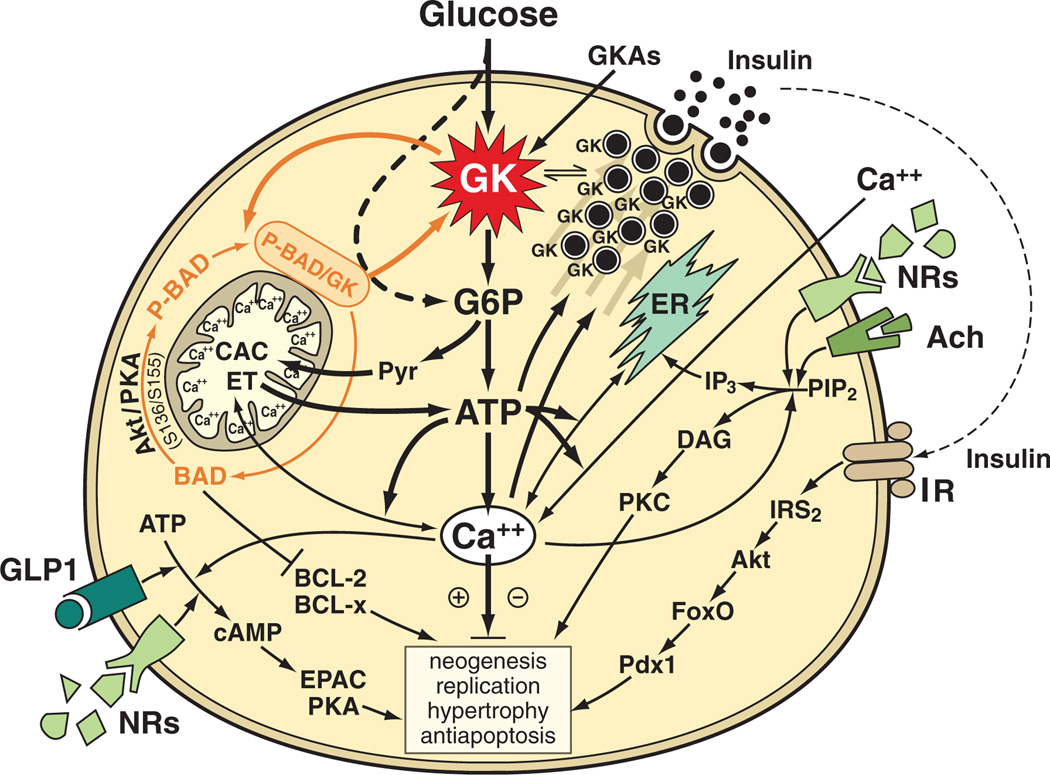
This brief discussion focusses on the role of GK as glucose sensor of the pancreatic β-cell in man and common laboratory animals (figure 8). The emphasis of the minimal model depicted here is on the central signalling pathway initiated by GK-mediated glucose phosphorylation and culminating in the generation of ATP, increased intracellular calcium and activation of protein kinase A, endoplasmic reticulum and protein kinase C [19,25,48]. This axial signalling system is regulated by receptor-mediated signalling pathways activated physiologically by acetylcholine, glucagon-like peptide 1 (GLP-1) and insulin. It is important that this regulation of the β-cell by neurons, hormones and perhaps directly by G-protein-coupled transmembrane NRs – as discussed above [39,42,43] – depends absolutely on active glycolysis and glucose oxidation. Insulin biosynthesis and secretion and processes governing the turnover and number of β-cells are the end targets of these signalling systems. GKAs thus affect this complex system profoundly.
Figure 8.
Hypothetical involvement of glucokinase (GK) and therefore glucokinase activators (GKAs) in the preservation and growth promotion of pancreatic β-cells. The interaction between GK and protein factors governing apoptosis [(BCL-2, BCL-XL, BAD, BAK, BAX) the latter two proapoptotic factors situated furthest downstream in the pathway but not shown] is highlighted. The P-BAD/GK complex and its association with mitochondria is shown [53,58]. For more details on P-BAD formation and the targeting of the proapoptotic factor to mitochondria and the ER, see [57,59]. The glucose and thus GK dependency of glucagon-like peptide-1 (GLP-1), insulin and acetylcholine initiated signalling processes is sketched in. It is hypothesized that activation of these pathways and their enhancement by GKAs protects β-cells from proapoptotic diabetogenic factors and enhances β-cell replication or neogenesis. It is further speculated that persistent deviations of free intracellular calcium from a basal set point may favour apoptosis but that transient, perhaps oscillatory changes of calcium may be beneficial. Note that for simplicity’s sake, the well established PKA, EPAC and PKC signalling in insulin release is not shown. Selected abbreviations: Akt or PKB, protein kinase B; Ach, acetylcholine; BAD, P-BAD, BCL-2 and BCL-x, mediators of apoptosis and antiapoptosis; CAC, citric acid cycle; DAG, diacylglycerol; ER, endoplasmic reticulum; EPAC, Rap GTPase guanine nucleotide exchange factor; ET, electron transport and oxidative phosphorylation; FoxO, mediator of insulin and insulin like growth factor signalling; GLP-1, glucagon like peptide 1; IRS-2, insulin receptor substrate 2; IP3, trisphosphoinositol; NRs, G-protein-coupled nutrient receptors; Pdx-1 or IPF-1, insulin promoter factor 1; PIP2, phosphotidyl inositol biphosphate; PKA, protein kinase A; PKC, protein kinase C; Pyr, pyruvate. Modified from [24].
Three aspects of this model require additional discussion: regulation of constitutive expression of islet cell GK, direct interactions of GK with other islet cell proteins and organelles and possible causes of limited durability of GKA action in patients with T2DM.
The nature of regulation of islet cell GK is a matter of debate. GK expression is controlled by a single gene with two tissue-specific promoters [49]: the upstream neuroendocrine promoter thought to govern constitutive expression in glucose sensor cells and the downstream hepatic promoter governing insulin-dependent expression of the enzyme in liver. Furthermore, it is important to realize that the enzyme is present in α- and in β-cells and very likely also in D-cells. GK activity was comparable in islet tissue of control rats and of rats made severely diabetic with alloxan or streptozotocin both with and without insulin treatment [30] and was found to be present in sorted α-cells [50]. Islet GK was found to be inducible by the competitive not phosphorylated inhibitor mannoheptulose [20,24]. GK mRNA levels and GK expression changes of islet tissue are not correlated [24]. Glucose stabilizes recombinant GK in thermolability tests and this effect is enhanced by GKAs [20,23] and as shown here by the observation that a combination of high glucose and a GKA fully repairs defective β-cell functions in GK-deficient mouse islets. It is worthy of note that 25 mM glucose alone when present during the organ culture step is not sufficient to repair the defect in pancreatic islets of K140E/K140E ENU-mutated mice (F. M. Matschinsky and collaborators, unpublished results). We interpret available data to imply that GK expression in islet cells is constitutive and is strongly influenced through stabilization by binding of substrate and allosteric activators (GKAs). Thus, ligand binding per se is thought to induce islet cell GK. Others believe that GK expression is primarily regulated by insulin [51]. The observations related above speak against this view. We speculate that the repair of β-cell function of islets from the GK-K140E/K140E and GK-P417R/P417R diabetic mice by culturing in high glucose plus GKA as illustrated by figures 4 and 5 is because of stabilization and induction of the GK protein by the ligands [23]. It can be extrapolated that such a mechanism is also operative in the therapeutic application of GKAs in T2DM. The results also imply that glucose and GKAs would induce GK in the α-cells and other GK-expressing cells and may thus influence its putative role as glucose sensor in these cells.
GKA actions can be fully comprehended only if the complex interactions of GK with other cellular constituents are also considered. In the β-cell, these include the direct protein/protein interaction with the bifunctional enzyme F6P-2-kinase/F2,6P2-2-phosphatase [52] and with the proapoptotic factor BAD [53] and also the close association with mitochondria [54] and insulin secretory granules [55]. In the liver, one needs to consider the interaction with GK regulatory protein [56], which sequesters the enzyme to the nucleus. Information on this complex aspect of GK regulation for GKA pharmacology is very limited. The proposed GK/BAD interaction and mitochondrial association of this protein complex is briefly discussed here as one important example (figure 8).
BAD, a universally expressed protein involved in the regulation of apoptotic cell death [57], was found to exist in a multiprotein complex which contained GK [53,54] and was discovered to be associated with hepatocyte and islet cell mitochondria [53,54,58]. Islets of BAD knockout mice had a 70% reduction of GK activity and defects of calcium metabolism and glucose-stimulated insulin release [53]. These findings lead to the proposal that BAD might serve a dual role, first as regulator of apoptosis and second as critical determinant of β-cell GK levels [53,59]. It remains to be fully explored whether and, if so, how GKAs affect this BAD/GK link of glucose metabolism and apoptotic processes. For example, are the β-cell GK level and activity direct functions of the cell’s content and phosphorylation state of BAD as suggested [53] or are the two molecules regulated independently? In any case, a critical evaluation of the published data suggests to us the possibility of an alternative interpretation with relevance to this discussion. It is important to note that the reported phenotype of BAD KO mice [53,60] differs greatly from that of mice with pancreatic β-cell GK deficiency and also of severe universal GK deficiency [31,61]. BAD KO mice are apparently not hyperglycemic [53,60] and, even more remarkable, their pancreas shows a very significant expansion of islet numbers when maintained on a high fat diet, an adaptive response that seems to be in compatible with the reported decrease of islet GK levels [61]. Furthermore, the secretory and calcium responses of isolated islets to glucose are only marginally abnormal in BAD KO mice, which is also inconsistent with the reported three fourth loss of GK compared to results with islets that have similar GK deficiencies. We speculate that the islet hyperplasia could be explained by the deinhibition of antiapoptotic factors as a result of the BAD KO (see figure 8). We further speculate that in the normal β-cell increased sequestration and neutralization of phosphorylated BAD in a mitochondrial multiprotein complex including GK may be in part responsible for the commonly observed β-cell expansion in diet induced obesity models as a result of enhanced antiapoptosis. It needs to be tested whether GKAs facilitate formation of the proposed GK/BAD complex. It is also necessary to explore whether GKAs affect the association of GK with the insulin secretory granule and, if so, how (see figure 8). Future experiments will undoubtedly settle some of the tantalizing question raised here.
In an attempt to determine the significance of the failed clinical phase II trial with the GKA MK-0941 [16] three issues need to be considered, one pertaining to limited durability of the blood glucose lowering effect and the others addressing the increase of the systolic blood pressure and the development of hypertriglyceridemia. It is perhaps important to realize that the patients participating in this trial had the disease for an average duration of 12 years, had an average HbA1c of 9%, received an average daily dose of 45 U of insulin glargine and many of them were treated with 1.5 g/day or more of metformin indicating a relatively advanced stage of the disease probably associated with severe β-cell impairment. It is possible that merely activating glucose metabolism by a GKA at this stage of the disease may be detrimental to β-cells already in overdrive due to high blood glucose. Perhaps using a low dose GKA regimen combined with activation of the GLP-1 receptor would be a better approach because both impaired bioenergetics and second messenger signalling might be improved synergistically at lower drug concentrations and overstimulation by glucose might be avoided. The elevation of serum triglycerides is almost certainly the result of the hepatic action of GKAs and could perhaps also be reduced by lowering the dose. Finally, it is difficult to explain the rise of the systolic blood pressure. This very surprising side effect may be unique for MK-0941 and results from its chemistry or its metabolism and may be unrelated to the basic mechanism of action of GKAs. It cannot be excluded, however, that it is caused by activation of GK-expressing cells that might be involved in the regulation of the vasculature [62].
We estimate that about 150 patents for distinct GKAs have been filed during the last 12 years and there is good evidence that applications for new ones are still being submitted which indicates that GK activation continues to be considered as a viable approach to develop a new therapy for diabetes mellitus. We also conclude from experimental examples presented here and the evaluation of the literature that GKAs have a unique potential as antidiabetic agents. These deliberations illustrate, however, that progress in this endeavour requires an intensified research effort to fully understand the complex role of GK in health and disease so that the still hypothetical potential of GKAs may be convincingly assessed.
Acknowledgements
We gratefully acknowledge the critical contribution to this study by the team of the Human Islet Resource Center at University of Pennsylvania under Dr A. Naji’s leadership. This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants DK-22122 (to F. M. M), by the Institute for Diabetes, Obesity, and Metabolism (via NIDDK Grant DK-19525) and an ADA grant (to N. M. D).
Footnotes
Conflict of Interests
The authors declare no conflict of interest.
References
- 1.Matschinsky FM, Magnuson MA, Zelent D, et al. The network of glucokinase-expressing cells in glucose homeostasis and the potential of glucokinase activators for diabetes therapy. Diabetes. 2006;55:1–12. [PubMed] [Google Scholar]
- 2.Matschinsky FM, Porte D. Glucokinase activators (GKAs) promise a new pharmacotherapy for diabetics. F1000 Med Rep. 2010;2:43. doi: 10.3410/M2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuesta-Munoz AL, Huopio H, Otonkoski T, et al. Severe persistent hyperinsulinemic hypoglycemia due to a de novo glucokinase mutation. Diabetes. 2004;53:2164–2168. doi: 10.2337/diabetes.53.8.2164. [DOI] [PubMed] [Google Scholar]
- 4.Njolstad PR, Sovik O, Cuesta-Munoz A, Bjorkhaug L, et al. Neonatal diabetes mellitus due to complete glucokinase deficiency. N Engl J Med. 2001;344:1588–1592. doi: 10.1056/NEJM200105243442104. [DOI] [PubMed] [Google Scholar]
- 5.Njolstad PR, Sagen JV, Bjorkhaug L, et al. Permanent neonatal diabetes caused by glucokinase deficiency: inborn error of the glucose-insulin signaling pathway. Diabetes. 2003;52:2854–60. doi: 10.2337/diabetes.52.11.2854. [DOI] [PubMed] [Google Scholar]
- 6.Glaser B, Kesavan P, Heyman M, Davis E, et al. Familial hyperinsulinism caused by an activating glucokinase mutation. N Engl J Med. 1998;338:226–230. doi: 10.1056/NEJM199801223380404. [DOI] [PubMed] [Google Scholar]
- 7.Christesen HB, Jacobsen BB, Odili S, et al. The second activating glucokinase mutation (A456V): implications for glucose homeostasis and diabetes therapy. Diabetes. 2002;51:1240–1246. doi: 10.2337/diabetes.51.4.1240. [DOI] [PubMed] [Google Scholar]
- 8.Gloyn AL, Noordam K, Willemsen MA, et al. Insights into the biochemical and genetic basis of glucokinase activation from naturally occurring hypoglycemia mutations. Diabetes. 2003;52:2433–2440. doi: 10.2337/diabetes.52.9.2433. [DOI] [PubMed] [Google Scholar]
- 9.Cuesta-Munoz AL, Tuomi T, Cobo-Vuilleumier N, et al. Clinical heterogeneity in monogenic diabetes caused by mutations in the glucokinase gene (GCK-MODY) Diabetes Care. 2010;33:290–292. doi: 10.2337/dc09-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Froguel P, Vaxillaire M, Sun F, et al. Close linkage of glucokinase locus on chromosome 7p to early-onset non-insulin-dependent diabetes mellitus. Nature. 1992;356:162–164. doi: 10.1038/356162a0. [DOI] [PubMed] [Google Scholar]
- 11.Hattersley AT, Turner RC, Permutt MA, et al. Linkage of type 2 diabetes to the glucokinase gene. Lancet. 1992;339:1307–1310. doi: 10.1016/0140-6736(92)91958-b. [DOI] [PubMed] [Google Scholar]
- 12.Grimsby J, Sarabu R, Corbett WL, et al. Allosteric activators of glucokinase: potential role in diabetes therapy. Science. 2003;301:370–373. doi: 10.1126/science.1084073. [DOI] [PubMed] [Google Scholar]
- 13.Bonadonna RC, Kapitza C, Heise T, et al. Glucokinase activator R04389620 improves beta cell function and plasma glucose fluxes in patients with type 2 diabetes. Diabetologia. 2008;51(Suppl. 1):S371. [Google Scholar]
- 14.Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nat Rev Drug Discov. 2009;8:399–416. doi: 10.1038/nrd2850. [DOI] [PubMed] [Google Scholar]
- 15.Zhi J, Zhai S, Mulligan ME, Grimsby J, et al. A novel glucokinase activator RO4389620 improved fasting and postprandial plasma glucose in type 2 diabetic patients. Diabetologia. 2008;51(Suppl. 1):S23. [Google Scholar]
- 16.Meininger GE, Scott R, Alba M, et al. Effects of MK-0941, a novel glucokinase activator, on glycemic control in insulin-treated patients with type 2 diabetes. Diabetes Care. 2011;34:2560–2566. doi: 10.2337/dc11-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matschinsky FM. Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45:223–241. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- 18.Matschinsky FM. Glucokinase, glucose homeostasis, and diabetes mellitus. Curr Diab Rep. 2005;5:171–176. doi: 10.1007/s11892-005-0005-4. [DOI] [PubMed] [Google Scholar]
- 19.Cardenas ML, Cornish-Bowden A, Ureta T. Evolution and regulatory role of the hexokinases. Biochim Biophys Acta. 1998;1401:242–264. doi: 10.1016/s0167-4889(97)00150-x. [DOI] [PubMed] [Google Scholar]
- 20.Zelent D, Najafi H, Odili S, et al. Glucokinase and glucose homeostasis: proven concepts and new ideas. Biochem Soc Trans. 2005;33:306–310. doi: 10.1042/BST0330306. [DOI] [PubMed] [Google Scholar]
- 21.Lin SX, Neet KE. Demonstration of a slow conformational change in liver glucokinase by fluorescence spectroscopy. J Biol Chem. 1990;265:9670–9675. [PubMed] [Google Scholar]
- 22.Kamata K, Mitsuya M, Nishimura T, Eiki J, Nagata Y. Structural basis for allosteric regulation of the monomeric allosteric enzyme human glucokinase. Structure. 2004;12:429–438. doi: 10.1016/j.str.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Zelent B, Buettger C, Grimsby J, et al. Thermal stabilty of glucokinase (GK) as influenced by the substrate glucose, an allosteric glucokinase activator drug (GKA) and the osmolytes glycerol and urea. Biochim Biophys Acta. 2012;1824:769–784. doi: 10.1016/j.bbapap.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matschinsky FM, Zelent B, Doliba NM, et al. Research and development of glucokinase activators for diabetes therapy: theoretical and practical aspects. Handb Exp Pharmacol. 2011;203:357–401. doi: 10.1007/978-3-642-17214-4_15. [DOI] [PubMed] [Google Scholar]
- 25.Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- 26.Kawamori D, Welters HJ, Kulkarni RN. Molecular pathways underlying the pathogenesis of pancreatic alpha-cell dysfunction. Adv Exp Med Biol. 2010;654:421–445. doi: 10.1007/978-90-481-3271-3_18. [DOI] [PubMed] [Google Scholar]
- 27.Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB. Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol. 2003;5:330–335. doi: 10.1038/ncb951. [DOI] [PubMed] [Google Scholar]
- 28.Walker JN, Ramracheya R, Zhang Q, Johnson PR, Braun M, Rorsman P. Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Diabetes Obes Metab. 2011;13(Suppl. 1):95–105. doi: 10.1111/j.1463-1326.2011.01450.x. [DOI] [PubMed] [Google Scholar]
- 29.Matschinsky FM, Pagliara AS, Hover BA, Pace CS, Ferrendelli JA, Williams A. Hormone secretion and glucose metabolism in islets of Langerhans of the isolated perfused pancreas from normal and streptozotocin diabetic rats. J Biol Chem. 1976;251:6053–6061. [PubMed] [Google Scholar]
- 30.Bedoya FJ, Oberholtzer JC, Matschinsky FM. Glucokinase in B-cell-depleted islets of Langerhans. J Histochem Cytochem. 1987;35:1089–1093. doi: 10.1177/35.10.3305701. [DOI] [PubMed] [Google Scholar]
- 31.Fenner D, Odili S, Hong HK, et al. Generation of N-ethyl-N-nitrosourea (ENU) diabetes models in mice demonstrates genotype-specific action of glucokinase activators. J Biol Chem. 2011;286:39560–39572. doi: 10.1074/jbc.M111.269100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 33.Riedel AA, Heien H, Wogen J, Plauschinat CA. Loss of glycemic control in patients with type 2 diabetes mellitus who were receiving initial metformin, sulfonylurea, or thiazolidinedione monotherapy. Pharmacotherapy. 2007;27:1102–1110. doi: 10.1592/phco.27.8.1102. [DOI] [PubMed] [Google Scholar]
- 34.Ohyama S, Takano H, Iino T, et al. A small-molecule glucokinase activator lowers blood glucose in the sulfonylurea-desensitized rat. Eur J Pharmacol. 2010;640:250–256. doi: 10.1016/j.ejphar.2010.04.054. [DOI] [PubMed] [Google Scholar]
- 35.Li C, Buettger C, Kwagh J, Matter A, et al. A signaling role of glutamine in insulin secretion. J Biol Chem. 2004;279:13393–13401. doi: 10.1074/jbc.M311502200. [DOI] [PubMed] [Google Scholar]
- 36.Sivitz WI. Lipotoxicity and glucotoxicity in type 2 diabetes. Effects on development and progression. Postgrad Med. 2001;109(55–9):63–64. doi: 10.3810/pgm.2001.04.908. [DOI] [PubMed] [Google Scholar]
- 37.Del Prato S. Role of glucotoxicity and lipotoxicity in the pathophysiology of Type 2 diabetes mellitus and emerging treatment strategies. Diabet Med. 2009;26:1185–1192. doi: 10.1111/j.1464-5491.2009.02847.x. [DOI] [PubMed] [Google Scholar]
- 38.Doliba NM, Qin W, Vatamaniuk MZ, et al. Restitution of defective glucose-stimulated insulin release of sulfonylurea type 1 receptor knockout mice by acetylcholine. Am J Physiol Endocrinol Metab. 2004;286:E834–E843. doi: 10.1152/ajpendo.00292.2003. [DOI] [PubMed] [Google Scholar]
- 39.Wellendorph P, Johansen LD, Brauner-Osborne H. Molecular pharmacology of promiscuous seven transmembrane receptors sensing organic nutrients. Mol Pharmacol. 2009;76:453–465. doi: 10.1124/mol.109.055244. [DOI] [PubMed] [Google Scholar]
- 40.De Leon DD, Li C, Delson MI, Matschinsky FM, Stanley CA, Stoffers DA. Exendin-(9–39) corrects fasting hypoglycemia in SUR-1−/− mice by lowering cAMP in pancreatic beta-cells and inhibiting insulin secretion. J Biol Chem. 2008;283:25786–25793. doi: 10.1074/jbc.M804372200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Leon D, Stanley CA. Pathophysiology of diffuse ATP-sensitive potassium channel hyperinsulinism. In: Stanley C, De Leon DD, editors. Monogenic Hyperinsulinemic Hypoglycemia Disorders. Front Diabetes. Basel: Karger; 2012. pp. 18–29. [Google Scholar]
- 42.Kyriazis GA, Soundarapandian MM, Tyrberg B. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc Natl Acad Sci USA. 2012;109:E524–E532. doi: 10.1073/pnas.1115183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kojima I, Nakagawa Y. The role of the sweet taste receptor in enteroendocrine cells and pancreatic beta-cells. Diabetes Metab J. 2011;35:451–457. doi: 10.4093/dmj.2011.35.5.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doliba NM, Qin W, Najafi H, et al. Glucokinase activation repairs defective bioenergetics of islets of Langerhans isolated from type 2 diabetics. Am J Physiol Endocrinol Metab. 2012;302:E87–E102. doi: 10.1152/ajpendo.00218.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ehses JA, Boni-Schnetzler M, Faulenbach M, Donath MY. Macrophages, cytokines and beta-cell death in Type 2 diabetes. Biochem Soc Trans. 2008;36:340–342. doi: 10.1042/BST0360340. [DOI] [PubMed] [Google Scholar]
- 46.Matschinsky FM, Ellerman JE. Metabolism of glucose in the islets of Langerhans. J Biol Chem. 1968;243:2730–2736. [PubMed] [Google Scholar]
- 47.Bedoya FJ, Wilson JM, Ghosh AK, Finegold D, Matschinsky FM. The glucokinase glucose sensor in human pancreatic islet tissue. Diabetes. 1986;35:61–67. doi: 10.2337/diab.35.1.61. [DOI] [PubMed] [Google Scholar]
- 48.Leech CA, Dzhura I, Chepurny OG, et al. Molecular physiology of glucagon-like peptide-1 insulin secretagogue action in pancreatic beta cells. Prog Biophys Mol Biol. 2011;107:236–247. doi: 10.1016/j.pbiomolbio.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iynedjian PB. Mammalian glucokinase and its gene. Biochem J. 1993;293:1–13. doi: 10.1042/bj2930001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heimberg H, De Vos A, Moens K, et al. The glucose sensor protein glucokinase is expressed in glucagon-producing alpha-cells. Proc Natl Acad Sci USA. 1996;93:7036–7041. doi: 10.1073/pnas.93.14.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leibiger IB, Walther R, Pett U, Leibiger B. Positive and negative regulatory elements are involved in transcriptional control of the rat glucokinase gene in the insulin producing cell line HIT M2.2.2. FEBS Lett. 1994;337:161–166. doi: 10.1016/0014-5793(94)80265-3. [DOI] [PubMed] [Google Scholar]
- 52.Payne VA, Arden C, Lange AJ, Agius L. Contributions of glucokinase and phosphofructokinase-2/fructose bisphosphatase-2 to the elevated glycolysis in hepatocytes from Zucker fa/fa rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R618–R625. doi: 10.1152/ajpregu.00061.2007. [DOI] [PubMed] [Google Scholar]
- 53.Danial NN, Walensky LD, Zhang CY, et al. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med. 2008;14:144–153. doi: 10.1038/nm1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Danial NN, Gramm CF, Scorrano L, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- 55.Miwa I, Toyoda Y, Yoshie S. Glucokinase in beta-cell insulin secretory granules. In: Matschinsky F, Magnuson MA, editors. Glucokinase and Glycemic Disease: From Basics to Novel Therapeutics. Basel: Karger; 2004. pp. 350–359. [Google Scholar]
- 56.Vandercammen A, Van Schaftingen E. The mechanism by which rat liver glucokinase is inhibited by the regulatory protein. Eur J Biochem. 1990;191:483–489. doi: 10.1111/j.1432-1033.1990.tb19147.x. [DOI] [PubMed] [Google Scholar]
- 57.Shamas-Din A, Brahmbhatt H, Leber B, Andrews DW. BH3-only proteins: Orchestrators of apoptosis. Biochim Biophys Acta. 2011;1813:508–520. doi: 10.1016/j.bbamcr.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 58.Arden C, Baltrusch S, Agius L. Glucokinase regulatory protein is associated with mitochondria in hepatocytes. FEBS Lett. 2006;580:2065–2070. doi: 10.1016/j.febslet.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 59.Danial NN. BAD: undertaker by night, candyman by day. Oncogene. 2008;27(Suppl. 1):S53–S70. doi: 10.1038/onc.2009.44. [DOI] [PubMed] [Google Scholar]
- 60.Osundiji MA, Godes ML, Evans ML, Danial NN. BAD modulates counterregulatory responses to hypoglycemia and protective glucoprivic feeding. PLoS One. 2011;6:e28016. doi: 10.1371/journal.pone.0028016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Terauchi Y, Takamoto I, Kubota N, et al. Glucokinase and IRS-2 are required for compensatory beta cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest. 2007;117:246–257. doi: 10.1172/JCI17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rudic RD, McNamara P, Reilly D, et al. Bioinformatic analysis of circadian gene oscillation in mouse aorta. Circulation. 2005;112:2716–2724. doi: 10.1161/CIRCULATIONAHA.105.568626. [DOI] [PubMed] [Google Scholar]
- 63.Doliba NM, Qin W, Vatamaniuk MZ, et al. Cholinergic regulation of fuel-induced hormone secretion and respiration of SUR1−/− mouse islets. Am J Physiol Endocrinol Metab. 2006;291:E525–E535. doi: 10.1152/ajpendo.00579.2005. [DOI] [PubMed] [Google Scholar]