Abstract
Objective This study aims to assess the hearing outcomes of patients undergoing surgical management of petrous apex cholesterol granuloma and to discuss the role of otic capsule–sparing approaches in drainage of petrous apex cholesterol granulomas.
Design Retrospective case series.
Setting Tertiary care medical center.
Participants Eight patients underwent surgery for presumed or definitive cholesterol granuloma between 2002 and 2011 and met the inclusion criteria for this study.
Main Outcome Measures Pre- and postoperative audiogram results as measured by pure tone thresholds and word recognition scores.
Results Four patients (50%) demonstrated improvement in speech discrimination. One patient had an increase from 0 to 67% in word recognition scores. Four patients (50%) demonstrated worsening of pure tone thresholds, including two patients with anacusis.
Conclusion Perilabyrinthine drainage of petrous apex cholesterol granulomas may result in hearing preservation or hearing improvement, even in the setting of otic capsule erosion. Patients should be counseled about the potential risk of significant hearing loss.
Keywords: cholesterol granuloma, petrous apex, hearing outcomes
Introduction
Petrous apex lesions are a challenge to manage given their proximity to critical structures such as the otic capsule, Eustachian tube, carotid artery, and jugular bulb. Hearing loss may result through the progression of the disease and erosion of the otic capsule, eighth cranial nerve compression, release of local inflammatory mediators, or vascular compression of the inferior cochlear vein adjacent to the cochlear aqueduct.1 Management options for petrous apex cholesterol granuloma (PACG) include observation with serial imaging, surgical excision, or marsupialization. Marsupialization is most commonly used to manage symptomatic patients and includes otic capsule sparing options for patients with serviceable hearing.2 3 Patients with nonserviceable hearing are typically managed with translabyrinthine or transotic approaches.4 The objective of the present study is to determine whether there is value in utilizing otic capsule–sparing approaches in patients with PACG regardless of their preoperative hearing status. This study also sought to assess predictors of hearing loss based on preoperative computed tomography (CT) imaging.
Patients and Methods
Institutional Review Board approval was obtained from UT Southwestern Medical Center for retrospective review of all patients who underwent surgical drainage of a PACG between January 2002 and May 2011. A total of 34 patients were identified. Eight patients met the inclusion criteria of having otic capsule–sparing surgeries with pre- and postoperative audiograms, operative reports, and CT imaging available for review. The rather large percentage of patients that did not meet the inclusion criteria stemmed from lack of available postoperative audiograms in the majority of patients. We attribute this to the large amount of itinerant surgeries done in a tertiary care facility and a tendency to follow-up with their referring institutions. Three frequency (500, 1,000, and 2,000 Hz) pure tone averages (PTA), speech reception threshold (SRT), and word recognition scores (WRS) were recorded for each patient before and after surgery. Primary and recurrent cases of PACG were included in this study. Patient demographics, cholesterol granuloma (CG) location, surgical method, audiologic data, and surgical complications were collected. Results are presented in a scatterplot format in accordance with the recommended format by Gurgel et al for posttreatment audiologic results after an intervention, including WRS and PTA findings.5 No change in hearing was defined as a less than 10% change between pre- and postoperative WRS and less than 10 dB change between pre- and postoperative PTA.5
The infracochlear approach typically employs a postauricular approach with wide elevation of a superiorly based tympanomeatal flap. The inferior aspect of the external auditory canal and osseous annulus is widened with an otologic drill. The vertical petrous carotid artery and jugular bulb are identified along with the inferior basal turn of the cochlea. The triangle of bone between the aforementioned structures is progressively opened until the fibrous wall of the PACG is encountered. Following the vertical and horizontal petrous carotid artery reduces the risk of a cerebrospinal fluid leak secondary to violation of the posterior fossa dura. The fibrous wall of the PACG is widely opened, allowing drainage of the cyst.6
The infralabyrinthine approach starts with a postauricular incision followed by a complete canal wall up mastoidectomy. The vertical facial nerve, sigmoid sinus, jugular bulb, and posterior semicircular canal are identified. Bone removal proceeds between these structures until the cyst wall is encountered and is opened widely. A high-riding jugular bulb may prevent adequate exposure and should be assessed by preoperative imaging.6
Results
Eight patients underwent otic capsule–sparing approaches to the petrous apex for drainage of PACG between February 2008 and March 2011. The cohort consisted of six men and two women with an average age of 50.7 years (range, 16–73 years). Five patients underwent an infracochlear approach. Two patients underwent an infralabyrinthine approach. One patient underwent a combined infracochlear–infralabyrinthine approach. All surgical approaches were for primary PACG except one surgical case (patient 4) in which an infracochlear approach was utilized (see Table 1).
Table 1. Patient summary.
| Patient | Sex | Age (y) | Side | Size (cm) | IAC erosion | Otic capsule erosion | Cochlear aqueduct erosion | Approach | Preop | Postop | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTA (dB) | SRT (dB) | WRS (%) | PTA (dB) | SRT (dB) | WRS (%) | |||||||||
| 1 | M | 66 | L | 3.4 × 3.2 | Yes | No | Yes | Infralabyrinthine | 45 | 55 | 0 | 35 | 35 | 64 |
| 2 | M | 56 | R | 1.4 × 2.2 | Yes | No | Yes | Infralabyrinthine | 10 | 5 | 96 | 10 | 10 | 100 |
| 3 | M | 56 | R | 2.1 × 2.4 | No | No | No | Infracochlear | 31 | 10 | 92 | 36 | 25 | 88 |
| 4 | M | 43 | R | 2.0 × 2.7 | Yes | No | No | Infracochlear | 10 | 10 | 100 | 95 | NR | NR |
| 5 | M | 68 | R | 1.2 × 2.0 | Yes | Yes | Yes | Infracochlear | 42 | 30 | 84 | 25 | 30 | 100 |
| 6 | F | 16 | L | 2.2 × 1.8 | No | No | No | Infracochlear | 8 | 10 | 100 | 12 | 15 | 100 |
| 7 | M | 73 | R | 2.6 × 2.8 | Yes | No | Yes | Infracochlear, Infralabyrinthine | 48 | 40 | 64 | 105 | NR | NR |
| 8 | F | 28 | R | 2.4 × 2.9 | Yes | Yes | Yes | Infracochlear | 34 | 24 | 84 | 15 | 25 | 100 |
Abbreviations: F, female; IAC, internal auditory canal; L, left; M, male; Postop, postoperative; Preop, preoperative; PTA, pure tone averages; R, right; SRT, speech reception threshold; WRS, word recognition scores.
Presenting Symptoms
Presenting symptoms included hearing loss in four patients (50%). Three patients (38%) complained of headache. Two patients (25%) showed symptoms of imbalance. One patient (12.5%) presented with House–Brackmann II/VI facial paresis, facial hyperkinesis, and hemifacial hypesthesia. One patient (12.5%) presented with ocular complaints.
Radiographic Findings
Preoperatively, six of eight patients (75%) showed evidence of internal auditory canal (IAC) erosion, five of eight (63%) showed erosion of cochlear aqueduct, and two of eight (25%) showed otic capsule erosion (Fig. 1). We defined otic capsule erosion as thinning or absence of bone over the cochlea, vestibule, semicircular canals, or vestibular and cochlear aqueducts. There was no correlation with radiographic findings and clinical presentation. The patient with facial paresis had evidence of IAC erosion on preoperative imaging. The mean size of PACG was 2.0 × 2.5 cm (range, 1.2–3.4 cm × 1.8–3.2 cm). Six of eight (75%) were right-sided lesions. The size of the PACG did not correlate with clinical symptoms or hearing loss.
Fig. 1.
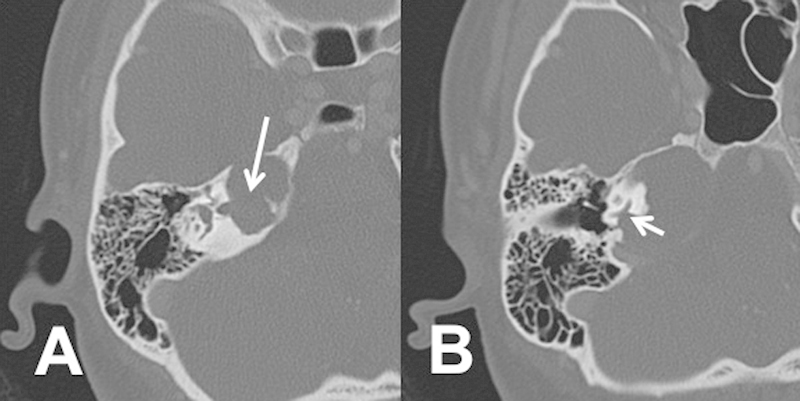
Axial computed tomography scan showing (A) internal auditory canal erosion (arrow marks erosion into the internal auditory canal) and (B) cochlea erosion (arrow mark erosion into the medial aspect of the inferior basal turn of the cochlea).
Hearing Outcomes
Four of eight patients (50%) demonstrated improvement in WRS after surgical drainage. One of eight patients (13%) showed no change in WRS while three patients (37%) showed a decline. Two of eight patients (25%) experienced complete loss of hearing. One patient with IAC erosion showed improvement in WRS from 0 to 64% after drainage of the PACG. PTA improved in three patients (37%), showed no change in one patient (13%), and declined in four patients (50%) (Fig. 2).
Fig. 2.
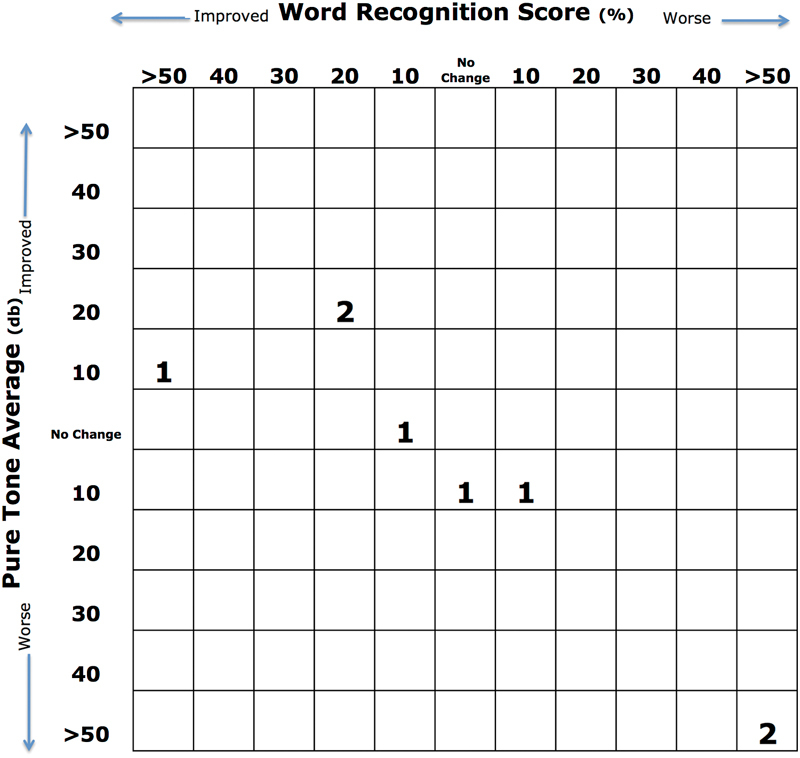
Scatterplot showing postoperative changes in hearing.
In the two patients (patients 4 and 7) who developed anacusis after surgery, there were no intraoperative findings to explain the hearing loss (Table 1). Patient 4 was the only revision surgery in this series and had been previously treated with an infracochlear approach. Postoperative imaging failed to identify a violation of the otic capsule or other radiographic findings to explain the anacusis. Patient 7 underwent a combined infracochlear–infralabyrinthine approach. The PACG and drainage pathway were noted to traverse the cochlear aqueduct. Mean lesion size for the two patients that developed anacusis was 2.3 cm × 2.7 cm.
Complications
An intraoperative CSF leak occurred in patient 1 which was repaired at the time of surgery with no further complications. Another subject developed postoperative surgical site cellulitis that resolved with oral antibiotics.
Discussion
Manasse was the first to describe temporal bone, CGs in the late 19th century.7 CGs form in well-aerated regions of the temporal bone and are composed of a thick fibrous capsule typically containing brownish or yellowish gelatinous contents. Primary CGs commonly form in the petrous apex, but can also occur in the middle ear and mastoid.6 Secondary CG in a postsurgical mastoid are occasionally encountered by surgeons during revision and second-look procedures. PACG are uncommon as an estimated 30% of the population is estimated to have a pneumatized petrous apex, which appears to be a precursor for such lesions.8 9
Clinical presentation of PACG is variable with the most commonly reported being headaches (32–80%), imbalance (24–82%), hearing loss (20–64%), facial paresthesia (6–30%), and facial paresis or hyperkinesis (4–23%).2 6 10 11 12 Mechanisms for hearing loss include compression of the eighth cranial nerve, erosion of the otic capsule resulting in a cochlear or semicircular canal fistula, release of local inflammatory mediators, or vascular compression.1 2
There are two prevailing theories on the pathogenesis of CG formation. Hemorrhage and subsequent anaerobic breakdown of hemoglobin, formation of cholesterol crystals, and subsequent foreign body giant cell reaction is a necessary step in both theories. The first theory is the classic obstruction-vacuum theory which proposes that negative pressure occurs from obstruction and isolation of petrous apex air cells leading to transudative hemorrhage from mucosal surfaces.11 The exposed marrow theory ascribes the hemorrhage to close approximation of an aerated petrous apex air cell to bone marrow in the clivus.13 The bone marrow coapts with overlying mucosa which then has a tendency toward hemorrhage, clot formation, and obstruction of the petrous apex. Enlargement of the cyst is from repeated hemorrhage caused by an inflammatory granulomatous reaction and from small vessel proliferation.13
PACG has a pathognomonic appearance on magnetic resonance imaging (MRI) in most patients. Differential diagnoses of petrous apex lesions include asymmetric pneumatization, petrous apex effusion, cholesteatoma, petrous apicitis, arachnoid cyst, carotid artery aneurysm, chondroid tumors, chordoma, schwannoma, and metastasis.6 MRI of PACG typically demonstrates a smooth-walled lesion that is hyperintense on both T1 and T2 imaging. CT imaging demonstrates absence of air-cell septation and smooth erosion of the surrounding bone. Lesions are more commonly found in the anterior portion of the petrous apex. Involvement of the otic capsule, IAC, and labyrinth is occasionally identified.
Surgical approaches for symptomatic patients with intact hearing include infracochlear, infralabyrinthine, middle fossa, and transsphenoidal. Limitations of the infralabyrinthine approach include a narrow surgical corridor from a high jugular bulb. Middle fossa approaches do not allow opportunity for simple drainage into an air-containing space and are therefore less favored. Transsphenoidal approaches are usually limited to PACG that are adjacent to the sphenoid sinus unless the petrous carotid artery is mobilized.14 Brackmann and Toh reported using the transcanal infracochlear approach in 18 of 22 patients with intact hearing.2 In most patients without serviceable hearing, translabyrinthine or transotic approaches have traditionally been preferred for improved access. We adhere to the traditional definition of serviceable hearing as PTA ≤ 50 dB and WRS ≥ 50%.
It is known that hearing loss is a complication of otic capsule–sparing approaches. Reports of hearing loss in otic capsule–sparing approaches vary in frequency. In the study by Brackmann and Toh, only 1 of 24 patients lost serviceable hearing after an otic capsule–sparing approach.2 In a six-patient series by Gherini et al, they reported hearing loss in one of six patients who underwent a combined middle fossa–infralabyrinthine approach.15 In our series, two of the eight patients developed anacusis. One patient underwent a combined infracochlear–infralabyrinthine approach and the other patient underwent a revision infracochlear approach. It is unclear whether combined approaches have a higher rate of hearing loss. One potential theory for postoperative hearing loss with the use of otic capsule–sparing approaches is compromise of arterial or venous cochlear blood flow. The vein of the cochlear aqueduct mediates venous drainage of the cochlea, which is in the surgical corridor of the both the infralabyrinthine and infracochlear approaches.16
Improvement in hearing was documented on one patient with nonserviceable hearing in our cohort. Improvement in hearing has been previously documented in otic capsule–sparing approaches. One patient in the study by Gherini et al showed hearing improvement.15 This has led us to challenge the presumption that transotic or translabyrinthine approaches should be used for patients without serviceable hearing. Brackmann noted that in patients with other cranial neuropathies, 88.9% of patients with preoperative dysfunction improved after surgery.2 This report along with previous studies lend further support that cochlear nerve function may be recovered after adequate drainage of PACG.
Conclusion
In patients with nonserviceable hearing, translabyrinthine or transotic approaches are typically advocated. In this series, however, we present a case of a patient with a WRS of 0% that showed improvement to 64% following drainage of PACG. WRS improved in 50% of the cases. There was no correlation between hearing loss and preoperative CT findings. As a result of these findings, even in cases of absent WRS preoperatively, we advocate hearing preservation approaches for PACG. We promote adequate counseling for all patients including the risk of complete hearing loss, which may occur even without violation of the otic capsule.
References
- 1.Watanabe Y, Nakashima T, Yanagita N. Venous communications of the cochlea after acute occlusion of the vein of the cochlear aqueduct. Arch Otorhinolaryngol. 1988;245(6):340–343. doi: 10.1007/BF00457990. [DOI] [PubMed] [Google Scholar]
- 2.Brackmann D E, Toh E H. Surgical management of petrous apex cholesterol granulomas. Otol Neurotol. 2002;23(4):529–533. doi: 10.1097/00129492-200207000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Ghorayeb B Y, Jahrsdoerfer R A. Subcochlear approach for cholesterol granulomas of the inferior petrous apex. Otolaryngol Head Neck Surg. 1990;103(1):60–65. doi: 10.1177/019459989010300109. [DOI] [PubMed] [Google Scholar]
- 4.Gacek R R Diagnosis and management of primary tumors of the petrous apex Ann Otol Rhinol Laryngol 197584(1 PT. 2 SUPPL 18, 1pt. 2 suppl 18):1–20. [DOI] [PubMed] [Google Scholar]
- 5.Gurgel R K, Jackler R K, Dobie R A, Popelka G R. A new standardized format for reporting hearing outcome in clinical trials. Otolaryngol Head Neck Surg. 2012;147(5):803–807. doi: 10.1177/0194599812458401. [DOI] [PubMed] [Google Scholar]
- 6.Isaacson B Kutz J W Roland P S Lesions of the petrous apex: diagnosis and management Otolaryngol Clin North Am 2007403479–519., viii [DOI] [PubMed] [Google Scholar]
- 7.Manasse P. Wiesbaden: JF Bergman; 1917. Otitis media catarrhalis chronica (otitis media chronica fibrosa) pp. 46–53. [Google Scholar]
- 8.Myerson M C, Rubin J, Gilbert J G. Anatomic studies of the petrous portion of the temporal bone. Arch Otolaryngol. 1934;20:195–210. [Google Scholar]
- 9.Roland P S, Meyerhoff W L, Judge L O, Mickey B E. Asymmetric pneumatization of the petrous apex. Otolaryngol Head Neck Surg. 1990;103(1):80–88. doi: 10.1177/019459989010300112. [DOI] [PubMed] [Google Scholar]
- 10.Brodkey J A, Robertson J H, Shea J J III, Gardner G. Cholesterol granulomas of the petrous apex: combined neurosurgical and otological management. J Neurosurg. 1996;85(4):625–633. doi: 10.3171/jns.1996.85.4.0625. [DOI] [PubMed] [Google Scholar]
- 11.Castillo M P, Samy R N, Isaacson B, Roland P S. Petrous apex cholesterol granuloma aeration: does it matter? Otolaryngol Head Neck Surg. 2008;138(4):518–522. doi: 10.1016/j.otohns.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Thedinger B A, Nadol J B Jr, Montgomery W W, Thedinger B S, Greenberg J J. Radiographic diagnosis, surgical treatment, and long-term follow-up of cholesterol granulomas of the petrous apex. Laryngoscope. 1989;99(9):896–907. doi: 10.1288/00005537-198909000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Jackler R K Cho M A new theory to explain the genesis of petrous apex cholesterol granuloma Otol Neurotol 200324196–106., discussion 106 [DOI] [PubMed] [Google Scholar]
- 14.Gore M R, Zanation A M, Ebert C S, Senior B A. Cholesterol granuloma of the petrous apex. Otolaryngol Clin North Am. 2011;44(5):1043–1058. doi: 10.1016/j.otc.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Gherini S G, Brackmann D E, Lo W W, Solti-Bohman L G. Cholesterol granuloma of the petrous apex. Laryngoscope. 1985;95(6):659–664. doi: 10.1288/00005537-198506000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Mom T, Chazal J, Gabrillargues J, Gilain L, Avan P. Cochlear blood supply: an update on anatomy and function. Fr ORL. 2005;88:81–88. [Google Scholar]


