Abstract
The mechanisms by which angiotensin II (AngII) elevates blood pressure and enhances end-organ damage appear to be distinct. However, the signal transduction cascade by which AngII specifically mediates vascular remodeling such as medial hypertrophy and perivascular fibrosis remains incomplete. We have previously shown that AngII-induced epidermal growth factor receptor (EGFR) transactivation is mediated by metalloprotease ADAM17, and that this signaling is required for vascular smooth muscle cell hypertrophy but not for contractile signaling in response to AngII. Recent studies have implicated endoplasmic reticulum (ER) stress in hypertension. Interestingly, EGFR is capable of inducing ER stress. The aim of this study was to test the hypothesis that activation of EGFR and ER stress are critical components required for vascular remodeling but not hypertension induced by AngII. Mice were infused with AngII for 2 weeks with or without treatment of EGFR inhibitor, erlotinib, or ER chaperone, 4-phenylbutyrate. AngII infusion induced vascular medial hypertrophy in the heart, kidney and aorta, and perivascular fibrosis in heart and kidney, cardiac hypertrophy, and hypertension. Treatment with Erlotinib as well as 4-phenylbutyrate attenuated vascular remodeling and cardiac hypertrophy but not hypertension. In addition, AngII infusion enhanced ADAM17 expression, EGFR activation and ER/oxidative stress in the vasculature, which were diminished in both erlotinib-treated and 4-phenylbutyrate-treated mice. ADAM17 induction and EGFR activation by AngII in vascular cells was also prevented by inhibition of EGFR or ER stress. In conclusion, AngII induces vascular remodeling by EGFR activation and ER stress via a signaling mechanism involving ADAM17 induction independent of hypertension.
Keywords: Hypertension, Angiotensin II, Vascular Smooth Muscle, Signal Transduction, Hypertrophy, Fibrosis
Introduction
The renin angiotensin system has been strongly implicated in hypertension and its complications. Importantly, it has been suggested that the mechanisms by which angiotensin II (AngII) elevates blood pressure and enhances end-organ damage may be distinct1. Vascular remodeling associated with hypertension has been strongly implicated in end-organ damage and associated with poor cardiovascular outcomes2, 3. The remodeling predisposes to end-organ damage and pharmacological intervention in vascular remodeling should have special clinical efficacy for prevention of hypertensive complications2, 3. However, the exact signal transduction cascade by which AngII mediates vascular remodeling such as medial hypertrophy and perivascular fibrosis remains insufficiently understood. Therefore, the rationale of the present study is to explore the signal transduction mechanism of AngII required for the initiation of vascular remodeling but not hypertension, and to test its functional relevance in order to seek a novel treatment for hypertensive complications.
AngII mediates vascular smooth muscle cell (VSMC) contraction via Gq-mediated intracellular Ca2+ elevation and G12/13-mediated Rho kinase activation4. We have shown in vitro that Gq- and metalloprotease ADAM17-mediated epidermal growth factor receptor (EGFR) “trans”-activation via heparin-binding EGF-like growth factor (HB-EGF) shedding is required for extracellular signal-regulated kinase activation and VSMC hypertrophy but not for intracellular Ca2+ elevation or Rho kinase activation5–7. Also, EGFR activity and ADAM17 expression are enhanced in the neointima after angioplasty, and dominant-negative ADAM17 gene-transfer prevents the EGFR activation and neointimal hyperplasia8. Others have shown that the EGFR activation mediates AngII-induced reactive oxygen species (ROS) generation in VSMCs9, and EGFR antisense10 or ADAM17 interfering RNA11 can suppress AngII-induced cardiac hypertrophy. Data from mice having mutant EGFR further support the role of EGFR in AngII associated cardiac remodeling12. However, whether an EGFR inhibitor such as erlotinib utilized for human cancer treatments13 has therapeutic potential against hypertensive vascular remodeling remains unclear.
Literature increasingly suggests that prolonged ER stress and the subsequent unfolded protein response (UPR) likely contribute to the development and progression of cardiovascular diseases such as heart failure and atherosclerosis14, 15. While the downstream consequences of prolonged ER stress generally involve UPR specific gene programs16, ER stress appears critical for enhancement of ROS in many organ and cell systems including VSMCs14, 17. AngII has been shown to enhance ER stress in vitro and in vivo18, 19, potentially mediating enhancement of oxidative stress and subsequent target organ damage. Supplementation of ER chaperone 78-kDa glucose-regulated protein (GRP78) into the subfornical organ of the brain has been demonstrated to be effective in attenuating AngII-induced ER stress and hypertension20. In addition, recent evidence suggests a link between EGFR and ER stress21.
Taking the above information together, we have tested our hypothesis that activation of EGFR and ER stress are critical components required for vascular remodeling but not hypertension induced by AngII utilizing mice treated with an EGFR inhibitor or ER stress inhibitor. Our findings support this hypothesis and further demonstrate a novel concept of ADAM17 induction by EGFR via ER stress, potentially amplifying hypertensive end-organ damage mediated by the renin angiotensin system.
Methods
Extended methods are provided in the online supplement.
Results
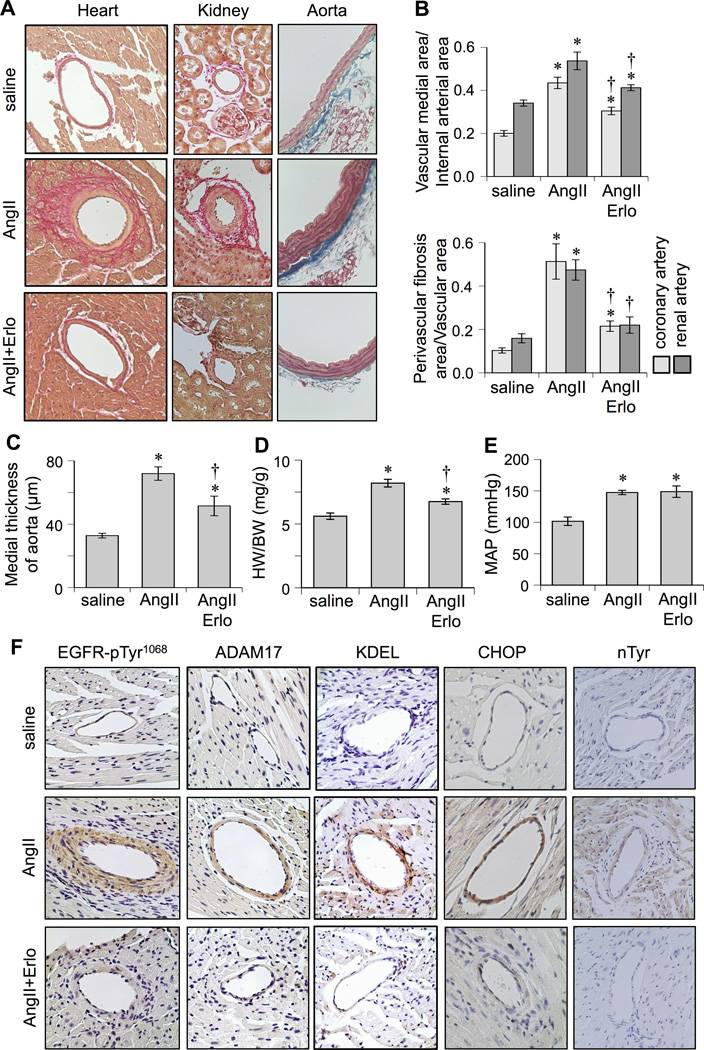
To test for the role of EGFR activation in AngII-induced vascular remodeling, AngII-infused mice were treated with or without an EGFR kinase inhibitor, erlotinib. 2 weeks of AngII infusion in control mice caused vascular medial hypertrophy in coronary arteries, renal arteries, and aortas that was markedly (but not completely) prevented in mice treated with erlotinib. Perivascular fibrosis in coronary and renal arteries induced by AngII infusion was partially or completely prevented in erlotinib-treated mice, respectively (Figure 1A–1C). AngII-induced cardiac hypertrophy assessed by heart weight to body weight ratio (Figure 1D) and echocardiogram (Supplemental Table S1) was partially or completely attenuated in erlotinib-treated mice, respectively. In contrast, hypertension was induced in both non-treated and erlotinib-treated mouse groups infused with AngII (Figure 1E and Supplemental Table S2). In addition, body weight and heart rate remained the same among the three animal groups (Supplemental Table S2).
Figure 1.
Effects of EGFR inhibitor, erlotinib, on cardiovascular remodeling induced by AngII. C57Bl/6 mice were infused with saline (n=8) for 2 weeks, or AngII (1 µg/kg/min) for 2 weeks with (n=8) or without (n=8) treatment of erlotinib (10 mg/kg/day intraperitoneal injection). Hearts and kidneys were stained with Sirius red and aortas were stained with Masson trichrome (Mean±SEM). A: Representative staining (200x) is presented. B: Quantification of medial area to internal arterial area of the coronary and renal arteries, and quantification of perivascular fibrosis area to vascular area of these arteries. C: Quantification of medial thickness of the thoracic aorta. D: Heart weight (HW) body weight (BW) ratio. E: Mean arterial pressure (MAP) was evaluated by telemetry. F: Heart sections were immuno-stained with antibodies as indicated (n=4). Antibodies against KDEL and CHOP were used to assess ER stress. Antibody against nitro-tyrosine (nTyr) was used to assess oxidative stress. *p<0.05 compared with control saline infusion. †p<0.05 compared with AngII infusion.
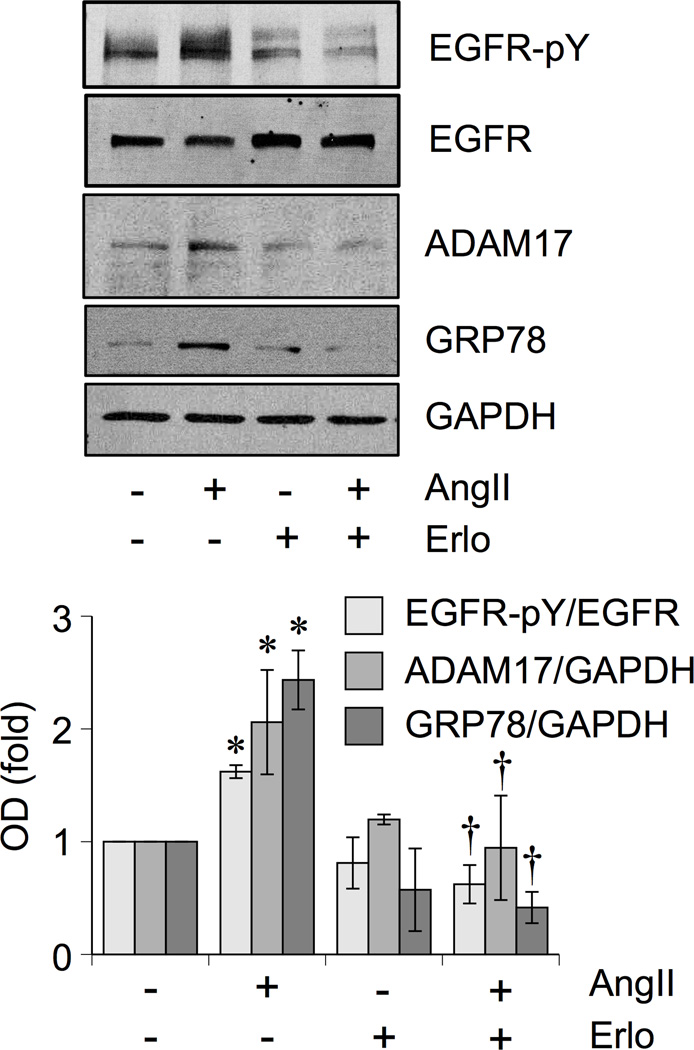
The AngII-induced vascular remodeling in control mice was associated with vascular EGFR activation, ER stress and oxidative stress assessed by immunohistochemistry. These AngII responses were markedly attenuated in mice treated with erlotinib (Figure 1F and Supplemental Figure S1). We were unable to assess active EGFR in the myocyte area of the heart due to lack of any significant staining in response to AngII infusion. ADAM17 expression was barely detectable in heart tissue but was significantly induced upon AngII infusion in the coronary arteries. No such induction was seen in mice treated with erlotinib. Erlotinib also inhibited EGFR activation, GRP78 protein induction, ADAM17 protein induction, and promoter activation in VSMCs stimulated with AngII (Figure 2 and Supplemental Figure S2A).
Figure 2.
EGFR inhibitor attenuated induction of ADAM17 and GRP78 in response to AngII in VSMCs. Rat VSMCs were pretreated with or without Erlotinib (1 µmol/L for 30 min) and stimulated with AngII (100 nmol/L) for 18 hours. The cell lysates were analyzed by immunoblotting as indicated (means ± SEM, n=4 in each group). *p<0.05 compared with basal. †p<0.05 compared with AngII stimulation.
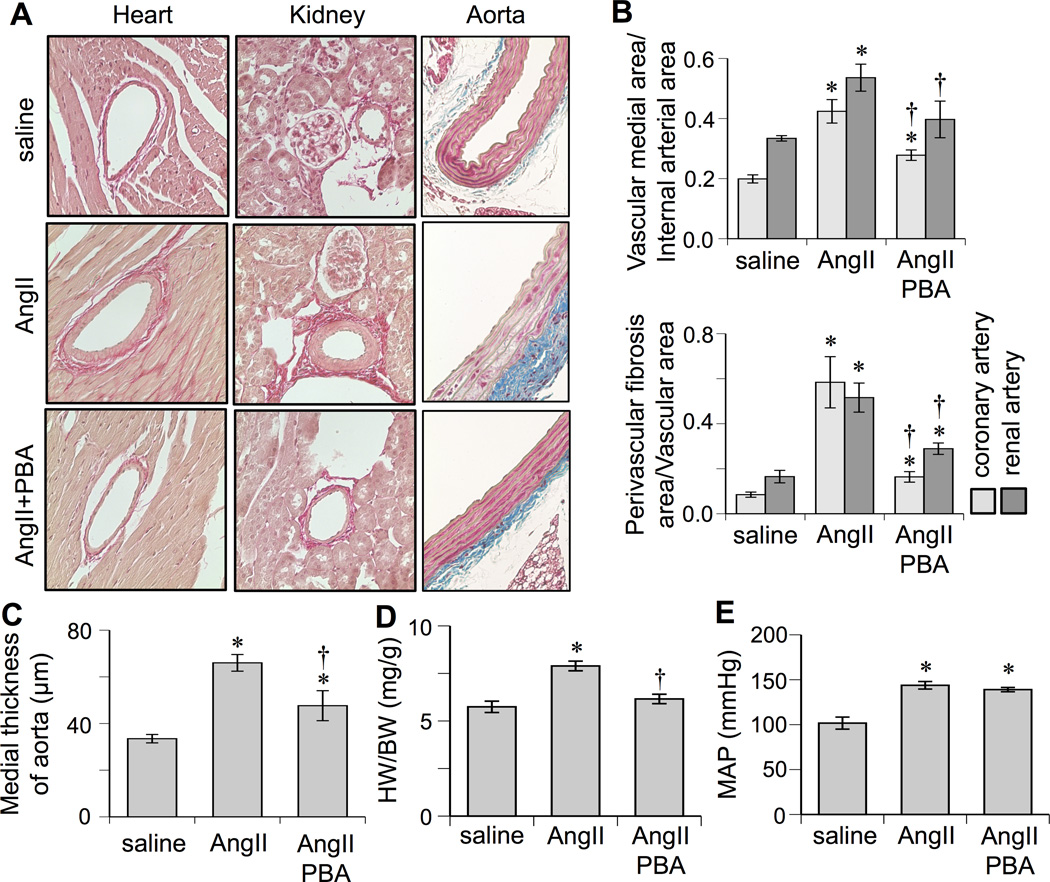
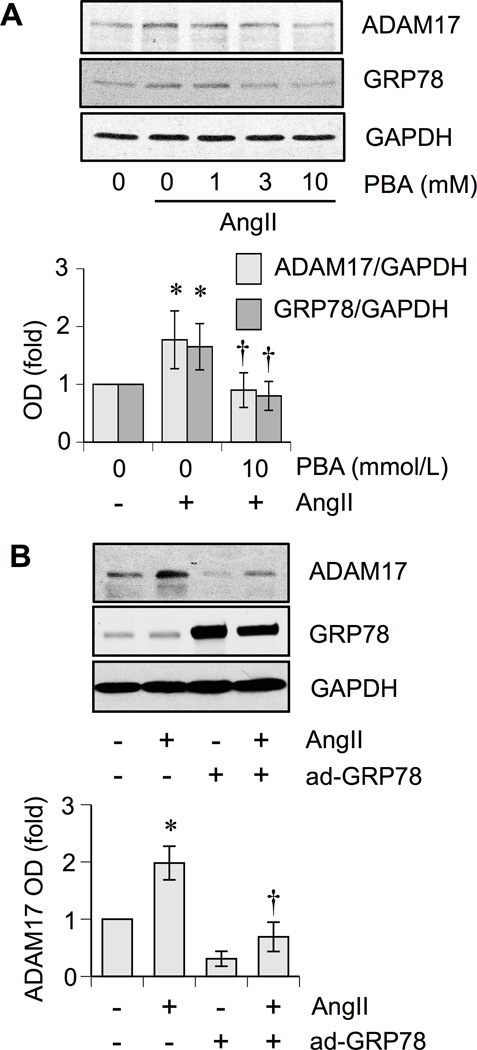
To test for the role of ER stress in AngII-induced vascular remodeling, AngII-infused mice were treated with a chemical ER chaperone, 4-phenylbutyrate (PBA). PBA prevented AngII-induced vascular hypertrophy, perivascular fibrosis, and cardiac hypertrophy markedly (but not fully except in renal arterial fibrosis) but not hypertension (Figure 3 and Supplemental Table S1 and S2). In addition, AngII-induced vascular ADAM17 induction, EGFR activation, and enhancement of ER/oxidative stress were prevented by PBA treatment (Supplemental Figure S3). PBA inhibited AngII-induced ADAM17 protein expression and promoter activity in cultured VSMCs (Figure 4A and and Supplemental Figure S2B). Gene-transfer of ER chaperone, GRP78, inhibited AngII-induced ADAM17 protein expression (Figure 4B). PBA also attenuated AngII-induced EGFR activation in VSMCs (Supplemental Figure S2C)
Figure 3.
Effects of ER stress inhibitor, PBA, on cardiovascular remodeling induced by AngII. C57Bl/6 mice were infused with saline (n=8) for 2 weeks, or AngII for 2 weeks with (n=6) or without (n=8) PBA treatment (1g/kg/day in drinking water). Hearts and kidneys were stained with Sirius red and aortas were stained with Masson trichrome (Mean±SEM). A: Representative staining (200×) is presented. B: Quantification of medial area to internal arterial area of the coronary and renal arteries, and quantification of perivascular fibrosis area to vascular area of these arteries. C: Quantification of medial thickness of the thoracic aorta. D: Heart weight (HW) body weight (BW) ratio. E: Mean arterial pressure (MAP) was evaluated by telemetry. *p<0.05 compared with control saline infusion. †p<0.05 compared with AngII infusion.
Figure 4.
Inhibition of ER stress attenuated AngII-induced ADAM17 induction in VSMCs. A. Rat VSMCs were pretreated with or without PBA (1 – 10 mmol/L for 30 min) and were stimulated with AngII (100 nmol/L) for 18 h. The cell lysates were analyzed by immunoblotting as indicated (means ± SEM, n=4 in each group). B. Rat VSMC infected with adenovirus encoding GRP78 or control LacZ (100 moi) were stimulated with AngII (100 nmol/L) for 24 h. The cell lysates were analyzed by immunoblotting as indicated (means ± SEM, n=4 in each group). *p<0.05 compared with basal. †p<0.05 compared with AngII stimulation.
Discussion
The major finding of the present study is that AngII-induced vascular hypertrophy and perivascular fibrosis were attenuated in mice treated with inhibitors of EGFR and ER stress, and that these protective effects were independent of hypertension. The suppression of AngII-induced vascular hypertrophy in erlotinib-treated mice is in line with our past in vitro observations that genetic ADAM17 silencing or inhibition of EGFR transactivation prevented the hypertrophic responses in cultured VSMCs5, 7. Moreover, mice with sm22α-promoter dependent EGFR silencing have less base-line arterial wall to lumen ratio while blood pressure increases to the same extent as wild type upon acute AngII infusion22, thus supporting the role of EGFR in vascular hypertrophy.
It is intriguing that pharmacological EGFR inhibition also prevented perivascular fibrosis induced by AngII, as low expression of ADAM17 under normal conditions and enhanced expression in areas of interstitial fibrosis damaged human kidneys have been reported23. Additionally, AngII-induced renal interstitial fibrosis can be inhibited in proximal tubule specific EGFR null mice or with erlotinib treatment24, and cardiac specific HB-EGF transgenic mice develop cardiac fibrosis25. In our control model of 2 week AngII infusion, interstitial fibrosis within the heart was too marginal to be quantitatively evaluated. However, it is likely that the paracrine production of HB-EGF and activation of EGFR via activation of ADAM17 in VSMCs, as well as other cell types, may be critical for development of overall tissue fibrosis associated with hypertension.
The present study demonstrating predominantly vascular ADAM17 induction and EGFR activation suggests a vascular contribution to cardiac hypertrophy via EGFR transactivation induced by AngII. It should be noted that vascular smooth muscle (but not cardiac myocyte)-targeted Gq inhibition attenuates cardiac hypertrophy in AngII-infused mice26. However, cardiac myocyte-targeted expression of dnEGFR inhibited cardiac hypertrophy induced by AngII12. Cardiac specific deletion of an ER stress sensor, double stranded RNA-activated protein kinase R-like ER kinase exacerbates pressure overload-induced cardiac hypertrophy27. Thus, interdependence for vascular and cardiac hypertrophy is most likely. Although vascular EGFR-silenced mice develop cardiac hypertrophy and hypotension with aging22, this phenotype could be due to cardiac EGFR silencing since the sm22α-promoter also drives significant deletion of EGFR in cardiomyocytes22. Indeed recent studies have reported a cardioprotective role for EGFR28, 29. However, partially contrasting findings were observed in mice regarding cardiac hypertrophy, contractility and apoptosis under the conditions of chronic isoproterenol infusion with co-treatment of EGFR inhibitors28, 30, albeit using distinct inhibitors and administrative routes. Therefore, further research is needed to clarify the roles of cell type-specific EGFR transactivation induced by distinct G protein-coupled receptors in cardiac pathophysiology such as those utilizing both cardiomyocyte and VSMC EGFR-deficient mice.
Although ER stress has been implicated in cardiovascular diseases14, limited information has been available about its role in hypertension and associated complications. It has been recently reported that ER stress inhibitors attenuate AngII-induced aortic apoptotic and fibrotic responses in rats31. Our data further suggest a potential prevention of hypertensive cardiovascular remodeling (but not hypertension) by reducing ER stress through inhibition of the ADAM17/EGFR axis of AngII signal transduction. However, PBA treatment was reported to attenuate hypertension in AngII-infused mice19, 32. Brain-selective treatment of ER stress inhibitor, tauroursodeoxycholic acid, also attenuates AngII-induced hypertension in mice20. It is possible that the anti-hypertensive effect of PBA might be overridden in the present study since a higher concentration of AngII was infused. In addition, mechanical stretch of VSMCs, which is enhanced during hypertension, may lead to EGFR activation33 and ER stress34 in these cells. However, this may not be the case in our study since hypertension was unaltered with either treatment. It should also be noted that most of the remodeling assessments were not completely inhibited by erlotinib or PBA. As EGFR activation or ER stress was completely suppressed by the corresponding inhibitor, respectively, it is likely that there is a minor but still important signaling mechanism causing cardiovascular remodeling independently from the EGFR/ER stress cascade.
Inhibition of ADAM17 induction with EGFR inhibition or ER stress inhibition suggests the presence of a feed-forward ADAM17 signal amplification, which seems to involve transcriptional up-regulation of ADAM17. Indeed, the ADAM17 promoter has functional ER stress responsible elements35. We have recently demonstrated that ADAM17 mRNA induction in aorta in response to AngII infusion was inhibited by erlotinib treatment36. Hypoxia inducible factor (HIF)-1α appears to mediate ADAM17 promoter activation by AngII37, and AngII is reported to activate HIF-1α through EGFR transactivation in VSMCs38. ER stress may also be involved in HIF-1α activation as reported in the vascular endothelial growth factor promoter39. Thus, AngII induction of ADAM17 via EGFR activation may involve downstream signal crosstalk between HIF-1α and ER stress.
It has been reported that activation of vascular EGFR or ER stress causes endothelial cell dysfunction19, 21, which could be very important to enhance vascular remodeling in response to AngII. Aldosterone antagonism also prevents AngII-dependent vascular and cardiac fibrosis40. While the role of EGFR in aldosterone-induced cardiovascular remodeling remains controversial41, it will be interesting to test the causal role of ER stress in aldosterone-induced vascular remodeling in the future.
Perspectives
EGFR signal transduction appears to be essential for cardiovascular remodeling associated with ER/oxidative stress but not for hypertension in mice with AngII infusion. The signal seems to include a feed forward mechanism involving vascular ADAM17 induction via ER stress acting upon its gene promoter, which enhances EGFR ligand production and subsequent EGFR activation and vascular remodeling (Supplemental Figure S4). ER stress also causes ROS generation, enhancing EGFR activation. Additional research in this cascade is warranted in order to seek for alternative or additive treatments against hypertension and its complications.
Supplementary Material
Novelty and Significance.
What is new?
Analyses of blood pressure and vascular pathology in the heart, kidney and aorta with intervention established a role for EGFR and ER stress in AngII-induced pathological vascular remodeling independent of hypertension in mice.
The concept of the feed-forward induction of vascular ADAM17 to amplify the EGFR pathway and subsequent vascular remodeling was presented.
What is relevant?
Results indicating prevention of vascular remodeling but not hypertension by erlotinib or PBA provide a foundation to seek a potential add-on therapy to current pressure lowering treatments for hypertension.
The vascular restricted EGFR signal transduction highlights the importance of vascular pathology for subsequent tissue dysfunction in hypertension.
Summary
In AngII-infused mice, vascular hypertrophy and perivascular fibrosis were prevented by pharmacological inhibition of EGFR activity and ER stress. AngII infusion showed vascular ADAM17 induction, EGFR activation and ER stress, which were attenuated by respective inhibitors. Cultured vascular cells were utilized to confirm the potential feed-forward mechanism of ADAM17 induction through transcriptional activation.
Acknowledgments
We thank Kunie Eguchi for technical assistance and support.
Sources of Funding
This work was supported by National Institute of Health grants, HL076770 (S.E.) and HL105414 (D.G.T.), and by American Heart Association grants, 13GRNT17060036 (S.E.).
Footnotes
Disclosures
None.
References
- 1.Kang N, Walther T, Tian XL, Bohlender J, Fukamizu A, Ganten D, Bader M. Reduced hypertension-induced end-organ damage in mice lacking cardiac and renal angiotensinogen synthesis. J Mol Med (Berl) 2002;80:359–366. doi: 10.1007/s00109-002-0326-6. [DOI] [PubMed] [Google Scholar]
- 2.Gibbons GH. Angiotensin-converting enzyme inhibition and vascular structure in hypertension. J Cardiovasc Pharmacol. 1991;18(Suppl 7):S19–S24. [PubMed] [Google Scholar]
- 3.Briet M, Schiffrin EL. Treatment of arterial remodeling in essential hypertension. Curr Hypertens Rep. 2013;15:3–9. doi: 10.1007/s11906-012-0325-0. [DOI] [PubMed] [Google Scholar]
- 4.Kanaide H, Ichiki T, Nishimura J, Hirano K. Cellular mechanism of vasoconstriction induced by angiotensin II: it remains to be determined. Circ Res. 2003;93:1015–1017. doi: 10.1161/01.RES.0000105920.33926.60. [DOI] [PubMed] [Google Scholar]
- 5.Ohtsu H, Dempsey PJ, Frank GD, Brailoiu E, Higuchi S, Suzuki H, Nakashima H, Eguchi K, Eguchi S. ADAM17 mediates epidermal growth factor receptor transactivation and vascular smooth muscle cell hypertrophy induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2006;26:e133–e137. doi: 10.1161/01.ATV.0000236203.90331.d0. [DOI] [PubMed] [Google Scholar]
- 6.Ohtsu H, Higuchi S, Shirai H, Eguchi K, Suzuki H, Hinoki A, Brailoiu E, Eckhart AD, Frank GD, Eguchi S. Central role of Gq in the hypertrophic signal transduction of angiotensin II in vascular smooth muscle cells. Endocrinology. 2008;149:3569–3575. doi: 10.1210/en.2007-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott KJ, Bourne AM, Takayanagi T, Takaguri A, Kobayashi T, Eguchi K, Eguchi S. ADAM17 silencing by adenovirus encoding miRNA-embedded siRNA revealed essential signal transduction by angiotensin II in vascular smooth muscle cells. J Mol Cell Cardiol. 2013;62:1–7. doi: 10.1016/j.yjmcc.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takaguri A, Kimura K, Hinoki A, Bourne AM, Autieri MV, Eguchi S. A disintegrin and metalloprotease 17 mediates neointimal hyperplasia in vasculature. Hypertension. 2011;57:841–845. doi: 10.1161/HYPERTENSIONAHA.110.166892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 10.Kagiyama S, Eguchi S, Frank GD, Inagami T, Zhang YC, Phillips MI. Angiotensin II-induced cardiac hypertrophy and hypertension are attenuated by epidermal growth factor receptor antisense. Circulation. 2002;106:909–912. doi: 10.1161/01.cir.0000030181.63741.56. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Oka T, Chow FL, Cooper SB, Odenbach J, Lopaschuk GD, Kassiri Z, Fernandez-Patron C. Tumor necrosis factor-alpha-converting enzyme is a key regulator of agonist-induced cardiac hypertrophy and fibrosis. Hypertension. 2009;54:575–582. doi: 10.1161/HYPERTENSIONAHA.108.127670. [DOI] [PubMed] [Google Scholar]
- 12.Zhai P, Galeotti J, Liu J, Holle E, Yu X, Wagner T, Sadoshima J. An angiotensin II type 1 receptor mutant lacking epidermal growth factor receptor transactivation does not induce angiotensin II-mediated cardiac hypertrophy. Circ Res. 2006;99:528–536. doi: 10.1161/01.RES.0000240147.49390.61. [DOI] [PubMed] [Google Scholar]
- 13.Steins M, Thomas M, Geissler M. Erlotinib. Recent Results Cancer Res. 2014;201:109–123. doi: 10.1007/978-3-642-54490-3_6. [DOI] [PubMed] [Google Scholar]
- 14.Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010;107:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res. 2010;107:1071–1082. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- 16.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 18.Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004;110:705–712. doi: 10.1161/01.CIR.0000137836.95625.D4. [DOI] [PubMed] [Google Scholar]
- 19.Kassan M, Galan M, Partyka M, Saifudeen Z, Henrion D, Trebak M, Matrougui K. Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice. Arterioscler Thromb Vasc Biol. 2012;32:1652–1661. doi: 10.1161/ATVBAHA.112.249318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young CN, Cao X, Guruju MR, Pierce JP, Morgan DA, Wang G, Iadecola C, Mark AL, Davisson RL. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest. 2012;122:3960–3964. doi: 10.1172/JCI64583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galan M, Kassan M, Choi SK, Partyka M, Trebak M, Henrion D, Matrougui K. A novel role for epidermal growth factor receptor tyrosine kinase and its downstream endoplasmic reticulum stress in cardiac damage and microvascular dysfunction in type 1 diabetes mellitus. Hypertension. 2012;60:71–80. doi: 10.1161/HYPERTENSIONAHA.112.192500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreier B, Rabe S, Schneider B, Bretschneider M, Rupp S, Ruhs S, Neumann J, Rueckschloss U, Sibilia M, Gotthardt M, Grossmann C, Gekle M. Loss of epidermal growth factor receptor in vascular smooth muscle cells and cardiomyocytes causes arterial hypotension and cardiac hypertrophy. Hypertension. 2013;61:333–340. doi: 10.1161/HYPERTENSIONAHA.112.196543. [DOI] [PubMed] [Google Scholar]
- 23.Melenhorst WB, Visser L, Timmer A, van den Heuvel MC, Stegeman CA, van Goor H. ADAM17 upregulation in human renal disease: a role in modulating TGF-alpha availability? Am J Physiol Renal Physiol. 2009;297:F781–F790. doi: 10.1152/ajprenal.90610.2008. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Chen JK, Nagai K, Plieth D, Tan M, Lee TC, Threadgill DW, Neilson EG, Harris RC. EGFR signaling promotes TGFbeta-dependent renal fibrosis. J Am Soc Nephrol. 2012;23:215–224. doi: 10.1681/ASN.2011070645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lian H, Ma Y, Feng J, Dong W, Yang Q, Lu D, Zhang L. Heparin-binding EGF-like growth factor induces heart interstitial fibrosis via an Akt/mTor/p70s6k pathway. PLoS One. 2012;7:e44946. doi: 10.1371/journal.pone.0044946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keys JR, Greene EA, Koch WJ, Eckhart AD. Gq-coupled receptor agonists mediate cardiac hypertrophy via the vasculature. Hypertension. 2002;40:660–666. doi: 10.1161/01.hyp.0000035397.73223.ce. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Kwak D, Lu Z, Xu X, Fassett J, Wang H, Wei Y, Cavener DR, Hu X, Hall J, Bache RJ, Chen Y. Endoplasmic reticulum stress sensor protein kinase R-like endoplasmic reticulum kinase (PERK) protects against pressure overload-induced heart failure and lung remodeling. Hypertension. 2014;64:738–744. doi: 10.1161/HYPERTENSIONAHA.114.03811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grisanti LA, Talarico JA, Carter RL, Yu JE, Repas AA, Radcliffe SW, Tang HA, Makarewich CA, Houser SR, Tilley DG. beta-Adrenergic receptor-mediated transactivation of epidermal growth factor receptor decreases cardiomyocyte apoptosis through differential subcellular activation of ERK1/2 and Akt. J Mol Cell Cardiol. 2014;72:39–51. doi: 10.1016/j.yjmcc.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grisanti LA, Repas AA, Talarico JA, Gold JI, Carter RL, Koch WJ, Tilley DG. Temporal and gefitinib-sensitive regulation of cardiac cytokine expression via chronic beta-adrenergic receptor stimulation. Am J Physiol Heart Circ Physiol. 2015;308:H316–H330. doi: 10.1152/ajpheart.00635.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spitler KM, Webb RC. Endoplasmic reticulum stress contributes to aortic stiffening via proapoptotic and fibrotic signaling mechanisms. Hypertension. 2014;63:e40–e45. doi: 10.1161/HYPERTENSIONAHA.113.02558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang B, Wang S, Wang Q, Zhang W, Viollet B, Zhu Y, Zou MH. Aberrant endoplasmic reticulum stress in vascular smooth muscle increases vascular contractility and blood pressure in mice deficient of AMP-activated protein kinase-alpha2 in vivo. Arterioscler Thromb Vasc Biol. 2013;33:595–604. doi: 10.1161/ATVBAHA.112.300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwasaki H, Eguchi S, Ueno H, Marumo F, Hirata Y. Mechanical stretch stimulates growth of vascular smooth muscle cells via epidermal growth factor receptor. Am J Physiol Heart Circ Physiol. 2000;278:H521–H529. doi: 10.1152/ajpheart.2000.278.2.H521. [DOI] [PubMed] [Google Scholar]
- 34.Cheng WP, Hung HF, Wang BW, Shyu KG. The molecular regulation of GADD153 in apoptosis of cultured vascular smooth muscle cells by cyclic mechanical stretch. Cardiovasc Res. 2008;77:551–559. doi: 10.1093/cvr/cvm057. [DOI] [PubMed] [Google Scholar]
- 35.Rzymski T, Petry A, Kracun D, Riess F, Pike L, Harris AL, Gorlach A. The unfolded protein response controls induction and activation of ADAM17/TACE by severe hypoxia and ER stress. Oncogene. 2012;31:3621–3634. doi: 10.1038/onc.2011.522. [DOI] [PubMed] [Google Scholar]
- 36.Obama T, Tsuji T, Kobayashi T, Fukuda Y, Takayanagi T, Taro Y, Kawai T, Forrester SJ, Elliott KJ, Choi ET, Daugherty A, Rizzo V, Eguchi S. Epidermal Growth Factor Receptor Inhibitor Protects Abdominal Aortic Aneurysm in a Mouse Model. Clin Sci (Lond) 2015;128:559–565. doi: 10.1042/CS20140696. [DOI] [PubMed] [Google Scholar]
- 37.Obama T, Takayanagi T, Kobayashi T, Bourne AM, Elliott KJ, Charbonneau M, Dubois CM, Eguchi S. Vascular induction of a disintegrin and metalloprotease 17 by angiotensin II through hypoxia inducible factor 1alpha. Am J Hypertens. 2015;28:10–14. doi: 10.1093/ajh/hpu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lauzier MC, Page EL, Michaud MD, Richard DE. Differential regulation of hypoxia-inducible factor-1 through receptor tyrosine kinase transactivation in vascular smooth muscle cells. Endocrinology. 2007;148:4023–4031. doi: 10.1210/en.2007-0285. [DOI] [PubMed] [Google Scholar]
- 39.Pereira ER, Frudd K, Awad W, Hendershot LM. Endoplasmic reticulum (ER) stress and hypoxia response pathways interact to potentiate hypoxia-inducible factor 1 (HIF-1) transcriptional activity on targets like vascular endothelial growth factor (VEGF) J Biol Chem. 2014;289:3352–3364. doi: 10.1074/jbc.M113.507194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luther JM, Luo P, Wang Z, Cohen SE, Kim HS, Fogo AB, Brown NJ. Aldosterone deficiency and mineralocorticoid receptor antagonism prevent angiotensin II-induced cardiac, renal, and vascular injury. Kidney Int. 2012;82:643–651. doi: 10.1038/ki.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griol-Charhbili V, Fassot C, Messaoudi S, Perret C, Agrapart V, Jaisser F. Epidermal growth factor receptor mediates the vascular dysfunction but not the remodeling induced by aldosterone/salt. Hypertension. 2011;57:238–244. doi: 10.1161/HYPERTENSIONAHA.110.153619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.