Abstract
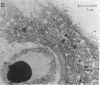
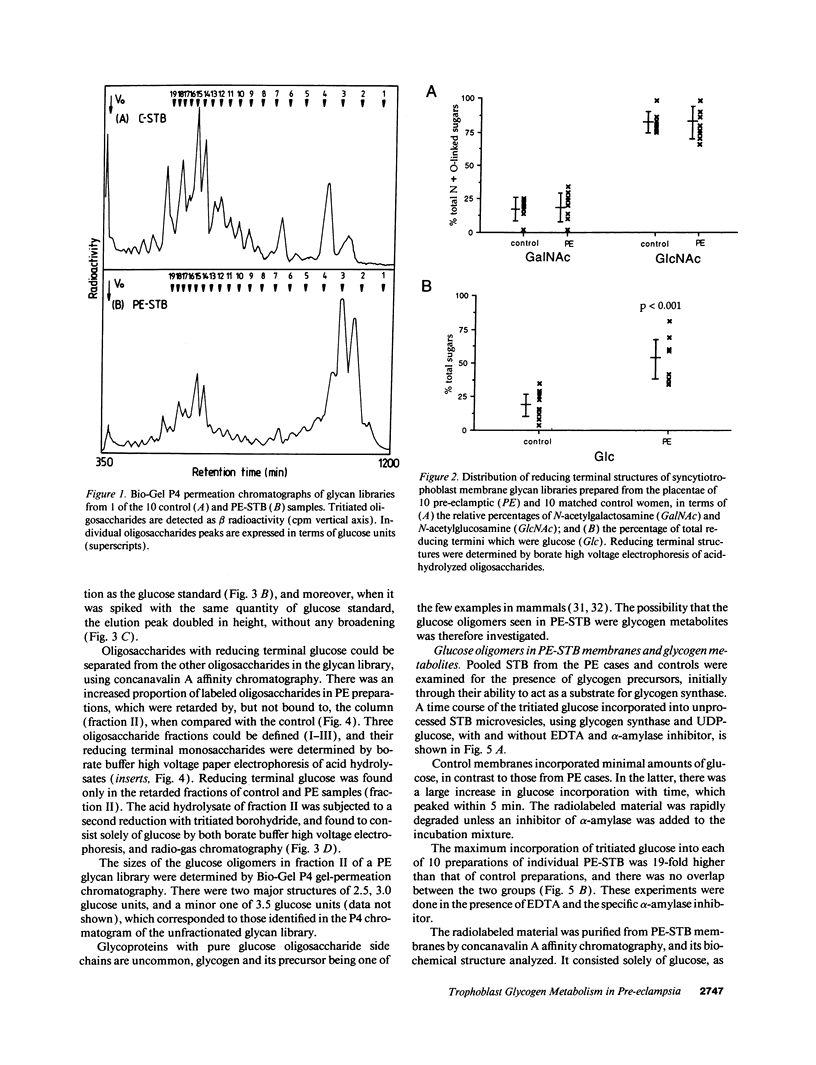
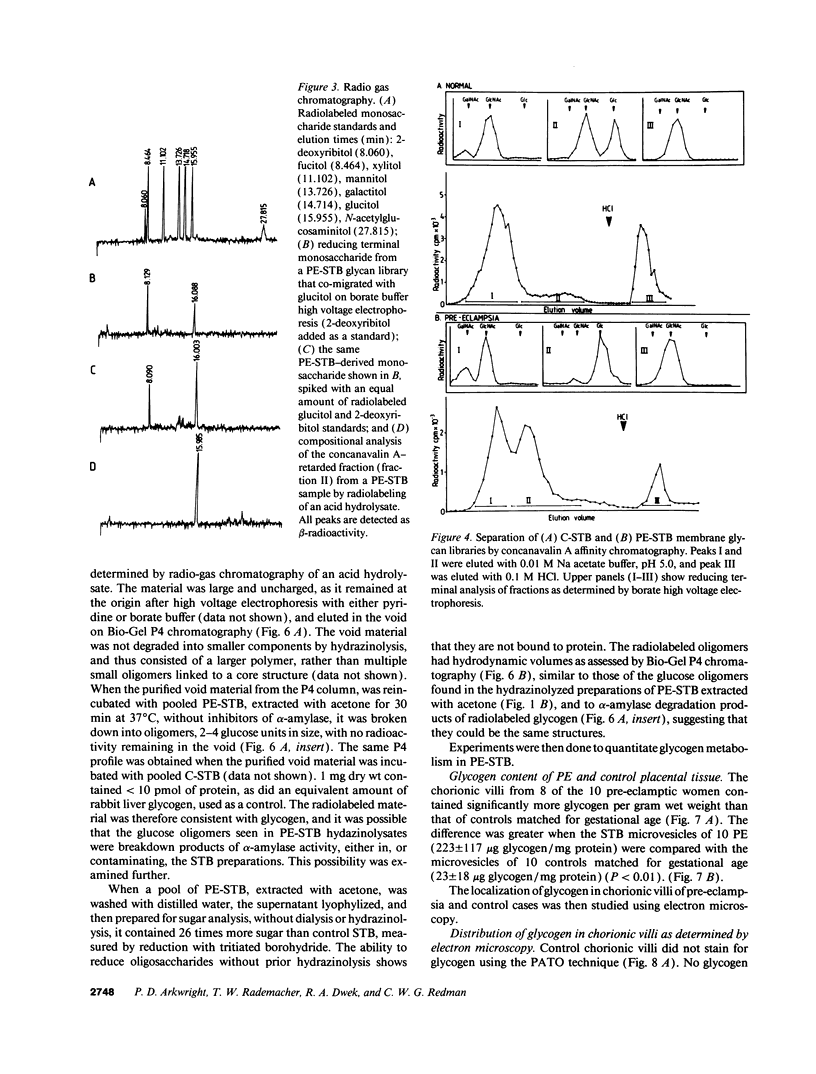
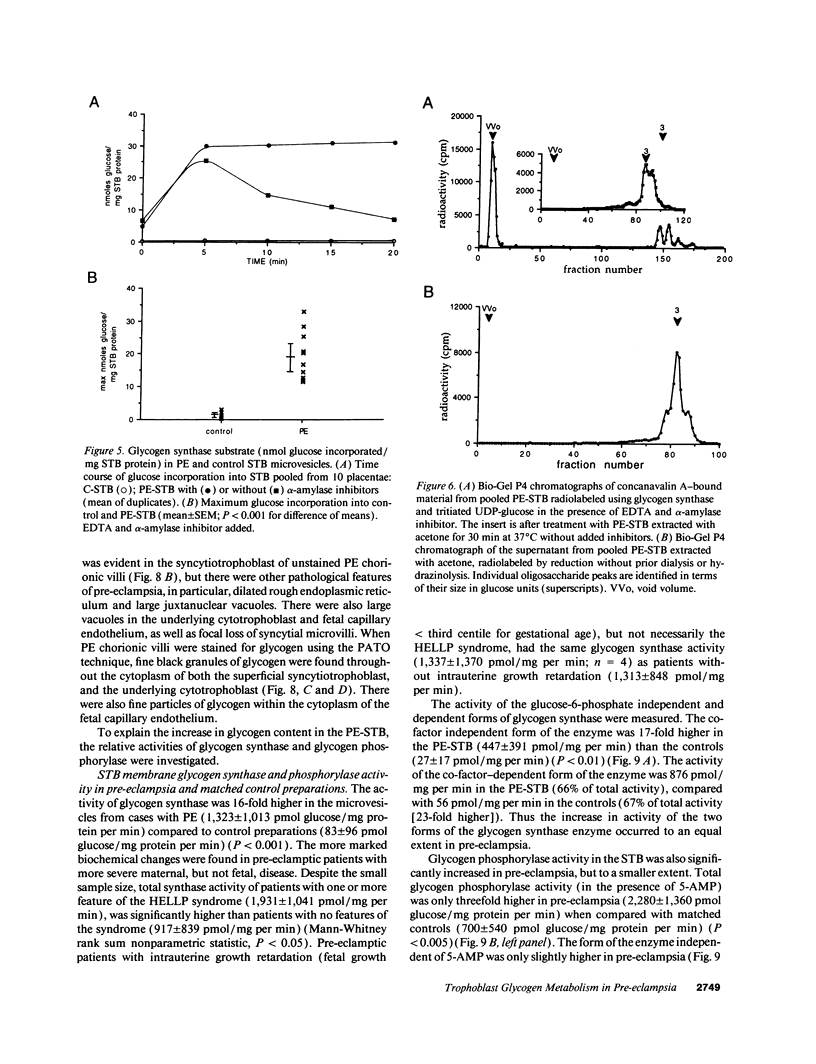
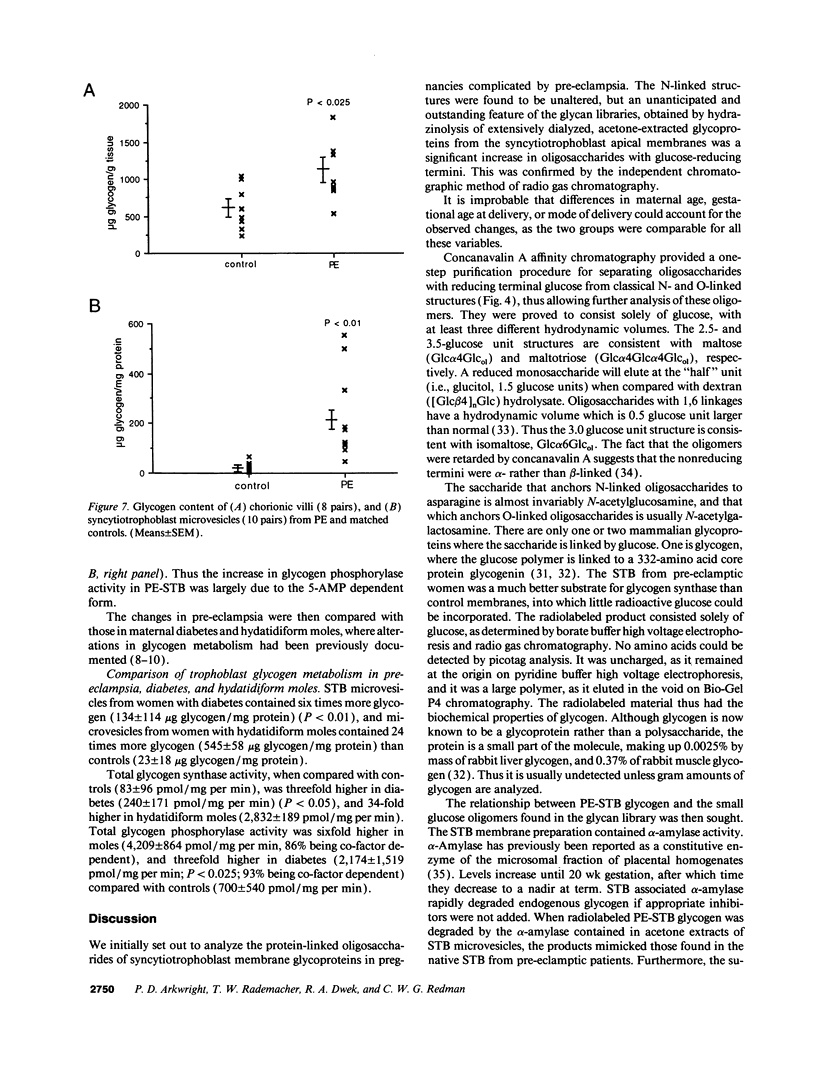
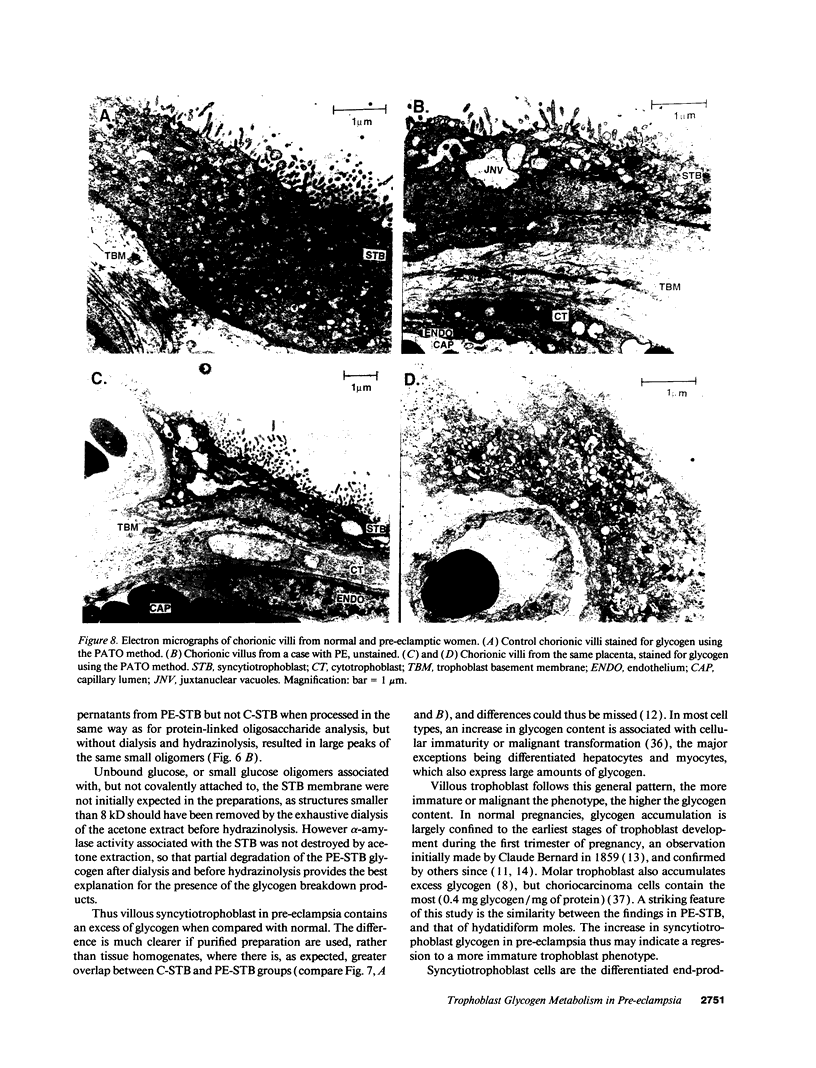
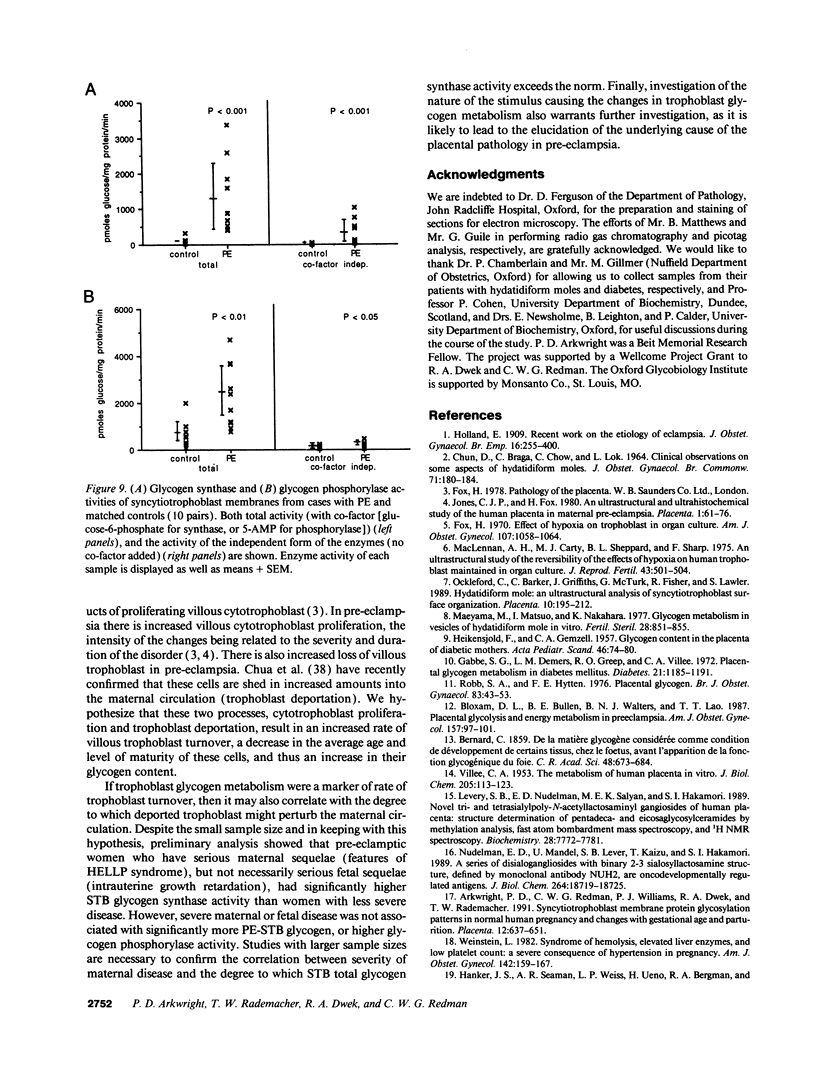
Pre-eclampsia is a placental disorder, but until now, biochemical details of dysfunction have been lacking. During an analysis of the oligosaccharide content of syncytiotrophoblast microvesicles purified from the placental chorionic villi of 10 primigravid women with proteinuric pre-eclampsia, we found an excess of glycogen breakdown products. Further investigation revealed a 10-fold increase in glycogen content (223 +/- 117 micrograms glycogen/mg protein), when compared with controls matched for gestational age at delivery (23 +/- 18 micrograms glycogen/mg protein) (P < 0.01). This was confirmed by examination of electron micrographs of chorionic villous tissue stained for glycogen. The increase in glycogen content was associated with 16 times more glycogen synthase (1,323 +/- 1,013 relative to 83 +/- 96 pmol glucose/mg protein per min) (P < 0.001), and a threefold increase in glycogen phosphorylase activity (2,280 +/- 1,360 relative to 700 +/- 540 pmol glucose/mg protein per min; P < 0.05). Similar changes in glycogen metabolism were found in trophoblast microvesicles derived from hydatidiform moles. Glycogen accumulation in villous syncytiotrophoblast may be a metabolic marker of immaturity of this cell which is unable to divide. The implications of these findings with regard to the pathogenesis of pre-eclampsia are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arkwright P. D., Redman C. W., Williams P. J., Dwek R. A., Rademacher T. W. Syncytiotrophoblast membrane protein glycosylation patterns in normal human pregnancy and changes with gestational age and parturition. Placenta. 1991 Nov-Dec;12(6):637–651. doi: 10.1016/0143-4004(91)90498-5. [DOI] [PubMed] [Google Scholar]
- Ashford D., Dwek R. A., Welply J. K., Amatayakul S., Homans S. W., Lis H., Taylor G. N., Sharon N., Rademacher T. W. The beta 1----2-D-xylose and alpha 1----3-L-fucose substituted N-linked oligosaccharides from Erythrina cristagalli lectin. Isolation, characterisation and comparison with other legume lectins. Eur J Biochem. 1987 Jul 15;166(2):311–320. doi: 10.1111/j.1432-1033.1987.tb13516.x. [DOI] [PubMed] [Google Scholar]
- Bloxam D. L., Bullen B. E., Walters B. N., Lao T. T. Placental glycolysis and energy metabolism in preeclampsia. Am J Obstet Gynecol. 1987 Jul;157(1):97–101. doi: 10.1016/s0002-9378(87)80354-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CHUN D., BRAGA C., CHOW C., LOK L. CLINICAL OBSERVATIONS ON SOME ASPECTS OF HYDATIDIFORM MOLES. J Obstet Gynaecol Br Commonw. 1964 Apr;71:180–184. doi: 10.1111/j.1471-0528.1964.tb04263.x. [DOI] [PubMed] [Google Scholar]
- Chua S., Wilkins T., Sargent I., Redman C. Trophoblast deportation in pre-eclamptic pregnancy. Br J Obstet Gynaecol. 1991 Oct;98(10):973–979. doi: 10.1111/j.1471-0528.1991.tb15334.x. [DOI] [PubMed] [Google Scholar]
- Ferguson D. J., Birch-Andersen A., Hutchison W. M., Siim J. C. Cytochemical electron microscopy on polysaccharide granules in the endogenous forms of Eimeria brunetti. Acta Pathol Microbiol Scand B. 1977 Aug;85(4):241–248. doi: 10.1111/j.1699-0463.1977.tb01969.x. [DOI] [PubMed] [Google Scholar]
- Fox H. Effect of hypoxia on trophoblast in organ culture. A morphologic and autoradiographic study. Am J Obstet Gynecol. 1970 Aug 1;107(7):1058–1064. doi: 10.1016/0002-9378(70)90629-0. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., MERRICK J. M. PROTEIN-CARBOHYDRATE INTERACTION. I. THE INTERACTION OF POLYSACCHARIDES WITH CONCANAVALIN A. Biochim Biophys Acta. 1965 Jan 4;97:68–76. doi: 10.1016/0304-4165(65)90270-9. [DOI] [PubMed] [Google Scholar]
- Gabbe S. G., Demers L. M., Greep R. O., Villee C. A. Placental glycogen metabolism in diabetes mellitus. Diabetes. 1972 Dec;21(12):1185–1191. doi: 10.2337/diab.21.12.1185. [DOI] [PubMed] [Google Scholar]
- HANKER J. S., SEAMAN A. R., WEISS L. P., UENO H., BERGMAN R. A., SELIGMAN A. M. OSMIOPHILIC REAGENTS: NEW CYTOCHEMICAL PRINCIPLE FOR LIGHT AND ELECTRON MICROSCOPY. Science. 1964 Nov 20;146(3647):1039–1043. doi: 10.1126/science.146.3647.1039. [DOI] [PubMed] [Google Scholar]
- HEIJKENSKJOLD F., GEMZELL C. A. Glycogen content in the placenta in diabetic mothers. Acta Paediatr. 1957 Jan;46(1):74–80. doi: 10.1111/j.1651-2227.1957.tb08633.x. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Chen C. H., Robinson J. C. Glycogen synthesis by choriocarcinoma cells in vitro. Active synthesis in the presence of the glucose 6-phosphate-dependent form of glycogen synthase. J Biol Chem. 1978 Apr 25;253(8):2596–2603. [PubMed] [Google Scholar]
- Jones C. J., Fox H. An ultrastructural and ultrahistochemical study of the human placenta in maternal pre-eclampsia. Placenta. 1980 Jan-Mar;1(1):61–76. doi: 10.1016/s0143-4004(80)80016-6. [DOI] [PubMed] [Google Scholar]
- Levery S. B., Nudelman E. D., Salyan M. E., Hakomori S. Novel tri-and tetrasialosylpoly-N-acetyllactosaminyl gangliosides of human placenta: structure determination of pentadeca- and eicosaglycosylceramides by methylation analysis, fast atom bombardment mass spectrometry, and 1H NMR spectroscopy. Biochemistry. 1989 Sep 19;28(19):7772–7781. doi: 10.1021/bi00445a037. [DOI] [PubMed] [Google Scholar]
- MacLennan A. H., Carty M. J., Sheppard B. L., Sharp F. An ultrastructural study of the reversibility of the effects of hypoxia on human trophoblast maintained in organ culture. J Reprod Fertil. 1975 Jun;43(3):501–504. doi: 10.1530/jrf.0.0430501. [DOI] [PubMed] [Google Scholar]
- Maeyama M., Matsuo I., Nakahara K. Glycogen metabolism in vesicles of hydatidiform mole in vitro. Fertil Steril. 1977 Aug;28(8):851–855. doi: 10.1016/s0015-0282(16)42740-8. [DOI] [PubMed] [Google Scholar]
- Nudelman E. D., Mandel U., Levery S. B., Kaizu T., Hakomori S. A series of disialogangliosides with binary 2----3 sialosyllactosamine structure, defined by monoclonal antibody NUH2, are oncodevelopmentally regulated antigens. J Biol Chem. 1989 Nov 5;264(31):18719–18725. [PubMed] [Google Scholar]
- O'Connor C. M., McGeeney K. F. Interaction of human alpha-amylases with inhibitors from wheat flour. Biochim Biophys Acta. 1981 Apr 14;658(2):397–405. doi: 10.1016/0005-2744(81)90310-7. [DOI] [PubMed] [Google Scholar]
- O'Donnell M. D., McGeeney K. F. Purification and properties of an alpha-amylase inhibitor from wheat. Biochim Biophys Acta. 1976 Jan 23;422(1):159–169. doi: 10.1016/0005-2744(76)90016-4. [DOI] [PubMed] [Google Scholar]
- Ockleford C., Barker C., Griffiths J., McTurk G., Fisher R., Lawler S. Hydatidiform mole: an ultrastructural analysis of syncytiotrophoblast surface organization. Placenta. 1989 Mar-Apr;10(2):195–212. doi: 10.1016/0143-4004(89)90040-4. [DOI] [PubMed] [Google Scholar]
- Robb S. A., Hytten F. E. Placental glycogen. Br J Obstet Gynaecol. 1976 Jan;83(1):43–53. doi: 10.1111/j.1471-0528.1976.tb00729.x. [DOI] [PubMed] [Google Scholar]
- Rousset M., Zweibaum A., Fogh J. Presence of glycogen and growth-related variations in 58 cultured human tumor cell lines of various tissue origins. Cancer Res. 1981 Mar;41(3):1165–1170. [PubMed] [Google Scholar]
- Seligman A. M., Hanker J. S., Wasserkrug H., Dmochowski H., Katzoff L. Histochemical demonstration of some oxidized macromolecules with thiocarbohydrazide (tch) or thiosemicarbazide (TSC) and osmium tetroxide. J Histochem Cytochem. 1965 Nov-Dec;13(8):629–639. doi: 10.1177/13.8.629. [DOI] [PubMed] [Google Scholar]
- Smith C. H., Nelson D. M., King B. F., Donohue T. M., Ruzycki S., Kelley L. K. Characterization of a microvillous membrane preparation from human placental syncytiotrophoblast: a morphologic, biochemical, and physiologic, study. Am J Obstet Gynecol. 1977 May 15;128(2):190–196. doi: 10.1016/0002-9378(77)90686-x. [DOI] [PubMed] [Google Scholar]
- Smith N. C., Brush M. G., Luckett S. Preparation of human placental villous surface membrane. Nature. 1974 Nov 22;252(5481):302–303. doi: 10.1038/252302b0. [DOI] [PubMed] [Google Scholar]
- Smythe C., Cohen P. The discovery of glycogenin and the priming mechanism for glycogen biogenesis. Eur J Biochem. 1991 Sep 15;200(3):625–631. doi: 10.1111/j.1432-1033.1991.tb16225.x. [DOI] [PubMed] [Google Scholar]
- Tan A. W., Nuttall F. Q. Characteristics of the dephosphorylated form of phosphorylase purified from rat liver and measurement of its activity in crude liver preparations. Biochim Biophys Acta. 1975 Nov 20;410(1):45–60. doi: 10.1016/0005-2744(75)90206-5. [DOI] [PubMed] [Google Scholar]
- Thakur A. N., Sheth A. R., Thanavala Y. M., Rao S. S., Purandare M. Alpha-amylase activity of human placenta. Indian J Biochem Biophys. 1975 Mar;12(1):68–70. [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- VILLEE C. A. The metabolism of human placenta in vitro. J Biol Chem. 1953 Nov;205(1):113–123. [PubMed] [Google Scholar]
- Van Handel E. Estimation of glycogen in small amounts of tissue. Anal Biochem. 1965 May;11(2):256–265. doi: 10.1016/0003-2697(65)90013-8. [DOI] [PubMed] [Google Scholar]
- Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: a severe consequence of hypertension in pregnancy. Am J Obstet Gynecol. 1982 Jan 15;142(2):159–167. doi: 10.1016/s0002-9378(16)32330-4. [DOI] [PubMed] [Google Scholar]
- Whelan W. J. The initiation of glycogen synthesis. Bioessays. 1986 Sep;5(3):136–140. doi: 10.1002/bies.950050312. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Mizuochi T., Kobata A. Analysis of oligosaccharides by gel filtration. Methods Enzymol. 1982;83:105–126. doi: 10.1016/0076-6879(82)83008-5. [DOI] [PubMed] [Google Scholar]