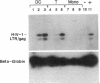
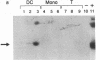
Abstract
The rate and efficiency of key steps in the life cycle of the human immunodeficiency virus type 1 was examined in three primary cell types, T cells, monocytes, and T helper dendritic cells using the same quantity of virus involved and same cell number. The results show that viral DNA synthesis proceeds much more rapidly and efficiently in primary T helper dendritic cell populations than in primary T cell and monocyte populations. The increased rate of virus DNA synthesis is attributable either to an increase in the efficiency and the rate of uptake of the virus particles by the T helper dendritic cells, as compared with that in other cell types, or to an increased efficiency and rate of viral DNA synthesis in the T helper dendritic cells. In the subsequent phase of viral expression the appearance of spliced viral mRNA products also occur more rapidly in cultures of primary-blood-derived T helper dendritic cells than is the case in primary T cells and monocytes. The increased efficiency of the early steps of HIV-1 replication in primary-blood-derived T helper dendritic cells than in other blood-derived mononuclear cells raises the possibility that these cells play a central role in HIV-1 infection and pathogenesis.
Full text
PDF

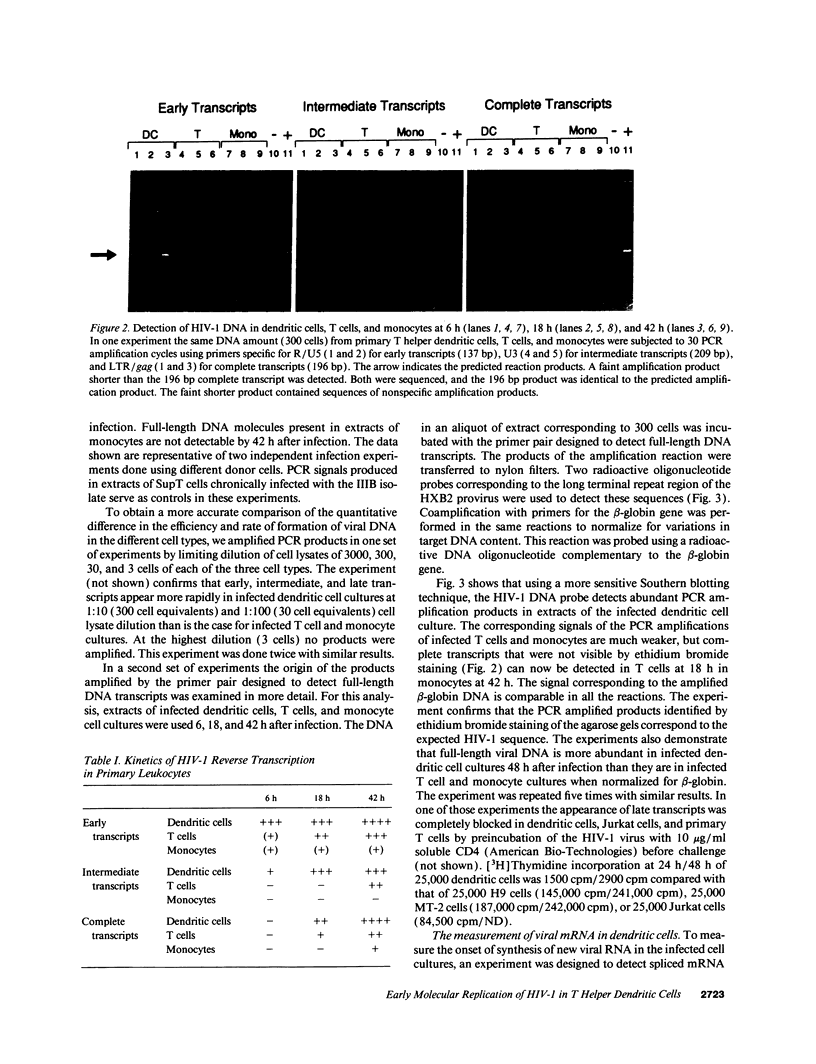
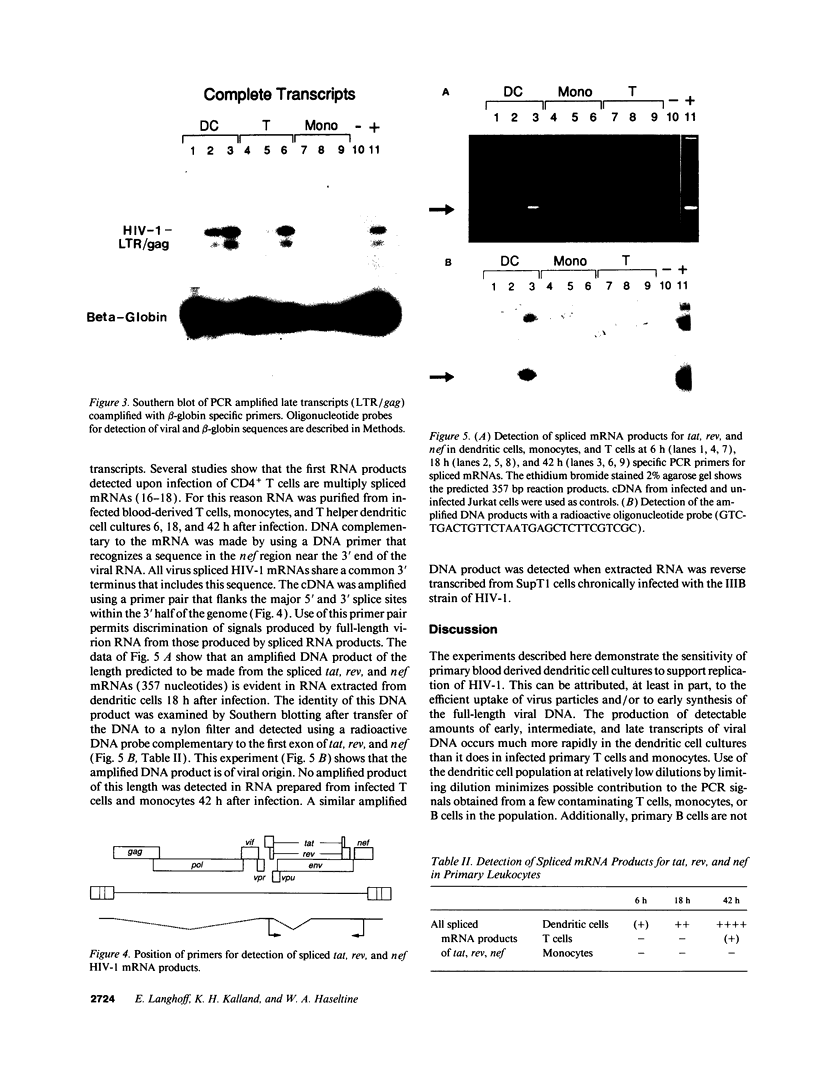


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo S. J., Weitsman S., Rosenblatt J. D., Chen I. S. Analysis of rev gene function on human immunodeficiency virus type 1 replication in lymphoid cells by using a quantitative polymerase chain reaction method. J Virol. 1989 Nov;63(11):4875–4881. doi: 10.1128/jvi.63.11.4875-4881.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BADER J. P. THE REQUIREMENT FOR DNA SYNTHESIS IN THE GROWTH OF ROUS SARCOMA AND ROUS-ASSOCIATED VIRUSES. Virology. 1965 Jun;26:253–261. doi: 10.1016/0042-6822(65)90272-2. [DOI] [PubMed] [Google Scholar]
- Bader J. P. Virus-induced transformation without cell division. Science. 1973 Jun 8;180(4090):1069–1071. doi: 10.1126/science.180.4090.1069. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Cameron P. U., Forsum U., Teppler H., Granelli-Piperno A., Steinman R. M. During HIV-1 infection most blood dendritic cells are not productively infected and can induce allogeneic CD4+ T cells clonal expansion. Clin Exp Immunol. 1992 May;88(2):226–236. doi: 10.1111/j.1365-2249.1992.tb03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron P. U., Freudenthal P. S., Barker J. M., Gezelter S., Inaba K., Steinman R. M. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992 Jul 17;257(5068):383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clapham P. R., Weber J. N., Whitby D., McIntosh K., Dalgleish A. G., Maddon P. J., Deen K. C., Sweet R. W., Weiss R. A. Soluble CD4 blocks the infectivity of diverse strains of HIV and SIV for T cells and monocytes but not for brain and muscle cells. Nature. 1989 Jan 26;337(6205):368–370. doi: 10.1038/337368a0. [DOI] [PubMed] [Google Scholar]
- Haase A. T. Pathogenesis of lentivirus infections. Nature. 1986 Jul 10;322(6075):130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A. Molecular biology of the human immunodeficiency virus type 1. FASEB J. 1991 Jul;5(10):2349–2360. doi: 10.1096/fasebj.5.10.1829694. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Hirsch M. S. Infection of monocyte/macrophages by human T lymphotropic virus type III. J Clin Invest. 1986 May;77(5):1712–1715. doi: 10.1172/JCI112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalland K. H., Langhoff E., Bos H. J., Göttlinger H., Haseltine W. A. Rex-dependent nucleolar accumulation of HTLV-I mRNAs. New Biol. 1991 Apr;3(4):389–397. [PubMed] [Google Scholar]
- Kim S. Y., Byrn R., Groopman J., Baltimore D. Temporal aspects of DNA and RNA synthesis during human immunodeficiency virus infection: evidence for differential gene expression. J Virol. 1989 Sep;63(9):3708–3713. doi: 10.1128/jvi.63.9.3708-3713.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima I., Yamamoto K., Sato K., Nakajima Y., Nakajima T., Maruyama I., Osame M., Nishioka K. Detection of human T cell lymphotropic virus type I proviral DNA and its gene expression in synovial cells in chronic inflammatory arthropathy. J Clin Invest. 1991 Oct;88(4):1315–1322. doi: 10.1172/JCI115436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight S. C., Macatonia S. E., Patterson S. HIV I infection of dendritic cells. Int Rev Immunol. 1990;6(2-3):163–175. doi: 10.3109/08830189009056627. [DOI] [PubMed] [Google Scholar]
- Langhoff E., Steinman R. M. Clonal expansion of human T lymphocytes initiated by dendritic cells. J Exp Med. 1989 Jan 1;169(1):315–320. doi: 10.1084/jem.169.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhoff E., Terwilliger E. F., Bos H. J., Kalland K. H., Poznansky M. C., Bacon O. M., Haseltine W. A. Replication of human immunodeficiency virus type 1 in primary dendritic cell cultures. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7998–8002. doi: 10.1073/pnas.88.18.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. P., Steinman R. M., Witmer-Pack M., Hankins D. F., Morris P. J., Austyn J. M. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990 Nov 1;172(5):1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn R. M., Efstratiadis A., O'Connell C., Maniatis T. The nucleotide sequence of the human beta-globin gene. Cell. 1980 Oct;21(3):647–651. doi: 10.1016/0092-8674(80)90428-6. [DOI] [PubMed] [Google Scholar]
- Leonard J., Khillan J. S., Gendelman H. E., Adachi A., Lorenzo S., Westphal H., Martin M. A., Meltzer M. S. The human immunodeficiency virus long terminal repeat is preferentially expressed in Langerhans cells in transgenic mice. AIDS Res Hum Retroviruses. 1989 Aug;5(4):421–430. doi: 10.1089/aid.1989.5.421. [DOI] [PubMed] [Google Scholar]
- Lewis P., Hensel M., Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 1992 Aug;11(8):3053–3058. doi: 10.1002/j.1460-2075.1992.tb05376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X. B., Heimer J., Rekosh D., Hammarskjöld M. L. U1 small nuclear RNA plays a direct role in the formation of a rev-regulated human immunodeficiency virus env mRNA that remains unspliced. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7598–7602. doi: 10.1073/pnas.87.19.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatonia S. E., Cruickshank J. K., Rudge P., Knight S. C. Dendritic cells from patients with tropical spastic paraparesis are infected with HTLV-1 and stimulate autologous lymphocyte proliferation. AIDS Res Hum Retroviruses. 1992 Sep;8(9):1699–1706. doi: 10.1089/aid.1992.8.1699. [DOI] [PubMed] [Google Scholar]
- Markowicz S., Engleman E. G. Granulocyte-macrophage colony-stimulating factor promotes differentiation and survival of human peripheral blood dendritic cells in vitro. J Clin Invest. 1990 Mar;85(3):955–961. doi: 10.1172/JCI114525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal J. S., Mawle A., Cort S. P., Nicholson J. K., Cross G. D., Scheppler-Campbell J. A., Hicks D., Sligh J. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985 Nov;135(5):3151–3162. [PubMed] [Google Scholar]
- Montagnier L., Gruest J., Chamaret S., Dauguet C., Axler C., Guétard D., Nugeyre M. T., Barré-Sinoussi F., Chermann J. C., Brunet J. B. Adaptation of lymphadenopathy associated virus (LAV) to replication in EBV-transformed B lymphoblastoid cell lines. Science. 1984 Jul 6;225(4657):63–66. doi: 10.1126/science.6328661. [DOI] [PubMed] [Google Scholar]
- Parmentier H. K., van Wichen D., Sie-Go D. M., Goudsmit J., Borleffs J. C., Schuurman H. J. HIV-1 infection and virus production in follicular dendritic cells in lymph nodes. A case report, with analysis of isolated follicular dendritic cells. Am J Pathol. 1990 Aug;137(2):247–251. [PMC free article] [PubMed] [Google Scholar]
- Poznansky M. C., Walker B., Haseltine W. A., Sodroski J., Langhoff E. A rapid method for quantitating the frequency of peripheral blood cells containing HIV-1 DNA. J Acquir Immune Defic Syndr. 1991;4(4):368–373. [PubMed] [Google Scholar]
- Steinman R. M., Lustig D. S., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. 3. Functional properties in vivo. J Exp Med. 1974 Jun 1;139(6):1431–1445. doi: 10.1084/jem.139.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thali M., Olshevsky U., Furman C., Gabuzda D., Li J., Sodroski J. Effects of changes in gp120-CD4 binding affinity on human immunodeficiency virus type 1 envelope glycoprotein function and soluble CD4 sensitivity. J Virol. 1991 Sep;65(9):5007–5012. doi: 10.1128/jvi.65.9.5007-5012.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. B., Matthews T. J., Cullen B. R., Malim M. H. Productive human immunodeficiency virus type 1 (HIV-1) infection of nonproliferating human monocytes. J Exp Med. 1991 Dec 1;174(6):1477–1482. doi: 10.1084/jem.174.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Haislip A. M., Krogstad P., Chen I. S. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992 Mar;66(3):1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]