Abstract
Objectives To identify factors associated with disease-specific survival (DSS) in intestinal and nonintestinal sinonasal adenocarcinoma.
Design Retrospective review.
Setting Surveillance Epidemiology and End Results database.
Participants Adult patients with sinonasal adenocarcinoma.
Main Outcome Measures DSS.
Results We identified 325 patients; of these, 300 had the nonintestinal type and 25 had intestinal type histologies. The 5-year DSS rates for patients who had no treatment, radiation (RT), surgery, and surgery and postoperative RT were 42.5, 46.1, 85.6, and 72.6%, respectively (log-rank test; p < 0.001). Black race, age ≥ 75 years, paranasal sinus involvement, and high grade were independently associated with decreased DSS. Compared with RT, surgery (hazard ratio [HR]: 0.34; 95% confidence interval [CI]: 0.15–0.77), and adjuvant RT (HR: 0.47; 95% CI, 0.26–0.86) were associated with improved DSS.
Conclusions There is no difference in prognosis between intestinal and nonintestinal subtypes of sinonasal adenocarcinoma. Treatment with surgery alone or adjuvant RT is associated with a more favorable prognosis.
Keywords: sinonasal, adenocarcinoma, intestinal type, nonintestinal type, survival
Introduction
Sinonasal tumors are rare and represent ∼ 3% of all head and neck tumors.1 2 Sinonasal adenocarcinoma is the second most common sinonasal malignancy and represents ∼ 10 to 20% of all sinonasal malignancies.2 3 Although other sinonasal histologies have not been associated with any improvement in overall survival over the past several decades, adenocarcinoma has been shown to have improved 5-year survival rates, from 49.6% in 1973 to 66.7% in 2001.3
Sinonasal adenocarcinoma can be categorized into intestinal and nonintestinal types. Intestinal-type adenocarcinomas are associated with wood and leather dust exposure. No known environmental factors are associated with nonintestinal-type adenocarcinomas.2 4 5 Currently, there is no standardized treatment algorithm for sinonasal adenocarcinomas.3 6
To our knowledge, most of the experience in the literature with prognostic factors for survival in sinonasal adenocarcinoma is from case reports and single-institution studies.1 6 7 8 9 A prior national study on the incidence and survival of sinonasal adenocarcinoma aggregated adenocarcinoma with salivary gland and neuroendocrine carcinomas.3 Our study is the largest study to date on nonsalivary sinonasal adenocarcinoma. The purpose of our study was to characterize prognostic factors associated with disease-specific survival (DSS) in nonsalivary sinonasal adenocarcinoma.
Materials and Methods
Data Source and Study Participants
Our study used data from the Surveillance Epidemiology and End Results (SEER) database for the period from 1988 to 2010. The SEER database encompasses 20 geographic regions that represent ∼ 28% of the U.S. population.10 These regions include Alaska, Arizona, Atlanta, greater California, Connecticut, the Cherokee Tribal Jurisdictional Service Area in Oklahoma, Detroit, greater Georgia, rural Georgia, Hawaii, Iowa, Kentucky, Los Angeles, Louisiana, New Jersey, New Mexico, San Francisco-Oakland, San Jose-Monterey, Seattle-Puget Sound, and Utah.10 Incidence rates were age adjusted to U.S. population data from 2000 and calculated based on SEER-9 regions (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco, Seattle, and Utah). These SEER-9 regions comprise nearly 10% of the U.S. population.
We identified patients using the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) topography (C-30.0: nasal cavity; C-31.0–3, 31.8–9: paranasal sinuses) and histology codes (intestinal-type adenocarcinoma: 8144; nonintestinal-type adenocarcinoma: 8140).
Demographic variables of interest included patient gender, age, and race. Pathologic variables included extent of disease and American Joint Committee on Cancer (AJCC) 6th edition stage groups. Extent of disease was classified into localized, regional, and distant spread. AJCC staging was available for a subset of patients diagnosed from 2004 to 2010 and grouped into stages I, II, III, and IV.
Clinical variables included year of diagnosis, surgical resection, radiation therapy (RT), and survival time. Surgery and RT were categorized into four groups: no treatment, RT only, surgery alone, and surgery and postoperative RT. Survival time in years was calculated from the date of diagnosis to death, the last date the patient was known to be alive, or December 31, 2010, whichever came first. DSS rates were determined.
Statistical Analysis
Chi-square and Student t tests were used to analyze categorical and continuous variables, respectively. Kaplan-Meier survival analysis was used for univariate analysis of survival, and the log-rank test was used to determine statistical significance. Cox univariate and multivariate regression was used to identify risk factors for disease-specific mortality. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for the strength of association between each prognostic factor and survival. All tests were two sided, and p < 0.05 was considered statistically significant.
Incidence rates and tests of trends were determined using SEER*Stat (v.7.1.0; Information Management Services, Inc., Silver Spring, Maryland, United States). The remaining data analysis was performed using Statistical Package for the Social Sciences (SPSS) software (v.21.0; SPSS Inc., Chicago, Illinois, United States). The SEER database is publicly available, and all patient information is deidentified; therefore, our study was granted an exemption from our institutional review board.
Results
We identified 325 patients with sinonasal adenocarcinoma in the SEER database between 1988 and 2010. The incidence of sinonasal adenocarcinoma in the United States was 1.7 per 10 million persons in 2010.
Patient Characteristics
Patients with sinonasal adenocarcinoma were primarily men (57.5%) and white (80.6%), and the mean age at diagnosis was 66.6 years (Table 1). Most of our cohort had paranasal sinus primaries (61.8%), and the remaining patients had nasal cavity primaries (38.2%). Nearly half of all paranasal sinus primaries were located in the maxillary sinus (49.3%). Histologically, most of the tumors were the nonintestinal type (92.3%); the remaining were the intestinal type (7.7%)
Table 1. Baseline characteristics of patients with sinonasal adenocarcinoma, 1988 to 2010.
| Characteristic | N = 325 | %a |
|---|---|---|
| Site | ||
| Nasal cavity | 124 | 38.2 |
| Maxillary sinus | 99 | 30.5 |
| Ethmoid sinus | 61 | 18.8 |
| Frontal/Sphenoid sinus | 13 | 4.0 |
| Other | 28 | 8.6 |
| Gender | ||
| Men | 187 | 57.5 |
| Women | 138 | 42.5 |
| Age, y | ||
| < 51 | 71 | 21.8 |
| 51–66 | 83 | 25.5 |
| 66–74 | 80 | 24.6 |
| > 75 | 91 | 28.0 |
| Race | ||
| White | 262 | 80.6 |
| Black | 43 | 13.2 |
| Other | 20 | 6.2 |
| Grade | ||
| Low grade | 171 | 52.6 |
| High grade | 100 | 30.8 |
| Unknown | 54 | 16.6 |
| Histology | ||
| Nonintestinal type | 300 | 92.3 |
| Intestinal type | 25 | 7.7 |
| Extent of disease | ||
| Localized | 91 | 30.8 |
| Regional | 153 | 51.9 |
| Distant | 51 | 17.3 |
| Stageb | ||
| Stage I | 36 | 28.6 |
| Stage II | 14 | 11.1 |
| Stage III | 20 | 15.9 |
| Stage IV | 41 | 32.5 |
| Unknown | 15 | 11.9 |
| Treatment | ||
| None | 33 | 10.6 |
| Radiation | 53 | 17.0 |
| Surgery | 104 | 33.4 |
| Both | 121 | 38.9 |
| Year of diagnosis | ||
| 1988–1992 | 34 | 10.5 |
| 1993–1998 | 60 | 18.5 |
| 1999–2004 | 106 | 32.6 |
| 2004–2010 | 125 | 38.5 |
Percentages are column percentages and may not add up to 100 because of rounding.
Stage available for cases diagnosed in 2004–2010.
In our cohort, most patients received either surgery alone (33.4%) or surgery and postoperative RT (38.9%). Compared with patients with low-grade tumors, those with high-grade tumors were more likely to have postoperative RT (34.3% versus 49.5%; p< 0.001).
Survival Analysis
On univariate analysis, paranasal sinus primaries, age ≥ 75 years, black race, high grade, and regional or distant spread of disease were associated with compromised survival (Table 2). Kaplan-Meier analysis of DSS by race demonstrated that 5-year DSS was 75.1, 73.1, and 44.6% for patients whose race was white, other, and black, respectively (p < 0.001). Compared with RT, both surgery (HR: 0.17; 95% CI, 0.09–0.35) and postoperative RT (HR: 0.44; 95% CI, 0.25–0.76) were associated with improved DSS. The 5-year DSS rates for patients who had surgery alone or postoperative RT were 85.6% and 72.6%, respectively; patients who underwent RT alone had 5-year rates of 46.1% (p < 0.001) (Fig. 1). There was no difference in survival based on year of diagnosis or histologic subtype. The 5-year DSS for the nonintestinal type was 71.2 and 69.3% for the intestinal type (p = 0.86) (Fig. 2).
Table 2. Univariate analysis.
| Hazard ratio | 95% confidence interval | p value | |
|---|---|---|---|
| Site | |||
| Nasal cavity | 1 | Reference | |
| Maxillary sinus | 3.99 | 2.16–7.38 | < 0.001 |
| Ethmoid sinus | 2.83 | 1.41–5.68 | 0.004 |
| Frontal sinus | 10.50 | 3.44–32.09 | < 0.001 |
| Sphenoid sinus | 2.04 | 0.27–15.56 | 0.49 |
| Other | 3.55 | 1.54–8.23 | 0.003 |
| Gender | |||
| Male | 1 | Reference | |
| Female | 0.97 | 0.63–1.50 | 0.89 |
| Age group, y | |||
| <51 | 1 | Reference | |
| 51–65 | 1.92 | 1.00–3.68 | 0.05 |
| 66–74 | 1.45 | 0.72–2.94 | 0.30 |
| 75+ | 2.18 | 1.12–4.25 | 0.02 |
| Race | |||
| White | 1 | Reference | |
| Black | 2.75 | 1.66–4.55 | < 0.001 |
| Other | 0.86 | 0.31–2.38 | 0.77 |
| Grade | |||
| Low grade | 1 | Reference | |
| High grade | 4.15 | 2.52–6.83 | < 0.001 |
| Unknown | 2.13 | 1.12–4.03 | 0.02 |
| Histology | |||
| Nonintestinal type | 1 | Reference | |
| Intestinal type | 1.07 | 0.49–2.33 | 0.86 |
| Extent of disease | |||
| Localized | 1 | Reference | |
| Regional | 3.45 | 1.73–6.85 | < 0.001 |
| Distant | 5.59 | 2.61–11.99 | < 0.001 |
| Treatment | |||
| None | 1 | Reference | |
| Radiation | 0.84 | 0.32–2.16 | 0.71 |
| Surgery | 0.14 | 0.05–0.41 | < 0.001 |
| Surgery and radiation | 0.38 | 0.15–0.94 | 0.04 |
| Year of diagnosis | |||
| 1988–1992 | 1 | Reference | |
| 1993–1998 | 0.84 | 0.41–1.71 | 0.63 |
| 1999–2004 | 0.71 | 0.36–1.39 | 0.32 |
| 2004–2010 | 0.65 | 0.32–1.36 | 0.25 |
Fig. 1.
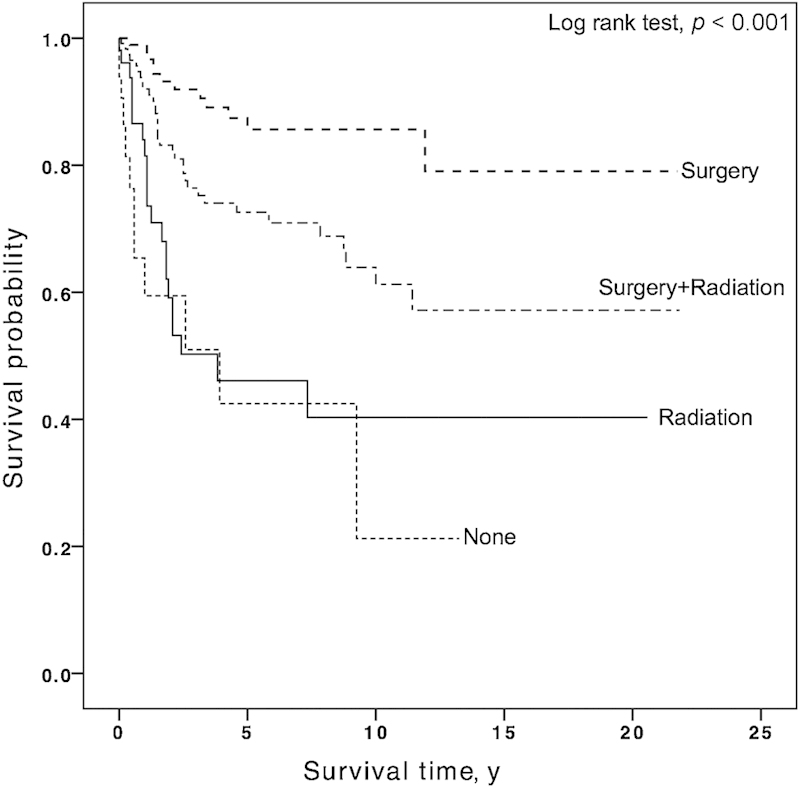
The 5-year disease-specific survival rates for patients who had no treatment, radiation therapy (RT), surgery, and adjuvant RT were 42.5, 46.1, 85.6, and 72.6%, respectively (p < 0.001).
Fig. 2.
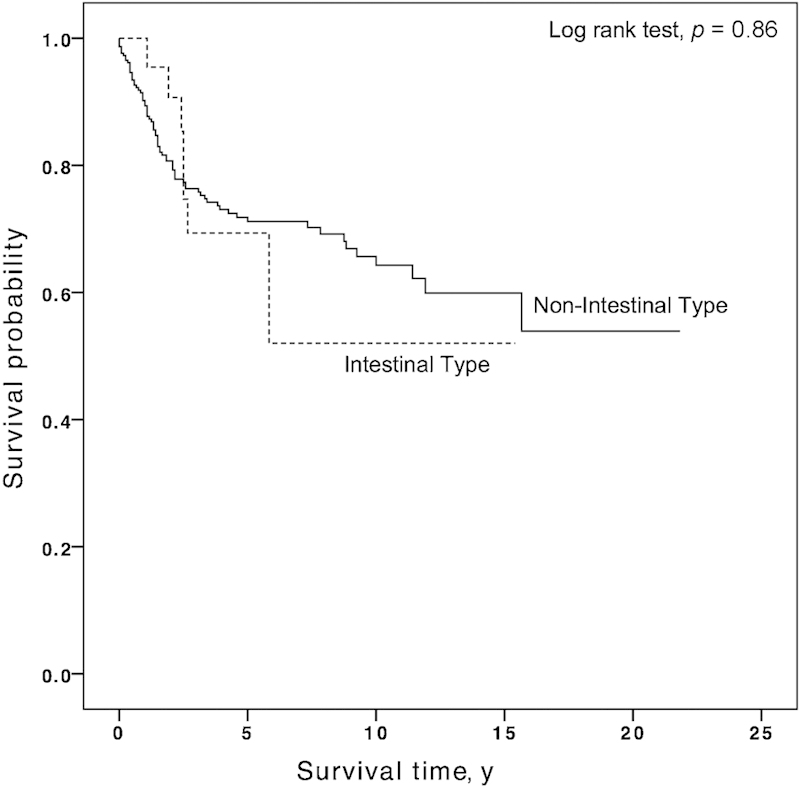
Kaplan-Meier analysis of patients with sinonasal adenocarcinoma by histologic subtype. The 5-year disease-specific survival rate was 71.2% for the nonintestinal type and 69.3% for the intestinal type (p = 0.86).
After adjustment in our multivariate analysis, paranasal sinus involvement, age ≥ 75 years, black race, and high grade continue to be independent risk factors for increased disease-specific mortality (Fig. 3). Compared with RT, both surgery (HR: 0.28; 95% CI, 0.09–0.87) and postoperative RT (HR: 0.37; 95% CI, 0.14–0.99) were associated with improved DSS. Stratified multivariate analysis of a subset of only surgical patients demonstrated no difference in survival in those who had postoperative RT compared with those who had surgery alone (HR: 0.59; 95% CI, 0.36–1.80). A subanalysis of 126 patients diagnosed from 2004 to 2011 who had AJCC stage data demonstrated that after controlling for demographics, AJCC stage, grade, histology, and tumor site, there was no difference between surgery alone and surgery and postoperative RT (HR: 1.22; 95% CI, 0.54–2.77).
Fig. 3.
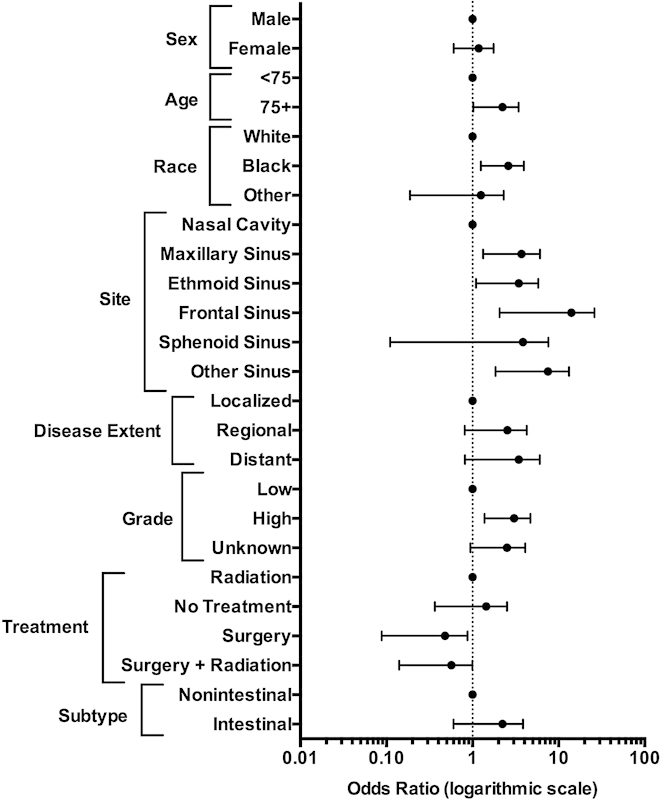
Multivariate analysis of disease-specific mortality. Reference categories are the first category in each group.
Discussion
To our knowledge, our study is the first population-level analysis of predictors of survival in sinonasal adenocarcinoma. We did not observe any difference in survival by year of survival or between intestinal and nonintestinal subtypes. Paranasal sinus involvement, age ≥ 75 years, black race, and high-grade tumors were identified as independent risk factors. Over a third of patients received adjuvant RT; however, there was no difference in survival between patients who received surgery alone and those who had adjuvant RT. This likely represents a selection bias, with patients with higher risk disease treated more frequently with radiation.
In our cohort, nearly 30% of our patients were ≥ 75 years of age, which was an independent risk factor for decreased DSS. There is a paucity of data on the association between age and DSS in sinonasal adenocarcinoma. In an analysis of 50 patients with sinonasal adenocarcinoma, Heffner et al determined that patients with high-grade adenocarcinoma were more likely to be older and male than those with low-grade tumors.8 We did not observe any difference in grade between our two age groups. The mean age of our cohort was 67 years. This was slightly older than a recent single-institution study of sinonasal adenocarcinoma by Bhayani et al that identified a mean age of 57 years in their cohort of 66 patients.6
We observed that year of diagnosis was not associated with DSS from 1988 to 2010. Turner and Reh conducted an analysis of patients with sinonasal cancer and reported an improvement in 5-year survival rates from 49.6% to 66.7% over 28 years, from 1973 to 2001.3 However, a meta-analysis of 25 patients with sinonasal cancer treated from 1975 to 1994 reported no change in survival for sinonasal adenocarcinoma over those 4 decades.11 Our results in a more recent cohort demonstrate that survival for sinonasal adenocarcinoma remains stable.
We demonstrated that there was no difference in DSS between patients with intestinal-type and those with nonintestinal-type histologies. This is the first study to analyze DSS between these two histologic subtypes. The 5-year DSS rates for the intestinal-type adenocarcinoma was reported to range from 40 to 60%.12 There are no reports in the literature specifically regarding survival for nonintestinal-type sinonasal adenocarcinoma.
Prior studies have shown that the incidence of sinonasal malignancies have decreased among blacks, but survival also has decreased.3 We observed that black race was independently associated with worse DSS. Similar results were shown in sinonasal squamous cell carcinoma, where blacks had worse 20-year survival than whites (19 vs. 31%).13 Differences in outcomes by race with head and neck cancer have been attributed partly to differences in socioeconomic status and access to health care.14
There is no standardized treatment algorithm for sinonasal adenocarcinoma, and patients are most often treated with a combination of surgery and/or RT. In our study, surgery was independently associated with improved survival over RT alone, but there was no difference in survival between patients who had surgery alone and those who had adjuvant RT. The improved survival associated with surgery may represent a selection bias, however, we controlled for demographics, clinical, and pathologic characteristics in our analysis. A review of 220 patients with sinonasal tumors by Dulguerov et al reported that the 5-year DSS rates for patients who had RT alone, surgery alone, and adjuvant RT were 46, 79, and 66%, respectively.11 We reported similar 5-year DSS rates of 46, 86, and 73%, respectively.
The SEER database is a well-validated national database, allowing us to overcome institutional and geographic biases and analyze a rare cancer.15 Limitations to our study include those inherent to the use of a large database, such as misclassification and coding errors. However, the SEER database has been shown to have > 97% completeness in terms of case ascertainment and reporting by participating hospitals.16 This database does not include information on patient comorbidities, the rationale for choosing a specific treatment modality, surgical margin status, chemotherapy, and disease recurrence. However, there are no established guidelines for the use of postoperative chemotherapy in sinonasal adenocarcinoma. The experience in the literature with postoperative chemotherapy for sinonasal adenocarcinoma is limited to case reports17 and small case series.18 Another limitation is the lack of centralized review by an experienced head and neck pathologist.
Sinonasal adenocarcinoma is the second most common sinonasal malignancy and carries a favorable prognosis when treated with surgery and/or postoperative RT. Our findings also demonstrate that age ≥ 75 years, black race, paranasal sinus location, and high grade are poor prognostic features that can be used to identify high-risk patients. There is no difference in survival between the intestinal and nonintestinal histologic subtypes. Future investigation of access to care as well as treatment intensification in this subset of patients with high-risk features is needed to improve outcomes.
Funding
This study was supported by the James G. Hirsch, MD Endowed Medical Student Research Fellowship at Yale University School of Medicine.
References
- 1.Ganly I, Patel S G, Singh B. et al. Craniofacial resection for malignant paranasal sinus tumors: report of an International Collaborative Study. Head Neck. 2005;27(7):575–584. doi: 10.1002/hed.20165. [DOI] [PubMed] [Google Scholar]
- 2.International Academy of Pathology, World Health Organization, International Agency for Research on Cancer . Lyon, France: IARC Press; 2005. Pathology and Genetics of Head and Neck Tumours. [Google Scholar]
- 3.Turner J H, Reh D D. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34(6):877–885. doi: 10.1002/hed.21830. [DOI] [PubMed] [Google Scholar]
- 4.Haerle S K, Gullane P J, Witterick I J, Zweifel C, Gentili F. Sinonasal carcinomas: epidemiology, pathology, and management. Neurosurg Clin N Am. 2013;24(1):39–49. doi: 10.1016/j.nec.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Luce D, Leclerc A, Bégin D. et al. Sinonasal cancer and occupational exposures: a pooled analysis of 12 case-control studies. Cancer Causes Control. 2002;13(2):147–157. doi: 10.1023/a:1014350004255. [DOI] [PubMed] [Google Scholar]
- 6.Bhayani M K Yilmaz T A Sweeney A et al. Sinonasal adenocarcinoma: a 16-year experience at a single institution Head Neck 2014. Available at: http://onlinelibrary.wiley.com/doi/10.1002/hed.23485/pdf. Accessed January 5, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Suarez C, Llorente J L, Fernandez De Leon R, Maseda E, Lopez A. Prognostic factors in sinonasal tumors involving the anterior skull base. Head Neck. 2004;26(2):136–144. doi: 10.1002/hed.10358. [DOI] [PubMed] [Google Scholar]
- 8.Heffner D K, Hyams V J, Hauck K W, Lingeman C. Low-grade adenocarcinoma of the nasal cavity and paranasal sinuses. Cancer. 1982;50(2):312–322. doi: 10.1002/1097-0142(19820715)50:2<312::aid-cncr2820500225>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Shah J P, Kraus D H, Bilsky M H, Gutin P H, Harrison L H, Strong E W. Craniofacial resection for malignant tumors involving the anterior skull base. Arch Otolaryngol Head Neck Surg. 1997;123(12):1312–1317. doi: 10.1001/archotol.1997.01900120062010. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program brochure Available at: http://seer.cancer.gov/about/factsheets/SEER_brochure.pdf. Accessed September 13, 2013
- 11.Dulguerov P, Jacobsen M S, Allal A S, Lehmann W, Calcaterra T. Nasal and paranasal sinus carcinoma: are we making progress? A series of 220 patients and a systematic review. Cancer. 2001;92(12):3012–3029. doi: 10.1002/1097-0142(20011215)92:12<3012::aid-cncr10131>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.Lund V J, Stammberger H, Nicolai P. et al. European Rhinologic Society Advisory Board on Endoscopic Techniques in the Management of Nose, Paranasal Sinus and Skull Base Tumours. European position paper on endoscopic management of tumours of the nose, paranasal sinuses and skull base. Rhinol Suppl. 2010;(22):1–143. [PubMed] [Google Scholar]
- 13.Sanghvi S, Khan M N, Patel N R, Yeldandi S, Baredes S, Eloy J A. Epidemiology of sinonasal squamous cell carcinoma: a comprehensive analysis of 4994 patients. Laryngoscope. 2014;124(1):76–83. doi: 10.1002/lary.24264. [DOI] [PubMed] [Google Scholar]
- 14.Ragin C C, Langevin S M, Marzouk M, Grandis J, Taioli E. Determinants of head and neck cancer survival by race. Head Neck. 2011;33(8):1092–1098. doi: 10.1002/hed.21584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harlan L C, Hankey B F. The surveillance, epidemiology, and end-results program database as a resource for conducting descriptive epidemiologic and clinical studies. J Clin Oncol. 2003;21(12):2232–2233. doi: 10.1200/JCO.2003.94.023. [DOI] [PubMed] [Google Scholar]
- 16.Zippin C, Lum D, Hankey B F. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995;76(11):2343–2350. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Trinkaus M E, Coleman A, D'Costa I, Sigston E, Rischin D. Recurrent intestinal type sinonasal adenocarcinoma treated with FOLFOX6 chemotherapy: case report and review of literature. Int J Case Rep Images. 2012;3(8):17–20. [Google Scholar]
- 18.Licitra L, Suardi S, Bossi P. et al. Prediction of TP53 status for primary cisplatin, fluorouracil, and leucovorin chemotherapy in ethmoid sinus intestinal-type adenocarcinoma. J Clin Oncol. 2004;22(24):4901–4906. doi: 10.1200/JCO.2004.05.071. [DOI] [PubMed] [Google Scholar]


