Abstract
Shear stress regulates endothelial nitric oxide and superoxide (O2−·) production, implicating the role of NADPH oxidase activity. It is unknown whether shear stress regulates the sources of reactive species production, consequent low-density lipoprotein (LDL) modification, and initiation of inflammatory events. Bovine aortic endothelial cells (BAECs) in the presence of 50 μg/mL of native LDL were exposed to (1) pulsatile flow with a mean shear stress (τave) of 25 dyne/cm2 and (2) oscillating flow at τave of 0. After 4 hours, aliquots of culture medium were collected for high-performance liquid chromatography analyses of electronegative LDL species, described as LDL− and LDL2−. In response to oscillatory shear stress, gp91phox mRNA expression was upregulated by 2.9±0.3-fold, and its homologue, Nox4, by 3.9±0.9-fold (P<0.05, n=4), with a corresponding increase in O2−· production rate. The proportion of LDL− and LDL2− relative to static conditions increased by 67±17% and 30±7%, respectively, with the concomitant upregulation of monocyte chemoattractant protein-1 expression and increase in monocyte/BAEC binding (P<0.05, n=5). In contrast, pulsatile flow downregulated both gp91phox and Nox4 mRNA expression (by 1.8±0.2-fold and 3.0±0.12-fold, respectively), with an accompanying reduction in O2−· production, reduction in the extent of LDL modification (51±12% for LDL− and 30±7% for LDL2−), and monocyte/BAEC binding. The flow-dependent LDL oxidation is determined in part by the NADPH oxidase activity. The formation of modified LDL via O2−· production may also affect the regulation of monocyte chemoattractant protein-1 expression and monocyte/BAEC binding.
Keywords: shear stress, NADPH oxidase, LDL oxidation, Nox4, gp91phox
Oscillatory shear stress, cyclic strain, and oxidized LDL increase vascular oxidative stress.1,2 Activation of the renin-angiotensin system and shear stress enhance the vascular production of reactive oxygen species (ROS), including superoxide (O2−·) and hydrogen peroxide (H2O2), in part through the activation of membrane-bound NADH/NADPH oxidases present in vascular smooth muscle cells, endothelium, fibroblasts, and phagocytic mononuclear cells.1-4 Increasing evidence suggests that ROS inactivates nitric oxide (NO), leading to the formation of peroxynitrite (OONO−) and other oxidants.5,6 Thus, the enhanced production of ROS impairs endothelium-dependent vasodilation, NO bioavailability, and antiinflammatory responses.
Low-density lipoprotein (LDL) oxidation is one of the fundamental processes in atherogenesis. LDL particles trapped within the subendothelial space undergo oxidative modification, resulting in the formation of minimally modified LDL (MM-LDL) and highly oxidized LDL. Specifically, MM-LDL induces expression of monocyte chemoattractant protein (MCP)-1 on endothelial cells (ECs), leading to monocyte binding and chemotaxis and subsequent trans-endothelial migration.7,8 The major fraction, native LDL, represents ≈90% to 99% of the total LDL. LDL−, found in plasma in vivo, is a minimally oxidized subspecies of LDL (resembling MM-LDL) and is characterized by its greater negative charge and oxidized status. LDL− represents 0.2% to 8% of LDL and is associated with an increased risk of atherosclerosis.9,10 However, LDL2−, which harbors a more electronegative charge than LDL−, constitutes 0.1% to 1% of total LDL and seems to be a more oxidized subfraction of LDL.11 A recent report has elaborated the characteristics of modified LDL species based on electronegative charge and their oxidation characteristics.12 Three possible sources proposed for LDL− are the following: (1) oxidation of LDL entrapped in the arterial wall,13 (2) ingestion of oxidants or generation from postprandial lipoprotein remnants,14 and (3) oxidation in plasma.15
The NADH/NADPH oxidases are multimeric enzymes composed of plasma membrane–associated proteins as well as cytosolic factors. For the phagocytic-type NADPH oxidase, the plasma membrane–associated proteins gp91phox and p22phox comprise the flavocytochrome b558 complex, which forms the catalytic subunit of the oxidase. The cytosolic subunits, including p47phox, p67phox, and the G-protein Rac, provide regulatory function.16,17 Several Nox proteins, including gp91phox and Nox4, may contribute to increased intracellular oxidative stress in vascular endothelium; however, the relative contribution of Nox subunits to O2−· production, as a source of oxidants, remains to be determined.18
Shear stress, the tangential drag force of blood passing along the surface of the endothelium,19 imparts profound effects on EC function.20-22 Around arterial bends and branches in which the inflammatory responses prevail, the fluid mechanical environment is distinct from the laminar pulsatile environment present in the long, straight sections of the vessel wall. Oscillatory flow with a time-averaged shear stress of zero, characteristic of flow separation points in the arterial branches, upregulates the atherogenic activities of ECs.23-25 Therefore, the characteristics of arterial wall shear stress can influence the recruitment of leukocytes that are fundamental to the initiation of immune responses.26,27
An emerging hypothesis for atherosclerosis is that oxidative stress induces aberrant behavior of the vascular endothelium. 28,29 Accordingly, the characteristics of shear stress influence oxidative activity that is characterized by increased NADPH oxidase activity and greater production of modified LDL. One consequence of this is the oxidation of LDL to more atherogenic particles that initiate the formation of early atherosclerotic lesions, particularly at the curvatures and lateral walls of vascular branching points.28 Thus, the magnitude, frequency, direction, as well as temporal and spatial components of shear stress may play a distinct role in the production of oxidative stress and oxidatively modified LDL.
Vascular cells use ROS and reactive nitrogen species (RNS) to modify LDL. ECs produce ROS and RNS from the enzymes and NO synthase and by specific homologues of NADPH oxidase (gp91phox, Nox1, and Nox4).30 We hypothesize that NADPH oxidases are capable of producing high levels of ROS in blood vessels in response to specific stimuli arising from flow conditions. Consequently, more modified LDL species are formed under oscillatory flow conditions that favor ROS production, whereas lower levels of LDL modification occur in response to pulsatile flow conditions that produce fewer ROS.
We demonstrate that the characteristics of shear force experienced by ECs play a critical role in the production of O2−· through the expression of 91phox and its homologue, Nox4, modifying the capacity of ECs to either prevent or promote oxidative modification of LDL. Oscillatory flow (bidirectional net zero forward flow), which occurs at the arterial bifurcations, induces greater NADPH oxidase activities and O2−· production and thus enhances LDL oxidation and upregulation of inflammatory markers. In contrast, pulsatile flow (unidirectional positive net forward flow), which occurs in the straight part of vessel, favors downregulated NADPH oxidase activity that may decrease LDL oxidation. Furthermore, we observed relatively higher levels of Nox4 than gp91phox mRNA expression in response to flow conditions, confirming the former as the major NADPH oxidase homolog in ECs.
Materials and Methods
A Novel Pulsatile Flow System
The pulsatile flow system, which was mounted on the inverted microscope (Olympus FV-IX701), was designed to generate welldefined temporal shear stress gradients (see Figure 1 in the online data supplement, available at http://www.circresaha.org).31,32 Micro Electro Mechanical Systems were used to monitor real-time shear stress.33 The theoretical formulation for the pulsatile flow generated by this flow channel can be accessed online at http://ojps.aip.org/ abme.34 The pH of the circulating DMEM culture medium was monitored and kept at 7.4 (Accumet AP) using 5% CO2. The flow system allows for real-time recording of shear stress and monitoring of ECs and monocyte binding under both pulsatile and oscillatory flow conditions.
Flow Experiments to Measure Oxidatively Modified LDL
Confluent BAEC monolayers between 3 and 6 passages (see the online data supplement for EC culture) were plated on glass slides and incubated in DMEM (no phenol red), 10% FBS, 1% penicillin-streptomycin, and 0.05% Fungizone. Cells were subjected to pulsatile or oscillatory flow in the presence of 50 μg/mL of native LDL in the flow channel at 60 cycles/min (1 Hz). Two flow conditions were studied, pulsatile flow at ∂τ/∂ t=293 dyne/cm2 per second with the time-averaged shear stress (τave)=25 dyne/cm2 and oscillating flow between ±3.0 dyne/cm2 with τave=0 dyne/cm2. After 4 hours, the medium was aliquoted to determine the extent of LDL modification, as described below. At 4 and 8 hours, BAECs were collected for the real-time reverse transcriptase–polymerase chain reaction (RT-PCR) for measurement of GP91phox, MCP-1, and endothelial NO synthase (eNOS) mRNA expression.
Measurement of Extracellular Superoxide Formation
The production of O2−· was measured by cytochrome c reduction rates, as described previously35,36 (see the online data supplement).
Intracellular O2−· Production Measurements
Dihydroethidium (DHE) was used to localize intracellular O2−· production, as previously described37 (see the online data supplement).
Separation of LDL Subspecies by High-Performance Liquid Chromatography
Venous blood was obtained at the Atherosclerosis Research Unit from fasting adult human volunteers under institutional review board approval. Plasma was pooled and immediately separated by centrifugation at 1500g for 10 minutes at 4°C. LDL (δ=1.019 to 1.063 g/mL) was isolated from freshly separated plasma by preparative ultracentrifugation using a Beckman L8-55 ultracentrifuge and a SW-41 rotor. The technique used for separating LDL was similar to that described previously38 (see the online data supplement).
Quantitative Real-Time RT-PCR
After BAECs were exposed to the flow conditions, total RNA was isolated using RNeasy kit (Qiagen). Real-time RT-PCR was performed according to the recommendations of PE Biosystems Taq-Man PCR Core Reagent Kit.39 TaqMan probes40 were used for added specificity and sensitivity (see the online data supplement).
Cloning of a Bovine Nox4 cDNA Fragment From BAECs
Superscript II RT (Invitrogen) was used to prepare cDNA from 5 μg total BAEC RNA. PCR primers n4+1 and n4−2 were designed to anneal to the coding region of human, rat, and mouse Nox4 sequences (see the online data supplement).
Western Blot Analysis of gp91phox
BAEC protein was size separated in 10% SDS BioRad polyacrylamide electrophoresis gel (BioRad) and electro-transferred to a PVDF membrane (Millipore) (see the online data supplement).
Real-Time Monocyte/Endothelial Cell Interactions in Response to Flow Conditions
Monocytes were isolated using a modification of the Recalde method, as previously described,41 from healthy volunteers with institutional review board approval. Freshly isolated monocytes (105 monocytes/cm3) were introduced into the testing channel under flow conditions, as described above for LDL oxidation (see the online data supplement).
Incubation of BAECs With 2-Deoxyglucose
To demonstrate that NADPH oxidase mediates the extent of LDL oxidation in response to oscillatory flow, we incubated BAECs with 2-deoxyglucose (2-DOG) to block the pentose shunt pathway for NADPH production (see the online data supplement). Once the BAEC monolayers became confluent, growth media was changed to glucose-free media comprised of glucose-free DMEM, 15% heat-inactivated FBS, 100 U/mL penicillin-streptomycin, 0.05% amphotericin B, and 10 mmol/L 2-deoxy-d-glucose (Sigma). Confluent BAEC monolayers were incubated overnight (>12 hours) before the flow exposure and measurement of extracellular O2−· formation.
Statistical Analysis
Data are expressed as mean±SD and compared among separate experiments. For comparisons between two groups, statistical analysis was performed using the two-sample independent-groups t test. Comparisons of multiple mean values were made by one-way ANOVA, and statistical significance among multiple groups was determined using the Tukey procedure (for pairwise comparisons of means between static-like and pulsatile flow conditions). P values of <0.05 are considered statistically significant.
An expanded Materials and Methods section can be found in the online data supplement, available at http://www.circresaha.org.
Results
Relative Expression of NADPH Oxidase Subunit gp91phox and Its Homologue, Nox4, in Response to Pulsatile Versus Oscillatory Flow
Pulsatile versus oscillatory flow had different effects on the relative induction of NADPH subunit gp91phox mRNA expression in BAECs (Figure 1). In response to pulsatile flow (25 dyne/cm2, 1 Hz), gp91phox was downregulated relative to static conditions by 44±4% at 4 hours and 33±5% at 8 hours (P<0.05, n=5). In contrast, oscillatory flow (±3 dyne/cm2, 1 Hz, τave±0) induced sustained upregulation of gp91phox expression by 34±5% at 4 hours and by 32±3% at 8 hours (P<0.05, n=5) (Figure 1B). Western blot analysis reflected the relative degree of protein expression of gp91phox in response to pulsatile versus oscillatory flow conditions (Figure 1C).
Figure 1.
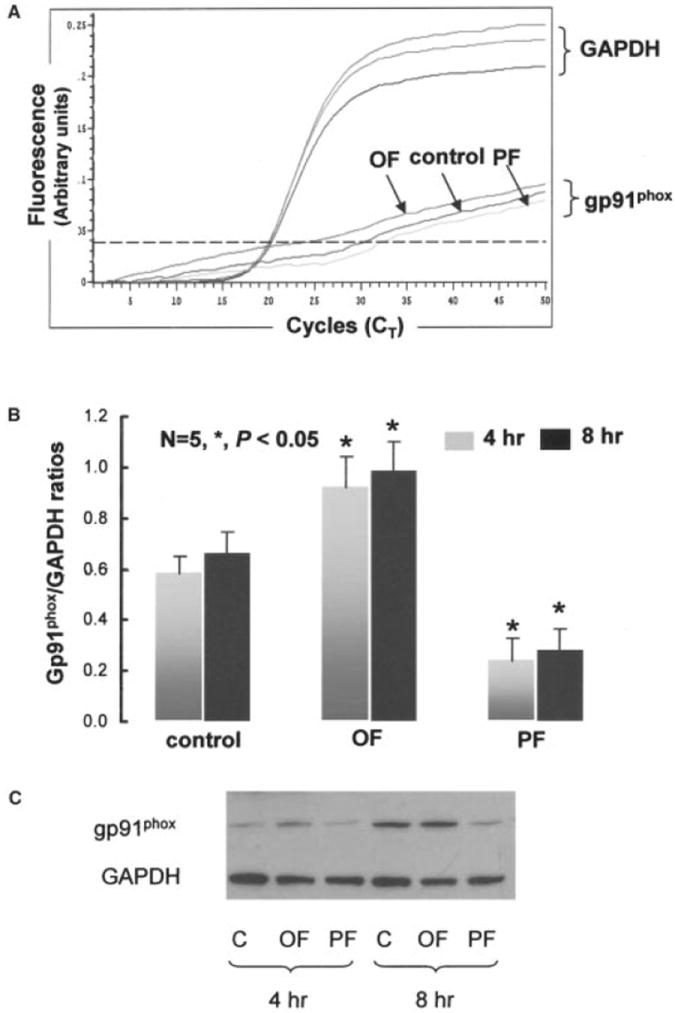
A, Fluorescent signals versus PCR cycles of gp91phox versus GAPDH. B, Relative NADPH subunit gp91phox mRNA expression normalized to GAPDH in response to pulsatile flow (PF) and oscillatory flow (OF) at 4 and 8 hours. C, Western blots of gp91phox at 4 and 8 hours.
It is unknown whether gp91phox and its homolog, Nox4, are both responsive to pulsatile shear stress. We performed quantitative real-time RT-PCR and compared the relative mRNA expression between Nox4 and gp91phox. We observed a relatively higher level of Nox4 than that of gp91phox mRNA expression by fluorescent intensity and CT cycles (Figure 2A). Pulsatile flow downregulated both gp91phox and Nox4 mRNA expression (by 1.8±0.2-fold and 3.0±0.12-fold, respectively, n=4, P<0.05), whereas oscillatory flow upregulated the expression of both genes (gp91phox by 2.9±0.3-fold, Nox4 by 3.9±0.9-fold, n=4, P<0.05) (Figure 2B). Our findings suggest that shear stress modulates O2−· formation mediated by NADPH oxidase complex. Both gp91phox and Nox4 are expressed in BAECs and responsive to the characteristics of shear stress.
Figure 2.
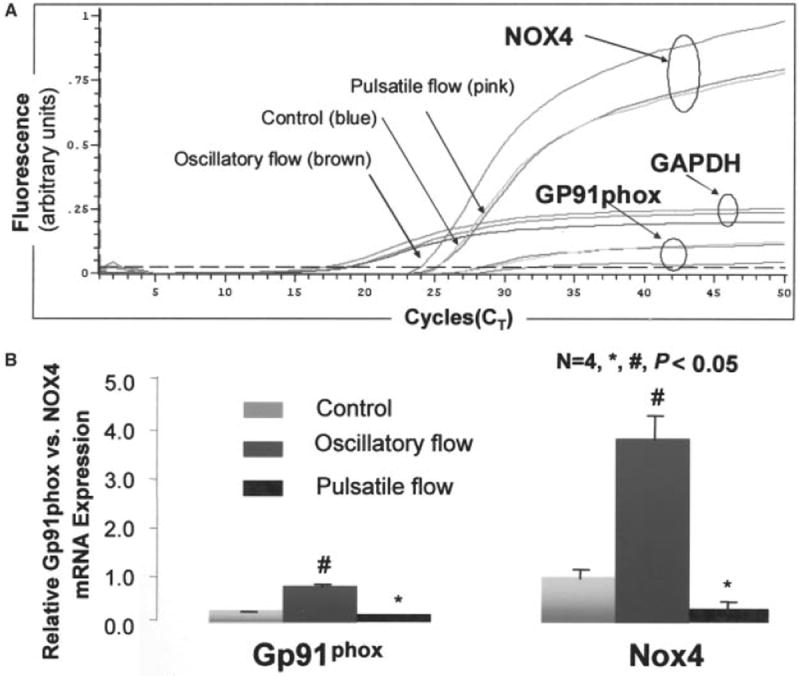
A, Fluorescence signal versus cycle number for Nox4 and gp91phox and GAPDH. B, Bar graphs show relative mRNA expression for gp91phox and Nox4 in response to pulsatile versus oscillatory flow conditions. Values are expressed as mean±SEM. #P<0.05 gp91phox vs control; *P<0.05 Nox4 vs control.
Extracellular O2−· Production in Response to Pulsatile Versus Oscillatory Flow Conditions
Using the cytochrome c reduction assay, the level of extracellular O2−· production in BAECs was measured in static cultures (control) and under the two flow conditions (Figure 3). O2−· production in BAECs remained relatively unchanged at 1 hour after exposure to both oscillatory shear stress (±3 dyne/cm2, 1 Hz) and pulsatile shear stress (25 dyne/cm2, 1 Hz). However, the rate of O2−· production remained steady under static conditions (P>0.05, n=5), whereas the rates of production in response to oscillatory versus pulsatile flow diverged steadily starting at 2 hours of exposure (P<0.05, n=5). The specificity of reduction by O2−· was established by comparing production rates in the presence and absence of SOD (60 μg/mL). The relative levels of O2−· production were found to correlate with Nox4 mRNA expression (Figure 2B) and gp91phox gene and protein expression in BAECs exposed to the flow conditions (Figure 1).
Figure 3.
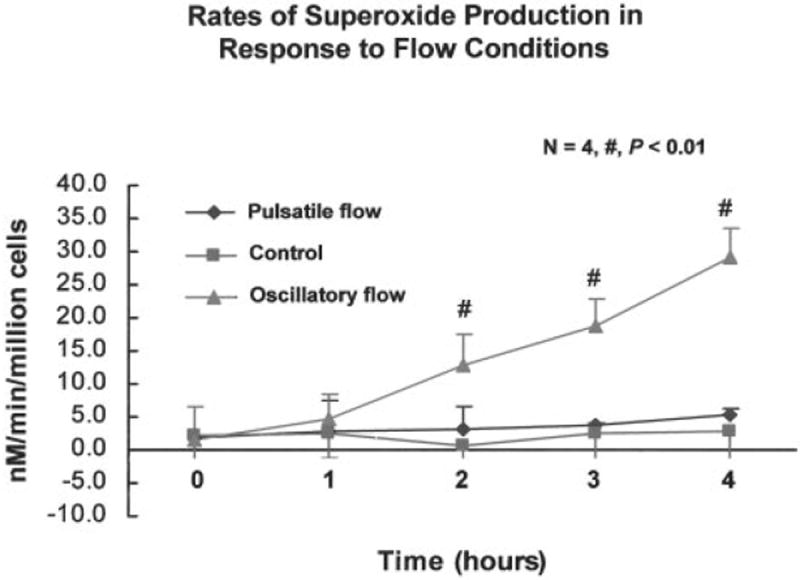
Extracellular superoxide–measured production in BAECs by cytochrome c reduction assay. Rate of superoxide production remained unchanged under the static state (control). However, the rates in response to oscillatory versus pulsatile shear stress diverged starting at 2 hours of exposure.
Intracellular O2−· Production by DHE
To complement the cytochrome c reduction assay, measurements of intracellular O2−· production were performed using DHE that specifically reacts with O2−· to form the red fluorescent compound ethidium.42 Representative fluorescent photomicrographs of DHE-stained BAECs in response to pulsatile and oscillatory flow and control cultures are shown in Figure 4. Ethidium binds irreversibly to double-stranded DNA, appearing as fluorescent staining in the nuclei (Figure 4B). DHE-stained fluorescent images show that increased intracellular O2−· levels are present at significantly higher intensity in the BAECs exposed to oscillatory flow than to pulsatile flow. This is consistent with previous observations showing significant extracellular O2−· production in response to oscillatory flow.
Figure 4.
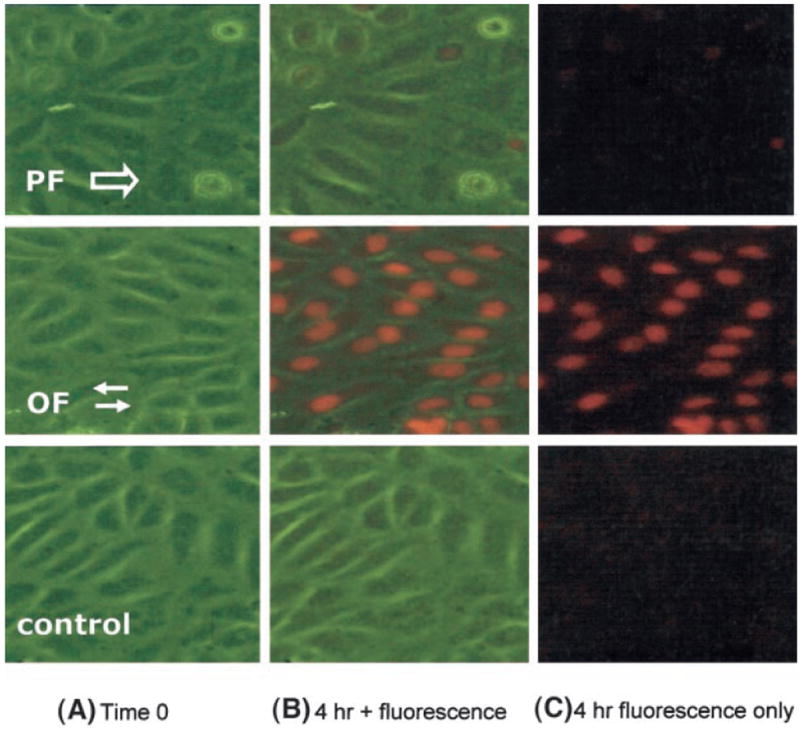
Real-time intracellular superoxide production in response to flow measured by DHE. A, At time zero, column A illustrates BAECs under 3 conditions: pulsatile flow (PF), oscillatory flow (OF), and static state (control). B, Real-time merged images of phase and fluorescence at 4 hours demonstrate the localization of red fluorescence in the nuclei. In the presence of superoxide, DHE was converted to ethidium, which intercalated into the double-stranded DNA in the nuclei. C, Real-time fluorescent microscopy underscores the superoxide production as red fluorescence.
Correlation of Shear Stress–Induced O2−· Production With the Extent of LDL Oxidation
Pulsatile flow significantly reduced the ratios of oxidatively modified LDL species relative to static conditions by 51±12% for LDL− and 30±7% for LDL−2, whereas oscillating flow increased these modified LDLs by 67±17% and 30±7%, respectively (P<0.05, n=5) (Figure 5). The change in LDL modification coincided with the downregulation of gp91phox and Nox4 mRNA expression and the relative rates of O2−· production in response to pulsatile flow and upregulation in response to oscillatory flow conditions (Figures 2 and 3).
Figure 5.
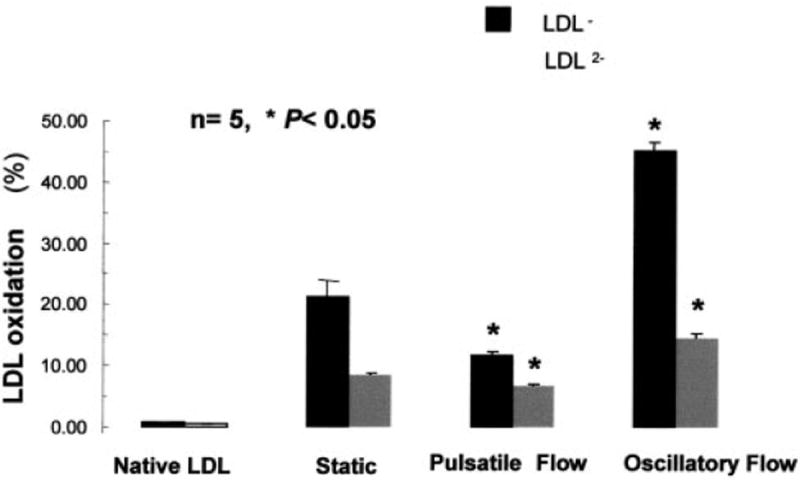
Flow regulation of native LDL oxidation. Pulsatile flow significantly reduced the ratios of oxidatively modified forms of LDL relative to static conditions by 51±12% for LDL− and 30±7% for LDL2−, whereas oscillating flow increased LDL oxidation by 67±17% and 30±7%, respectively (*P<0.05, n=5).
Correlation of Oxidatively Modified LDL With the Relative MCP mRNA Expression and Monocyte/BAEC Binding
The extent of oxidatively modified LDL coincided with MCP-1 expression and the subsequent monocyte/BAEC binding. Introduction of pulsatile flow with ∂τ/∂t=293 dyne/cm2 per second and τave=25 dyne/cm2 resulted in downregulation of MCP-1 mRNA expression by 2-fold compared with no-flow conditions. In contrast, reversing oscillating flow induced upregulation of MCP-1 expression by 8.5-fold compared with the no-flow conditions (Figure 6B). The extent of LDL modification also coincided with the numbers of monocyte binding to BAECs (control, 8±2 monocytes per high power field; pulsatile flow, 4±1; oscillatory flow alone, 29±3; P<0.05, n=5) (Figure 6A). Therefore, the extent of LDL modification coincided with the level of MCP-1 expression and the numbers of monocyte/BAEC binding.
Figure 6.
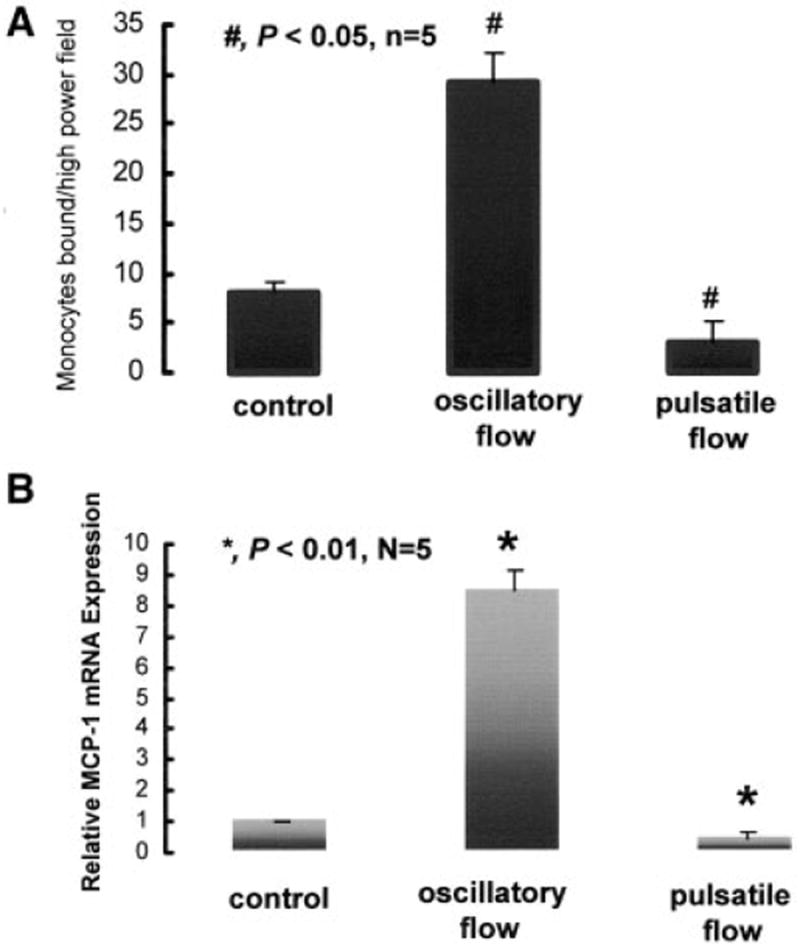
Monocytes establishing solid adhesion to ECs were captured in response to pulsatile flow and oscillatory flow. A, Numbers of monocytes bound per high-power field in response to control (static condition), oscillatory, and pulsatile flow at 4 hours. B, Relative changes in MCP-1 mRNA expression.
eNOS mRNA Expression in Response to Flow Condition
Although the induction of eNOS expression in response to oscillatory flow remains controversial, it seems to depend on the magnitude of shear stress, the frequency of oscillation, and the duration of flow exposure (Figure 7). This observation suggested flow conditions may influence oxidatively modified LDL formation by regulating the relative levels of eNOS and gp91phox expression and activity.
Figure 7.
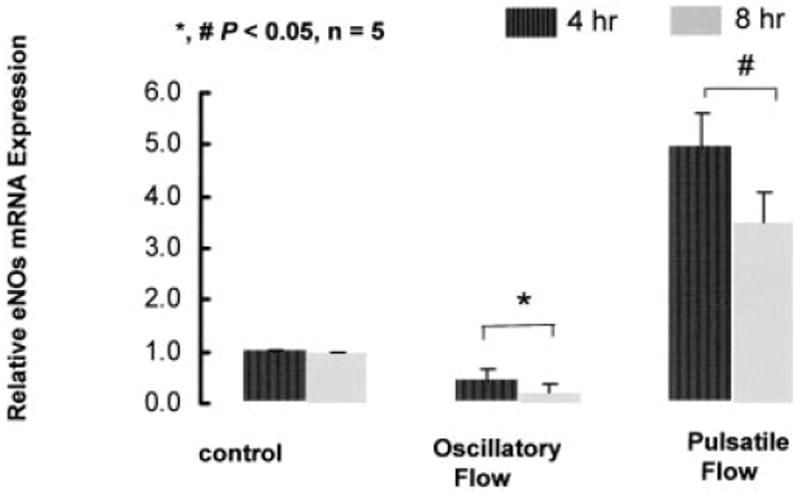
Flow regulation of eNOS mRNA expression normalized with GAPDH in response to control, pulsatile flow, and oscillatory flow at 4 and 8 hours.
Effects of 2-Deoxyglucose on Superoxide Production
To demonstrate that NADPH oxidase mediates the extent of LDL oxidation in response to pulsatile versus oscillatory flow, we incubated BAECs with 2-DOG to block pentose shunt pathway for NADPH production. The limitation in NADPH created by 2-DOG resulted in a strong reduction in O2−· production, as would be anticipated by the substrate requirements of NADPH oxidase activity (Figure 8A). Incubating BAECs with 2-DOG significantly reduced the rates of O2−· formation between the control and 2-DOG samples, oscillatory flow, and oscillatory flow plus 2-DOG samples. The treatment of BAECs with 2-DOG also reduced the amount of LDL− formation (Figure 8B). Although oscillatory flow significantly increased the extent of LDL oxidation, LDL− formation was attenuated in the 2-DOG–treated BAECs, suggesting the role of NADPH as an important cofactor for O2−· production and subsequent LDL oxidation.
Figure 8.
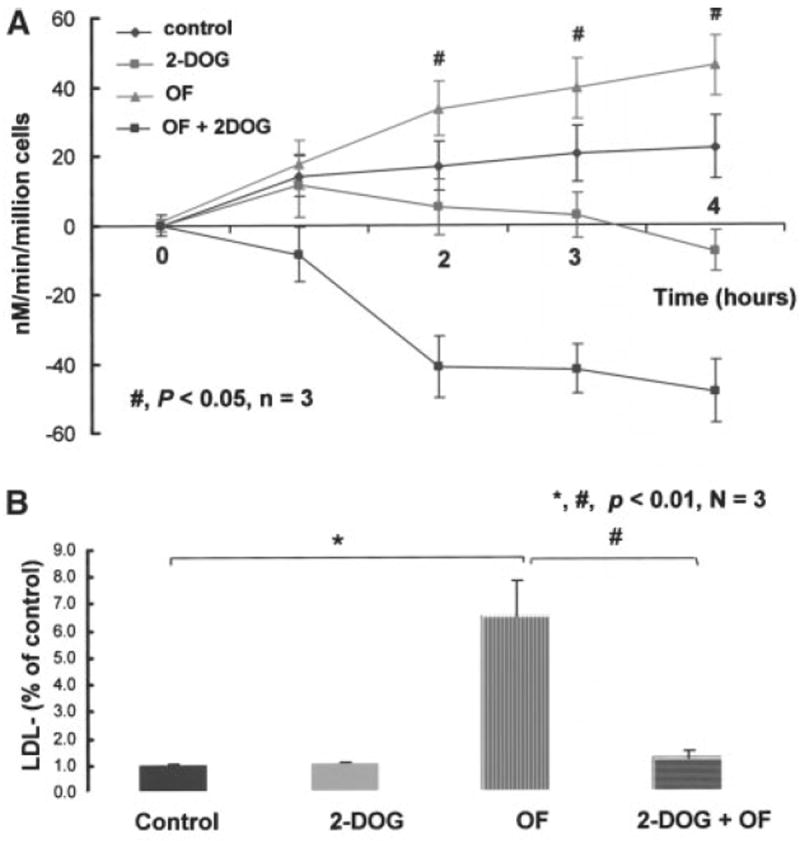
Rates of superoxide production in the presence of 2-DOG between static and oscillatory flow (OF) conditions. A, BAECs were incubated with 10 mmol/L of 2-DOG for 12 hours before SOD inhibited ferric cytochrome c reduction assay. The presence of 2-DOG significantly reduced the rates of superoxide formation between the control and 2-DOG samples, OF, and OF+2-DOG samples. The negative rates of superoxide production were attributable to the use of SOD. B, LDL oxidation in response to OF. The presence of 2-DOG significantly attenuated the extent of LDL oxidation, suggesting the role of NADPH as an important cofactor for superoxide production and the subsequent LDL oxidation.
Discussion
Increasing evidence has shown that NADPH oxidase, a multicomponent enzyme complex, is the major source of ROS in the vessel wall.16,37 The present study suggests that induction of both gp91phox and Nox4 expression regulates the activity of NADPH oxidase in vascular endothelium, which in turn modulates the relative amounts of LDL− and LDL−2 · formation. The characteristics of shear stress activate the NADPH oxidase system and enhance the cell-mediated oxidation of LDL.
Shear stress may regulate the balance between the expression of NADPH oxidase subunits and eNOS. Oscillatory flow induces greater oxidative stress by O2−· production and thus enhances LDL oxidation and upregulation of inflammatory markers. In contrast, pulsatile flow favors upregulation of atheroprotective and antioxidant genes that prevent LDL oxidation.43 We observed that both O2−· production and the extent of modified LDL (LDL− and LDL2−) were associated with the expression of gp91phox subunit of the NADPH oxidase activity, implicating this enzyme system in determining ROS generation and oxidative modification of target molecules under various flow conditions.
LDL from different batches contained different levels of LDL−, which, along with other properties, would contribute to the extent of the LDL oxidation. However, in this study, LDL particles were derived from pooled plasma of healthy subjects. Our goal was to compare the levels of LDL oxidation in response to two distinct flow patterns. Each set of flow experiments (pulsatile versus oscillatory flow) was performed using the same LDL sample.
As observed in our laboratory and reported by Rueckschloss et al,44 the NADPH oxidase subunits p22phox and p47phox are expressed at similar levels in both ECs and leukocytes, whereas gp91phox is expressed at a much lower level in ECs than in leukocytes, as determined by real-time RT-PCR (Figure 2A). We have observed a relatively higher level of Nox4 than that of gp91phox mRNA expression by the quantitative real-time RT-PCR method (Figure 2B). Our findings suggest that shear stress modulates O2−· formation mediated by the NADPH oxidase complex. Both gp91phox and Nox4 are expressed in BAECs and responsive to the characteristics of shear stress; however, Nox4 seems to be the dominant source of NADPH oxidase-derived O2−·. Based on real-time RT-PCR data, Nox4 expression is ≈4 times greater than gp91phox. Assuming that both gp91phox and Nox4 are equal in their capacity to generate O2−·, we estimate that Nox4 contributes ≈4 times more to O2−· production. Several Nox proteins, including gp91phox and Nox4, may contribute to increased intracellular oxidative stress. Monocytes express almost exclusively gp91phox, whereas the most abundant gp91phox homologue in ECs, smooth muscle cells, and fibroblasts is Nox4.45 In atherosclerotic arteries, gp91phox is localized to the central region of plaque shoulder. Nox4 expression is evident in the media and in the intima surrounding the central core of the plaque. In response to oxidized LDL, gp91phox is expressed at a much lower level in ECs than in leukocytes.44
DHE staining showed a significant level of intracellular O2−· production in response to oscillatory flow. This finding, taken together with results from cytochrome c reduction assay for extracellular O2−· production, suggests that vascular endothelium and NADPH oxidase subunits, gp91phox and Nox4 expression, generate the bulk of O2−·. It is possible that the increased NADPH oxidase–derived O2−· levels in BAECs consume flow-induced endothelium-derived NO, decreasing its signaling function with increased formation of other oxidants, such as ONOO−. There is also evidence for mitochondria as a source of cytosolic O2−· that may transit via the outer membrane voltage-dependent anion channels.46
A subclass of LDL described on the basis of its greater electronegativity and oxidative status has been previously characterized.9,38,47 These particles, which were referred to as LDL−, are enriched in lipid peroxides and other peroxidation products compared with the bulk of the unmodified, native LDL recovered from human plasma. We have demonstrated that LDL− is a major carrier of lipid hydroperoxides and cholesterol oxides associated with plasma LDL and that LDL− may arise from oxidative events in the vasculature.48 Decreased rates of O2−· production in glucose-free medium containing 2-DOG additionally implicate NADPH as an important cofactor for NADPH oxidase activity and the necessity of this activity to produce O2−· and modify LDL. The variation in LDL− levels formed by BAECs under pulsatile versus oscillatory flow conditions points to a role for NADPH oxidase activation in cell-mediated LDL modification.
The electronegative and oxidative status of LDL impacts the regulation of MCP-1 expression and monocyte/EC binding, key factors in the pathogenesis of inflammatory processes associated with atherogenesis. LDL oxidation and inflammation are based on seminal observations that LDL must be oxidatively modified for it to be taken up by macrophages.49,50 At the arterial bifurcations or branching point, ECs become hyperpermeable in the presence of hyperlipoproteinemia, which may favor intimal uptake and retention of LDL, resulting in local oxidative degradation of trapped LDL.51-53 LDL particles trapped within the subendothelial space undergo oxidative modification, resulting in the formation of MM-LDL and highly oxidized LDL. MM-LDL and LDL− induce expression of MCP-1 on ECs,9,54 leading to monocyte binding, chemotaxis, and subsequent trans-endothelial migration.7,54
Conclusion
Pulsatile (positive net forward flow) versus oscillatory (zero net forward flow) flow regulates the relative induction of the NADPH subunits, gp91phox and its homologue Nox4 mRNA, which affects the conversion of native LDL to more electronegative LDL particles, namely, LDL− and LDL−2. The modification of LDL seems to be NADPH oxidase dependent, the activity of which is influenced by the nature of shear forces. Pulsatile versus oscillatory shear stress regulates the production of ROS and RNS and the extent of LDL modification. The electronegative status of LDL may subsequently affect the regulation of MCP-1 expression and monocyte/EC binding as important inflammatory and proatherogenic events.
Supplementary Material
Acknowledgments
T.K.H. is supported by the American Heart Association Beginning Grant-in-Aid (0265166U) and grant HL50350 from the NIH, National Institutes of Health Career Research Award (K08 HL068689- 01A1) and a grant from the National Heart Foundation/American Health Assistance Foundation (H2003-028). We would like to express gratitude to Dr Howard Hodis of University of Southern California Atherosclerosis Research Unit for providing sample plasma for LDL isolation.
References
- 1.Dzau VJ. Theodore Cooper Lecture. Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37:1047–1052. doi: 10.1161/01.hyp.37.4.1047. [DOI] [PubMed] [Google Scholar]
- 2.Hsieh HJ, Cheng CC, Wu ST, Chiu JJ, Wung BS, Wang DL. Increase of reactive oxygen species (ROS) in endothelial cells by shear flow and involvement of ROS in shear-induced c-fos expression. J Cell Physiol. 1998;175:156–162. doi: 10.1002/(SICI)1097-4652(199805)175:2<156::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 3.Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res. 2002;90:e58–e65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- 4.Nickenig G, Harrison DG. The AT1-type angiotensin receptor in oxidative stress and atherogenesis, part I: oxidative stress and atherogenesis. Circulation. 2002;105:393–396. doi: 10.1161/hc0302.102618. [DOI] [PubMed] [Google Scholar]
- 5.Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37:1053–1059. doi: 10.1161/01.hyp.37.4.1053. [DOI] [PubMed] [Google Scholar]
- 6.Irani K. Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res. 2000;87:179–183. doi: 10.1161/01.res.87.3.179. [DOI] [PubMed] [Google Scholar]
- 7.Berliner JA, Territo MC, Sevanian A, Ramin S, Kim JA, Bamshad B, Esterson M, Fogelman AM. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest. 1990;85:1260–1266. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berliner JA, Mohamad N, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ. Atherosclerosis: basic mechanisms: oxidation, inflammation, and genetics. Circulation. 1995;91:2488–2496. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- 9.Sevanian A, Hwang J, Hodis H, Cazzolato G, Avogaro P, Bittolo-Bon G. Contribution of an in vivo oxidized LDL to LDL oxidation and its association with dense LDL subpopulations. Arterioscler Thromb Vasc Biol. 1996;16:784–793. doi: 10.1161/01.atv.16.6.784. [DOI] [PubMed] [Google Scholar]
- 10.Austin MA. Small, dense low-density lipoprotein as a risk factor for coronary heart disease. Int J Clin Lab Res. 1994;24:187–192. doi: 10.1007/BF02592460. [DOI] [PubMed] [Google Scholar]
- 11.Fabjan JS, Abuja PM, Schaur RJ, Sevanian A. Hypochlorite induces the formation of LDL−, a potentially atherogenic low density lipoprotein subspecies. FEBS Lett. 2001;499:69–72. doi: 10.1016/s0014-5793(01)02523-6. [DOI] [PubMed] [Google Scholar]
- 12.Yang CY, Raya JL, Chen HH, Chen CH, Abe Y, Pownall HJ, Taylor AA, Smith CV. Isolation, characterization, and functional assessment of oxidatively modified subfractions of circulating low-density lipoproteins. Arterioscler Thromb Vasc Biol. 2003;23:1083–1090. doi: 10.1161/01.ATV.0000071350.78872.C4. [DOI] [PubMed] [Google Scholar]
- 13.Henriksen T, Mahoney EM, Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc Natl Acad Sci U S A. 1981;78:6499–6503. doi: 10.1073/pnas.78.10.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rong JX, Rangaswamy S, Shen L, Dave R, Chang YH, Peterson H, Hodis HN, Chisolm GM, Sevanian A. Arterial injury by cholesterol oxidation products causes endothelial dysfunction and arterial wall cholesterol accumulation. Arterioscler Thromb Vasc Biol. 1998;18:1885–1894. doi: 10.1161/01.atv.18.12.1885. [DOI] [PubMed] [Google Scholar]
- 15.Stone WL, Heimberg M, Scott RL, LeClair I, Wilcox HG. Altered hepatic catabolism of low-density lipoprotein subjected to lipid peroxidation in vitro. Biochem J. 1994;297:573–579. doi: 10.1042/bj2970573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- 17.Jones SA, O’Donnell VB, Wood JD, Broughton JP, Hughes EJ, Jones OT. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol. 1996;271:H1626–H1634. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- 18.Sorescu D, Weiss D, Lassègue B, Clempus RE, Szöcs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of Nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 19.Fung YC, Liu SQ. Elementary mechanics of the endothelium of blood vessels. J Biomech Eng. 1993;115:1–12. doi: 10.1115/1.2895465. [DOI] [PubMed] [Google Scholar]
- 20.DePaola N, Gimbrone MA, Jr, Davies PF, Dewey CF., Jr Vascular endothelium responds to fluid shear stress gradients. Arterioscler Thromb. 1992;12:1254–1257. doi: 10.1161/01.atv.12.11.1254. published erratum appears in Arterioscler Thromb. 1993;13:465. [DOI] [PubMed] [Google Scholar]
- 21.Frangos JA, Huang TY, Clark CB. Steady shear and step changes in shear stimulate endothelium via independent mechanisms: superposition of transient and sustained nitric oxide production. Biochem Biophys Res Commun. 1996;224:660–665. doi: 10.1006/bbrc.1996.1081. [DOI] [PubMed] [Google Scholar]
- 22.Helmlinger G, Berk BC, Nerem RM. Calcium responses of endothelial cell monolayers subjected to pulsatile and steady laminar flow differ. Am J Physiol. 1995;269:C367–C375. doi: 10.1152/ajpcell.1995.269.2.C367. [DOI] [PubMed] [Google Scholar]
- 23.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation: positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985;5:293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 24.Ravensbergen J, Ravensbergen JW, Krijger JK, Hillen B, Hoogstraten HW. Localizing role of hemodynamics in atherosclerosis in several human vertebrobasilar junction geometries. Arterioscler Thromb Vasc Biol. 1998;18:708–716. doi: 10.1161/01.atv.18.5.708. [DOI] [PubMed] [Google Scholar]
- 25.Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF. Carotid bifurcation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53:502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]
- 26.Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 27.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. J Am Med Assoc. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 28.Steinberg D, Witztum JL. Is the oxidative modification hypothesis relevant to human atherosclerosis? Do the antioxidant trials conducted to date refute the hypothesis? Circulation. 2002;105:2107–2111. doi: 10.1161/01.cir.0000014762.06201.06. [DOI] [PubMed] [Google Scholar]
- 29.Landmesser U, Harrison DG. Oxidant stress as a marker for cardiovascular events: Ox marks the spot. Circulation. 2001;104:2638–2640. [PubMed] [Google Scholar]
- 30.Szocs K, Lassegue B, Sorescu D, Hilenski LL, Valppu L, Couse TL, Wilcox JN, Quinn MT, Lambeth JD, Griendling KK. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler Thromb Vasc Biol. 2002;22:21–27. doi: 10.1161/hq0102.102189. [DOI] [PubMed] [Google Scholar]
- 31.Nerem RM, Alexander RW, Chappell DC, Medford RM, Varner SE, Taylor WR. The study of the influence of flow on vascular endothelial biology. Am J Med Sci. 1998;316:169–175. doi: 10.1097/00000441-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Hsiai TK, Cho SK, Reddy S, Hama S, Navab M, Demer LL, Honda HM, Ho CM. Pulsatile flow regulates monocyte adhesion to oxidized lipidinduced endothelial cells. Arterioscler Thromb Vasc Biol. 2001;21:1770–1776. doi: 10.1161/hq1001.097104. [DOI] [PubMed] [Google Scholar]
- 33.Hsiai TK, Cho SK, Wong PK, Ing M, Salazar A, Sevanian A, Navab M, Demer LL, Ho CM. Monocyte recruitment to endothelial cells in response to oscillatory shear stress. FASEB J. 2003;17:1648–1657. doi: 10.1096/fj.02-1064com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsiai T, Cho SK, Honda HM, Hama S, Navab M, Demer LL, Ho CM. Endothelial cell dynamics under pulsating flow: significance of high versus low shear stress slew rates. Ann Biomed Eng. 2002;30:646–656. doi: 10.1114/1.1484222. [DOI] [PubMed] [Google Scholar]
- 35.Hwang J, Wang J, Morazzoni P, Hodis HN, Sevanian A. The phytoestrogen equol increases nitric oxide availability by inhibiting superoxide production: an antioxidant mechanism for cell-mediated LDL modification. Free Radic Biol Med. 2003;34:1271–1282. doi: 10.1016/s0891-5849(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 36.De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxideproducing NADH oxidase. Circ Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 37.De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxideproducing NADH oxidase. Circ Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 38.Hodis HN, Kramsch DM, Avogaro P, Bittolo-Bon G, Cazzolato G, Hwang J, Peterson H, Sevanian A. Biochemical and cytotoxic characteristics of an in vivo circulating oxidized low density lipoprotein (LDL−) J Lipid Res. 1994;35:669–677. [PubMed] [Google Scholar]
- 39.Chu Y, Heistad DD, Knudtson KL, Lamping KG, Faraci FM. Quantification of mRNA for endothelial NO synthase in mouse blood vessels by real-time polymerase chain reaction. Arterioscler Thromb Vasc Biol. 2002;22:611–616. doi: 10.1161/01.atv.0000012663.85364.fa. [DOI] [PubMed] [Google Scholar]
- 40.Walker NJ. A technique whose time has come. Science. 2002;296:557–559. doi: 10.1126/science.296.5567.557. [DOI] [PubMed] [Google Scholar]
- 41.Fogelman AM, Elahi F, Sykes K, Van Lenten BJ, Territo MC, Berliner JA. Modification of the Recalde method for the isolation of human monocytes. J Lipid Res. 1988;29:1243–1247. [PubMed] [Google Scholar]
- 42.Benov L, Sztejnberg L, Fridovich I. Critical evaluation of the use of hydroethidine as a measure of superoxide anion radical. Free Radic Biol Med. 1998;25:826–831. doi: 10.1016/s0891-5849(98)00163-4. [DOI] [PubMed] [Google Scholar]
- 43.Gimbrone MA, Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci. 2000;902:230–239. doi: 10.1111/j.1749-6632.2000.tb06318.x. [DOI] [PubMed] [Google Scholar]
- 44.Rueckschloss U, Galle J, Holtz J, Zerkowski HR, Morawietz H. Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: antioxidative potential of hydroxymethylglutaryl coenzyme A reductase inhibitor therapy. Circulation. 2001;104:1767–1772. doi: 10.1161/hc4001.097056. [DOI] [PubMed] [Google Scholar]
- 45.Sorescu D, Weiss D, Lassègue B, Clempus RE, Szöcs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of Nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 46.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;21:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 47.Sevanian A, Seraglia R, Traldi P, Rossato P, Ursini F, Hodis H. Analysis of plasma cholesterol oxidation products using gas and high-performance liquid chromatography/mass spectrometry. Free Radic Biol Med. 1994;17:397–409. doi: 10.1016/0891-5849(94)90166-x. [DOI] [PubMed] [Google Scholar]
- 48.Sevanian A, Bittolo-Bon G, Cazzolato G, Hodis H, Hwang J, Zamburlini A, Maiorino M, Ursini F. LDL− is a lipid hydroperoxide-enriched circulating lipoprotein. J Lipid Res. 1997;38:419–428. [PubMed] [Google Scholar]
- 49.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol: modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 50.Witztum JL. The oxidation hypothesis of atherosclerosis. Lancet. 1994;344:793–795. doi: 10.1016/s0140-6736(94)92346-9. [DOI] [PubMed] [Google Scholar]
- 51.Buchanan JR, Jr, Kleinstreuer C, Truskey GA, Lei M. Relation between non-uniform hemodynamics and sites of altered permeability and lesion growth at the rabbit aorto-celiac junction. Atherosclerosis. 1999;143:27–40. doi: 10.1016/s0021-9150(98)00264-0. [DOI] [PubMed] [Google Scholar]
- 52.Deng X, Marois Y, How T, Merhi Y, King M, Guidoin R, Karino T. Luminal surface concentration of lipoprotein (LDL) and its effect on the wall uptake of cholesterol by canine carotid arteries. J Vasc Surg. 1995;21:135–145. doi: 10.1016/s0741-5214(95)70252-0. [DOI] [PubMed] [Google Scholar]
- 53.Henry PD, Chen CH. Inflammatory mechanisms of atheroma formation: influence of fluid mechanics and lipid-derived inflammatory mediators. Am J Hypertens. 1993;6:328S–334S. doi: 10.1093/ajh/6.11.328s. [DOI] [PubMed] [Google Scholar]
- 54.Cushing SD, Berliner JA, Valente AJ, Territo MC, Navab M, Parhami F, Gerrity R, Schwartz CJ, Fogelman AM. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990;87:5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


