Abstract
Thick ascending limbs (THALs) reabsorb 25–30% of the filtered NaCl. About 50–70% is reabsorbed via the transcellular pathway and 30–50% is reabsorbed through the Na-selective paracellular pathway. Nitric oxide (NO) inhibits transepithelial Na reabsorption, but its effects on the paracellular pathway are unknown. We hypothesized that NO decreases the selectivity of the paracellular pathway in THALs via cGMP-dependent protein kinase (PKG). To assess relative Na/Cl permeability ratios (PNa/PCl), we perfused rat THALs and measured the effect of reducing bath NaCl on transepithelial voltage, creating dilution potentials, with vehicle, NO donors and endogenous NO. PNa/PCl was calculated using the Goldman-Hodgkin-Katz equation. Reducing bath Na/Cl to 16/8; 32/24; and 64/56 mmol/l created dilution potentials of −13.6 ± 2.2, −10.8 ± 3.0, and −6.1 ± 0.9 mV, respectively. Calculated PNa/PCls were 2.0 ± 0.2, 2.2 ± 0.5, and 1.9 ± 0.2. The NO donor spermine NONOate (SPM; 200 μmol/l) blunted the dilution potential caused by 32/24 mmol/l Na/Cl from −11.1 ± 2.1 to −6.5 ± 1.6 mV (p<0.004) and PNa/PCl from 2.2 ± 0.4 to 1.5± 0.2. Nitroglycerin (200 μmol/l), another NO donor, also reduced PNa/PCl. Controls showed no significant changes. Dibutyryl-cGMP decreased dilution potentials from −13.4 ± 2.9 to −7.5 ± 1.8 mV (n=6, p<0.01). PKG inhibition with KT5823 (4μM) blocked the effect of SPM, while phosphodiesterase 2 inhibition did not. Endogenously-produced NO mimicked the effect of the NO donors. Conclusion: NO reduces the selectivity of the paracellular pathway in thick ascending limbs via cGMP and PKG.
Keywords: sodium transport, permeability, hypertension, kidney, NO
Thick ascending limbs reabsorb about 30% of the filtered load of NaCl 1, 2. About 50–70% of the Na traverses the transcellular pathway, entering the cell via Na/K/2Cl cotransport and Na/H exchange, and exiting via Na/K ATPase. Transcellular NaCl reabsorption generates a lumen-positive potential that drives Na reabsorption via the paracellular pathway 3. Up to 50% of the total Na reabsorbed by thick ascending limbs traverses the paracellular pathway 4. The paracellular pathway in thick ascending limbs is selective for Na over Cl with a Na/Cl permeability ratio (PNa/PCl) of about 2 3, 5, 6.
Renal nitric oxide causes natriuresis and diuresis 7-9. A major part of this is due to inhibition of NaCl reabsorption by the thick ascending limb 10. We have shown that NO inhibits net NaCl 11 and Na bicarbonate 12 reabsorption in this segment. We have also shown that NO inhibits Na/K/2Cl cotransport 13 and Na/H exchange activity 14. The former was due to an increase in cGMP, activation of phosphodiesterase 2, and consequent decreases in cAMP 15. The latter was also due to an increase in cGMP with subsequent activation of cGMP-dependent protein kinase (PKG) rather than phosphodiesterase 2 12. Although as much as 50% of reabsorbed Na traverses the paracellular pathway, the effects of NO on transport via this route are unknown.
Even though there have been no studies of the effect of NO on the properties of the paracellular pathway in native renal epithelia, several reports have shown that it regulates the permeability and selectivity of the paracellular pathway in other cells. NO increases the permeability of the paracellular pathway in endothelial cells 16 as the initiating step in angiogenesis 17,18. NO has also been shown to reduce the assembly of the proteins that form the paracellular pathway of Sertoli cells via a cGMP and PKG-dependent process 19. Finally, in intestinal epithelial cells NO has been reported to both increase and decrease total permeability and permselectivity of the paracellular pathway in both physiological and pathological states 20-24.
We hypothesized that NO reduces the permselectivity of the paracellular pathway in thick ascending limbs via a process involving cGMP and PKG.
Methods
An expanded Methods section is available in the online-only Data Supplement. Please see http://hyper.ahajournals.org.
Animals
We used male Sprague-Dawley rats (Charles River Breeding Laboratories, Wilmington, MA) weighing 110–150 g for this study. Animals were maintained on a diet containing 0.22% sodium and 1.1% potassium (Purina, Richmond, IN) for at least 4 days. All protocols involving animals were approved by the Institutional Animal Care and Use Committee (IACUC) of Case Western Reserve University in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Results
Effect of different NaCl concentrations on dilution potential and PNa/PCl
To set the most adequate experimental conditions for subsequent protocols, we first measured the dilution potentials generated by replacing the basolateral solution for one containing either 16/8; 32/24; or 64/56 mmol/l Na/Cl. The dilution potentials were − 13.6 ± 2.2 mV (n=5), −10.8 ± 3.0 mV (n=6), and −6.1 ± 0.9 mV (n=4), respectively. The calculated PNa/PCls were 2.0 ± 0.2, 2.2 ± 0.5, and 1.9 ± 0.2. We decided to use the solution containing 32/24 mmol/l Na/Cl in all subsequent experiments.
Effect of NO donors on the permselectivity of the paracellular pathway
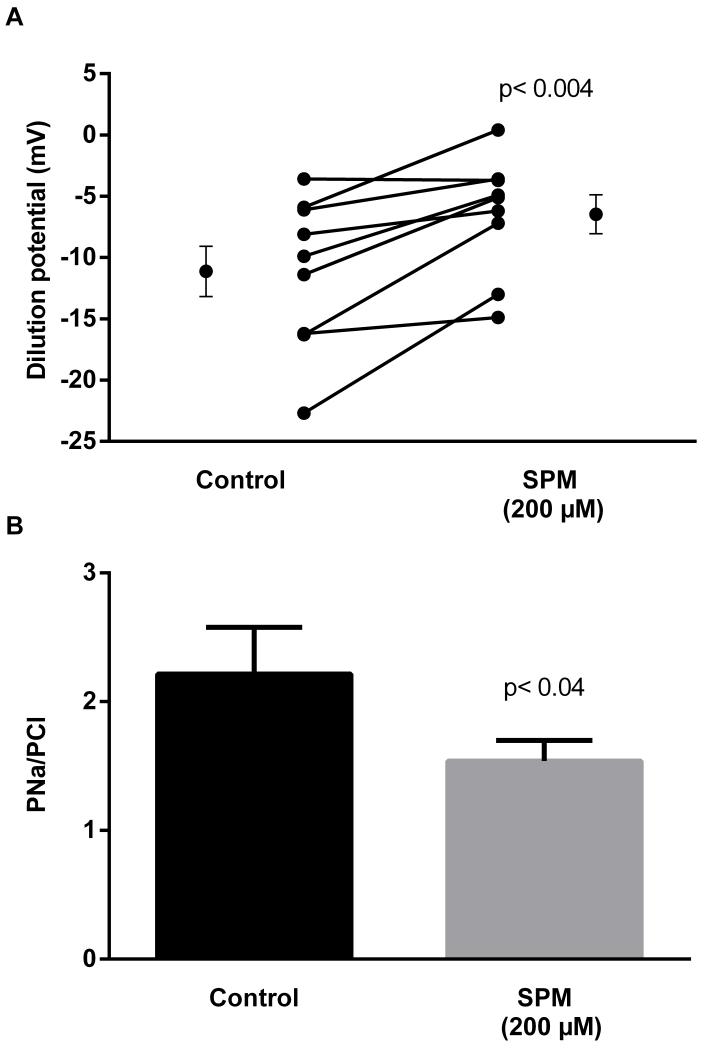
We first evaluated the effect of 200 μmol/l spermine NONOate, an NO donor, on the dilution potential caused by reducing the bath to 32/24 mmol/l Na/Cl and the calculated PNa/PCl. During the control period, the dilution potential was −11.1 ± 2.1 mV. After the addition of spermine NONOate to the bath, the dilution potential was −6.5 ± 1.6 mV, a reduction of 41.4% (Fig 1; p <0.004; n=9). The calculated PNa/PCl in the control period was 2.2 ± 0.4, while that after spermine NONOate was 1.5 ± 0.2 (p< 0.04). Controls showed no significant changes with time.
Figure 1.
Effect of the NO donor spermineNONOate (SPM) on dilution potentials evoked by 32/24 mmol/l Na/Cl and calculated PNa/PCl. Individual experiments and means ± SEM are depicted (n=9).
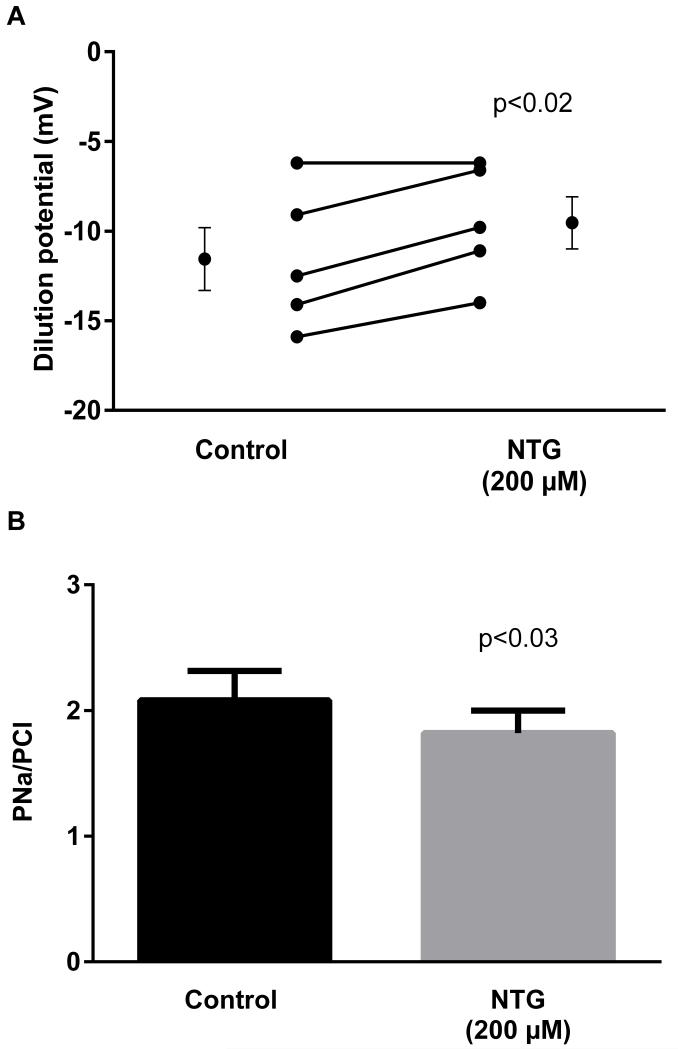
A second NO donor, nitroglycerin (200 μmol/l), was also used. During the control period, the dilution potential was −11.6 ± 1.7 mV. After the addition of nitroglycerin, the dilution potential was −9.5 ± 1.5 mV, a reduction of 18.1% (Fig 2; p<0.02; n=5). The calculated PNa/PCl in the control period was 2.1 ± 0.2, while that after nitroglycerin was 1.8 ± 0.2 (p< 0.03).
Figure 2.
Effect of nitroglycerin (NTG) on dilution potentials evoked by 32/24 mmol/l Na/Cl and calculated PNa/PCl. Individual experiments and means ± SEM are depicted (n=5).
Effect of endogenously-produced NO on the permselectivity of the paracellular pathway
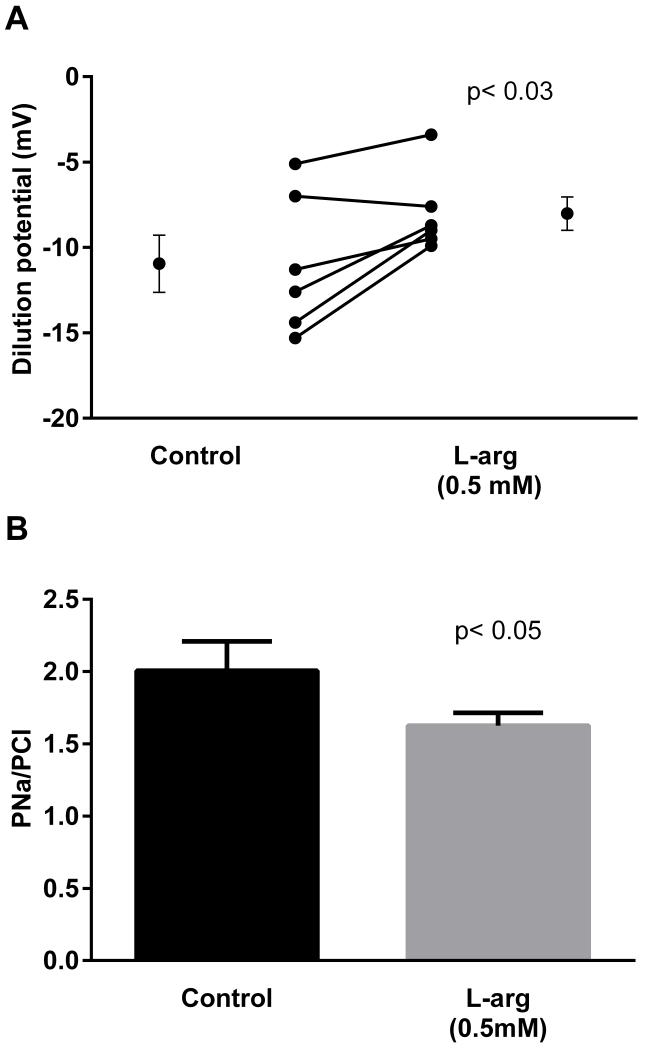
Thick ascending limbs produce NO from L-arginine. Thus we next tested whether endogenously-produced NO reduced the permselectivity of the paracellular pathway. During the control period, the dilution potential was −11.0 ± 1.7 mV. When 0.5 mM L-arginine was added to stimulate NO production, the dilution potential was −8.0 ± 1.0 mV (Fig 3; n=6; p<0.03), a 27.3% reduction. The calculated PNa/PCl was 2.0 ± 0.2 in the control period, while that after L-arginine was 1.6 ± 0.1 (p<0.05).
Figure 3.
Effect of L-arginine (L-arg) on dilution potentials evoked by 32/24 mmol/l Na/Cl and calculated PNa/PCl. Individual experiments and means ± SEM are depicted (n=6).
Signaling cascade mediating the effect of NO on the paracellular pathway
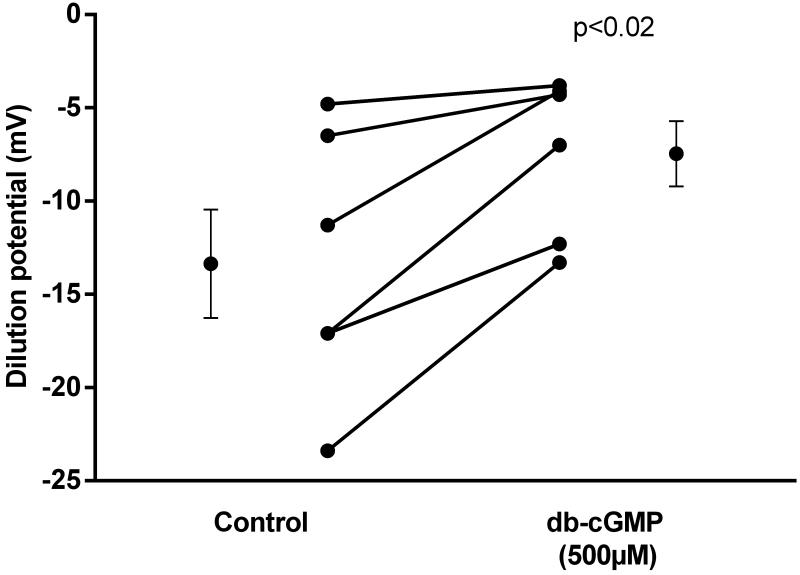
NO exerts most of its effects via cGMP. Thus we tested whether cGMP mediates its effects on PNa/PCl. We found that during the control period, the dilution potential was −13.4 ± 2.9 mV. In the presence of the membrane-permeant cGMP analog, dibutyryl-cGMP (500 μmol/l), the dilution potential was −7.5 ± 1.8 mV, a reduction of 44% (Fig 4; p< 0.02; n=6). Controls showed no significant changes with time.
Figure 4.
Effect of the second messenger cGMP on dilution potentials evoked by 32/24 mmol/l Na/Cl. db-cGMP, dibutyryl-cGMP. Individual experiments and means ± SEM are depicted (n=6).
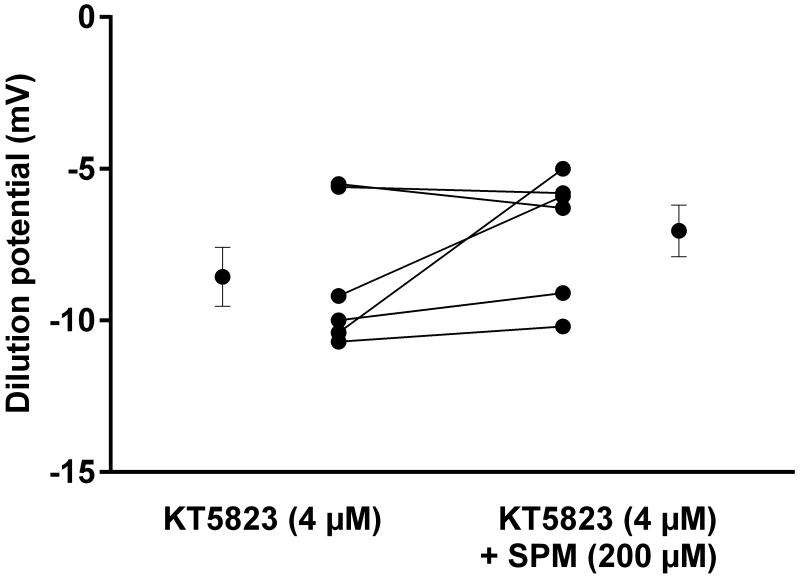
cGMP can activate two downstream mediators, PKG 12 and phosphodiesterase 2 15. Thus, we next tested which of these mediates the actions of NO. First we studied the effect of the PKG inhibitor KT5823 (4 μmol/l). During the control period, in the presence of KT 5823 the dilution potential was −8.6 ± 0.1 mV. When spermine NONOate was added, the dilution potential was −7.1 ± 0.9 mV, not significantly different (Fig 5, n=6). KT 5823 alone did not affect the dilution potential.
Figure 5.
Effect of spermine NONOate (SPM) on dilution potentials evoked by 32/24 mmol/l Na/Cl in the presence of PKG inhibitor KT5823. Individual experiments and means ± SEM are depicted (n=6).
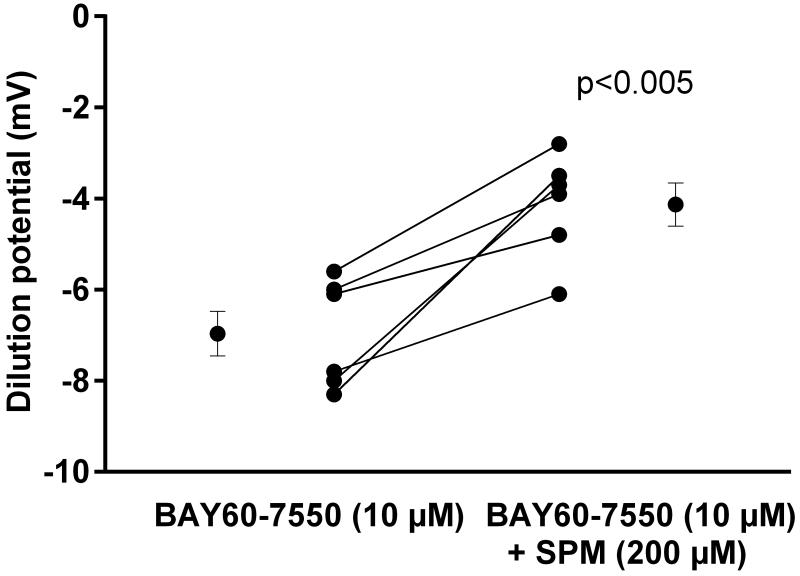
Next we tested the effect of phosphodiesterase 2-specific inhibitor BAY60-7550 (10 μmol/l). The dilution potential in the control period with BAY60-7550 present was − 7.0 ± 0.5 mV. After the addition of spermine NONOate (200 μmol/l), the dilution potential was −4.1 ± 0.5 mV (Fig 6; n=6; p<0.005), a reduction of 41.4%. Time controls showed no significant differences.
Figure 6.
Effect of SPM on dilution potentials evoked by 32/24 mmol/l Na/Cl in the presence of the phosphodiesterase inhibitor BAY60-7550. Individual experiments and means ± SEM are depicted (n=6).
Discussion
Our hypothesis was that NO decreases the permselectivity of the paracellular pathway in thick ascending limbs. We found that: 1) the PNa/PCl (a measure of permselectivity of the paracellular pathway) of perfused thick ascending limbs measured under different conditions was about 2; 2) NO donors reduce PNa/PCl; 3) endogenously-produced NO reduces the PNa/PCl of the paracellular pathway; 4) cGMP mimicks the effects of the NO donors; 5) inhibiting PKG blocks the effect of NO; and 6) inhibiting phosphodiesterase 2 does not block the effect of NO. These are the first results showing that both exogenously-added and endogenously-produced NO reduces the permselectivity of the paracellular pathway in the thick ascending limb, or any nephron segment. They are also the first data showing that cGMP and PKG mediate these effects.
We studied the effects of NO on the paracellular pathway by measuring Na/Cl dilution potentials and calculating PNa/PCl. The ionic permeability of the paracellular pathway is a result of charge selectivity and steric hindrance. Dilution potentials result from the differential permeabilities of Na and Cl of the paracellular pathway. Thus they are a direct measure of a physiologically relevant parameter which takes into account both charge selectivity and steric hindrance. We found that the PNa/PCl was about 2 under a variety of conditions and did not change significantly with time. Our results are comparable to those previously reported 3, 5, 6 and indicate that the paracellular pathway of thick ascending limbs is selective for Na over Cl by a factor of 2. The fact that PNa/PCl was stable over time indicates that cell viability was maintained throughout the protocol. This is consistent with our previous data which show that isolated, perfused thick ascending limbs remain viable for 85-90 min 12, 15, 25, 26.
NO has been shown to reduce net transepithelial NaCl reabsorption 10, 27 and Na/K/2 Cl cotransport activity 13 in thick ascending limbs. However its effect on the paracellular pathway, which is response for as much as 50% of Na reabsorption, is unknown. Our data show that the NO donor spermine NONOate decreased both the magnitude of the Na/Cl dilution potential and the calculated PNa/PCl. Nitroglycerin had a similar effect.
The fact that two chemically distinct NO donors similarly reduced PNa/PCl is strong evidence that the effect was due to NO and not a byproduct of either spermine NONOate’s or nitroglycerin’s metabolism/degradation. The larger inhibitory effect observed with spermine NONOate despite using equimolar concentrations of both compounds can most likely be explained by the fact that while spermine NONOate spontaneously degrades releasing NO with a half-time of 40 min in aqueous solutions, nitroglycerin needs to be metabolized to produce NO. The concentration of spermine NONOate we used produces an NO concentration of about 1.6 μM. This is within the physiological range of 0.6 to 9 μM previously reported for the renal medulla 28, 29.
Since thick ascending limbs produce NO and physiologically relevant concentrations of NO from donors reduced PNa/PCl, we next tested whether the results observed with exogenously-added NO were mimicked by endogenously produced NO. We found that endogenously-produced NO significantly decreased both dilution potentials and PNa/PCl.
We tested the effects of endogenously produced NO on PNa/PCl by first depleting tubules of L-arginine by perfusing and bathing them in L-arginine-free solution, and then adding it back to the bath. This protocol works because the Y+ transporter that moves L-arginine into the cell will also remove it from the cell if the chemical gradient favors exit. Since the bath is constantly exchanged, the concentration of L-arginine in the bath is effectively zero. Thus the chemical gradient always favors L-arginine exit from the cell. When L-arginine is added back to the basolateral bath NO production begins because NOS is already activated by luminal flow and only lacks substrate to produce NO 30.
Our finding that addition of L-arginine to the bath reduces PNa/PCl and the conclusion that this is due to NO is based on our previously published work showing that: 1) L-arginine stimulates NO production 31; 2) L-arginine inhibits net NaCl reabsorption and this is blocked by L-NAME, an inhibitor of NOS; 3) D-arginine does not mimic the effects of L-arginine on net NaCl reabsorption 32. If L-arginine per se rather than NO were affecting the paracellular pathway in the experiments reported here, we would have been able to observe this effect in the previously reported experiments in which net NaCl reabsorption was measured. Such an effect would have reduced net reabsorption, not been inhibitable by L-NAME, and may have been mimicked by D-arginine.
The concentration of L-arginine in the outer medulla of the kidney is not clear. Recently it has been reported that the concentration in the entire kidney may be as high as 0.5 mM 33. If this value is accepted for whole kidney, one would expect that the concentration in the outer medulla could be considerably greater. Thus, the concentration of L-arginine we used approximates physiological concentrations 34.
In our experimental design luminal flow serves as the stimulus for NO production 30, 34. Flow in the nephron is not static but oscillates 35-37, and in some nephron segments it stops periodically 35, 37. Thus, it is likely that the NO signaling also oscillates and therefore the effect of NO on the paracellular pathway is presumably always present but variable.
There are at least three possible explanations for the fact that NO reduces PNa/PCl. First, PNa may be reduced. Second, PCl may be increased. Third, both PNa and PCl may be affected in opposite ways. Given that NO reduces net transepithelial NaCl reabsorption, it is likely that NO decreases PNa rather than increasing PCl. Such an effect would be expected to contribute to the reduction in net NaCl reabsorption caused by NO.
The permselectivity of the paracellular pathway is due to the claudin family of proteins. Thick ascending limbs express a number of claudins including claudin−3, −10, − 16, −18 and −19 38. Recently it was reported that claudin−10b, an isoform of claudin−10, is the primary regulator of Na selectivity of the paracelular pathway 38. Thus, NO may be affecting this protein.
Although we have previously shown that NO inhibits active, transcellular NaCl reabsorption 13, 14, 32, this is likely to have a negligible effect on the dilution potentials and calculated PNa/PCl in our experiments because of the nature of our experimental design. The dilution potentials that we used to calculate PNa/PCl in the presence of NO take into account any effect NO may have on the spontaneously developed transepithelial voltage due to active transport. Furthermore, since we diluted the concentration of NaCl in the bath rather than in the luminal perfusion solution, Na/K/2 Cl cotransport activity is not diminished by the reduction in NaCl concentration, and, in fact, if anything it would be enhanced. This would lead to an underestimation of the true dilution potential, and thus our experimental design is conservative by nature. We do not think this is a major issue, however, because our calculated control PNa/PCl ratios are consistent with those previously reported in the literature 3, 5, 6, 39.
Our study is the first to investigate whether NO regulates the permselectivity of the paracellular pathway in isolated, perfused tubules. Besides our study, there is little evidence regarding the effects of NO on paracellular permeability in the kidney. As far as we know, there is only a single report by Liang et al 40. These authors showed a dose-dependent effect of NO on paracellular permeability of immortalized opossum kidney cells in culture. In this study high concentrations of the NO donor Na nitroprusside increased permeability, increased lipid peroxidation, and reduced ATP levels 40. Given the long incubation times used in this study and the fact that the effects were reversed by superoxide dismutase, it is unclear whether the results were due to NO per se or ONOO−, produced when NO and O2− react.
Outside the kidney NO has been shown to regulate the permeability of the paracellular pathway in numerous systems. In epithelial cells of the gastrointestinal tract, NO has been shown to both increase and decrease permeability 20-23, 41-45. The precise explanation for these data is unclear; however it may be related to the source of the NO, and therefore its concentration. Most studies in which constitutive NO synthases (cNOSs), including neuronal NO synthase (nNOS) and endothelial NO synthase (eNOS), report that NO decrease permeability 46. In contrast, when inducible NO synthase (iNOS) is activated, NO appears to increase permeability 20-22, 45, 46. It is known that cNOSs produce low, levels of NO while iNOS is a high output enzyme involved in pathophysiological disorders 47, 48. Thus low levels of NO produced by cNOS play a crucial role in the maintenance and regulation of normal gut function, whereas lack of cNOS activity or excess of iNOS activity leads to gut inflammation 46.
cGMP mediates the effects of NO on Na transport in both proximal tubules 49-51 and thick ascending limbs 11, 52. We found that NO reduces the permselectivity of the paracellular pathway via cGMP. Our data show that the membrane permeant cGMP analog dibutyryl-cGMP reproduced the effects of NO indicate that cGMP mediates the first step of the signaling pathway. These data are consistent with our previous data showing that NO inhibits net bicarbonate and NaCl reabsorption via cGMP in this nephron segment. They are also consistent with our data showing that NO reduces NHE3 and NKCC2 activity via cGMP. In fact, the magnitude of the decrease in the dilution potentials was larger, probably due to the fact that a relatively high concentration of the second messenger was used to assure a visible result in the case of an effect.
Both cGMP-dependent and independent pathways have been linked to changes in permeability in a variety of cell types. Increased cAMP levels have been consistently shown to preserve paracellular barrier integrity 53-55. Specifically in the kidney, cAMP has been shown to mediate changes in paracellular permeability in the proximal tubule, leading to changes in transport 56. The relation between cGMP levels and changes in the paracellular pathway is not as clear. Increases in cGMP have shown to both increase and decrease permeability and permselectivity in different cell types 57-60. Our results showing that cGMP regulates the paracellular pathway in the thick ascending limb are in agreement with other reports showing a NO/cGMP cascade involved in the decrease of permeability in endothelial cells 57, 58, as well as permeability and permselectivity 23, 24 of the intestinal barrier.
The effects of NO on the paracellular pathway in other cells are mediated by PKG; however, NO inhibits NaCl reabsorption by thick ascending limbs via phosphodiesterase 2. As a result, we next evaluated which mediates the actions of NO on the permselectivity of the paracellular pathway in isolated thick ascending limbs. We found that PKG mediates the effects of NO on the paracellular pathway, based on the fact that the effect of NO was prevented in the presence of a PKG inhibitor. In contrast, when we used a phosphodiesterase 2 inhibitor, NO still reduced PNa/PCl. Together, these data indicate that PKG rather than phosphodiesterase 2 mediates the effect of NO on the permselectivity of the paracellular pathway in thick ascending limbs. These data are consistent with our previous data showing that NO reduces bicarbonate reabsorption in this segment via PKG but differ from our results showing that phosphodiesterase 2 mediates the inhibitory action of NO on Cl reabsorption. The current results are in agreement with those of others who have implicated PKG as the downstream mediator of the effects of NO on the permeability of the paracellular pathway 18, 19.
Perspectives.
In summary, our results show that NO reduces the permselectivity of the paracellular pathway in thick ascending limbs via cGMP and PKG. The data presented in this report may provide evidence of an additional antihypertensive action exerted by NO in the thick ascending limb. To our knowledge it is the first work showing a regulatory effect of NO on the permselectivity of the paracellular pathway in native renal tubules.
Novelty and significance.
What Is New?
Nitric oxide decreases the permselectivity of the paracellular pathway in thick ascending limbs
This mechanism is mediated by cGMP and PKG
PDE2 does not mediate this process
What Is Relevant?
We have previously shown that NO decreases net NaCl and Na bicarbonate reabsorption in thick ascending limb by inhibiting transcellular Na/K/2Cl cotransport and Na/H exchange activity. Up to 50% of reabsorbed Na traverses the paracellular pathway. In this manuscript we show that NO inhibits the permselectivity of the paracellular pathway and its mechanism of action, which may provide evidence of an additional antihypertensive action exerted by NO in this segment.
Acknowledgments
Sources of funding: This work was in part supported by NIH HL 28982
Footnotes
Disclosures: None
References
- 1.Burg MB. Thick ascending limb of Henle’s loop. Kidney International. 1982;22:454–464. doi: 10.1038/ki.1982.198. [DOI] [PubMed] [Google Scholar]
- 2.Burg M, Good D. Sodium chloride coupled transport in mammalian nephrons. Annu Rev Physiol. 1983;45:533–547. doi: 10.1146/annurev.ph.45.030183.002533. [DOI] [PubMed] [Google Scholar]
- 3.Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs. II. ADH enhancement of transcellular NaCl cotransport; origin of transepithelial voltage. The American Journal of Physiology. 1981;241:F432–442. doi: 10.1152/ajprenal.1981.241.4.F432. [DOI] [PubMed] [Google Scholar]
- 4.Hebert SC, Andreoli TE. Control of NaCl transport in the thick ascending limb. The American Journal of Physiology. 1984;246:F745–756. doi: 10.1152/ajprenal.1984.246.6.F745. [DOI] [PubMed] [Google Scholar]
- 5.Burg MB, Green N. Function of the thick ascending limb of Henle’s loop. The American Journal of Physiology. 1973;224:659–668. doi: 10.1152/ajplegacy.1973.224.3.659. [DOI] [PubMed] [Google Scholar]
- 6.Greger R. Cation selectivity of the isolated perfused cortical thick ascending limb of Henle’s loop of rabbit kidney. Pflugers Archiv: European Journal of Physiology. 1981;390:30–37. doi: 10.1007/BF00582707. [DOI] [PubMed] [Google Scholar]
- 7.Manning RD, Jr., Hu L. Nitric oxide regulates renal hemodynamics and urinary sodium excretion in dogs. Hypertension. 1994;23:619–625. doi: 10.1161/01.hyp.23.5.619. [DOI] [PubMed] [Google Scholar]
- 8.Majid DS, Navar LG. Nitric oxide in the control of renal hemodynamics and excretory function. American Journal of Hypertension. 2001;14:74S–82S. doi: 10.1016/s0895-7061(01)02073-8. [DOI] [PubMed] [Google Scholar]
- 9.Ortiz PA, Garvin JL. Role of nitric oxide in the regulation of nephron transport. American Journal of Physiology. Renal Physiology. 2002;282:F777–784. doi: 10.1152/ajprenal.00334.2001. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Rojas JM, Kassem KM, Beierwaltes WH, Garvin JL, Herrera M. Nitric oxide produced by endothelial nitric oxide synthase promotes diuresis. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2010;298:R1050–1055. doi: 10.1152/ajpregu.00181.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia NH, Plato CF, Stoos BA, Garvin JL. Nitric oxide-induced inhibition of transport by thick ascending limbs from dahl salt-sensitive rats. Hypertension. 1999;34:508–513. doi: 10.1161/01.hyp.34.3.508. [DOI] [PubMed] [Google Scholar]
- 12.Ortiz PA, Garvin JL. Autocrine effects of nitric oxide on HCO(3)(−) transport by rat thick ascending limb. Kidney International. 2000;58:2069–2074. doi: 10.1111/j.1523-1755.2000.00379.x. [DOI] [PubMed] [Google Scholar]
- 13.Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na(+)-K(+)-2Cl(−) cotransporter activity. American Journal of Physiology. Renal Physiology. 2001;281:F819–825. doi: 10.1152/ajprenal.2001.281.5.F819. [DOI] [PubMed] [Google Scholar]
- 14.Garvin JL, Hong NJ. Nitric oxide inhibits sodium/hydrogen exchange activity in the thick ascending limb. The American Journal of Physiology. 1999;277:F377–382. doi: 10.1152/ajprenal.1999.277.3.F377. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz PA, Garvin JL. NO inhibits NaCl absorption by rat thick ascending limb through activation of cGMP-stimulated phosphodiesterase. Hypertension. 2001;37:467–471. doi: 10.1161/01.hyp.37.2.467. [DOI] [PubMed] [Google Scholar]
- 16.Yang S, Chen Y, Deng X, Jiang W, Li B, Fu Z, Du M, Ding R. Hemoglobin-induced nitric oxide synthase overexpression and nitric oxide production contribute to blood-brain barrier disruption in the rat. Journal of Molecular Neuroscience: MN. 2013;51:352–363. doi: 10.1007/s12031-013-9990-y. [DOI] [PubMed] [Google Scholar]
- 17.Michel CC, Curry FE. Microvascular permeability. Physiological Reviews. 1999;79:703–761. doi: 10.1152/physrev.1999.79.3.703. [DOI] [PubMed] [Google Scholar]
- 18.Wu HM, Huang Q, Yuan Y, Granger HJ. VEGF induces NO-dependent hyperpermeability in coronary venules. The American Journal of Physiology. 1996;271:H2735–2739. doi: 10.1152/ajpheart.1996.271.6.H2735. [DOI] [PubMed] [Google Scholar]
- 19.Lee NP, Cheng CY. Regulation of sertoli cell tight junction dynamics in the rat testis via the nitric oxide synthase/soluble guanylate cyclase/3′,5′-cyclic guanosine monophosphate/protein kinase G signaling pathway: An in vitro study. Endocrinology. 2003;144:3114–3129. doi: 10.1210/en.2002-0167. [DOI] [PubMed] [Google Scholar]
- 20.Takizawa Y, Kitazato T, Ishizaka H, Kamiya N, Tomita M, Hayashi M. Effect of aminoguanidine on ischemia/reperfusion injury in rat small intestine. Biological & Pharmaceutical Bulletin. 2011;34:1737–1743. doi: 10.1248/bpb.34.1737. [DOI] [PubMed] [Google Scholar]
- 21.Han X, Uchiyama T, Sappington PL, Yaguchi A, Yang R, Fink MP, Delude RL. NAD+ ameliorates inflammation-induced epithelial barrier dysfunction in cultured enterocytes and mouse ileal mucosa. The Journal of Pharmacology and Experimental Therapeutics. 2003;307:443–449. doi: 10.1124/jpet.103.056556. [DOI] [PubMed] [Google Scholar]
- 22.Cenac N, Garcia-Villar R, Ferrier L, Larauche M, Vergnolle N, Bunnett NW, Coelho AM, Fioramonti J, Bueno L. Proteinase-activated receptor-2-induced colonic inflammation in mice: Possible involvement of afferent neurons, nitric oxide, and paracellular permeability. Journal of Immunology. 2003;170:4296–4300. doi: 10.4049/jimmunol.170.8.4296. [DOI] [PubMed] [Google Scholar]
- 23.Schleiffer R, Raul F. Prophylactic administration of L-arginine improves the intestinal barrier function after mesenteric ischaemia. Gut. 1996;39:194–198. doi: 10.1136/gut.39.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trischitta F, Pidala P, Faggio C. Nitric oxide modulates ionic transport in the isolated intestine of the eel, anguilla anguilla. Comp Biochem Phys A. 2007;148:368–373. doi: 10.1016/j.cbpa.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 25.Plato CF, Pollock DM, Garvin JL. Endothelin inhibits thick ascending limb chloride flux via et(b) receptor-mediated no release. American journal of physiology. Renal Physiology. 2000;279:F326–333. doi: 10.1152/ajprenal.2000.279.2.F326. [DOI] [PubMed] [Google Scholar]
- 26.Silva GB, Ortiz PA, Hong NJ, Garvin JL. Superoxide stimulates nacl absorption in the thick ascending limb via activation of protein kinase c. Hypertension. 2006;48:467–472. doi: 10.1161/01.HYP.0000236646.83354.51. [DOI] [PubMed] [Google Scholar]
- 27.Plato CF, Shesely EG, Garvin JL. Enos mediates l-arginine-induced inhibition of thick ascending limb chloride flux. Hypertension. 2000;35:319–323. doi: 10.1161/01.hyp.35.1.319. [DOI] [PubMed] [Google Scholar]
- 28.Zou AP, Cowley AW., Jr Nitric oxide in renal cortex and medulla. An in vivo microdialysis study. Hypertension. 1997;29:194–198. doi: 10.1161/01.hyp.29.1.194. [DOI] [PubMed] [Google Scholar]
- 29.Jin C, Hu C, Polichnowski A, Mori T, Skelton M, Ito S, Cowley AW., Jr Effects of renal perfusion pressure on renal medullary hydrogen peroxide and nitric oxide production. Hypertension. 2009;53:1048–1053. doi: 10.1161/HYPERTENSIONAHA.109.128827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortiz PA, Hong NJ, Garvin JL. Luminal flow induces enos activation and translocation in the rat thick ascending limb. American Journal of Physiology. Renal Physiology. 2004;287:F274–280. doi: 10.1152/ajprenal.00382.2003. [DOI] [PubMed] [Google Scholar]
- 31.Ortiz PA, Garvin JL. Interaction of o(2)(−) and no in the thick ascending limb. Hypertension. 2002;39:591–596. doi: 10.1161/hy0202.103287. [DOI] [PubMed] [Google Scholar]
- 32.Plato CF, Stoos BA, Wang D, Garvin JL. Endogenous nitric oxide inhibits chloride transport in the thick ascending limb. The American Journal of Physiology. 1999;276:F159–163. doi: 10.1152/ajprenal.1999.276.1.F159. [DOI] [PubMed] [Google Scholar]
- 33.Tain YL, Leu S, Wu KL, Lee WC, Chan JY. Melatonin prevents maternal fructose intake-induced programmed hypertension in the offspring: Roles of nitric oxide and arachidonic acid metabolites. Journal of Pineal Research. 2014;57:80–89. doi: 10.1111/jpi.12145. [DOI] [PubMed] [Google Scholar]
- 34.Ortiz PA, Hong NJ, Wang D, Garvin JL. Gene transfer of eNOS to the thick ascending limb of eNOS-ko mice restores the effects of L-arginine on NaCl absorption. Hypertension. 2003;42:674–679. doi: 10.1161/01.HYP.0000085561.00001.81. [DOI] [PubMed] [Google Scholar]
- 35.Dwyer TM, Schmidt-Nielsen B. The renal pelvis: Machinery that concentrates urine in the papilla. News in Physiological Sciences: an International Journal of Physiology produced jointly by the International Union of Physiological Sciences and the American Physiological Society. 2003;18:1–6. doi: 10.1152/nips.1416.2002. [DOI] [PubMed] [Google Scholar]
- 36.Holstein-Rathlou NH, Marsh DJ. A dynamic model of the tubuloglomerular feedback mechanism. The American Journal of Physiology. 1990;258:F1448–1459. doi: 10.1152/ajprenal.1990.258.5.F1448. [DOI] [PubMed] [Google Scholar]
- 37.Sakai T, Craig DA, Wexler AS, Marsh DJ. Fluid waves in renal tubules. Biophysical Journal. 1986;50:805–813. doi: 10.1016/S0006-3495(86)83521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breiderhoff T, Himmerkus N, Stuiver M, Mutig K, Will C, Meij IC, Bachmann S, Bleich M, Willnow TE, Muller D. Deletion of claudin-10 (cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis. Proc Natl Acad Sci USA. 2012;109:14241–14246. doi: 10.1073/pnas.1203834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs 1. Functional nephron heterogeneity and ADH-stimulated NaCl cotransport. The American Journal of Physiology. 1981;241:F412–F431. doi: 10.1152/ajprenal.1981.241.4.F412. [DOI] [PubMed] [Google Scholar]
- 40.Liang M, Knox FG. Nitric oxide enhances paracellular permeability of opossum kidney cells. Kidney International. 1999;55:2215–2223. doi: 10.1046/j.1523-1755.1999.00465.x. [DOI] [PubMed] [Google Scholar]
- 41.Katsube T, Tsuji H, Onoda M. Nitric oxide attenuates hydrogen peroxide-induced barrier disruption and protein tyrosine phosphorylation in monolayers of intestinal epithelial cell. Biochimica et Biophysica Acta. 2007;1773:794–803. doi: 10.1016/j.bbamcr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Kanwar S, Tepperman BL, Payne D, Sutherland LR, Kubes P. Time course of nitric oxide production and epithelial dysfunction during ischemia/reperfusion of the feline small intestine. Circulatory Shock. 1994;42:135–140. [PubMed] [Google Scholar]
- 43.Kubes P. Nitric oxide modulates epithelial permeability in the feline small intestine. The American Journal of Physiology. 1992;262:G1138–1142. doi: 10.1152/ajpgi.1992.262.6.G1138. [DOI] [PubMed] [Google Scholar]
- 44.Takizawa Y, Kishimoto H, Kitazato T, Tomita M, Hayashi M. Effects of nitric oxide on mucosal barrier dysfunction during early phase of intestinal ischemia/reperfusion. European Journal of Pharmaceutical Sciences: Official Journal of the European Federation for Pharmaceutical Sciences. 2011;42:246–252. doi: 10.1016/j.ejps.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 45.Han X, Fink MP, Delude RL. Proinflammatory cytokines cause no*-dependent and -independent changes in expression and localization of tight junction proteins in intestinal epithelial cells. Shock. 2003;19:229–237. doi: 10.1097/00024382-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Miller MJ, Chotinaruemol S, Sadowska-Krowicka H, Kakkis JL, Munshi UK, Zhang XJ, Clark DA. Nitric oxide: The jekyll and hyde of gut inflammation. Agents and Actions. 1993;39:C180–182. doi: 10.1007/BF01972759. Spec No. [DOI] [PubMed] [Google Scholar]
- 47.Forstermann U, Closs EI, Pollock JS, Nakane M, Schwarz P, Gath I, Kleinert H. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension. 1994;23:1121–1131. doi: 10.1161/01.hyp.23.6.1121. [DOI] [PubMed] [Google Scholar]
- 48.Pautz A, Art J, Hahn S, Nowag S, Voss C, Kleinert H. Regulation of the expression of inducible nitric oxide synthase. Nitric oxide: Biology and Chemistry / Official Journal of the Nitric Oxide Society. 2010;23:75–93. doi: 10.1016/j.niox.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 49.Guzman NJ, Fang MZ, Tang SS, Ingelfinger JR, Garg LC. Autocrine inhibition of Na+/K(+)-ATPase by nitric oxide in mouse proximal tubule epithelial cells. The Journal of Clinical Investigation. 1995;95:2083–2088. doi: 10.1172/JCI117895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roczniak A, Burns KD. Nitric oxide stimulates guanylate cyclase and regulates sodium transport in rabbit proximal tubule. The American Journal of Physiology. 1996;270:F106–115. doi: 10.1152/ajprenal.1996.270.1.F106. [DOI] [PubMed] [Google Scholar]
- 51.Wang T. Nitric oxide regulates HCO3− and Na+ transport by a cGMP-mediated mechanism in the kidney proximal tubule. The American Journal of Physiology. 1997;272:F242–248. doi: 10.1152/ajprenal.1997.272.2.F242. [DOI] [PubMed] [Google Scholar]
- 52.Grandes S, Gallego MJ, Riesco A, Lopez Farre A, Millas I, Casado S, Hernando L, Caramelo C. Mechanisms of renal effects of different agents stimulating production of cgmp. The American Journal of Physiology. 1991;261:H1109–1114. doi: 10.1152/ajpheart.1991.261.4.H1109. [DOI] [PubMed] [Google Scholar]
- 53.Aslam M, Tanislav C, Troidl C, Schulz R, Hamm C, Gunduz D. cAMP controls the restoration of endothelial barrier function after thrombin-induced hyperpermeability via rac1 activation. Physiological Reports. 2014;2:e12175. doi: 10.14814/phy2.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adamson RH, Liu B, Fry GN, Rubin LL, Curry FE. Microvascular permeability and number of tight junctions are modulated by cAMP. The American Journal of Physiology. 1998;274:H1885–1894. doi: 10.1152/ajpheart.1998.274.6.H1885. [DOI] [PubMed] [Google Scholar]
- 55.Yuan SY. Protein kinase signaling in the modulation of microvascular permeability. Vascular Pharmacology. 2002;39:213–223. doi: 10.1016/s1537-1891(03)00010-7. [DOI] [PubMed] [Google Scholar]
- 56.Lorentz WB., Jr The effect of cyclic AMP and dibutyryl cyclic AMP on the permeability characteristics of the renal tubule. The Journal of Clinical Investigation. 1974;53:1250–1257. doi: 10.1172/JCI107671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Westendorp RG, Draijer R, Meinders AE, van Hinsbergh VW. Cyclic-GMP-mediated decrease in permeability of human umbilical and pulmonary artery endothelial cell monolayers. Journal of Vascular Research. 1994;31:42–51. doi: 10.1159/000159030. [DOI] [PubMed] [Google Scholar]
- 58.Draijer R, Atsma DE, van der Laarse A, van Hinsbergh VW. cGMP and nitric oxide modulate thrombin-induced endothelial permeability. Regulation via different pathways in human aortic and umbilical vein endothelial cells. Circulation Research. 1995;76:199–208. doi: 10.1161/01.res.76.2.199. [DOI] [PubMed] [Google Scholar]
- 59.Gorodeski GI. NO increases permeability of cultured human cervical epithelia by cGMP-mediated increase in g-actin. Am J Physiol-Cell Ph. 2000;278:C942–C952. doi: 10.1152/ajpcell.2000.278.5.C942. [DOI] [PubMed] [Google Scholar]
- 60.Surapisitchat J, Jeon KI, Yan C, Beavo JA. Differential regulation of endothelial cell permeability by cGMP via phosphodiesterases 2 and 3. Circulation Research. 2007;101:811–818. doi: 10.1161/CIRCRESAHA.107.154229. [DOI] [PubMed] [Google Scholar]