Abstract
Background
The performance of nationwide studies of chronic otitis media (COM) in adults has been insufficient in Korea. We evaluated the prevalence and risk factors of COM in Korea.
Methods
This study was conducted using data from the fifth Korean National Health and Nutrition Examination Survey (n = 23,621). After excluding the subjects under 20 year old and suffered from cancers, 16,063 patients were evaluated for COM. Participants underwent a medical interview, physical examination, endoscopic examination, and blood and urine test. COM was diagnosed by trained residents in the Department of Otorhinolaryngology using an ear, nose, and throat questionnaire and otoendoscopy findings. Data on the presence and absence of COM were collected. Multivariate logistic regression analyses were performed to identify its risk factors.
Results
Of the 16,063 participants aged above 20 year old, the weighted prevalence of COM was 3.8%. In the multivariate analyses, the following factors showed high odds ratios (ORs) for COM: pulmonary tuberculosis (adjusted OR, 1.78; 95% confidence interval [CI], 1.06-3.01), chronic rhinosinusitis (adjusted OR, 1.87; 95% CI, 1.17-2.98), mild hearing impairment (adjusted OR, 1.95; 95% CI, 1.34-2.85), moderate hearing impairment (adjusted OR, 4.00; 95% CI, 2.21-7.22), tinnitus (adjusted OR, 1.82; 95% CI, 1.34-2.49), increased hearing thresholds in pure tone audiometry in the right ear (adjusted OR, 1.02; 95% CI, 1.01-1.03), and left ear (adjusted OR, 1.03; 95% CI, 1.02-1.04). The following factors showed low odds ratios for COM: hepatitis B (adjusted OR, 0.28; 95% CI, 0.08-0.94) and rhinitis (adjusted OR, 0.60; 95% CI, 0.42-0.88). In addition, high levels of vitamin D, lead, and cadmium, EQ-5D index; and low red blood cell counts were associated with development of COM (Student’s t-test, P < 0.01).
Conclusions
Our population-based study showed that COM is not rare in Korea, and its development may be associated with various host and environmental factors. Further research on its relationships and the pathogenesis are needed.
Introduction
Chronic otitis media (COM) is characterized by inflammation of the middle ear that results in long-term or permanent changes in the tympanic membrane. These changes include perforation, atelectasis, retraction, tympanosclerosis, and cholesteatoma. COM can be classified based on whether it involves active inflammation or is associated with a cholesteatoma [1]. This disorder is a major cause of acquired hearing loss, especially in developing countries, and is a major disease entity in the field of otolaryngology. It often requires expensive treatment and ear surgery, and can induce severe or fatal complications such as mastoiditis, facial nerve palsy, labyrinthitis, petrositis, brain abscessation, meningitis, and thrombophlebitis. COM also decreases patients’ quality of life. Chronic active or suppurative otitis media affects 65 to 330 million people worldwide, and more than half of these patients have significant hearing impairment. Worldwide, COM is responsible for an estimated 28,000 deaths annually, and is associated with a disease burden involving more than 2 million individuals daily [2].
Many previous studies have investigated the prevalence and risk factors of COM. Its reported prevalence in Southeast Asia, Africa, and Western Pacific countries is 2–4%, and that in North America and European countries is < 2%. Risk factors of COM include low socioeconomic status, malnutrition, high number of children in the household, family history, and passive exposure to smoking [3]. However, most studies have involved children, and have confined the study of otitis media to chronic suppurative otitis media, acute otitis media, or otitis media with effusion [4–13]. Moreover, the effects of various host and environmental factors have not been well defined. Information on the risk factors of COM would contribute to effective treatment and control of this disease.
Here, we identified the prevalence and risk factors of COM in Korea through analysis of data collected by medical interviews, endoscopic examinations, pure tone audiometry, and blood tests that included heavy metal levels.
Materials and Methods
Population
This study used the data of the fifth Korean National Health and Nutrition Examination Survey (KNHANES). This survey collected information, such as health and nutritional status, from a representative sample of the general Korean population to monitor the prevalence and control, and to reveal the risk factors, of certain chronic diseases. The KNHANES included information on the presence and absence of COM, which was diagnosed by otolaryngology residents.
In total, 23,621 individuals (8,313 in 2010, 7,887 in 2011, 7,421 in 2012) agreed to participate in the health surveys and underwent medical checkups that included ear, nose, and throat (ENT) examinations. Patients aged < 20 years (n = 5,744), those with cancer (n = 641), and those with a missed COM diagnosis (those in whom otoendoscopy was not performed because of the individual’s refusal or missed examination) (n = 1,173) were excluded. Finally, 16,063 individuals were analyzed in this study. The average patient age was 50.2 ± 16.3 years (range, 20–97 years), and the male: female ratio 1.00:1.35. Written informed consent was obtained from all of the participants prior to the survey. This study was approved by the Institutional Review Board of the Seoul National University Hospital (1409–079–609).
ENT evaluation, medical history, and clinical examination
The diagnosis of COM was determined by trained residents using a systematic ENT questionnaire and the following otoendoscopy findings: tympanic membrane perforation and/or cholesteatoma, including congenital cholesteatoma, and a retraction pocket and/or otitis media with effusion, including patients with insertion of a ventilation tube. Because it is not easy to diagnose congenital cholesteatoma in adults and there is some debate about the definition of congenital cholesteatoma, we did not differentiate between cholesteatoma and congenital cholesteatoma in this study.
The prevalence of COM was analyzed in six different age groups, each covering a 10-year period, as well as between male and female patients.
Information on the patients’ socioeconomic status was investigated, including education level (less than middle school or beyond high school), income (< 25%, 25–50%, 50–75%, or > 75% according to the equivalized household income per month), occupation (white-collar: manager, professional, clerk, service/sales worker, unemployed, retired, student, or housewife; blue-collar: agriculture, forestry, fishery worker, craft and related trade worker, plant or machine operator or assembler, or simple laborer), residency (urban or rural area in accordance with the patient’s official address), and exposure to noise (earphone use in noisy situations or temporary exposure to noise). Information was also collected on each patient’s smoking status (nonsmoker, < 5 packs in their life; smoker, > 5 packs and currently smoking), alcohol drinking status (no, does not drink; yes, alcohol consumption one or more times per month during the past year), number of household members (1–2, 3–4, 5–6, or ≥ 7), and subjective health status (very good, good, average, poor, or very poor). Each patient’s body mass index was categorized as either < 25 or > 25 kg/m2.
Professional interviewers from the Korea Centers for Disease Control and Prevention provided a documented questionnaire and obtained the patients’ medical histories. Allergic rhinitis was diagnosed when patients had experienced symptoms of sneezing, rhinorrhea, nasal obstruction, and itching for the past year. The diagnosis of chronic rhinosinusitis was made when nasal polyps were observed during endoscopy or when more than one of the following symptoms was present: anterior/posterior nasal drip, nasal obstruction, facial pain/tenderness, and olfactory dysfunction for more than 3 months (anterior/posterior nasal drip or nasal obstruction should be included as a presenting symptom). Septal deviation was defined as asymmetrical displacement of the nasal septum to the left or right side of the nasal cavity after vasoconstriction of the nasal mucosa.
For otologic investigation, the patients were asked about their hearing and any symptoms of tinnitus by questionnaire, and physical examinations were conducted by the residents to assess the presence or absence of facial palsy and preauricular sinuses. In addition, pure tone audiometry was performed and calculated by averaging thresholds at 500 Hz, 1000 Hz, 2000 Hz, and 3000 Hz.
Blood samples were collected and analyzed in a single laboratory (Neodin Medical Institute, Seoul, Korea). The Euro Qol-5D (EQ-5D) is a standard tool used to measure patients’ health status in five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression [14,15]. Each dimension has three grades of severity: no problems (score of 1), moderate problems (score of 2), or serious problems (score of 3). The EQ-5D index is calculated from the EQ-5D score by applying a formula that assigns weights to each of the grades in each dimension. This formula differs among nations based on the value of the EQ-5D from population samples [16]. The KNHANES algorithm for calculating the EQ-5D index was applied in the present study; it ranged from 1 (best health) through 0 (equivalent to death) to—0.171 (worse than death).
Statistical analysis
The data were analyzed with SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA), which incorporates sample weights, and adjusts the analysis for the complex sample design of the survey. We used the KNHANES sampling weight variables along with a masked variance primary sampling unit and stratum variables. This adjustment allowed for extrapolation from the samples to the noninstitutionalized civilian Korean population as a whole. The survey sample weights were used in all of the analyses. Statistics were used to describe the general characteristics, medical conditions, otologic conditions, blood test results, and questionnaire results regarding quality of life of all of the samples according to COM. Data were tested for statistical significance by applying the chi-squared test for categorical variables and the Student’s t-test for continuous variables.
Logistic regression analysis was performed to identify risk factors independently associated with COM. Multiple logistic regression analyses were performed and included variables with P values < 0.2 in the univariate analysis to estimate their adjusted odds ratios (ORs) and 95% confidence intervals (CIs). However, the blood levels of heavy metals were excluded from the logistic regression analysis because the fifth KNHANES investigated these in few participants. A P value < 0.05 indicated statistical significance.
Results
Prevalence of COM
Among the 16,063 participants, the weighted prevalence of COM was 3.8% (tympanic perforation, 2.17%; cholesteatoma, 1.82%; otitis media with effusion, 0.68%), and those of the right, left, and both ears were 1.5%, 1.4%, and 0.9%, respectively.
The prevalence of COM according to the general characteristics of the participants is described in Table 1. Age, sex, education, residence, earphone use in noisy situations, number of household members, and subjective health status affected the prevalence of COM. An increased prevalence was associated with old age (P < 0.0001), female sex (P = 0.0287), lower education level (P < 0.0001), urban residence (P = 0.0239), not using earphones in noisy situations (P < 0.0001), fewer household members (P < 0.0001), and a poor subjective health status (P < 0.0001). There were no significant differences between male and female patients among the six different age groups, but the prevalence of COM tended to increase with age (Fig 1).
Table 1. Prevalence of chronic otitis media according to the general characteristics of KNHNES participants.
| COM weighted*, % (SE) | ||||
|---|---|---|---|---|
| Characteristics | Unweighted total number | No | Yes | P value |
| Overall | 16063 | 96.2 (0.2) | 3.8 (0.2) | |
| Affected ear | ||||
| Right | 98.5 (0.1) | 1.5 (0.1) | ||
| Left | 98.6 (0.1) | 1.4 (0.1) | ||
| Both | 99.1 (0.1) | 0.9 (0.1) | ||
| Age(year) | <0.0001 | |||
| 20–29 | 1797 | 98.9 (0.3) | 1.1 (0.3) | |
| 30–39 | 3154 | 98.1 (0.3) | 1.9 (0.3) | |
| 40–49 | 2989 | 97.0 (0.4) | 3.0 (0.4) | |
| 50–59 | 3105 | 94.2 (0.6) | 5.8 (0.6) | |
| 60–69 | 2632 | 93.3 (0.6) | 6.7 (0.6) | |
| ≥70 | 2386 | 91.5 (0.7) | 8.5 (0.7) | |
| Sex | 0.0287 | |||
| Male | 6830 | 96.7 (0.3) | 3.3 (0.3) | |
| female | 9233 | 95.8 (0.3) | 4.2 (0.3) | |
| Education | <0.0001 | |||
| ≤Middle school | 5492 | 93.1 (0.4) | 6.9 (0.4) | |
| ≥High school | 10140 | 97.6 (0.2) | 2.4 (0.2) | |
| Missing | 431 | |||
| Income | 0.1732 | |||
| <25% | 3891 | 96.0 (0.4) | 4.0 (0.4) | |
| 25–50% | 3959 | 96.5 (0.3) | 3.5 (0.3) | |
| 50–75% | 4008 | 95.8 (0.4) | 4.2 (0.4) | |
| ≥75% | 4012 | 96.8 (0.4) | 3.2 (0.4) | |
| missing | 193 | |||
| Occupation | 0.7594 | |||
| white collar | 12767 | 96.4 (0.2) | 3.6 (0.2) | |
| blue collar | 2828 | 96.2 (0.4) | 3.8 (0.4) | |
| Residence | 0.0239 | |||
| Rural | 12780 | 96.5 (0.2) | 3.5 (0.2) | |
| Urban | 3283 | 95.4 (0.4) | 4.6 (0.4) | |
| Body mass index(kg/m2) | 0.3783 | |||
| <25 | 10857 | 96.1 (0.2) | 3.9 (0.2) | |
| ≥25 | 5151 | 96.5 (0.3) | 3.5 (0.3) | |
| Missing | 55 | |||
| Noise | ||||
| Earphone use in noisy situation | <0.0001 | |||
| Yes | 1376 | 98.4 (0.4) | 1.6 (0.4) | |
| No | 14627 | 96.0 (0.2) | 4.0 (0.2) | |
| Missing | 60 | |||
| Temporary exposure to noise | 0.1384 | |||
| Yes | 3347 | 96.7 (0.4) | 3.3 (0.4) | |
| No | 12643 | 96.1 (0.2) | 3.9 (0.2) | |
| Smoking status | 0.0605 | |||
| No | 12398 | 96.1 (0.2) | 3.9 (0.2) | |
| Yes | 3245 | 96.9 (0.4) | 3.1 (0.4) | |
| Missing | 420 | |||
| Alcohol consumption | 0.0681 | |||
| No | 7242 | 95.9 (0.3) | 4.1 (0.3) | |
| Yes | 8335 | 96.6 (0.2) | 3.4 (0.2) | |
| Missing | 486 | |||
| Household members(persons) | <0.0001 | |||
| 1–2 | 5234 | 94.2 (0.4) | 5.8 (0.4) | |
| 3–4 | 8609 | 97.1 (0.2) | 2.9 (0.2) | |
| 5–6 | 2052 | 96.7 (0.5) | 3.3 (0.5) | |
| ≥7 | 146 | 98.1 (1.0) | 1.9 (1.0) | |
| Missing | 22 | |||
| Subjective health status | <0.0001 | |||
| Very good | 703 | 97.2 (0.7) | 2.8 (0.7) | |
| Good | 4560 | 97.1 (0.3) | 2.9 (0.3) | |
| Average | 7491 | 96.5 (0.3) | 3.5 (0.3) | |
| Poor | 2413 | 94.7 (0.6) | 5.3 (0.6) | |
| Very poor | 490 | 91.9 (1.3) | 8.1 (1.3) | |
| Missing | 406 | |||
COM, chronic otitis media; SE, standard error; KNHANES, Korean national health and nutrition examination survey;
*weighted for the multistage sampling design of KNHANES 2010~2012
Fig 1. Prevalence of chronic otitis media by age group and sex in Korean adults.
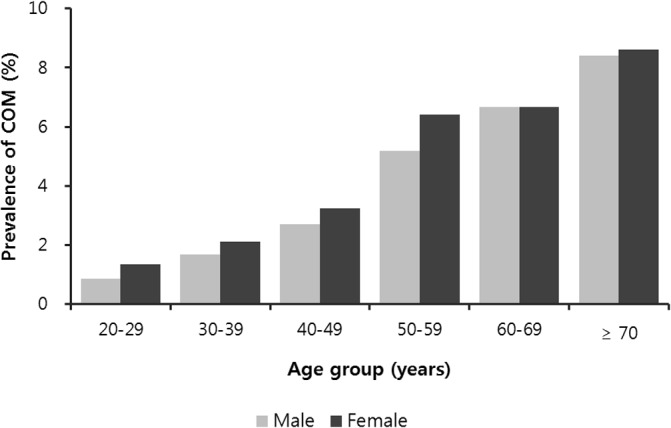
The prevalence of chronic otitis media increased with age in both sexes.
Table 2 shows that diverse medical conditions were associated with COM. Hypertension and diabetes mellitus were associated with an increased prevalence of COM. In contrast, hepatitis B and allergic rhinitis were associated with decreased prevalence of COM.
Table 2. Prevalence of chronic otitis media according to the medical conditions of KNHANES participants.
| COM weighted*, % (SE) | ||||
|---|---|---|---|---|
| Characteristics | Unweighted total number | No | Yes | P value |
| Hypertension | <.0001 | |||
| No | 10742 | 97.2 (0.2) | 2.8 (0.2) | |
| Yes | 4744 | 93.8 (0.5) | 6.2 (0.5) | |
| Diabetes mellitus | 0.0001 | |||
| No | 13228 | 96.6 (0.2) | 3.4 (0.2) | |
| Yes | 1460 | 93.1 (0.9) | 6.9 (0.9) | |
| Pulmonary Tuberculosis | 0.1219 | |||
| No | 14903 | 96.4 (0.2) | 3.6 (0.2) | |
| Yes | 750 | 94.7 (1.1) | 5.3 (1.1) | |
| Hepatitis B | 0.0004 | |||
| No | 15414 | 96.3 (0.2) | 3.7 (0.2) | |
| Yes | 237 | 98.6 (0.6) | 1.4 (0.6) | |
| Atopic dermatitis | 0.5667 | |||
| No | 15298 | 96.3 (0.2) | 3.7 (0.2) | |
| Yes | 353 | 97.2 (1.5) | 2.8 (1.5) | |
| Allergic rhinitis | 0.0019 | |||
| No | 12023 | 95.9 (0.2) | 4.1 (0.2) | |
| Yes | 4033 | 97.1 (0.3) | 2.9 (0.3) | |
| Chronic rhinosinusitis | 0.1657 | |||
| No | 14922 | 96.3 (0.2) | 3.7 (0.2) | |
| Yes | 928 | 95.1 (0.9) | 4.9 (0.9) | |
| Septal deviation | 0.2119 | |||
| No | 8155 | 96.5 (0.3) | 3.5 (0.3) | |
| Yes | 7701 | 96.0 (0.3) | 4.0 (0.3) | |
COM, chronic otitis media; SE, standard error; KNHANES, Korean national health and nutrition examination survey;
*weighted for the multistage sampling design of KNHANES 2010~2012
Several items relevant to otologic findings are described in Table 3. Subjective hearing discomfort and tinnitus were associated with COM. Several other items are shown in Table 4. Blood test analysis showed that COM was associated with decreased red blood cell counts (Student’s t-test, P < 0.0001) and increased levels of vitamin D, lead, and cadmium (Student’s t-test, P < 0.0172). In the pure tone audiometry examinations, COM was associated with an increased hearing threshold in both ears (Student’s t-test, P < 0.0001). With respect to health status, COM was associated with a high EQ-5D index (P < 0.0001).
Table 3. Prevalence of chronic otitis media according to otologic conditions of KNHANES participants.
| COM weighted*, % (SE) | ||||
|---|---|---|---|---|
| Characteristics | Unweighted total number | No | Yes | P value |
| Subjective hearing | <0.0001 | |||
| Not discomfort | 13715 | 97.5 (0.2) | 2.5 (0.2) | |
| A little discomfort | 1852 | 89.9 (1.0) | 10.1 (1.0) | |
| A lot of discomfort | 444 | 72.7 (2.9) | 27.3 (2.9) | |
| Cannot hearing anything | 45 | 76.0 (7.1) | 24.0 (7.1) | |
| Tinnitus | <0.0001 | |||
| Yes | 3593 | 93.3 (0.5) | 6.7 (0.5) | |
| No | 12425 | 97.1 (0.2) | 2.9 (0.2) | |
| Not remember | 38 | 96.6 (2.4) | 3.4 (2.4) | |
| Facial palsy(House-brackman grade) | 0.9230 | |||
| Ⅰ~Ⅱ | 16027 | 96.2 (0.2) | 3.8 (0.2) | |
| Right side Ⅲ~Ⅵ | 21 | 95.6 (4.4) | 4.4 (4.4) | |
| Left side Ⅲ~Ⅵ | 15 | 94.3 (5.4) | 5.7 (5.4) | |
| Preauricular sinus, right | 0.3345 | |||
| Normal | 15919 | 96.3 (0.2) | 3.7 (0.2) | |
| Abnormal | 144 | 93.9 (2.4) | 6.1 (2.4) | |
| Preauricular sinus, left | 0.1339 | |||
| Normal | 15913 | 96.2 (0.2) | 3.8 (0.2) | |
| Abnormal | 150 | 97.7 (1.0) | 2.3 (1.0) | |
KNHANES, Korean national health and nutrition examination survey;
*weighted for the multistage sampling design of KNHANES 2010~2012
Table 4. Prevalence of chronic otitis media according to blood test, hearing threshold, and questionnare of quality of life.
| COM weighted* (SE) | ||||
|---|---|---|---|---|
| Variable | Unweighted total number | No | Yes | P value |
| Blood test | ||||
| White blood cells (Thous/μL) | 15267 | 6.13 (0.02) | 6.18 (0.08) | 0.5260 |
| Red blood cells (Mil/μL) | 15267 | 4.64 (0.01) | 4.53 (0.02) | <.0001 |
| Platelet (Thous/μL) | 15267 | 255.36 (0.64) | 251.41 (2.63) | 0.1511 |
| Vitamin D (ng/mL) | 15322 | 17.24 (0.13) | 17.95 (0.32) | 0.0172 |
| Heavy metals | ||||
| Lead (μg/dL) | 5420 | 2.301 (0.019) | 2.659 (0.148) | 0.0163 |
| Mercury (μg/L) | 5420 | 4.387 (0.070) | 4.639 (0.255) | 0.3416 |
| Cadmium (μg/L) | 5420 | 1.084 (0.012) | 1.385 (0.061) | <.0001 |
| Zinc (μg/dL) | 1790 | 138.08 (1.79) | 134.21 (5.33) | 0.4436 |
| Average hearing threshold | ||||
| Right ear (dB) | 14983 | 13.7 (0.2) | 30.9 (1.1) | <0.0001 |
| Left ear (dB) | 14982 | 13.9 (0.2) | 32.9 (1.4) | <0.0001 |
| EQ-5D index | 15640 | 0.95 (0.00) | 0.93 (0.01) | <.0001 |
EQ-5D, Euro Qol-5D; KNHANES, Korean national health and nutrition examination survey;
*weighted for the multistage sampling design of KNHANES 2010~2012
Multivariate analyses of risk factors
The results of the multivariable-adjusted analyses between COM and several patient characteristics are shown in Table 5. A history of pulmonary tuberculosis (adjusted OR, 1.78; 95% CI, 1.06–3.01) and chronic rhinosinusitis (adjusted OR, 1.87; 95% CI, 1.17–2.98) were positively associated with COM. In contrast, hepatitis B (adjusted OR, 0.28; 95% CI, 0.08–0.94) and allergic rhinitis (adjusted OR, 0.60; 95% CI, 0.42–0.88) were negatively associated with COM. Among patients with no discomfort in subjective hearing, the risk of COM was significantly higher in those with slight discomfort (adjusted OR, 1.95; 95% CI, 1.34–2.85) and severe discomfort (adjusted OR, 4.00; 95% CI, 2.21–7.22). In addition, tinnitus was definitely associated with a higher risk of COM (adjusted OR, 1.82; 95% CI, 1.34–2.49). Compared to patients with normal hearing thresholds in pure tone audiometry, the ORs of COM with increased hearing thresholds were 1.02 and 1.03 in the right and left ear, respectively. With respect to health status, the EQ-5D index was positively associated with COM (adjusted OR, 6.25; 95% CI, 1.94–20.16).
Table 5. Adjusted odds ratio for the association between chronic otitis media and risk factors.
| Adjusted OR | 95% CI | P value | |
|---|---|---|---|
| Age(year) | |||
| 20–29 | 1.00 | ||
| 30–39 | 1.25 | (0.62–2.53) | 0.5334 |
| 40–49 | 1.73 | (0.75–3.97) | 0.1974 |
| 50–59 | 1.64 | (0.71–3.79) | 0.2464 |
| 60–69 | 0.70 | (0.28–1.75) | 0.4422 |
| ≥70 | 0.65 | (0.24–1.72) | 0.3837 |
| Sex (female) | 1.44 | (0.94–2.21) | 0.0967 |
| Education (≤Middle school) | 1.06 | (0.74–1.52) | 0.7598 |
| Income | |||
| <25% | 1.01 | (0.68–1.49) | 0.9750 |
| 25–50% | 0.96 | (0.63–1.45) | 0.8299 |
| 50–75% | 1.27 | (0.85–1.90) | 0.2375 |
| ≥75% | 1.00 | ||
| Residence (Urban) | 1.03 | (0.74–1.41) | 0.8820 |
| Earphone use in noisy situation | 0.94 | (0.44–2.01) | 0.8674 |
| Temporary exposure to noise | 1.00 | (0.68–1.46) | 0.9781 |
| Smoking status | 0.93 | (0.62–1.40) | 0.7266 |
| Alcohol consumption | 1.36 | (1.00–1.87) | 0.0530 |
| Household members (persons) | |||
| 1–2 | 1.42 | (0.37–5.47) | 0.6131 |
| 3–4 | 0.91 | (0.24–3.43) | 0.8936 |
| 5–6 | 1.19 | (0.29–4.84) | 0.8085 |
| ≥7 | 1.00 | ||
| Subjective health status | |||
| Very good | 1.00 | ||
| Good | 1.23 | (0.60–2.55) | 0.5745 |
| Average | 1.14 | (0.52–2.49) | 0.7519 |
| Poor | 1.45 | (0.63–3.33) | 0.3789 |
| Very poor | 1.60 | (0.67–3.86) | 0.2916 |
| Hypertension | 1.27 | (0.95–1.69) | 0.1086 |
| Diabetes mellitus | 1.31 | (0.89–1.94) | 0.1681 |
| Pulmonary tuberculosis | 1.78 | (1.06–3.01) | 0.0302 |
| Hepatitis B | 0.28 | (0.08–0.94) | 0.0395 |
| Allergic rhinitis | 0.60 | (0.42–0.88) | 0.0079 |
| Chronic rhinosinusitis | 1.87 | (1.17–2.98) | 0.0087 |
| Subjective hearing | |||
| Not discomfort | 1.00 | ||
| A little discomfort | 1.95 | (1.34–2.85) | 0.0005 |
| A lot of discomfort | 4.00 | (2.21–7.22) | <.0001 |
| Cannot hearing anything | 2.64 | (0.53–13.04) | 0.2336 |
| Tinnitus | |||
| Yes | 1.82 | (1.34–2.49) | 0.0002 |
| No | 1.00 | ||
| Not remember | 1.45 | (0.23–9.03) | 0.6882 |
| Preauricular sinus, left | 0.61 | (0.17–2.16) | 0.4448 |
| Average hearing threshold | |||
| Right ear (dB) | 1.02 | (1.01–1.03) | 0.0016 |
| Left (dB) | 1.03 | (1.02–1.04) | <.0001 |
| Blood test | |||
| Red blood cells | 0.93 | (0.62–1.42) | 0.7473 |
| Platelet | 1.00 | (1.00–1.00) | 0.9851 |
| Vitamin D (ng/mL) | 1.00 | (0.98–1.02) | 0.9018 |
| EQ-5D index | 6.25 | (1.94–20.16) | 0.0022 |
OR, odds ratio; CI, confidence interval; EQ-5D, Euro Qol-5D
Discussion
To the best of our knowledge, this is the first community-based study to examine the prevalence and risk factors of COM by assessment of clinical examination and laboratory test results among the Korean population. In addition, this study investigated the relationship between COM and the results of several blood tests, including blood levels of heavy metals. We believe that this study accurately represents the data of the Korean population, because the data from the KNHANES are comprehensive, statistically verified, and nationally representative [17].
The weighted prevalence of COM in Korean adults aged > 20 years was 3.8%. Generally, the prevalence of COM has been gradually declining worldwide. Many previous studies from several countries have reported that the prevalence of COM has been gradually declining on an annual basis. This decline has resulted from the wide use of antibiotics [18], improved nutrition and hygiene statuses secondary to economical growth, improved public welfare (i.e., coverage of universal health in Korea in 1989), and easy access to medical centers. Although the prevalence of COM is very diverse among different countries, our data are similar to those of East Asia (3.67%) according to the estimation released by the World Health Organization in 2004 [2,19]. Kim et al. [20,21] reported that the overall prevalence of otitis media in all of the age groups in Korea was 4.59% in 1981 and 2.85% in 1991, according to the results of nationwide surveys. Their study included both COM and otitis media with effusion. The prevalence of COM was higher in this study than in 1991. However, because the 1991 study was conducted for only 3 months (from July to October), the data may have been influenced by the season. Moreover, in the 1991 study, otorhinolaryngologists visited the subjects’ houses door to door and examined the tympanic membrane using an otoscope. In the present study, the participants visited the clinic and were examined with endoscopes by trained ENT residents. Therefore, an appropriate interpretation may be that an increase occurred in the rate of diagnosis of COM, and not the prevalence of COM.
We found high ORs for COM in relation to the following factors: hypertension, diabetes mellitus, pulmonary tuberculosis, chronic rhinosinusitis, mild/moderate hearing impairment, tinnitus, and increased hearing thresholds in pure tone audiometry. Many previous studies have shown that diabetes and cardiometabolic disease are associated with hearing loss [22–25], depending on the process of diabetic microangioapathy and macroangiopathy [23,26,27]. Moreover, otitis media is prevalent among people with high blood pressure and diabetes mellitus. Our result is in accordance with this study [28]. No previous studies have shown an association between pulmonary tuberculosis and COM. The association found in our study seems to reflect the special situation in Korea. Korea is endemic for pulmonary tuberculosis. Pulmonary tuberculosis might aggravate the patient’s general condition, which may be associated with the increased OR. The association between chronic rhinosinusitis and otitis media is well documented [29], and the findings in the present study are in accordance with previously established data. The polyps and swollen mucosa in patients with rhinosinusitis obstruct the eustachian tube orifice, leading to eustachian tube dysfunction. This is supported by the fact that the nasal cavity and eustachian tube share the same bacteria [29]. COM induces either conductive or sensorineural hearing loss. Our data also showed an association between subjective/objective hearing discomfort and COM, and also between tinnitus and COM.
In the present study, the ORs for COM were low in relation to hepatitis B and allergic rhinitis. Regarding hepatitis B, no previously published studies or other scientific data have reported this relationship. Further studies will be needed to investigate the cause of the relationship between hepatitis B and COM. Allergic rhinitis is a widely accepted risk factor for otitis media with effusion [30–34], but not for chronic suppurative otitis media [35]. The present study showed that allergic rhinitis was negatively associated with COM, although the precise mechanism underlying this association remains unclear. This study included more patients with chronic suppurative otitis media than with otitis media with effusion, because we only included adults > 20 years of age.
Most studies have suggested that otitis media has a negative effect on patients’ quality of life [36,37]. In the present study, quality of life showed a strong positive association with the prevalence of COM. Considering that one of the etiologies of COM is infection, and that infection is negatively associated with quality of life, our result opposes the commonly belief. This implies that the etiologies of COM are multifactorial, and that many critical factors other than infections influence COM. Interestingly, we found an association between heavy metals and COM. To the best of our knowledge, there have been no previous reports on this association. Although the reason for this association is unclear, it seems that high blood levels of heavy metals may destroy the middle ear mucosa or prevent the recovery of the tympanic membrane. However, these data were excluded from the multivariate analysis due to the reliability of the data because blood tests, including heavy metals, were only conducted in some cases.
This study had some limitations. First, COM is a generic term for all forms of middle ear inflammation. That is, it does not distinguish otitis media from effusion, cholesteatoma, and chronic suppurative otitis media. These conditions share some aspects of their pathogenesis, but differ with respect to other aspects. Second, because this was a cross-sectional study, the causative relationships between risk factors and COM may be difficult to determine. Third, an association between gastroesophageal reflux and otitis media in adults has not been proposed [38]. Gastric acid, biliary acid, and pepsin are refluxed into the esophagus by relaxation of the lower esophageal sphincter, and subsequently reach the nasopharynx, eustachian tube, and middle ear [39]. Although the casual link between gastroesophageal reflux and COM is not definitive, it might be clinically worthwhile to evaluate this relationship in the Korean population. If a sixth nationwide study can compensate for the weak points of the fifth study, solid data could be collected.
In conclusion, this population-based study revealed that 3.8% of the Korean population has COM. Various host factors, including chronic rhinosinusitis, hearing impairment, and tinnitus, were associated with risk of COM. Some environmental factors, such as high lead or cadmium, may also be associated with an increased risk of COM. Further studies are required to ascertain these associations.
Acknowledgments
We thank the Korea Centers for Disease Control and Prevention for providing the data used in this study.
Data Availability
The data are from the fifth Korean National Health and Nutrition Examination Survey, available via https://knhanes.cdc.go.kr/knhanes/index.do or Kyungwon Oh at kwoh27@korea.kr.
Funding Statement
This study was supported by the Korean Ministry of the Environment as part of “The Environmental Health Action Program” and the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2012R1A1A1013003). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Nadol JB Jr. Revision mastoidectomy. Otolaryngol Clin North Am. 2006;39: 723–740,vi-vii. [DOI] [PubMed] [Google Scholar]
- 2. Acuin J. Chronic suppurative otitis media: burden of illness and management options Child and Adolescent Health and Development Prevention of Blindness and Deafness. World Health Organization, Geneva, Switzerland: 2004. [Google Scholar]
- 3. Lasisi AO, Olaniyan FA, Muibi SA, Azeez IA, Abdulwasiu KG, Lasisi TJ, et al. Clinical and demographic risk factors associated with chronic suppurative otitis media. Int J Pediatr Otorhinolaryngol. 2007;71: 1549–1554. [DOI] [PubMed] [Google Scholar]
- 4. Stahlberg MR, Ruuskanen O, Virolainen E. Risk factors for recurrent otitis media. Pediatr Infect Dis. 1986;5: 30–32. [DOI] [PubMed] [Google Scholar]
- 5. Daly K, Giebink GS, Le CT, Lindgren B, Batalden PB, Anderson RS, et al. Determining risk for chronic otitis media with effusion. Pediatr Infect Dis J. 1988;7: 471–475. [DOI] [PubMed] [Google Scholar]
- 6. Fliss DM, Shoham I, Leiberman A, Dagan R. Chronic suppurative otitis media without cholesteatoma in children in southern Israel: incidence and risk factors. Pediatr Infect Dis J. 1991;10: 895–899. [DOI] [PubMed] [Google Scholar]
- 7. Kalm O, Johnson U, Prellner K. HLA frequency in patients with chronic secretory otitis media. Int J Pediatr Otorhinolaryngol. 1994;30: 151–157. [DOI] [PubMed] [Google Scholar]
- 8. Engel J, Mahler E, Anteunis L, Marres E, Zielhuis G. Why are NICU infants at risk for chronic otitis media with effusion? Int J Pediatr Otorhinolaryngol. 2001;57: 137–144. [DOI] [PubMed] [Google Scholar]
- 9. Ilicali OC, Keles N, De er K, Sa un OF, Guldiken Y. Evaluation of the effect of passive smoking on otitis media in children by an objective method: urinary cotinine analysis. Laryngoscope. 2001;111: 163–167. [DOI] [PubMed] [Google Scholar]
- 10. Keles B, Ozturk K, Gunel E, Arbag H, Ozer B. Pharyngeal reflux in children with chronic otitis media with effusion. Acta Otolaryngol. 2004;124: 1178–1181. [DOI] [PubMed] [Google Scholar]
- 11. Engel JA, Straetemans M, Zielhuis GA. Birth characteristics and recurrent otitis media with effusion in young children. Int J Pediatr Otorhinolaryngol. 2005;69: 533–540. [DOI] [PubMed] [Google Scholar]
- 12. Gozal D, Kheirandish-Gozal L, Capdevila OS, Dayyat E, Kheirandish E. Prevalence of recurrent otitis media in habitually snoring school-aged children. Sleep Med. 2008;9: 549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nelson HM, Daly KA, Davey CS, Himes JH, Synder DJ, Bartoshuk LM. Otitis media and associations with overweight status in toddlers. Physiol Behav. 2011;102: 511–517. 10.1016/j.physbeh.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 14. Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Medical Decision Making. 2006;26: 410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. EuroQol G. EuroQol—a new facility for the measurement of health-related quality of life. Health policy (Amsterdam, Netherlands). 1990;16: 199 [DOI] [PubMed] [Google Scholar]
- 16. Choo J, Jeon S, Lee J. Gender differences in health-related quality of life associated with abdominal obesity in a Korean population. BMJ. 2014;4:e003954 10.1136/bmjopen-2013-003954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park RJ, Moon JD. Prevalence and risk factors of tinnitus: the Korean National Health and Nutrition Examination Survey 2010–2011, a cross-sectional study. Clin Otolaryngol. 2014;39: 89–94. 10.1111/coa.12232 [DOI] [PubMed] [Google Scholar]
- 18. Lin YS, Lin LC, Lee FP, Lee KJ. The prevalence of chronic otitis media and its complication rates in teenagers and adult patients. Otolaryngol Head Neck Surg. 2009;140: 165–170. 10.1016/j.otohns.2008.10.020 [DOI] [PubMed] [Google Scholar]
- 19. Zhang Y, Xu M, Zhang J, Zeng L, Wang Y, Zheng QY. Risk factors for chronic and recurrent otitis media-a meta-analysis. PLoS One. 2014;9: e86397 10.1371/journal.pone.0086397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim CS, Chnag SO. Park HS. A statistical study of otitis media in Korea. The Korean Journal of Otol. 1981;24: 505–513. [Google Scholar]
- 21. Kim CS, Jung HW, Yoo KY. Prevalence of otitis media and allied diseases in Korea—results of a nation-wide survey, 1991. J Korean Med Sci. 1993;8: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito K, Naito R, Murofushi T, Iguchi R. Questionnaire and interview in screening for hearing impairment in adults. Acta Otolaryngol Suppl. 2007; 24–28. [DOI] [PubMed]
- 23. Kakarlapudi V, Sawyer R, Staecker H. The effect of diabetes on sensorineural hearing loss. Otol Neurotol. 2003;24: 382–386. [DOI] [PubMed] [Google Scholar]
- 24. Austin DF, Konrad-Martin D, Griest S, McMillan GP, McDermott D, Fausti S. Diabetes-related changes in hearing. Laryngoscope. 2009;119: 1788–1796. 10.1002/lary.20570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bainbridge KE, Cheng YJ, Cowie CC. Potential mediators of diabetes-related hearing impairment in the U.S. population: National Health and Nutrition Examination Survey 1999–2004. Diabetes Care. 2010;33: 811–816. 10.2337/dc09-1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wackym PA, Linthicum FH Jr. Diabetes mellitus and hearing loss: clinical and histopathologic relationships. Am J Otol. 1986:7: 176–182. [PubMed] [Google Scholar]
- 27. Smith TL, Raynor E, Prazma J, Buenting JE, Pillsbury HC. Insulin-dependent diabetic microangiopathy in the inner ear. Laryngoscope. 1995;105: 236–240. [DOI] [PubMed] [Google Scholar]
- 28.EHealthMe website. Available: http://www.ehealthme.com/cs/diabetes+mellitus/otitis+media. Accessed 15 February 2015.
- 29. Pagella F, Colombo A, Gatti O, Giourgos G, Matti E. Rhinosinusitis and otitis media: the link with adenoids. Int J Immunopathol Pharmacol. 2010;23: 38–40. [PubMed] [Google Scholar]
- 30. Doyle WJ. The link between allergic rhinitis and otitis media. Curr Opin Allergy Clin Immunol. 2002;2: 21–25. [DOI] [PubMed] [Google Scholar]
- 31. Luong A, Roland PS. The link between allergic rhinitis and chronic otitis media with effusion in atopic patients. Otolaryngol Clin North Am. 2008;41: 311–323, vi. 10.1016/j.otc.2007.11.004 [DOI] [PubMed] [Google Scholar]
- 32. Nguyen LH, Manoukian JJ, Tewfik TL, Sobol SE, Joubert P, Mazer BD, et al. Evidence of allergic inflammation in the middle ear and nasopharynx in atopic children with otitis media with effusion. J Otolaryngol. 2004;33: 345–351. [DOI] [PubMed] [Google Scholar]
- 33. Yeo SG, Park DC, Eun YG, Cha CI. The role of allergic rhinitis in the development of otitis media with effusion: effect on eustachian tube function. Am J Otolaryngol. 2007;28: 148–152. [DOI] [PubMed] [Google Scholar]
- 34. Doner F, Yariktas M, Demirci M. The role of allergy in recurrent otitis media with effusion. J Investig Allergol Clin Immunol. 2004;14: 154–158. [PubMed] [Google Scholar]
- 35. Bakhshaee M, Rajati M, Fereidouni M, Khadivi E, Varasteh A. Allergic rhinitis and chronic suppurative otitis media. Eur Arch Otorhinolaryngol. 2011;268: 87–91. 10.1007/s00405-010-1290-3 [DOI] [PubMed] [Google Scholar]
- 36. Paradise JL, Rockette HE, Colborn DK, Bernard BS, Smith CG, Kurs-Lasky M, et al. Otitis media in 2253 Pittsburgh-area infants: prevalence and risk factors during the first two years of life. Pediatrics. 1997;99: 318–333. [DOI] [PubMed] [Google Scholar]
- 37. Ah-Tye C, Paradise JL, Colborn DK. Otorrhea in young children after tympanostomy-tube placement for persistent middle-ear effusion: prevalence, incidence, and duration. Pediatrics. 2001;107: 1251–1258. [DOI] [PubMed] [Google Scholar]
- 38. Poelmans J, Tack J, Feenstra L. Chronic middle ear disease and gastroesophageal reflux disease: a causal relation? Otol Neurotol. 2001;22: 447–450. [DOI] [PubMed] [Google Scholar]
- 39. Sone M, Kato T, Nakashima T. Current concepts of otitis media in adults as a reflux-related disease. Otol Neurotol. 2013;34: 1013–1017. 10.1097/MAO.0b013e318299aa52 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are from the fifth Korean National Health and Nutrition Examination Survey, available via https://knhanes.cdc.go.kr/knhanes/index.do or Kyungwon Oh at kwoh27@korea.kr.


