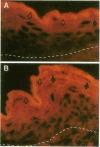
Abstract
The cutaneous permeability barrier to systemic water loss is mediated by hydrophobic lipids forming membrane bilayers within the intercellular domains of the stratum corneum (SC). The barrier emerges during day 20 of gestation in the fetal rat and is correlated with increasing SC thickness and increasing SC lipid content, the appearance of well-formed lamellar bodies in the epidermis, and the presence of lamellar unit structures throughout the SC. Because glucocorticoids accelerate lung lamellar body and surfactant maturation in man and experimental animals, these studies were undertaken to determine whether maternal glucocorticoid treatment accelerates maturation of the epidermal lamellar body secretory system. Maternal rats were injected with betamethasone or saline (control) on days 16-18, and pups were delivered prematurely on day 19. Whereas control pups exhibited immature barriers to transepidermal water loss (8.16 +/- 0.52 mg/cm2 per h), glucocorticoid-treated pups exhibited competent barriers (0.74 +/- 0.14 mg/cm2 per h; P < 0.001). Glucocorticoid treatment also: (a) accelerated maturation of lamellar body and SC membrane ultrastructure; (b) increased SC total lipid content twofold; and (c) increased cholesterol and polar ceramide content three- to sixfold. Thus, glucocorticoids accelerate the functional, morphological, and lipid biochemical maturation of the permeability barrier in the fetal rat.
Full text
PDF
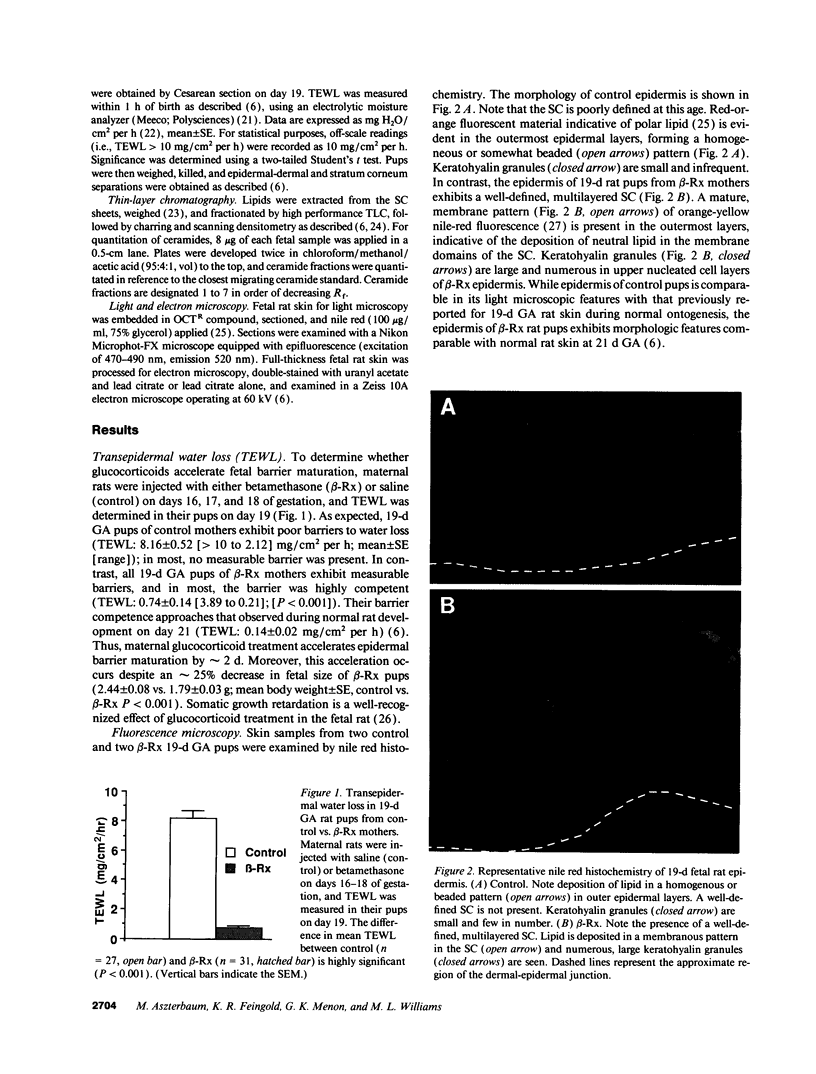



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aszterbaum M., Menon G. K., Feingold K. R., Williams M. L. Ontogeny of the epidermal barrier to water loss in the rat: correlation of function with stratum corneum structure and lipid content. Pediatr Res. 1992 Apr;31(4 Pt 1):308–317. doi: 10.1203/00006450-199204000-00002. [DOI] [PubMed] [Google Scholar]
- Ballard P. L. Hormonal regulation of pulmonary surfactant. Endocr Rev. 1989 May;10(2):165–181. doi: 10.1210/edrv-10-2-165. [DOI] [PubMed] [Google Scholar]
- Bourbon J. R., Farrell P. M., Doucet E., Brown D. J., Valenza C. Biochemical maturation of fetal rat lung: a comprehensive study including surfactant determination. Biol Neonate. 1987;52(1):48–60. doi: 10.1159/000242684. [DOI] [PubMed] [Google Scholar]
- Bowser P. A., Nugteren D. H., White R. J., Houtsmuller U. M., Prottey C. Identification, isolation and characterization of epidermal lipids containing linoleic acid. Biochim Biophys Acta. 1985 May 17;834(3):419–428. doi: 10.1016/0005-2760(85)90016-5. [DOI] [PubMed] [Google Scholar]
- Corbet A., Bucciarelli R., Goldman S., Mammel M., Wold D., Long W. Decreased mortality rate among small premature infants treated at birth with a single dose of synthetic surfactant: a multicenter controlled trial. American Exosurf Pediatric Study Group 1. J Pediatr. 1991 Feb;118(2):277–284. doi: 10.1016/s0022-3476(05)80502-5. [DOI] [PubMed] [Google Scholar]
- Elias P. M., Cooper E. R., Korc A., Brown B. E. Percutaneous transport in relation to stratum corneum structure and lipid composition. J Invest Dermatol. 1981 Apr;76(4):297–301. doi: 10.1111/1523-1747.ep12526137. [DOI] [PubMed] [Google Scholar]
- Elias P. M., Menon G. K. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991;24:1–26. doi: 10.1016/b978-0-12-024924-4.50005-5. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Man M. Q., Proksch E., Menon G. K., Brown B. E., Elias P. M. The lovastatin-treated rodent: a new model of barrier disruption and epidermal hyperplasia. J Invest Dermatol. 1991 Feb;96(2):201–209. doi: 10.1111/1523-1747.ep12461153. [DOI] [PubMed] [Google Scholar]
- Feingold K. R. The regulation and role of epidermal lipid synthesis. Adv Lipid Res. 1991;24:57–82. doi: 10.1016/b978-0-12-024924-4.50007-9. [DOI] [PubMed] [Google Scholar]
- Fowler S. D., Greenspan P. Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections: comparison with oil red O. J Histochem Cytochem. 1985 Aug;33(8):833–836. doi: 10.1177/33.8.4020099. [DOI] [PubMed] [Google Scholar]
- Frost P., Weinstein G. D., Bothwell J. W., Wildnauer R. Ichthyosiform dermatoses. 3. Studies of transepidermal water loss. Arch Dermatol. 1968 Sep;98(3):230–233. doi: 10.1001/archderm.98.3.230. [DOI] [PubMed] [Google Scholar]
- Ghadially R., Williams M. L., Hou S. Y., Elias P. M. Membrane structural abnormalities in the stratum corneum of the autosomal recessive ichthyoses. J Invest Dermatol. 1992 Dec;99(6):755–763. doi: 10.1111/1523-1747.ep12614489. [DOI] [PubMed] [Google Scholar]
- Grayson S., Johnson-Winegar A. G., Wintroub B. U., Isseroff R. R., Epstein E. H., Jr, Elias P. M. Lamellar body-enriched fractions from neonatal mice: preparative techniques and partial characterization. J Invest Dermatol. 1985 Oct;85(4):289–294. doi: 10.1111/1523-1747.ep12276826. [DOI] [PubMed] [Google Scholar]
- Grubauer G., Feingold K. R., Harris R. M., Elias P. M. Lipid content and lipid type as determinants of the epidermal permeability barrier. J Lipid Res. 1989 Jan;30(1):89–96. [PubMed] [Google Scholar]
- Hammarlund K., Sedin G. Transepidermal water loss in newborn infants. III. Relation to gestational age. Acta Paediatr Scand. 1979 Nov;68(6):795–801. doi: 10.1111/j.1651-2227.1979.tb08214.x. [DOI] [PubMed] [Google Scholar]
- Hammarlund K., Strömberg B., Sedin G. Heat loss from the skin of preterm and fullterm newborn infants during the first weeks after birth. Biol Neonate. 1986;50(1):1–10. doi: 10.1159/000242554. [DOI] [PubMed] [Google Scholar]
- Hawgood S., Benson B. J., Schilling J., Damm D., Clements J. A., White R. T. Nucleotide and amino acid sequences of pulmonary surfactant protein SP 18 and evidence for cooperation between SP 18 and SP 28-36 in surfactant lipid adsorption. Proc Natl Acad Sci U S A. 1987 Jan;84(1):66–70. doi: 10.1073/pnas.84.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q. C., Slotkin T. A. Effects of prenatal dexamethasone or terbutaline exposure on development of neural and intrinsic control of heart rate. Pediatr Res. 1989 Dec;26(6):554–557. doi: 10.1203/00006450-198912000-00005. [DOI] [PubMed] [Google Scholar]
- Hou S. Y., Mitra A. K., White S. H., Menon G. K., Ghadially R., Elias P. M. Membrane structures in normal and essential fatty acid-deficient stratum corneum: characterization by ruthenium tetroxide staining and x-ray diffraction. J Invest Dermatol. 1991 Feb;96(2):215–223. doi: 10.1111/1523-1747.ep12461361. [DOI] [PubMed] [Google Scholar]
- Kendig J. W., Notter R. H., Cox C., Reubens L. J., Davis J. M., Maniscalco W. M., Sinkin R. A., Bartoletti A., Dweck H. S., Horgan M. J. A comparison of surfactant as immediate prophylaxis and as rescue therapy in newborns of less than 30 weeks' gestation. N Engl J Med. 1991 Mar 28;324(13):865–871. doi: 10.1056/NEJM199103283241301. [DOI] [PubMed] [Google Scholar]
- Lampe M. A., Burlingame A. L., Whitney J., Williams M. L., Brown B. E., Roitman E., Elias P. M. Human stratum corneum lipids: characterization and regional variations. J Lipid Res. 1983 Feb;24(2):120–130. [PubMed] [Google Scholar]
- Madison K. C., Swartzendruber D. C., Wertz P. W., Downing D. T. Presence of intact intercellular lipid lamellae in the upper layers of the stratum corneum. J Invest Dermatol. 1987 Jun;88(6):714–718. doi: 10.1111/1523-1747.ep12470386. [DOI] [PubMed] [Google Scholar]
- Maurer A., Micheli J. L., Schütz Y., Freymond D., Jéquier E. Transepidermal water loss and resting energy expenditure in preterm infants. Helv Paediatr Acta. 1984 Dec;39(5-6):405–418. [PubMed] [Google Scholar]
- Menon G. K., Feingold K. R., Elias P. M. Lamellar body secretory response to barrier disruption. J Invest Dermatol. 1992 Mar;98(3):279–289. doi: 10.1111/1523-1747.ep12497866. [DOI] [PubMed] [Google Scholar]
- Menon G. K., Feingold K. R., Mao-Qiang M., Schaude M., Elias P. M. Structural basis for the barrier abnormality following inhibition of HMG CoA reductase in murine epidermis. J Invest Dermatol. 1992 Feb;98(2):209–219. doi: 10.1111/1523-1747.ep12555880. [DOI] [PubMed] [Google Scholar]
- Menon G. K., Feingold K. R., Moser A. H., Brown B. E., Elias P. M. De novo sterologenesis in the skin. II. Regulation by cutaneous barrier requirements. J Lipid Res. 1985 Apr;26(4):418–427. [PubMed] [Google Scholar]
- Nachman R. L., Esterly N. B. Increased skin permeability in preterm infants. J Pediatr. 1971 Oct;79(4):628–632. doi: 10.1016/s0022-3476(71)80311-6. [DOI] [PubMed] [Google Scholar]
- Navarro H. A., Lachowicz J., Bartolome J., Whitmore W. L., Slotkin T. A. Effects of prenatal dexamethasone on development of ornithine decarboxylase activity in brain and peripheral tissues of rats. Pediatr Res. 1988 Oct;24(4):465–469. doi: 10.1203/00006450-198810000-00009. [DOI] [PubMed] [Google Scholar]
- Ponec M., Weerheim A., Kempenaar J., Mommaas A. M., Nugteren D. H. Lipid composition of cultured human keratinocytes in relation to their differentiation. J Lipid Res. 1988 Jul;29(7):949–961. [PubMed] [Google Scholar]
- Rutter N., Hull D. Water loss from the skin of term and preterm babies. Arch Dis Child. 1979 Nov;54(11):858–868. doi: 10.1136/adc.54.11.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellhase D. E., Emrie P. A., Fisher J. H., Shannon J. M. Ontogeny of surfactant apoproteins in the rat. Pediatr Res. 1989 Sep;26(3):167–174. doi: 10.1203/00006450-198909000-00001. [DOI] [PubMed] [Google Scholar]
- Schurer N. Y., Elias P. M. The biochemistry and function of stratum corneum lipids. Adv Lipid Res. 1991;24:27–56. doi: 10.1016/b978-0-12-024924-4.50006-7. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Miyamura K., Kuroki Y. Appearance of surfactant proteins, SP-A and SP-B, in developing rat lung and the effects of in vivo dexamethasone treatment. Biochim Biophys Acta. 1991 Jan 4;1081(1):53–60. doi: 10.1016/0005-2760(91)90249-h. [DOI] [PubMed] [Google Scholar]
- Sicard R. E., Werner J. C. Dexamethasone induces a transient relative cardiomegaly in neonatal rats. Pediatr Res. 1992 Apr;31(4 Pt 1):359–363. doi: 10.1203/00006450-199204000-00011. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Fujita Y., Kogishi K. Reconstitution of tubular myelin from synthetic lipids and proteins associated with pig pulmonary surfactant. Am Rev Respir Dis. 1989 Jul;140(1):75–81. doi: 10.1164/ajrccm/140.1.75. [DOI] [PubMed] [Google Scholar]
- Swartzendruber D. C., Wertz P. W., Kitko D. J., Madison K. C., Downing D. T. Molecular models of the intercellular lipid lamellae in mammalian stratum corneum. J Invest Dermatol. 1989 Feb;92(2):251–257. doi: 10.1111/1523-1747.ep12276794. [DOI] [PubMed] [Google Scholar]
- Taeusch H. W., Jr, Wang N. S., Avery M. E. Studies on organ maturation: "skin age" as an indicator of "lung age" in fetal rabbits. Pediatrics. 1972 Mar;49(3):400–405. [PubMed] [Google Scholar]
- Takahashi A., Fujiwara T. Proteolipid in bovine lung surfactant: its role in surfactant function. Biochem Biophys Res Commun. 1986 Mar 13;135(2):527–532. doi: 10.1016/0006-291x(86)90026-4. [DOI] [PubMed] [Google Scholar]
- Van Golde L. M., Batenburg J. J., Robertson B. The pulmonary surfactant system: biochemical aspects and functional significance. Physiol Rev. 1988 Apr;68(2):374–455. doi: 10.1152/physrev.1988.68.2.374. [DOI] [PubMed] [Google Scholar]
- Vernon H. J., Lane A. T., Wischerath L. J., Davis J. M., Menegus M. A. Semipermeable dressing and transepidermal water loss in premature infants. Pediatrics. 1990 Sep;86(3):357–362. [PubMed] [Google Scholar]
- Wertz P. W., Downing D. T. Ceramides of pig epidermis: structure determination. J Lipid Res. 1983 Jun;24(6):759–765. [PubMed] [Google Scholar]
- Wertz P. W., Downing D. T., Freinkel R. K., Traczyk T. N. Sphingolipids of the stratum corneum and lamellar granules of fetal rat epidermis. J Invest Dermatol. 1984 Sep;83(3):193–195. doi: 10.1111/1523-1747.ep12263553. [DOI] [PubMed] [Google Scholar]
- Wilson D. R., Maibach H. I. Transepidermal water loss in vivo. Premature and term infants. Biol Neonate. 1980;37(3-4):180–185. doi: 10.1159/000241271. [DOI] [PubMed] [Google Scholar]