Abstract
X-linked acro-gigantism (X-LAG) is a new syndrome of pituitary gigantism, caused by microduplications on chromosome Xq26.3, encompassing the gene GPR101, which is highly upregulated in pituitary tumors. We conducted this study to explore the clinical, radiological and hormonal phenotype and responses to therapy in patients with X-LAG syndrome. The study included 18 patients (13 sporadic) with X-LAG and a microduplication in chromosome Xq26.3. All sporadic cases had unique duplications and the inheritance pattern in 2 families was dominant with all Xq26.3 duplication carriers being affected. Patients began to grow rapidly as early as 2–3 months of age (median 12 months). At diagnosis (median delay 27 months), patients had a median height and weight SDS score of >+3.9 SDS. Apart from the increased overall body size, the children had acromegalic symptoms including acral enlargement and facial coarsening. More than a third of cases had increased appetite. Patients had marked hypersecretion of GH/IGF-1 and prolactin, usually due to a pituitary macroadenoma or hyperplasia. Primary neurosurgical control was achieved with extensive anterior pituitary resection but postoperative hypopituitarism was frequent. Control with somatostatin analogs was not readily achieved despite moderate to high somatostatin receptor subtype-2 expression in tumor tissue. Postoperative adjuvant pegvisomant achieved control of IGF-1 all 5 cases in which it was employed. X-LAG is a new infant-onset gigantism syndrome that has a severe clinical phenotype leading to challenging disease management.
Keywords: gigantism, X chromosome, pituitary adenoma, pediatric, X-LAG syndrome, GPR101, FIPA
Introduction
Pituitary gigantism is a rare condition associated with hypersecretion of growth hormone (GH) by a pituitary tumor or hyperplasia prior to the complete fusion of growth plates, leading to pathological tall stature (Davies and Cheetham 2014; Eugster and Pescovitz 1999). Chronic GH hypersecretion of growth hormone leads to the development of signs and symptoms of acromegaly, which continue to develop after the attainment of final height. Although it is a well-recognized condition among the medical and general populations (de Herder 2015), the genetic etiology of pituitary gigantism remained poorly understood until recently. Pituitary gigantism can occur as a feature of a number of monogenic disorders. Approximately one third of acromegaly patients with germline mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene have gigantism (Beckers, et al. 2013), and additional etiologies include mosaicism for GNAS1 mutations in McCune Albright syndrome (MAS), PRKAR1A mutations in Carney complex or rare MEN1 mutations (Salenave, et al. 2014; Salpea and Stratakis 2014; Stratakis, et al. 2000).
We recently described a new disorder of X-linked acrogigantism (X-LAG) that is due to microduplications in chromosome Xq26.3 that involve the gene for the orphan G-protein coupled receptor (GPCR), GPR101, which is highly upregulated in pituitary tumors from affected patients (Trivellin, et al. 2014). X-LAG is a distinctive clinical entity that is characterized by excessive growth usually beginning during the first year of life in previously normal infants. The overgrowth is caused by GH hypersecretion from a pituitary macroadenoma or hyperplasia. X-LAG can occur as a sporadic condition or can present as familial isolated pituitary adenomas (FIPA) in acrogigantism kindreds. This newly described condition is rare and the phenotype incompletely characterized, particularly in terms of clinical responses to treatment. The aim of the current study was to better clinically characterize X-LAG in an expanded cohort of 18 affected patients.
Methods
The study population consisted of 18 patients with X-LAG syndrome, of whom 5 were familial and 13 were sporadic (basic diagnostic/genetic data presented on 13 cases in (Trivellin et al. 2014)). Patients underwent genetic diagnosis (peripheral blood DNA) using commercial array comparative genome hybridization (aCGH) and research-based high-definition array CGH and all tested patients had a microduplication of chromosome Xq26.3. Breakpoint analyses were performed using long-range PCR techniques, and fluorescent in situ hybridization (FISH) studies using Xq26.3 microduplication specific probes was also performed on peripheral blood leukocytes in a subset of patients as previously described (Trivellin et al. 2014). The study collected a comprehensive set of data on the demographic, clinical, radiological, hormonal, pathological and therapeutic outcomes in the patient population under the headings below (individual criteria are listed in Supplemental materials): demographics and background parameters; birth and family characteristics; growth disorder characteristics; symptoms and hormone disturbances; pituitary imaging characteristics; treatment; outcomes.
Pathology
Immunohistochemistry (IHC)
Staining of formalin fixed paraffin embedded (FFPE) tissue samples of pituitary tumors for pituitary hormones (GH, PRL, ACTH, FSH, LH, TSH), and growth hormone releasing hormone receptor (GHRH-R) were performed as previously described (Trivellin et al. 2014) (Magri, et al. 2010). Immunohistochemical stains for somatostatin receptors (SSTRs) were performed using an automated immunostainer (Ventana Medical Systems, Tucson, AZ, USA) as previously reported (Lee, et al. 2013). The SuperSentitive IHC detection system from BioGenex (Fremon, CA, USA) was used to visualize the antibody binding following the manufacturer’s instructions. Sections were counterstained with Mayer’s Haemalum, dehydrated and coverslipped. The primary antibodies directed against SSTR2 (clone UMB-1 reacting with the SSTR2a isoform, dilution: 1/500), SSTR3 (clone UMB-5, dilution: 1/750), SSTR5 (clone UMB-4, dilution: 1/75) were purchased from Abcam (Cambridge, MA, USA). Sections of normal pancreas were used as positive control and included in each run. Sections incubated without the primary antibody were included in each batch as a negative control. Immunostains were evaluated semi-quantitatively by two individuals on acquired images. An immunoreactive score (IRS) was recorded for each section as reported previously (Lee, et al. 2014). Briefly, the IRS was generated noting the intensity of the staining (no staining, 0; mild, 1; moderate, 2; strong, 3) and the percentage of cells showing membranous or cytoplasmic expression (no positive cells, 0; <10% of positive cells, 1; 10%–50% of positive cells, 2; 51%–80% of positive cells, 3; >80% of positive cells, 4). The overall IRS was calculated as [percentage of positive cells] × [intensity of staining]. We considered the staining as being negative for IRS 0 and 1, weakly positive for IRS 2 and 3, moderately positive for IRS 4–8, and strongly positive for IRS >8. Immunostains for anterior pituitary hormones, Ki67 (MIB1), and AIP, were performed as described previously (Jaffrain-Rea, et al. 2009; Villa, et al. 2011). usly (12,13). Briefly AIP/ARA9 antobody (Novus Biologicals) was used at a dilution of 1:2000 and processed on an automated protocol for Benchmark Ventana.
Statistics
Data were collated and expressed as medians and ranges. As the data were not normally distributed, comparisons of data between subgroups by sex were performed using the Mann-Whitney test.
Literature review
We undertook a comprehensive study of literature on gigantism from the extensive historical collection of one of the authors (WWdH) that includes images, medical information, family accounts, media reports and other items. These items formed the basis of a secondary search of the medical literature in terms of original scientific publications, books and manuscripts dealing with gigantism and acromegaly among children. Cases in which strong pictorial, medical and narrative evidence of onset of overgrowth during childhood (before age of 8) or where gigantism was already well established by height measurements before the age of 10 years were identified and data were extracted.
The study on gigantism and the genetic studies of patients with pituitary tumors was approved by the Ethics Committee of the Centre Hospitalier Universitaire de Liège, Belgium. The studies at the National Institutes of Health (NIH) Clinical Research Center were approved by the NICHD IRB under various protocols. Patients or parents/guardians provided informed consent; children over the age of 8 years at the NIH provided assent. The study was conducted according to the Declaration of Helsinki on the Ethical Principles for Medical Research Involving Human Subjects.
Results
Demographics and genetic diagnosis
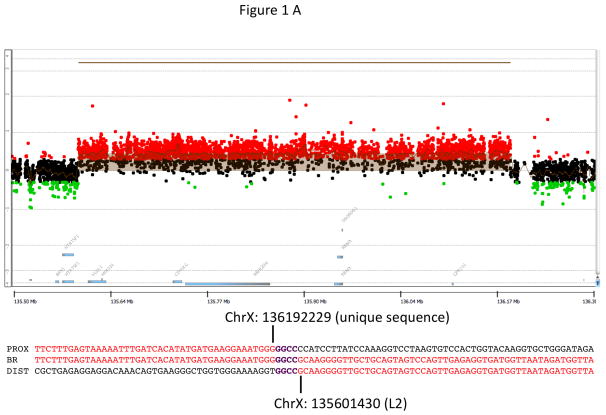
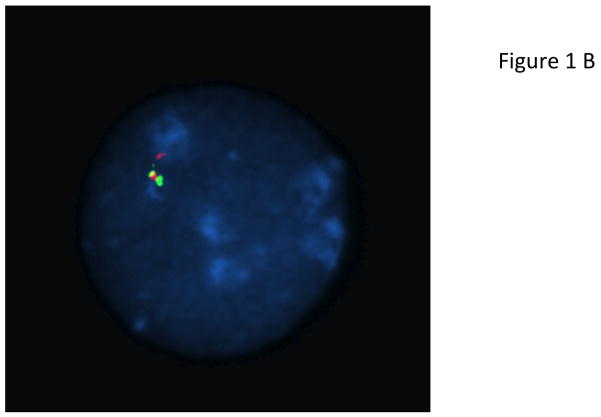
Most patients with X-LAG syndrome were sporadic (13/18; 72.2%). Patients usually presented in a sporadic setting (n=13; 72.2%), while two FIPA families had 3 and 2 affected members, respectively. The ethnic backgrounds were diverse: Caucasian Europeans, South East Asian, Indian subcontinent, First Nations Canadian, and Latino-Afro-Caribbean backgrounds were all seen. All patients came from non-consanguineous marriages. Apart from the familial cases there were no histories of growth disorders in any relatives of the affected patients. Duplications of an approximately 500 kb region on chromosome Xq26.3 (involving CD40L, ARHGEF6, RBMX, and GPR101) were seen in both new and previously reported cases (Figure 1) (Trivellin et al. 2014). Studied cases also shared the previously noted smallest regions of overlap on a high definition CGH array. Breakpoint analyses showed that all non-familial cases were unique rearrangements, which is likely to be mediated by a FoSTeS/MMBIR mechanism (Lee, et al. 2007; Zhang, et al. 2009). Duplications were visualized by FISH in all cases studied (Figure 1).
Figure 1.
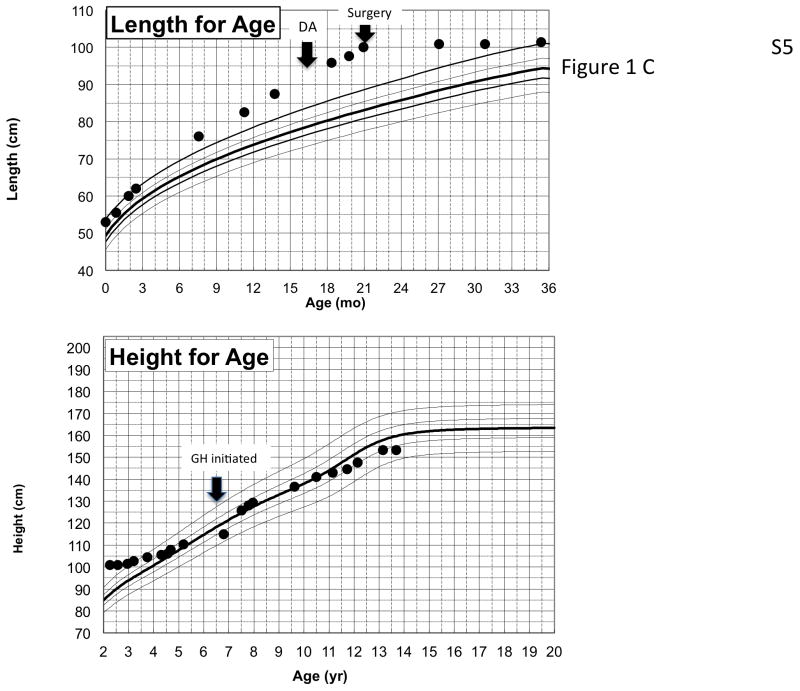
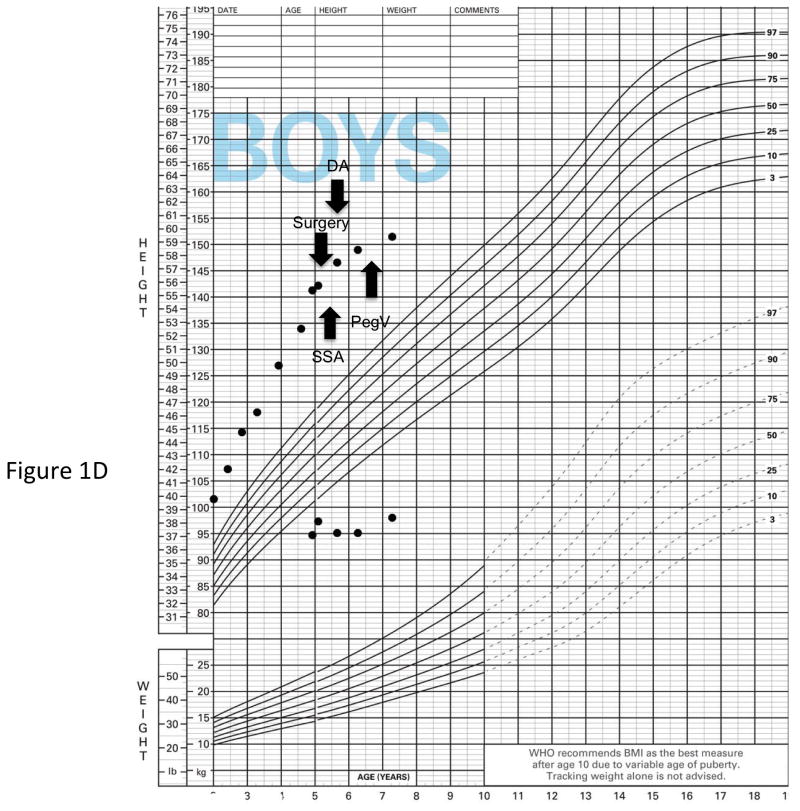
Genetic and growth features in patients with X-LAG syndrome. Panel A Representative chromosome Xq26.3 microduplication in female sporadic patient S10 on HD-CGH array showing the duplicated region (red) and details of the breakpoint regions of microhomology leading to the duplication (below in purple GGCC). FISH results for sporadic patient S1 (male) showing a duplication of the proximal and distal probes (red/green) in a representative leukocyte. Growth patterns during early life in sporadic patients S5 (female) and S1 (male). Panel C shows the growth in patient S5 that exceeded the 97 centile for length as early as 6 months of age; she was treated with cabergoline DA and then surgery, after which time the total resection of tumor was associated with flattening of her growth curve. She eventually was diagnosed with panhypopituitarism and diabetes insipidus and required GH supplementation as indicated. Panel D shows the early growth of patient S1, a sporadic male case that began between the ages of 9 and 12 months and continued despite subtotal surgical resection, SSA and DA; the introduction of pegvisomant (PegV) at the age of 6 led to a decrease in gain in height.
Growth and disease characteristics (Figure 1; Table 1)
Table 1.
Summary of characteristics of 18 patients with X-LAG syndrome.
| Study ID |
Sex | Age at start of abnormal growth/ age at diagnosis (months) |
GH | OGTT: GH Suppression |
IGF-1 (xULN) |
Prolactin | Max tumor/ hyperplasia diameter (mm) |
Treatment order | Pathology | Excessive GH/IGF-1 controlled |
Excessive growth controlled (age at control) |
Age at last follow up (years) |
Hypopituitarism (axes) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | M | 6/56 | ↑↑↑ | No | 4.4 | ↑↑↑ | 27 | Sx, SSA, DA, PV | PA; GH+/PRL+; Ki-67: 3% | Yes | Y (7 yr) | 7 | ACTH, TSH, DI |
| S2 | F | 12/40 | ↑ | ND | 4.9 | ↑ | 15 | Sx, DA, SSA, PV, Sx, SSA | PA; GH+/PRL+; Ki-67:<1% | Yes | Y (11 yr) | 18 | TSH, DI |
| S3 | F | 18/42 | ↑ | No | 2.4 | ↑↑ | 17 | Sx, DA, SSA, RTx (γ-knife) | PA; GH+/PRL+ | No | N | 7.5 | No |
| S4 | F | 2/23 | ↑↑ | ND | 5.2 | ↑↑ | global enlargement | DA, SSA | Pending surgery | No | N | 3.5 | No |
| S5 | F | 7/17 | ↑↑↑ | ND | 3.9 | ↑↑ | 19 | DA, Sx | PA; GH+/PRL+; Ki-67:<1% | Yes | Y (19 mo) | 13 | Panhypopituitarism plus DI |
| S6 | F | 6/36 | ↑↑↑ | Paradoxical increase | 3.1 | ↑↑ | 39 | DA, 2Sx, SSA, PV | PA; GH+/PRL+; Ki-67<1% | Yes | Y (5.5 yr) | 6 | DI |
| S7 | F | 36/63 | ↑ | N/A | N/A | 10 | Sx, SSA, DA, RTx (Conv.), Sx, SSA | PA; GH+/FSH+; Ki-67<1% | Yes | Y (8 yr) | 30 | TSH | |
| S8 | F | 11/33 | ↑↑↑ | Paradoxical increase | 3.3 | ↑ | 18 | DA, Sx, RTx, SSA | Eosinophilic adenoma | No | N | 8 | No |
| S9 | F | 3/33 | ↑↑ | No | 3.4 | ↑↑ | 18; general enlargement | DA, Sx | Global hyperplasia; Ki-67<1% | Yes | Y (21 mo) | 11 | Pahypopituitarism plus DI |
| S10 | F | 6/43 | ↑↑ | No | 3.3 | ↑↑↑ | 24 | DA, Sx, SSA, RTx (Conv.), PV | PA; GH+/PRL+ | Yes | Y (4.9 yr) | 11 | No |
| S11 | M | 48/67 | ↑↑↑ | No | 3.6 | ↑↑ | 32.5 | Sx | PA; GH+/PRL+ | No | N | 10 | No |
| S12 | F | 10/48 | ↑ | No | N/A | ↑ | 25 | Sx, SSA, DA, RTx (Conv.), PV | PA; GH+/PRL+ | Yes | Y (adult) | 35 | ACTH, TSH, LH |
| S13 | F | 48/91 | ↑ | Paradoxical increase | 2.6 | ↑ | 17; general enlargement | SSA, DA, Sx | Global hyperplasia (GH+/PRL+; TSH+); Ki-67<1%) | Yes* | N | 8 | N/A* |
| F1A | F | 12/31 | ↑↑ | No | N/A | ↑↑ | >10 | Sx | Eosinophilic adenoma | Yes | Y (5 yr) | 45 | ACTH, TSH, GH |
| F1B | M | 12/18 | ↑↑ | No | 3.3 | ↑↑↑ | 15 | SSA, DA, 3Sx | PA plus hyperplasia; GH+/PRL+ | Yes | Y (4yr) | 18 | ACTH, TSH, GH |
| F1C | M | 14/14 | ↑ | Paradoxical increase | 1.9 | ↑ | symmetric enlargement | DA, Sx | Global hyperplasia plus microadenoma (GH+/PRL+) | Yes | Y (4 yr) | 13 | ACTH, TSH |
| F2A | M | 48/96 | ↑ | No | N/A | ↑ | 19 | Sx | PA; GH+/PRL+ | Yes | Y (12 yr) | 23 | DI |
| F2B | F | Child/264 | ↑ | No | N/A | ↑ | >10 | Sx | PA; GH+/PRL+ | Yes | Y (adult) | 26┼ | ACTH, TSH, LH |
OGTT: Oral glucose tolerance test,; ULN: upper limit of the normal range; SSA: somatostatin analog; DA: dopamine agonist; PV: pegvisomant; RTx: radiotherapy; Conv.: conventional; PA: pituitary adenoma; DI: diabetes insipidus. For hormonal criteria: ↑: 2–10 × ULN; ↑↑: >10–50 × ULN; ↑↑↑: >50× ULN.
Patient died of sepsis aggravated by hypopituitarism;
insufficient follow up postoperatively (<1 month) to determine permanent pituitary hypofunction.
Patients were generally born at full-term (one sporadic case born at 35 weeks) from uncomplicated pregnancies. In all but two of the cases, the birth weight, length and head circumference was normal. One female patient, the mother of two familial cases, had a birth length in excess of the 97th centile, although the birth weight was normal, while another sporadic case was small for gestational age. In the X-LAG cohort overall the median age at the onset of rapid growth was 12 months (range: 2–48 months) and although females had a younger age at onset of rapid growth than males, this was not statistically significant (10.5 vs 14.0 months, respectively; p=NS). The median age at diagnosis was 41 months (range: 14–264 months), and again females were younger than males at diagnosis (40 vs. 56 months, respectively; p=NS). At diagnosis the median height SDS score was +3.9 (range: +1.9 to +7.6). The median weight SDS score was similarly increased at diagnosis: (+4.4; range: +3.0 to +9.3).
The presenting symptoms at diagnosis are shown as Table 2. Apart from abnormally increased growth in all patients, the most frequent complaints at presentation were typically acromegalic-type signs, such as, acral enlargement (38.9%) and coarsened facial features (33.3%). Some of the physical features and characteristics are illustrated in Figure 2. Interestingly, 7/18 (38.9%) patients had a preceding history of increased appetite or food seeking behavior, a feature that is not typical of other forms of gigantism. Four patients (22.2%) had acanthosis nigricans at diagnosis. At diagnosis fasting insulin levels were available in 12 patients, of whom 6 demonstrated fasting hyperinsulinemia (including all with acanthosis nigricans). No patient had diabetes mellitus at diagnosis or during follow-up. Neurological symptoms such as headache, visual field defects, ataxia and seizure occurred in one patient each.
Table 2.
Signs and symptoms reported in 18 patients with X-LAG syndrome at presentation
| Sign/symptom | Number of patients (%) |
|---|---|
| Abnormally increased growth | 18 (100)% |
| Acral enlargement | 7 (38.9%) |
| Increased appetite | 7 (38.9%) |
| Coarsened facial features | 6 (33.3%) |
| Acanthosis nigricans | 4 (22.2%) |
| Sleep apnea/ snoring | 3 (16.7%) |
| Excessive perspiration/body odor | 3 (16.7%) |
| Widening of interdental spaces | 3 (16.7%) |
| Abdominal distension/weight gain | 2 (11.1%) |
| Other (pubic hair, prognathism, ataxia, visual field defect, seizure, thickened skin, headache) | 1 each (5.6%) |
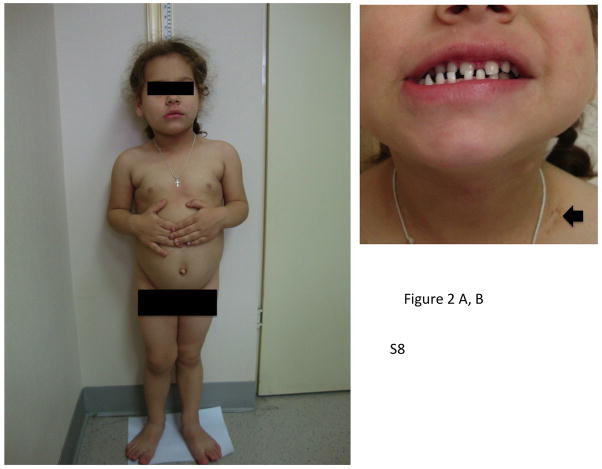
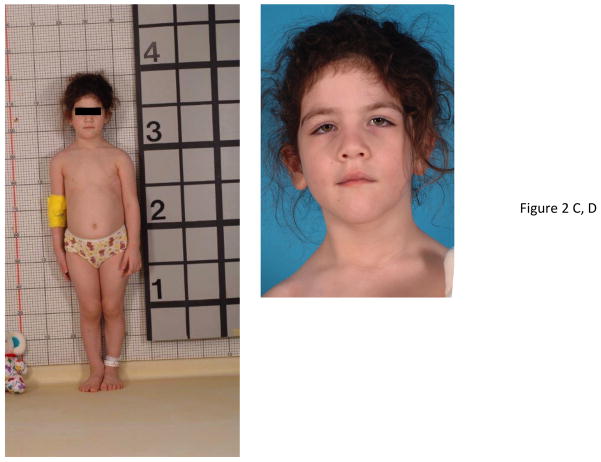
Figure 2.
Physical changes in representative cases of X-LAG syndrome with Xq26.3 microduplications. Patient S8 is shown at the age of 32 months at which time her height was >4 SDS (she had been growing at an abnormal rate since the age of 11 months). Of note are her large hands and feet and facial features that are moderately coarsened but well proportioned. Panel B shows widely spaced teeth and a patch of acanthosis nigricans on the left side of her neck. In Panel C, patient S9 who began to grow abnormally at the age of 3 months is pictured shortly after diagnosis (aged 33 months), when her height was >5 SDS and hands and feet are enlarged. Panel D shows her facial features, which include a large nose and prominent mandible and the impression of hypertelorism.
Radiological characteristics
MRI findings at diagnosis in a selection of the patient population are shown in Figure 3. There was some variability in the tumor sizes and appearances, from large homogeneous bean or peanut-shaped tumors, to those with diffuse pituitary enlargement suggestive of hyperplasia. All patients had pituitary abnormalities at diagnosis, of which most appeared to be a macroadenoma on MRI (14/18), while the remaining patients had pituitary enlargement without an identifiable adenoma. The median tumor diameter was 18 mm (range: 10 to 39 mm). Tumor extension to the optic chiasm was frequent, occurring in 12 patients, whereas invasion of the cavernous sinus was present in only 2 patients at diagnosis. There were no statistically significant differences between males and females.
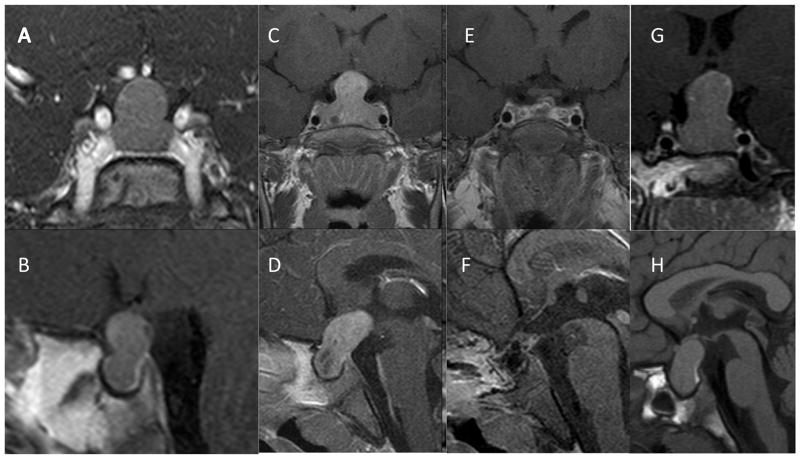
Figure 3.
T1 weighted gadolinium enhanced MRI images of female patient S9 at diagnosis (age 2 yr 11 mo) revealed a large, hypoattenuated hourglass (“peanut”) shaped mass within the sella with expansion of the diaphragma sella (A, B). Panels B and C show coronal and saggital T1 weighted post contrast images at diagnosis in female patient S6 (age 3 yr) showing a large pituitary mass with marked upward and posterior extension and areas of degenerative changes. Post operative images (E, F) from the same patient reveal that the adenoma has been visibly resected (hormonal and growth control, however, required SSA and pegvisomant). Panels G and H show coronal and saggital T1-weighted MRI images of female patient S10 at diagnosis (aged 3 yr) showing a large homogeneous pituitary mass extending superiorly and posteriorly (“bean shaped”); the posterior pituitary is clearly seen as a hyperattenuated posterior bright spot in Panel H.
Hormonal measures
GH levels were universally elevated in all patients at diagnosis. Of note, the random GH levels at diagnosis were usually markedly increased with a median level that was 52.5 times the ULN (range: 6 to 300-fold ULN). OGTT data were available in 14 cases; none showed GH suppression and 4 showed a paradoxical rise in GH during the test. IGF-1 levels were also elevated at diagnosis in the 13 cases with available data; the median fold increase above the ULN was 3.4 (range: 3.1 to 5.2-fold ULN). Prolactin levels at diagnosis were available in 17 patients and were also elevated (median 18-fold over the ULN (range: 2.0 to 90.8 fold)). No patient exhibited galactorrhea. Five patients had GHRH levels measured at diagnosis and in all cases these were in the middle of or just above the ULN. At diagnosis none of the patients exhibited hypopituitarism.
Therapeutic responses and outcomes
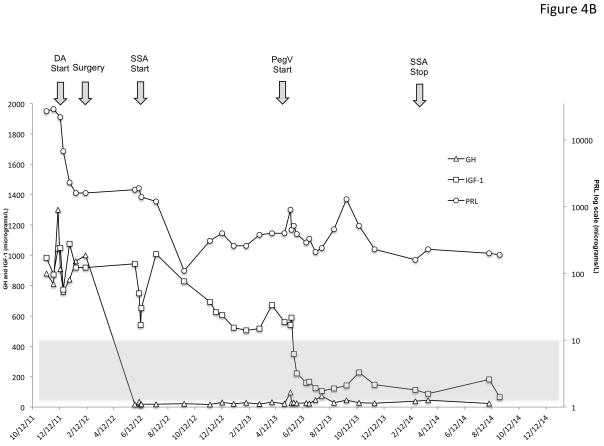
Management of the X-LAG patients was multimodal in the majority of cases and in many cases this was complex (Table 1), as illustrated in two cases in Figure 4. All but one of the 18 patients underwent neurosurgery, the remaining case is pending surgery.
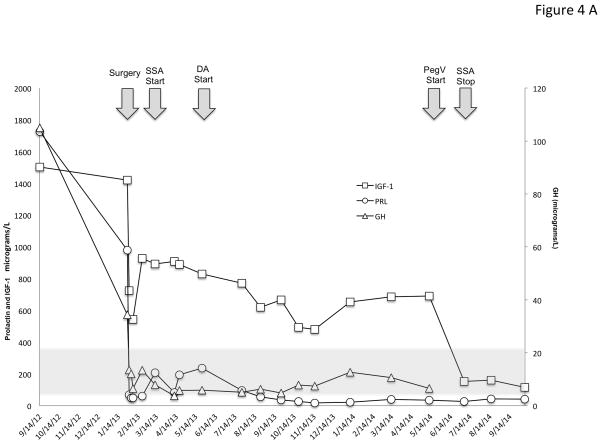
Figure 4.
Time course of the effects of treatment modalities on GH, IGF-1 and PRL in two representative patients with X-LAG syndrome. Panel A shows the evolution of sporadic patient S1, a male who was diagnosed at the age of 56 months and underwent primary neurosurgical treatment to grossly resect the visible tumor. This surgical intervention lead to marked decreases in GH and PRL, but IGF-1 remained very high (normal IGF-1 range shown as a grey shaded zone). Addition of a SSA (octreotide LAR 30 mg/month) and later a DA (cabergoline 0.25 mg 5× week) reduced the IGF-1 by about 50% from the postoperative level but as the IGF-1 remained elevated and overgrowth continued, pegvisomant (10mg/day) was eventually added and lead to a rapid decrease in IGF-1, which allowed the SSA to be withdrawn. In Panel B, a female sporadic case S6 first received a dopamine agonist and later underwent a neurosurgical intervention, which removed the majority of the visible tumor. The post-operative GH levels were decreased greatly but remained elevated to an extent that IGF-1 secretion was still greatly increased (normal range for IGF-1 shown as a grey shaded zone)and growth continued. Addition of a depot somatostatin analog (octreotide LAR 30mg/month) did not control IGF-1 alone or in combination with cabergoline, although IGF-1 levels showed approximately a 40% decrease from levels before SSA were started. Due to the lack of control and continuing somatic overgrowth, pegvisomant was added and rapidly brought IGF-1 levels and growth under control. As IGF-1 levels fell to near the lower limit of normal for the age of the patient (5 yr), the SSA was withdrawn and subsequent IGF-1 was in the middle of the normal range on pegvisomant 10mg/day.
Nine patients underwent medical therapy as the initial modality (DA: n=7; SSA: n=2) and none achieved control of either GH or PRL secretion despite the use of doses normally used in adult patients (SSA (duration 6–12 months): octreotide 40 mg/month; lanreotide autogel 90mg/month; DA (duration 3–6 months): 0.25 mg 3–5 times weekly). In these patients surgery was then employed (1–3 interventions) in all cases (apart from one patient awaiting surgery) and growth control was achieved in 3 cases but at the cost of hypopituitarism. Further use of SSA (dose: octreotide 30 mg/month; lanreotide 90 mg/month; duration: 6 mo-5 yr.) did not lead to control in the remaining cases. Radiotherapy (conventional; follow-up: 5–20 yr.) also did not itself lead to permanent control of GH hypersecretion. Addition of pegvisomant (10mg/day-20 mg 4 times/week) permitted control of IGF-1 and excessive growth in 3 previously operated patients who also had developed multiple pituitary axis deficiencies. Among the 9 patients that underwent primary surgical resection, 3 had immediate GH/PRL control and excessive growth was halted. The surgical control was followed in two cases by the development of panhypopituitarism and in one by diabetes insipidus. For those patients in whom growth and GH secretion were not controlled by their initial surgery, the subsequent management was complex. Use of SSA (octreotide 30–40mg/month depot, lanreotide 90 mg/month) and DA (0.25 mg2–7 times/week) postoperatively did not lead to hormonal or growth control, which was only achieved with combinations of radiotherapy (conventional or gamma-knife (follow-up <1 year-10 years), repeated surgery, SSA and finally pegvisomant (10–20mg/day). SSA use was associated with a median reduction in GH and IGF-1 of 37.5% and 15.2%, respectively, whether used as primary or secondary therapy but in no case was the reduction sufficient to bring patients under hormonal control. None of the patients receiving pasireotide experienced tumor expansion on therapy, even after >5 years of follow-up. Pasireotide was not available or was not used in the treatment of any of these patients.
Control of excessive growth was frequently accompanied by hypopituitarism (Table 1); one patient had <1 month of follow-up postoperatively and definitive growth control/hypopituitarism was difficult to determine. Four patients remain uncontrolled in terms of growth. One operated patient is awaiting availability of SSA and one patient is pending surgery but is receiving 100mcg sc daily octreotide plus DA; two other children are receiving 30–40 mg/month octreotide depot, DA, and have undergone radiotherapy (pegvisomant is not available in their jurisdiction).
Pathological characteristics
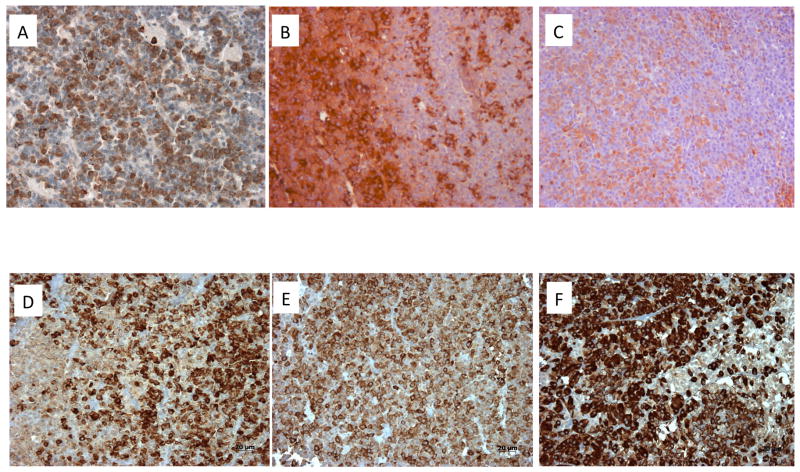
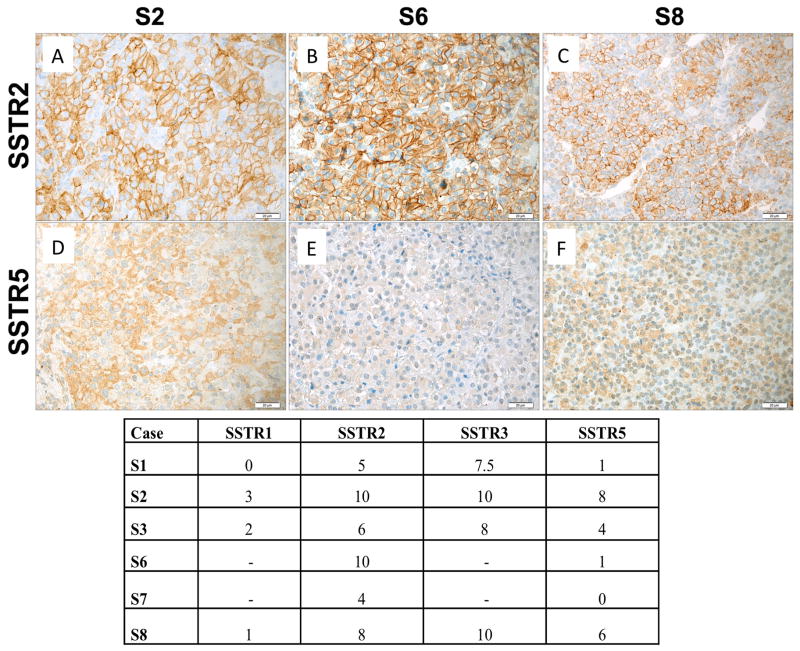
Overall, pituitary adenomas alone were seen in 12/17 operated cases, pituitary adenoma plus generalized hyperplasia in 2/16 cases and global hyperplasia alone in 2/16 cases. As shown in Table 1, resected tissues were usually positive on immunohistochemistry for both GH and PRL alone and most represented mixed GH-PRL secreting pituitary adenomas (Figure 5). Proliferation rates (Ki-67) were low (<1%) in tumors from 6 cases and was 3% in one adenoma. For six Xq26.3 microduplicated patients, sufficient pituitary tumor tissue was available to undertake somatostatin receptor (SSTR) immunohistochemistry. All six cases had moderate to high expression of SSTR2, the main target of somatostatin analogues such as octreotide and lanreotide, was variable but moderate to high in all cases (IRSs 4 to 10). Despite the presence of SSTR2 in these cases, none of the six experienced hormonal or growth control using a SSA, even at doses usually used in adults. Five of the six tumors stained positively for SSTR5, although at a lower level than for SSTR2. Four tumors were stained for SSTR1 and SSTR3, while for two cases only the level of SSTR2 and SSTR5 could be assessed (Figure 6). One of four tumors was negative for SSTR1. SSTR3 is also highly expressed (average IRS 8.8) in the analyzed tumors. AIP expression was preserved at a moderate to high level among these patients (Figure 5).
Figure 5.
Immunohistochemistry of pituitary adenomas from Xq26.3 microduplication cases. In case S1 (sporadic male) Panel A demonstrates strong GHRH-R staining (brown) in the pituitary adenoma using a C-terminal GHRH-R peptide (20× magnification). The tumor was a mixed GH and PRL adenoma and staining of consecutive slices demonstrated largely different cell populations that stained for GH (brown; Panel B) and PRL (brown; Panel C). SSTR immunohistochemistry was also performed on tumors from patients with the Xq26.3 microduplication. AIP staining (brown) shown in 3 pituitary tumors from X-LAG syndrome cases (Panels D–F)). In all 6 cases tested AIP staining was preserved at moderate to high levels and was predominantly cytoplasmic (20× magnification).
Figure 6.
SSTR immunohistochemistry was performed on tumors from patients with the Xq26.3 microduplication and results are shown in the tabulation (lower Panel). Expression of SSTR2 in representative tumor samples of three X-LAG patients are shown in Panels D-F and SSTR5 in Panels G-I. Immunoreactive scores for SSTR2 for all three samples illustrated were high, whereas SSTR5 scores varied from low (S6) to high (S2). Original magnification: ×400. Scale bar: 20 μm.
Discussion
X-LAG syndrome is a recently described cause of pituitary gigantism that is due to a microduplication in chromosome Xq26.3 encompassing GPR101; the latter gene is highly upregulated in pituitary tumors from patients (Trivellin et al. 2014). In this study we expand the number of described patients to 18 and focus for the first time on aspects of the clinical management of these patients. A number of factors related to X-LAG syndrome combine to make the clinical management of this condition particularly challenging. The age of onset of increased growth in X-LAG syndrome is significantly younger than other pituitary gigantism cases (Trivellin et al. 2014). In the current study we confirm that patients with the Xq26.3 microduplication can exhibit overgrowth as young as 2–3 months of age, despite being normally sized newborns. Across the cohort the onset of rapid abnormal growth always occurred before the age of 5 years, and in most patients it occurred before the end of the first year of life. As this is a newly described disease and as infant somatic overgrowth is rare and arguably less prioritized than failure to thrive, there was a delay in diagnosis in many cases. Overall, the median delay in diagnosis was 27 months. As a function of this delay, unchecked growth of the pituitary hyperplasia-adenoma led to most patients presenting with macroadenomas that had expanded to the level of the optic chiasm. Accompanying the tumoral/hyperplasia growth in X-LAG syndrome is very marked hypersecretion of GH/IGF-1 and PRL. The action of chronic and markedly elevated GH and IGF-1 on the young patients led to a phenotype of increased length/height (+3.9 SDS) and weight (+4.4 SDS) at diagnosis. Patients with X-LAG have a spectrum of signs and symptoms apart from the increased growth. Many of these echo the clinical features of GH excess seen in adult acromegaly, including acral enlargement and facial coarsening. Among the cohort the increase in foot size was remarked upon in many cases. In terms of facial changes, some patients appeared to exhibit hypertelorism as part of the overall coarsening of the nose and brow, which would differ from adult acromegaly. Prognathism itself was rare, but in three cases patients also developed a widening of the interdental spaces (Figure 2). Other acromegalic features such as increased perspiration/body odor, sleep apnea and skin thickening were also reported variably. Unusually, more than a third of patients had abnormally increased appetite, which has not been reported in association with gigantism previously. This and the presence of hyperinsulinemia and acanthosis nigricans suggests that there are metabolic effects that either stem from or accompany the process of somatic overgrowth in affected children. The appearance of acromegaly signs and symptoms in a young child should raise a high suspicion of an underlying Xq26.3 microduplication.
Treatment of pediatric patients with pituitary adenomas is complex, particularly in those-like the X-LAG syndrome cohort-where the pituitary tumor is large and the children are very young (Jane 2008). A desire for disease control must be acutely balanced against attempts to maintain normal function in other anterior pituitary axes while avoiding diabetes insipidus. The current study highlights for the first time the difficulties that have been encountered in treating pediatric gigantism patients with X-LAG syndrome. SSAs are the mainstay of medical treatment in GH excess, but in the setting of X-LAG syndrome their efficacy was poor. No patient achieved primary or secondary control of their disease or GH/IGF-1 with SSA, even when using adult doses in young children. We report new data on SSTR levels in pituitary adenomas from X-LAG patients, which indicate that the poor SSA responses to octreotide and lanreotide are not due to low SSTR2 expression, which was preserved in all cases. Indeed, in two cases, SSTR2 levels were very high despite no control of GH or IGF-1 with chronic SSA use at adult-type doses. The genomic abnormality encountered in X-LAG with tumoral GPR101 overexpression could, in turn, hypothetically promote growth by concomitantly down-regulating important signaling elements (e.g. ZAC1) linked to SSA effects (Theodoropoulou, et al. 2009). A similar but less dramatic situation has also been noted to occur in the statistically significantly decreased responses to SSA in patients with AIP mutation associated acromegaly (Chahal, et al. 2012; Daly, et al. 2010). However, staining for AIP, which can be reduced in tumors of acromegaly patients with poor SSA responses (Jaffrain-Rea, et al. 2013; Kasuki, et al. 2012), was preserved in X-LAG patients. Other SSTR were also preserved to a variable extent, including SSTR5 and SSTR3, which would suggest that treatment of patients with X-LAG by targeting these receptors with a multi-receptor SSA like pasireotide might be of clinical interest (Cuevas-Ramos and Fleseriu 2014; Eigler, et al. 2014). A medical option to achieve control of IGF-1 and physical overgrowth involved the use of pegvisomant, either alone or in combination with SSA/DA in a post-operative setting. This option, while useful, needs to be balanced against the lack of safety data for pegvisomant in the pediatric setting, although other cases of use in pediatrics use cases have been reported and in the current cohort no evidence of tumor expansion during pegvisomant monotherapy was noted (Daniel, et al. 2013; Goldenberg, et al. 2008; Mussig, et al. 2007; Rix, et al. 2005). DA alone was not sufficient to achieve control tumoral hyperprolactinemia in these cases. Neurosurgery was performed or planned in all cases and represents an effective option but was frequently associated with significant pituitary dysfunction (including GH deficiency). It should be noted that in cases where global pituitary hyperplasia is found, significant hypopituitarism is probably unavoidable when pursuing successful surgical resection of pathological tissue. On the other hand, very small residual tumor was capable of maintaining levels of GH/IGF-1 above normal for many years thereby necessitating chronic medical therapy. These residual tumor tissues did not regrow significantly, which might be due to the low proliferative index seen in most cases; radiotherapy could also have played a role in preventing tumor regrowth but the current cohort is probably still too limited to draw conclusions about the role of radiotherapy in X-LAG syndrome. To achieve control of pediatric overgrowth in the narrow window before this overgrowth became adult gigantism required recourse to radical surgery or multimodal combination therapy in the current cohort.
A unifying feature of the X-LAG syndrome is the strikingly early age at onset given that phenotypically the affected children are born normal and in sporadic cases come from normally sized families. The generally normal prenatal growth in these cases is probably due to the fact that in utero growth is mediated mainly by IGF-2. However, this may be in keeping with the ontogeny of the maturing hypothalamus and pituitary during late fetal and early childhood life. Activity of the fetal somatotrope axis is high at full term and GH secretion reaches one of the lifetime peaks on day 1 of post-natal life (Veldhuis 2012) (Coutant 2012). Crucially, somatotrope axis regulation in early neonatal life appears to promote the maintenance of GH secretory responses to GHRH (less desensitization) while this effect is accompanied by high levels of GHRH-R in infant animals and the induction by GHRH of its own receptor, GHRH-R, only during early life which later wanes and disappears (Collins, et al. 1996; Cuttler, et al. 1993; Cuttler, et al. 1995; Torronteras, et al. 2012; Torronteras, et al. 1996, 1997). These factors could represent a window during which increased GHRH secretion in a young patient could lead to upregulation of pituitary GHRH-R and subsequent cellular and hormonal over activity.
The etiology of the pituitary pathology remains a matter of debate. As we showed previously, of the 4 candidate coding genes contained within the common duplicated region, only GPR101 was upregulated at the level of pituitary tumor tissue, implicating a role for this GPCR in the pituitary itself (Trivellin et al. 2014). The finding that the GH/PRL hypersecretion was accompanied by normal or slightly elevated GHRH secretion (in the absence of peripheral sources on imaging scans) suggests that hypothalamic GHRH dysregulation also may play a part in the etiology. Previous studies in patients with disease phenotypes that closely resemble X-LAG syndrome also favor an important role for GHRH (Dubuis, et al. 1995; Moran, et al. 1990; Zimmerman, et al. 1993). Other factors support this hypothesis. The pathology seen has similarities to two situations caused by GHRH hypersecretion. Endocrine malignancies, such as bronchial or pancreatic neuroendocrine tumors (NETs), can rarely secrete GHRH. This situation leads to unregulated hyperstimulation of pituitary somatotropes and hyperplasia of these cells and concomitant GH/IGF-1 excess (Garby, et al. 2012; Zatelli, et al. 2005). Such NETs occurring as they do in adults, lead to a phenotype of acromegaly and are associated with highly increased GHRH levels in the peripheral blood. However, in cases where such patients have been mistakenly operated for their pituitary pathology before their peripheral GHRH source was identified, adenomatous changes are rarely encountered (Borson-Chazot, et al. 2012). This is in contrast to our infant-onset gigantism cohort in which hyperplasia occurs in a minority of cases and mixed GH and prolactin secreting adenomas predominate. The second relevant setting is that of GHRH-overexpressing transgenic mouse models (Hammer, et al. 1985). While these mice have elevated circulating GHRH levels, their pituitary and growth phenotypes are instructive. Such mice develop a spectrum of mixed GH and prolactin secreting pituitary adenomas, with or without accompanying somatotroph, lactotroph or mammosomatotroph hyperplasia (Asa, et al. 1990, 1992; Brar, et al. 1989; Mayo, et al. 1988; Stefaneanu, et al. 1989). It has been suggested that the process behind tumorigenesis in these GHRH transgenic mice involves an evolution from normal tissue through hyperplasia to adenoma formation (Lloyd, et al. 1992; Umemura, et al. 1995). Another element implicating GHRH in the etiology of this infant-onset gigantism syndrome is the widespread presence of GHRH-R in hyperplastic and adenomatous tissue, as compared with normal pituitary. Such presence of GHRH-R would seem to be a pre-requisite for a GHRH-driven disease particularly as local pituitary GHRH sources were not found on immunohistochemistry (Trivellin et al. 2014).
Most X-LAG syndrome patients were females and occurred sporadically in genetically normal families. While transmission was dominant in all 3 offspring in the 2 kindreds with X-LAG syndrome, currently only transmission from affected mother to affected son has been seen; the only unaffected offspring was a clinically and genetically normal female. There are no cases so far of disease transmission of affected male to offspring. The preponderance of cases of sporadic females might be arithmetical due to X chromosome number, but the potential negative effect of an Xq26.3 microduplication on the viability of hemizygous male embryos must be considered. The true genetic inheritance patterns in X-LAG syndrome will likely become apparent with identification of a larger cohort of patients.
X-LAG syndrome may explain the etiology of some of the most severe cases of gigantism. As we described previously, the medical literature from 1970 (advent of GH assays) contains reports of cases with similar clinical features to the X-LAG syndrome cohort (Trivellin et al. 2014), to which others can be added (AvRuskin, et al. 1973; Spence, et al. 1972). Other earlier cases of infant onset pituitary gigantism were well described medically (de Majo and Oñativia 1960; Hurxthal 1961; Todd 1958) and again mirror X-LAG syndrome closely. Parsing these historical reports and accompanying scientific observations suggest that many of the tallest patients in history had an early childhood onset gigantism phenotype that is highly reminiscent of X-LAG syndrome. As shown in Table 3, these cases occurred against a background of a normal family history and in some, a pituitary pathology was demonstrable. At present the clinical syndrome that most closely fits the etiology of these cases is X-LAG syndrome. These cases of extreme gigantism in adults underline the need for effective therapy to arrest tumor-induced growth during childhood.
Table 3.
Historical cases of extreme gigantism in which growth began in early childhood.
| Name | Country | Sex | Year of birth | Birth weight (kg) | Normal family history | Age at onset of abnormal growth (yr) | Final height (cm) |
|---|---|---|---|---|---|---|---|
| Martin Van Buren Bates1 | United States | M | 1837 | Normal | Y | 6 | 222 |
| Anna Haining Swan1 | Canada | F | 1846 | 8.1 | Y | <4 | 227 |
| Ella Kate Ewing | United States | F | 1872 | 3.4 | Y | 7 | 225 |
| Fedor Andreevich Machnow | Russia | M | 1878 | N/A | Y | 5 | 239 |
| Edouard Beaupré* | Canada | M | 1881 | 4.1 | Y | 3 | 251 |
| Joh(a)n Aasen* | United States | M | 1890 | N/A | N | <8 | 218 |
| Albert Johan Kramer* | Netherlands | M | 1897 | 8.5 | Y | <7 | 238 |
| Robert Pershing Wadlow* | United States | M | 1918 | 4.1 | Y | <3 | 272 |
| Cecil Boling | United States | M | 1920 | Normal | Y | <7 | 235 |
| Rigardus Rijnhout* | Netherlands | M | 1922 | Normal | Y | >3 | 238 |
| Dolores Ann Pullard+ | United States | F | 1946 | Normal | Y | 4 | 227 |
| Sandra Elaine (Sandy) Allen* | United States | F | 1955 | 2.95 | Y | 3 | 232 |
| Zeng Jinlian | China | F | 1965 | Normal | Y | <1 | 249 |
| Yao Defen* | China | F | 1972 | 2.8 | Y | <3 | 234 |
Swan and Bates were married and had two pregnancies; one female child was stillborn (weighed 8.1 kg) and later a son who died in early infancy was 10.8Kg and 76 cm in length when born.
cases for which pituitary pathology was reliably diagnosed/reported.
X-LAG syndrome is a new, rare disorder of pituitary gigantism associated with a phenotype of overgrowth due to mixed GH and PRL secreting pituitary adenomas/hyperplasia that usually begins during the first 2 years of life. Early recognition of these cases may be helped by better clinical awareness of the characteristic acromegalic symptomatology; diagnosis of the underlying Xq26.3 microduplication is readily achievable using commercial aCGH. Management of X-LAG syndrome is complicated by the high baseline levels of GH hypersecretion and therapeutic control of abnormal growth requires radical surgical resection or multimodal therapy including pegvisomant. SSA responses are poor despite the presence of moderate to high SSTR2 staining. The underlying pathophysiology of X-LAG remains to be explained fully, particularly the role of GPR101 in regulating tumor growth by modulating hypothalamic or pituitary signals.
Supplementary Material
Acknowledgments
Funding Statement: This study was supported by U.S. Department of Health and Human Services-National Institutes of Health-National Institute of Child Health and Human Development (Z01-HD008920); U.S. Department of Health and Human Services-National Institutes of Health-National Human Genome Research Institute (U54HG006542); Fonds D’Investissement de Recherche Scientifique, CHU de Liège, Jabbs Foundation; National Institute of Neurological Disorders and Stroke (NINDS) (R01 NS058529)
Footnotes
Disclosure statement: The authors have nothing to disclose
References
- Asa SL, Kovacs K, Stefaneanu L, Horvath E, Billestrup N, Gonzalez-Manchon C, Vale W. Pituitary mammosomatotroph adenomas develop in old mice transgenic for growth hormone-releasing hormone. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine. 1990;193:232–235. doi: 10.3181/00379727-193-3-rc1. [DOI] [PubMed] [Google Scholar]
- Asa SL, Kovacs K, Stefaneanu L, Horvath E, Billestrup N, Gonzalez-Manchon C, Vale W. Pituitary adenomas in mice transgenic for growth hormone-releasing hormone. Endocrinology. 1992;131:2083–2089. doi: 10.1210/endo.131.5.1425411. [DOI] [PubMed] [Google Scholar]
- AvRuskin TW, Sau K, Tang S, Juan C. Childhood acromegaly: successful therapy with conventional radiation and effects of chlorpromazine on growth hormone and prolactin secretion. The Journal of Clinical Endocrinology & Metabolism. 1973;37:380–388. doi: 10.1210/jcem-37-3-380. [DOI] [PubMed] [Google Scholar]
- Beckers A, Aaltonen LA, Daly AF, Karhu A. Familial isolated pituitary adenomas (FIPA) and the pituitary adenoma predisposition due to mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocrine Reviews. 2013;34:239–277. doi: 10.1210/er.2012-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borson-Chazot F, Garby L, Raverot G, Claustrat F, Raverot V, Sassolas G group GTE. Acromegaly induced by ectopic secretion of GHRH: a review 30 years after GHRH discovery. Annales d’Endocrinologie. 2012;73:497–502. doi: 10.1016/j.ando.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Brar AK, Brinster RL, Frohman LA. Immunohistochemical analysis of human growth hormone-releasing hormone gene expression in transgenic mice. Endocrinology. 1989;125:801–809. doi: 10.1210/endo-125-2-801. [DOI] [PubMed] [Google Scholar]
- Chahal HS, Trivellin G, Leontiou CA, Alband N, Fowkes RC, Tahir A, Igreja SC, Chapple JP, Jordan S, Lupp A, et al. Somatostatin Analogs Modulate AIP in Somatotroph Adenomas : The Role of the ZAC1 Pathway. The Journal of Clinical Endocrinology & Metabolism. 2012;97:1–10. doi: 10.1210/jc.2012-1111. [DOI] [PubMed] [Google Scholar]
- Collins BJ, Szabo M, Cuttler L. Differential desensitization response of the neonatal and adult rat somatotroph to growth hormone-releasing hormone and phorbol ester. Molecular and Cellular Endocrinology. 1996;117:75–81. doi: 10.1016/0303-7207(95)03731-4. [DOI] [PubMed] [Google Scholar]
- Coutant RB-NN. Endocrine Control and Regulation of Growth Hormone: An Overview. In: Preedy VR, editor. Handbook of Growth and Growth Monitoring in Health and Disease. Spinger Science+Business Media, LLC; 2012. pp. 73–92. [Google Scholar]
- Cuevas-Ramos D, Fleseriu M. Somatostatin receptor ligands and resistance to treatment in pituitary adenomas. Journal of Molecular Endocrinology. 2014;52:R223–240. doi: 10.1530/JME-14-0011. [DOI] [PubMed] [Google Scholar]
- Cuttler L, Collins BJ, Marone PA, Szabo M. The effect of isobutylmethylxanthine, forskolin, and cholera toxin on growth hormone release from pituitary cell cultures of perinatal and mature rats. Endocrine Research. 1993;19:33–46. doi: 10.3109/07435809309035406. [DOI] [PubMed] [Google Scholar]
- Cuttler L, Collins BJ, Szabo M. Ontogeny of the GH response to phorbol ester and phospholipase C in rat pituitary cells. The Journal of Endocrinology. 1995;145:307–314. doi: 10.1677/joe.0.1450307. [DOI] [PubMed] [Google Scholar]
- Daly AF, Tichomirowa MA, Petrossians P, Heliövaara E, Jaffrain-Rea M-L, Barlier A, Naves LA, Ebeling T, Karhu A, Raappana A, et al. Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: an international collaborative study. The Journal of Clinical Endocrinology & Metabolism. 2010;95:E373–383. doi: 10.1210/jc.2009-2556. [DOI] [PubMed] [Google Scholar]
- Daniel A, d’Emden M, Duncan E. Pituitary gigantism treated successfully with the growth hormone receptor antagonist, pegvisomant. Internal Medicine Journal. 2013;43:345–347. doi: 10.1111/imj.12077. [DOI] [PubMed] [Google Scholar]
- Davies JH, Cheetham T. Investigation and management of tall stature. Archives of Disease in Childhood. 2014;99:772–777. doi: 10.1136/archdischild-2013-304830. [DOI] [PubMed] [Google Scholar]
- de Herder WW. The History of Acromegaly. Neuroendocrinology. 2015 doi: 10.1159/000371808. [DOI] [PubMed] [Google Scholar]
- de Majo SF, Oñativia A. Acromegaly and gigantism in a boy:comparison with 3 overgrown nonacromegalic children. The Journal of Pediatrics. 1960;57:382–390. [Google Scholar]
- Dubuis JM, Deal CL, Drews RT, Goodyer CG, Lagace G, Asa SL, Van Vliet G, Collu R. Mammosomatotroph adenoma causing gigantism in an 8-year old boy: a possible pathogenetic mechanism. Clinical Endocrinology. 1995;42:539–549. doi: 10.1111/j.1365-2265.1995.tb02675.x. [DOI] [PubMed] [Google Scholar]
- Eigler T, Ben-Shlomo A, Zhou C, Khalafi R, Ren SG, Melmed S. Constitutive somatostatin receptor subtype-3 signaling suppresses growth hormone synthesis. Molecular Endocrinology. 2014;28:554–564. doi: 10.1210/me.2013-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugster EA, Pescovitz OH. Gigantism. The Journal of Clinical Endocrinology & Metabolism. 1999;84:4379–4384. doi: 10.1210/jcem.84.12.6222. [DOI] [PubMed] [Google Scholar]
- Garby L, Caron P, Claustrat F, Chanson P, Tabarin A, Rohmer V, Arnault G, Bonnet F, Chabre O, Christin-Maitre S, et al. Clinical characteristics and outcome of acromegaly induced by ectopic secretion of growth hormone-releasing hormone (GHRH): a French nationwide series of 21 cases. The Journal of Clinical Endocrinology & Metabolism. 2012;97:2093–2104. doi: 10.1210/jc.2011-2930. [DOI] [PubMed] [Google Scholar]
- Goldenberg N, Racine MS, Thomas P, Degnan B, Chandler W, Barkan A. Treatment of pituitary gigantism with the growth hormone receptor antagonist pegvisomant. The Journal of Clinical Endocrinology & Metabolism. 2008;93:2953–2956. doi: 10.1210/jc.2007-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer RE, Brinster RL, Rosenfeld MG, Evans RM, Mayo KE. Expression of human growth hormone-releasing factor in transgenic mice results in increased somatic growth. Nature. 1985;315:413–416. doi: 10.1038/315413a0. [DOI] [PubMed] [Google Scholar]
- Hurxthal LM. Pituitary gigantism in a child five years of age: effect of x-radiation, estrogen therapy and self-imposed starvation diet during an eleven-year period. The Journal of Clinical endocrinology and Metabolism. 1961;21:343–353. doi: 10.1210/jcem-21-3-343. [DOI] [PubMed] [Google Scholar]
- Jaffrain-Rea ML, Angelini M, Gargano D, Tichomirowa MA, Daly AF, Vanbellinghen JF, D’Innocenzo E, Barlier A, Giangaspero F, Esposito V, et al. Expression of aryl hydrocarbon receptor (AHR) and AHR-interacting protein in pituitary adenomas: pathological and clinical implications. Endocrine-related cancer. 2009;16:1029–1043. doi: 10.1677/ERC-09-0094. [DOI] [PubMed] [Google Scholar]
- Jaffrain-Rea ML, Rotondi S, Turchi A, Occhi G, Barlier A, Peverelli E, Rostomyan L, Defilles C, Angelini M, Oliva MA, et al. Somatostatin analogues increase AIP expression in somatotropinomas, irrespective of Gsp mutations. Endocrine-related Cancer. 2013;20:753–766. doi: 10.1530/ERC-12-0322. [DOI] [PubMed] [Google Scholar]
- Jane JA., Jr Management of pediatric sellar tumors. Pediatric Endocrinology Eeviews : PER. 2008;5(Suppl 2):720–726. [PubMed] [Google Scholar]
- Kasuki L, Vieira Neto L, Wildemberg LE, Colli LM, De Castro M, Takiya CM, Gadelha MR. AIP expression in sporadic somatotropinomas is a predictor of the response to octreotide LAR therapy independent of SSTR2 expression. Endocrine-related Cancer. 2012 doi: 10.1530/ERC-12-0020. [DOI] [PubMed] [Google Scholar]
- Lee JA, Carvalho CM, Lupski JR. A DNA replication mechanism for generating nonrecurrent rearrangements associated with genomic disorders. Cell. 2007;131:1235–1247. doi: 10.1016/j.cell.2007.11.037. [DOI] [PubMed] [Google Scholar]
- Lee M, Lupp A, Mendoza N, Martin NM, Beschorner R, Honegger J, Schlegel J, Shively T, Pulz E, Schulz S, et al. SSTR3 is a putative target for the medical treatment of gonadotroph adenomas of the pituitary. Endocrine-related Cancer. 2014 doi: 10.1530/ERC-14-0472. [DOI] [PubMed] [Google Scholar]
- Lee M, Marinoni I, Irmler M, Psaras T, Honegger JB, Beschorner R, Anastasov N, Beckers J, Theodoropoulou M, Roncaroli F, et al. Transcriptome analysis of MENX-associated rat pituitary adenomas identifies novel molecular mechanisms involved in the pathogenesis of human pituitary gonadotroph adenomas. Acta Neuropathologica. 2013;126:137–150. doi: 10.1007/s00401-013-1132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd RV, Jin L, Chang A, Kulig E, Camper SA, Ross BD, Downs TR, Frohman LA. Morphologic effects of hGRH gene expression on the pituitary, liver, and pancreas of MT-hGRH transgenic mice. An in situ hybridization analysis. The American Journal of Pathology. 1992;141:895–906. [PMC free article] [PubMed] [Google Scholar]
- Magri F, Villa C, Locatelli D, Scagnelli P, Lagonigro MS, Morbini P, Castellano M, Gabellieri E, Rotondi M, Solcia E, et al. Prevalence of double pituitary adenomas in a surgical series: Clinical, histological and genetic features. Journal of Endocrinological Investigation. 2010;33:325–331. doi: 10.3275/6716. [DOI] [PubMed] [Google Scholar]
- Mayo KE, Hammer RE, Swanson LW, Brinster RL, Rosenfeld MG, Evans RM. Dramatic pituitary hyperplasia in transgenic mice expressing a human growth hormone-releasing factor gene. Molecular Endocrinology. 1988;2:606–612. doi: 10.1210/mend-2-7-606. [DOI] [PubMed] [Google Scholar]
- Moran A, Asa S, Kovacs K, Horvath E, Singer W, Sagman U, Reubi J, Wilson C, Larson R, Pescovits O. Gigantism due to pituitary mammosomatotroph hyperplasia. 1990;323:322–327. doi: 10.1056/NEJM199008023230507. [DOI] [PubMed] [Google Scholar]
- Mussig K, Gallwitz B, Honegger J, Strasburger CJ, Bidlingmaier M, Machicao F, Bornemann A, Ranke MB, Haring HU, Petersenn S. Pegvisomant treatment in gigantism caused by a growth hormone-secreting giant pituitary adenoma. Experimental and Clinical Endocrinology & Diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2007;115:198–202. doi: 10.1055/s-2007-956172. [DOI] [PubMed] [Google Scholar]
- Rix M, Laurberg P, Hoejberg AS, Brock-Jacobsen B. Pegvisomant therapy in pituitary gigantism: successful treatment in a 12-year-old girl. European journal of endocrinology / European Federation of Endocrine Societies. 2005;153:195–201. doi: 10.1530/eje.1.01956. [DOI] [PubMed] [Google Scholar]
- Salenave S, Boyce AM, Collins MT, Chanson P. Acromegaly and McCune-Albright syndrome. The Journal of Clinical Endocrinology and Metabolism. 2014;99:1955–1969. doi: 10.1210/jc.2013-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpea P, Stratakis CA. Carney complex and McCune Albright syndrome: an overview of clinical manifestations and human molecular genetics. Molecular and Cellular Endocrinology. 2014;386:85–91. doi: 10.1016/j.mce.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence HJ, Trias EP, Raiti S. Acromegaly in a 9 and one-half-year-old boy. Pituitary function studies before and after surgery. American Journal of Diseases of Children. 1972;123:504–506. doi: 10.1001/archpedi.1972.02110110132018. [DOI] [PubMed] [Google Scholar]
- Stefaneanu L, Kovacs K, Horvath E, Asa SL, Losinski NE, Billestrup N, Price J, Vale W. Adenohypophysial changes in mice transgenic for human growth hormone-releasing factor: a histological, immunocytochemical, and electron microscopic investigation. Endocrinology. 1989;125:2710–2718. doi: 10.1210/endo-125-5-2710. [DOI] [PubMed] [Google Scholar]
- Stratakis CA, Schussheim DH, Freedman SM, Keil MF, Pack SD, Agarwal SK, Skarulis MC, Weil RJ, Lubensky IA, Zhuang Z, et al. Pituitary macroadenoma in a 5-year-old: an early expression of multiple endocrine neoplasia type 1. The Journal of Clinical Endocrinology and Metabolism. 2000;85:4776–4780. doi: 10.1210/jcem.85.12.7064. [DOI] [PubMed] [Google Scholar]
- Theodoropoulou M, Tichomirowa MA, Sievers C, Yassouridis A, Arzberger T, Hougrand O, Deprez M, Daly AF, Petrossians P, Pagotto U, et al. Tumor ZAC1 expression is associated with the response to somatostatin analog therapy in patients with acromegaly. International Journal of Cancer. 2009;125:2122–2126. doi: 10.1002/ijc.24602. [DOI] [PubMed] [Google Scholar]
- Todd RM. Acromegaly in a girl of 8 years. Archives of Disease in Childhood. 1958;33:49–54. doi: 10.1136/adc.33.167.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torronteras R, Canalejo A, Elsaesser F. Differential ontogenetic patterns of in vitro desensitization to GHRH in fetal and neonatal anterior pituitary. Neuroendocrinology. 2012;95:257–266. doi: 10.1159/000333779. [DOI] [PubMed] [Google Scholar]
- Torronteras R, Gracia-Navarro F, Elsaesser F. Different effects of somatostatin on in vitro growth hormone release in two porcine breeds with different growth rates. Journal of Neuroendocrinology. 1996;8:891–900. doi: 10.1111/j.1365-2826.1996.tb00817.x. [DOI] [PubMed] [Google Scholar]
- Torronteras R, Gracia-Navarro F, Elsaesser F. Control of growth hormone secretion from porcine fetal and neonatal pituitary tissue in vitro by growth hormone-releasing hormone, somatostatin, and insulin-like growth factor. Neuroendocrinology. 1997;65:117–128. doi: 10.1159/000127171. [DOI] [PubMed] [Google Scholar]
- Trivellin G, Daly AF, Faucz FR, Yuan B, Rostomyan L, Larco DO, Schernthaner-Reiter MH, Szarek E, Leal LF, Caberg JH, et al. Gigantism and acromegaly due to Xq26 microduplications and GPR101 mutation. The New England Journal of Medicine. 2014;371:2363–2374. doi: 10.1056/NEJMoa1408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura S, Oda K, Utsunomiya H, Sanno N, Itoh J, Katakami H, Osamura RY. Immunohistochemical characterization of “hyperplasia-adenoma sequence” in the pituitaries of transgenic mice expressing a human growth hormone-releasing factor gene. The Tokai Journal of Experimental and Clinical Medicine. 1995;20:71–79. [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A, Erickson E, Roelfsema F, Bowers CY. Lifetime Regulation of Growth Hormone (GH) Secretion. In: Fink G, Pfaff DW, Levine JW, editors. Handbook of Neuroendocrinology. 1. Academic Press; 2012. pp. 237–255. [Google Scholar]
- Villa C, Lagonigro MS, Magri F, Koziak M, Jaffrain-Rea ML, Brauner R, Bouligand J, Junier MP, Di Rocco F, Sainte-Rose C, et al. Hyperplasia-adenoma sequence in pituitary tumorigenesis related to aryl hydrocarbon receptor interacting protein gene mutation. Endocrine-related Cancer. 2011;18:347–356. doi: 10.1530/ERC-11-0059. [DOI] [PubMed] [Google Scholar]
- Zatelli MC, Maffei P, Piccin D, Martini C, Rea F, Rubello D, Margutti A, Culler MD, Sicolo N, degli Uberti EC. Somatostatin analogs in vitro effects in a growth hormone-releasing hormone-secreting bronchial carcinoid. The Journal of Clinical Endocrinology and Metabolism. 2005;90:2104–2109. doi: 10.1210/jc.2004-2156. [DOI] [PubMed] [Google Scholar]
- Zhang F, Khajavi M, Connolly AM, Towne CF, Batish SD, Lupski JR. The DNA replication FoSTeS/MMBIR mechanism can generate genomic, genic and exonic complex rearrangements in humans. Nature Genetics. 2009;41:849–853. doi: 10.1038/ng.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman D, Young WF, Jr, Ebersold MJ, Scheithauer BW, Kovacs K, Horvath E, Whitaker MD, Eberhardt NL, Downs TR, Frohman LA. Congenital gigantism due to growth hormone-releasing hormone excess and pituitary hyperplasia with adenomatous transformation. The Journal of Clinical Endocrinology and Metabolism. 1993;76:216–222. doi: 10.1210/jcem.76.1.8421089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.