Abstract
Carney Triad(CTr) describes the association of paragangliomas(PGL), pulmonary chondromas, and gastrointestinal(GI) stromal tumors(GISTs) with a variety of other lesions including pheochromocytomas and adrenocortical tumors. The gene(s) causing CTr remain(s) unknown. PGL and GISTs may be caused by loss-of-function mutations in succinate dehydrogenase (SDH) (a condition known as Carney-Stratakis syndrome (CSS)). Mitochondrial structure and function are abnormal in tissues carrying SDH defects but they have not been studied in CTr. For this study, we examined mitochondrial structure in human tumors and GI tissue(GIT) of mice with SDH deficiency. Tissues from 16 CTr tumors (n=12), and those with isolated GIST(n=1), with CSS caused by SDHC(n=1), SDHD(n=2) mutations were studied by electron microscopy (EM). GIT from mice with a heterozygous deletion in Sdhb (Sdhb+/−, n=4) were also studied by EM. CTr patients presented with mostly epithelioid GISTs that were characterized by plump cells containing a centrally located round nucleus and prominent nucleoli; these changes were almost identical to those seen in the GIST of patients with SDH. In tumor cells from patients, regardless of diagnosis or tumor type, cytoplasm contained increased mitochondria with “hypoxic” phenotype: mitochondria were devoid of cristae, exhibited structural abnormalities and of variable size. Occasionally, mitochondria were small/round, rarely thin, elongated with tubular cristae. Many mitochondria exhibited amorphous fluffy material with membranous whorls or cystic structures. Similar mitochondrial hypoxic phenotype was seen in Sdhb+/− mice. We conclude tissues from SDH-deficient tumors and mouse GIT and from CTr tumors shared identical abnormalities in mitochondrial structure and other features. Thus, the still elusive CTr defect(s) is(are) likely to affect mitochondrial function just like germline SDH-deficiency.
Keywords: GIST, mitochondria, Carney Triad, Succinate dehydrogenase
Introduction
Carney triad (CTr) is a syndrome that describes the association of paragangliomas (PGLs) with gastrointestinal stromal tumors (GISTs) and pulmonary chondromas (PCH); other lesions, including pheochromocytomas, esophageal leiomyomas and adrenocortical adenomas, have also been described (Carney, 1999, 2009; Stratakis and Carney, 2009). CTr is a novel form of multiple endocrine neoplasia (MEN) predominantly affecting females; it is caused by a yet unknown genetic defect (Matyakhina et al., 2007). The dyad of PGLs and GISTs (Carney–Stratakis syndrome, CSS) is inherited in an autosomal dominant manner relating to germline mutations in SDHB, SDHC and SDHD genes (but not KIT or PDFGRA) (Stratakis and Carney, 2009).
GISTs are the most common mesenchymal neoplasm of the gastrointestinal tract occurring in the stomach (60–70%) (Corless and Heinrich, 2008; El-Rifai et al., 2000) and small intestine (25–35%) (Corless and Heinrich, 2008; El-Rifai et al., 2000; Miettinen et al., 2006); they occur rarely in the large intestine or colon (5–10%) (Huang et al., 2006) and esophagus (Gouveia et al., 2005), typically later in life with only a few cases in the pediatric population and young adults (Janeway and Pappo, 2012; Kaemmer et al., 2009). GISTs are considered to originate from the interstitial cells of Cajal, the pacemaker cells that regulate peristalsis in the digestive tract (Parkin and Chugh, 2011). Worldwide, GISTs occur at an incidence of around 11 to 19.6 per million (Bulbul Dogusoy, 2012; Chan et al., 2006; Goettsch et al., 2005; Nilsson et al., 2005; Vukobrat-Bijedic et al., 2012), equating to 3,300 to 6,000 new cases reported annually in the United States.
Molecularly, most GISTs are driven by gain-of-function mutations in KIT or platelet-derived growth factor receptor-α (PDGFRA) (Lasota and Miettinen, 2008), however, a small subset of GISTs lack such mutations and are termed ‘wild-type’ (WT) GISTs. The latter constitute about 15% of GISTs that are identified in adult patients and more than half of the tumors seen in pediatric patients (Doyle et al., 2012; Heinrich et al., 2003; Hirota et al., 1998; Lasota and Miettinen, 2008). Recently, we identified succinate dehydrogenase (SDH) deficiency, which activates oncogenesis by inhibiting hypoxia-induced factor (HIF)-alpha prolyl hydroxylase (Lancaster, 2002) to be associated with most WT-GISTs at the protein level (Celestino et al., 2012; Gaal et al., 2011; Janeway et al., 2011; Stratakis and Carney, 2009). SDH consists of four subunits, encoded for by the SDHA, SDHB, SDHC, and SDHD, genes. Mutations in these genes, collectively known as SDHx, which were known to predispose individuals to hereditary PGL and pheochromocytomas, were additionally found to be responsible for 10 to 15% of WT-GISTs (Celestino et al., 2012): loss of SDHB immunostaining was seen in the majority of WT-GISTs studied to date (Celestino et al., 2012; Gaal et al., 2011; Janeway et al., 2011; Killian et al., 2013), suggesting SDH deficiency is present even in these WT-GISTs that do not harbor SDHx DNA defects, possibly due to SDHx epigenetic down regulation (Astuti et al., 2001; Velasco et al., 2005). SDH is involved in catalyzing the oxidation of succinate to fumarate in the Krebs cycle, and participates in oxidative phosphorylation (Lancaster, 2002). All SDH subunits are encoded for by nuclear genes and SDHx-deficient tumors bear inactivating germline mutations as well as loss of the corresponding normal allele (Astuti et al., 2001; Baysal et al., 2000; Burnichon et al., 2010; Celestino et al., 2012; Gill et al., 2011; Niemann and Muller, 2000; Velasco et al., 2005).
Histologically, GISTs consist of spindle cells, epithelioid cells, or a mixture of both, and typically express the KIT (c-KIT) protein (Miettinen and Lasota, 2006a). GISTs with mutations in PDGFRA are reported to be of gastric origin, are of epithelioid cell type, and have the KIT-positive phenotype (Lasota and Miettinen, 2008). At the ultrastructural level, examination of GISTs from different anatomic locations have been examined, with particular focus on the variability of tumor cells (ranging from non-specialized spindle cells with similarities to fibroblasts to smooth muscle cells exhibiting neuronal features) (Min and Leabu, 2006; Segal et al., 1994; Yantiss et al., 2002). These studies have helped to identify the many overlapping ultrastructural characteristics and have contributed to the better classification of this heterogeneous group of neoplasms. However, these studies have not examined the ultrastructural features of the mitochondria (whose integral role resides in cellular metabolism) in these tumors. Here we present ultrastructural evidence for significant abnormalities in the appearance of mitochondria in tumors from patients with CTr, similar to those seen in SDHB-deficient tumors; interestingly, tissue from mice with a heterozygous deletion in Sdhb also showed mitochondrial structural abnormalities, which has never been shown before.
Materials and Methods
Patient Case Evaluations
Sixteen cases were identified. Six (6) from Mayo Clinic, nine (9) from National Institutes of Health, and one (1) from la Timone University Hospital, Marseille, France, France (Taieb et al., 2012).
Tumors from a total of 16 patients were studied; the patient’s clinical data are presented in Table 1. There were 3 male and 13 female patients (with only one male patient with CTr). A total of 19 tumors were investigated: CTr-associated GISTs (n=13), PGL (n=1), and chondroma (n=1); a tumor from a patient with isolated GIST (n=1), and tumors from patients with the dyad (CSS), a GIST caused by SDHC (n=1), and a GIST (n=1) and a PGL (n=1) caused by an SDHD mutation, each from a sibling with CSS from the same family. Their mutations and pathology (with regards to SDHB immunohistochemistry) have been described by our laboratory previously (23, 24); all tumors demonstrated negative SDHB immunohistochemistry (data not shown and previously published (23, 24)).
Table 1.
Clinical and molecular findings in the patients that were included in the present investigation.
| Patient/Case No. | Age at diagnosis (years) | Sex | Clinical Presentation | Dyad/Triad | SDHx Mutation |
|---|---|---|---|---|---|
| 1 | 29 | F | PGL | Triad | No |
| Stomach GIST | Triad | None | |||
| 2 | 28 | F | Stomach GIST | Triad | None |
| Duodenal GIST | Triad | None | |||
| Stomach GIST | Triad | None | |||
| 3 | 20 | F | Stomach GIST | Triad | None |
| 4 | 15 | F | Stomach GIST | Triad | None |
| 5 | 17 | M | Stomach GIST | Triad | None |
| 6 | 52 | F | Stomach GIST | Triad | None |
| 7 | 15 | F | lung chondroma | Triad | None |
| 8 | 20 | F | Stomach + Duodenum GIST | Triad | None |
| 9 | 14 | F | GIST m, met + PGL m | Triad | None |
| 10 | 34 | F | GIST m, met | Triad | None |
| 11 | 12 | F | GIST + chondroma + PGL | Triad | None |
| 12 | 25 | F | GIST met + PGL + adrenocortical tumor | Triad | None |
| 13 | 18 | F | Stomach GIST | Dyad* | None |
| 14 | 9 | M | GSS m + PGL nf | Dyad* | SDHC c.405+1G>A (IVS5+1G>C)/p.Met136Leufs*3 |
| 15 | 20 | F | PGL m, nf | Isolated PGL | SDHD c.388insG / p.Ala130Glyfs*61 # |
| 16 | 16 | M | GSS multiobulated; PGL m, nf; Pigmented penile spots | Dyad* | SDHD c.388insG / p.Ala130Glyfs*61 # |
Abbreviations: m = multiple; nf = nonfunctioning; f = functioning; met = metastatic; PGL= paraganglioma; GIST = gastrointestinal stromal tumor; IVS5 = intervening sequence intron 5;
Dyad = the dyad of PGLs and GISTs or Carney-Stratakis syndrome (CSS);
These two patients also have a co-segregating variation in SDHB c.423+20T>A
Electron Microscopy
Nine cases were processed from fresh tissue samples and were diced into 1mm3 cubes, fixed in 2.5% glutaraldehyde, post-fixed in osmium, embedded in EPON and routinely processed for transmission electron microscopy. Another 7 cases were retrieved from formalin-fixed paraffin-embedded tissue; although these showed suboptimal preservation we could successfully make observations pertaining to mitochondrial ultrastructure. A total of 5–15 sections were analyzed per each patient sample.
Mouse Studies
Sdhb+/− mice (obtained from Dr. Maher) (Louis J. Maher III, 2011) were maintained on a C57BL/6 genetic background. Mice were sacrificed (CO2 inhalation) at 12 months of age (n=4; all female) and gastrointestinal tissue (GIT) was dissected. The small intestine (duodenum) was isolated and fixed in 2.5% glutaraldehyde, post-fixed in osmium, embedded in EPON and routinely processed for transmission electron microscopy (Laboratory of Pathology, National Cancer Institute (NCI), NIH, Bethesda, MD 20892, USA). All animal experiments were approved by the National Institute of Health Animal Ethics Committee (06-033).
Results
Ultrastructural findings in human tumors with SDH deficiency
The abnormalities of the tumor cells are described in comparison to normal controls. Our descriptions highlight the salient abnormal features of the various sets of our tumor specimens. Two patients with GISTs that harbored SDHB or SDHC mutations had similar mitochondrial morphology to that observed in CTr samples. A summary of mitochondrial ultrastructural features is presented below:
GISTs
GISTs were characterized by plump, epithelioid cells containing a centrally located round large nucleus with prominent nucleolus and diffused chromatin (Figure 1A) and a good number of morphologically abnormal mitochondria (Figure 1A–C). Most mitochondria contained remnants of cristae and hyaline aqueous solution. Throughout the cytoplasm glycogen granules were evident, which is a sign of hypoxic conditions and simultaneously present in cases of dysfunctional mitochondria. Cytoplasmic membranes were well defined and with frequent microvillus-like filopodia (Figure 1A, B and D). Few oval and spindle cells with indented nuclei, intracytoplasmic filamentous aggregates, slender surface filopodia and a few short intercellular attachments were present as well (Figure 1E and F). These polygonal and spindle cells were packed with abnormal cystic-looking mitochondria without cristae and with intra-mitochondrial membranous inclusions. Some mitochondria were small and round, or thin and elongated and many were enlarged with partial or complete loss of cristae exhibiting amorphous fluffy material with membranous whorls, or cystic structures (Figure 1G–I). No autophagy or mitophagy processing was seen. In general, the morphology of the mitochondria in GIST cells can be described as being very close to those of a primitive neoplasm.
Figure 1.
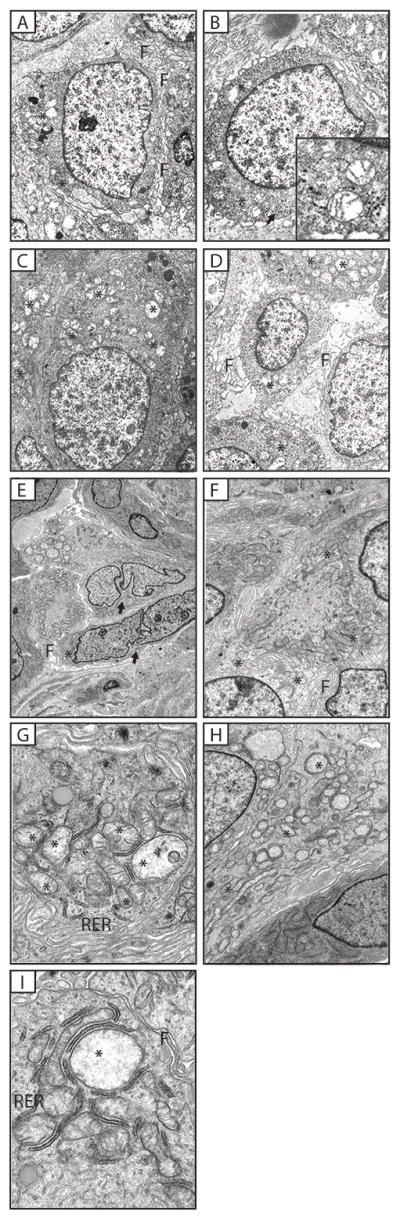
(A–D) Gastric stromal sarcoma (Case No. 1). Luxuriant filopodal cell borders (marked as F). Numerous glycogen granules (arrow) and prominent nucleoli (B). Mitochondria show abnormal cristae structure (*) (and inset B). Moderately increased numbers of mitochondria (C – D), all showing abnormal morphology; no cristae (*). (E – F) Gastric stromal sarcoma (Case No 2, specimen 6) Fewer numbers of oval and spindle cells with heavily lobulated nuclei (E, arrow) intracytoplasmic filamentous aggregates, slender surface filopodia (F) and a few short intercellular attachments were present. Polygonal and spindle cells were packed with cystic-looking mitochondria (*) without cristae and with intra-mitochondrial membranous inclusions (H, *). (G–I) CTr Gastrointestinal Stromal Tumors (Case no 2, specimen 6). Oval and spindle cells are in close apposition. Slender filopodia (F) are evident with few short intercellular attachments. The cytoplasm contains strands of rough endoplasmic reticulum (RER), branching ER, intermediate filaments and increased numbers of mitochondria (*). Mitochondria exhibit variable sizes and structural abnormalities – some are small and round, others are thin and elongated with tubular cristae. Many mitochondria have partial to complete loss of cristae and exhibit amorphous amorphous material containing membranous whorls or cystic structures.
PGLs
PGLs were characterized by scant cytoplasm, diffused chromatin, the presence of intracytoplasmic dense core secretory granules (Figure 2A and B) and, a large number of morphologically abnormal mitochondria. The latter was similar to the GISTs. However, unlike the GISTs, the mitochondria in the PGLs appeared more numerous and occupied most of the cytoplasm; they also exhibited larger size than those of GIST (Figure 3A–D). The cell membranes were occasionally degenerate and cells appeared as if they were multinucleated; nuclei had various shapes ranging from round to lobulated (Figure 3A). Again, no autophagy or mitophagy processing was seen. Adjacent endothelial cells did not have any of these mitochondrial abnormalities (images not shown here).
Figure 2.
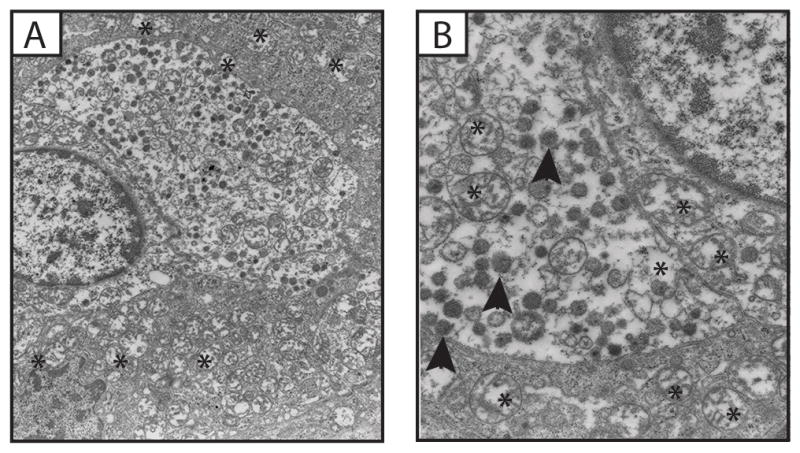
Ultrastructural features of a Paraganglioma (PGL) exhibiting many abnormal mitochondria (*) and dense core granules (arrowheads) (Case No 1).
Figure 3.
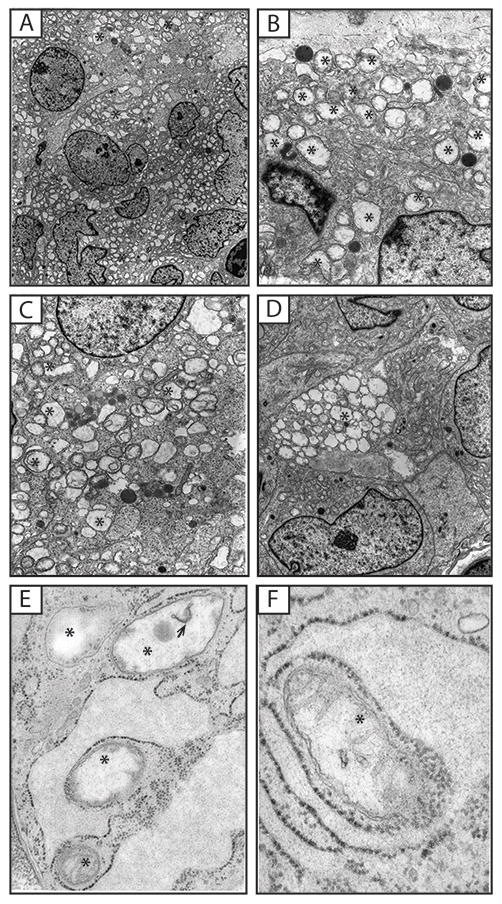
(A–D) Ultrastructural features of a Paraganglioma (PGL) (Case No 2) exhibiting polygonal and spindle cells packed with cystic-looking mitochondria (*) without cristae and with intra-mitochondrial membranous inclusions. The cell surfaces are smooth. (E–F) Lung chondroma, majority of mitochondria are devoid of cristae (*). Presence of leaky chromatin in the chondroma (E, arrow).
CTr Lung Chondroma
The only evaluated case of lung chondroma (Figure 3E and F) showed fibroblastic looking cells with dilated endoplasmic reticulum (ER). The mitochondria wer morphological abnormal, with degenerate inner membrane and absent cristae or only their remnants present (Figure 3E & F). Interestingly, we also detected presence of leaky extranuclear chromatin (Figure 3F).
Ultrastructural findings in mice with SDH deficiency (the Sdhb+/− mouse)
In mouse GIT, the duodenum in particular, Cajal cell morphology had similar characteristics to that of patients with epithelioid GISTs; cytoplasm contained numerous mitochondria that displayed highly abnormal morphology including disintegration of the inner membrane and lack of cristae, budding of mitophagic vesicles, and variability of sizes with some swelling that gave some round shape; the latter seemed to be due to accumulation of aqueous substance (Figure 4A and B). Chromatin appeared pycnotic and the nuclear membrane separating from cytoplasm or slightly degenerate (Figure 4A). Additionally, some short rough endoplasmic membrane strands present (Figure 4A & B), and lysosomes were visible (figure 4B).
Figure 4.
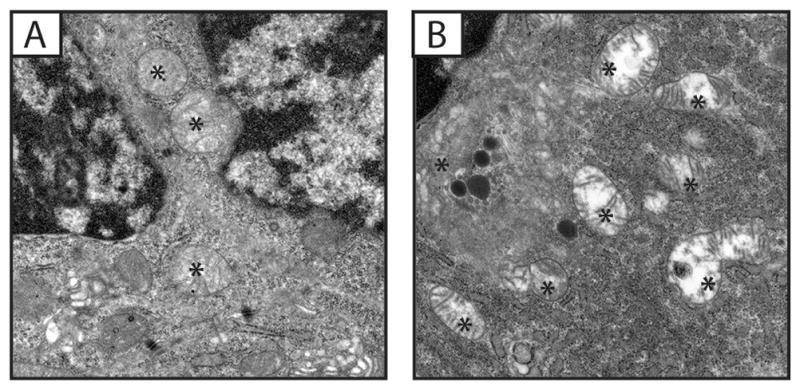
Ultrastructural features of (A) wild type and (B) Sdhb+/− 12-month old mouse duodenum. Mitochondria (*) in Sdhb+/− duodenum have partial to complete loss of cristae, compared to wild type.
Discussion
GISTs are the most frequent spindle cell tumor of the gastrointestinal tract, thought to arise from interstitial cells of Cajal (Min and Leabu, 2006). GISTs occur more frequently in the stomach (Durham et al., 2004); to a lesser extent (in approximately 30% of patients) GISTs can be found in the small bowel, and in 10% or fewer cases in the esophagus as well as rectum. GISTs exhibit heterogeneous ultrastructural features (Kindblom et al., 1998; Matsumoto et al., 1997; Park et al., 2004; Yantiss et al., 2002). Clinical studies to date have examined the histopathology, immunohistochemical, as well as genetic characteristics (Hirota and Isozaki, 2006; Miettinen and Lasota, 2006a, b; Paral et al., 2010), however, there have been no reported studies to date examining the ultrastructure of the mitochondrion in GISTs and their morphology in CTr.
In this study, we provide insight into mitochondrial ultrastructure in CTr and in GIST and PGL caused by SDH deficiency. We have identified that, in all clinical cases examined, mitochondria have a strikingly similar morphology – that of a hypoxic phenotype; generally lacking cristae, exhibit vacuoles, and varying in size; the more striking features reflect the severity/aggressiveness of the tumor. Patients with the dyad of PGL and GISTs (Carney-Stratakis syndrome or CSS) without other tumors harbor loss-of-function mutations in SDHx subunit genes. The current study shows that mitochondrial ultrastructure in tumors of these patients is almost identical to tumors from patients with CTr. Interestingly, we have recently found high succinate levels assessed by 1H high-resolution magic angle spinning nuclear magnetic resonance (HRMAS NMR) spectroscopy in 2 CTr-associated PGLs (Imperiale et al., 2015). CTr-related PGLs metabolomic profile was indeed consistent with a SDH deficiency.
We also utilized a mouse model with an Sdhb heterozygous deletion (Maher III et al., 2011). Unlike in humans (where there is high penetrance of PGL in individuals with SDHB mutations) no PGLs or GISTs or any other tumors have been detected in Sdhb+/− mice (42), except for the recent description by our laboratory of modest pituitary gland hyperplasia and increased growth hormone and prolactin secretion (Xekouki et al., 2015). However, as seen here, there are mitochondrial ultrastructure defects in the gastrointestinal cells of these mice. It is unclear how these defects influence disease progression; mitochondria play a central role in orchestrating many apoptotic processes (Newmeyer and Ferguson-Miller, 2003), but it is possible that mice require more genetic hits for GIST and PGLs to develop, SDH deficiency apparently is enough to produce a phenotype in the mouse pituitary gland, although tumors fail to develop there, too, unlike the situation in humans (Xekouki and Stratakis, 2012; Xekouki et al., 2015).
Our ultrastructural study indicates that mitochondria from both SDH-mutant tumors and those associated with CTr have considerable similarity, particularly with respect to their increase in numbers, size and loss or complete absence of cristae. Tumor cells have the ability to successfully escape hypoxia-mediated death as a result of lowered expression or mutation of p53 (Moll and Schramm, 1998). Under hypoxic conditions, mitochondria are unable to provide enough ATP for cell survival, therefore tumor cells must up-regulate the glycolytic pathway; this is facilitated by the induction of hypoxia-inducible factor 1 (HIF-1) (Wang and Semenza, 1993). Tumors from patients with SDHx mutations have, indeed, higher levels of HIF-1(Hagg and Wennstrom, 2005). Previous studies have proposed that enlarged mitochondria arise from HIF-1-induced fusion and that these enlarged mitochondria confer resistance to apoptosis (Chiche et al., 2010). We have seen a similar phenotype in human mutant SDHx-associated pituitary tumors (Xekouki et al., 2012; Xekouki and Stratakis, 2012).
Although further studies on live cells are mandated to identify the contribution of the abnormal mitochondria in CTr pathogenesis, this is yet another observation that endeavors to describe better the molecular etiology of the CTr. It is now tempting to speculate that the yet elusive gene(s) are involved somehow in mitochondrial function beyond the down-regulation of SDHB (Gaal et al., 2011; Janeway et al., 2011) and SDHC (Haller et al., 2014).
Acknowledgments
This investigation was funded by the Intramural Research Program of the Eunice Kennedy Shriver national Institute of Child Health & Human Development, Bethesda, MD, USA.
Footnotes
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
References
- Astuti D, Douglas F, Lennard TW, Aligianis IA, Woodward ER, Evans DG, Eng C, Latif F, Maher ER. Germline SDHD mutation in familial phaeochromocytoma. Lancet. 2001;357:1181–1182. doi: 10.1016/S0140-6736(00)04378-6. [DOI] [PubMed] [Google Scholar]
- Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers EN, Richard CW, 3rd, Cornelisse CJ, Devilee P, Devlin B. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–851. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- Bulbul Dogusoy G. Gastrointestinal stromal tumors: A multicenter study of 1160 Turkish cases. Turk J Gastroenterol. 2012;23:203–211. [PubMed] [Google Scholar]
- Burnichon N, Briere JJ, Libe R, Vescovo L, Riviere J, Tissier F, Jouanno E, Jeunemaitre X, Benit P, Tzagoloff A, Rustin P, Bertherat J, Favier J, Gimenez-Roqueplo AP. SDHA is a tumor suppressor gene causing paraganglioma. Human molecular genetics. 2010;19:3011–3020. doi: 10.1093/hmg/ddq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney JA. Gastric stromal sarcoma, pulmonary chondroma, and extra-adrenal paraganglioma (Carney Triad): natural history, adrenocortical component, and possible familial occurrence. Mayo Clin Proc. 1999;74:543–552. doi: 10.4065/74.6.543. [DOI] [PubMed] [Google Scholar]
- Carney JA. Carney triad: a syndrome featuring paraganglionic, adrenocortical, and possibly other endocrine tumors. The Journal of clinical endocrinology and metabolism. 2009;94:3656–3662. doi: 10.1210/jc.2009-1156. [DOI] [PubMed] [Google Scholar]
- Celestino R, Lima J, Faustino A, Maximo V, Gouveia A, Vinagre J, Soares P, Lopes JM. A novel germline SDHB mutation in a gastrointestinal stromal tumor patient without bona fide features of the Carney-Stratakis dyad. Fam Cancer. 2012;11:189–194. doi: 10.1007/s10689-011-9499-x. [DOI] [PubMed] [Google Scholar]
- Chan KH, Chan CW, Chow WH, Kwan WK, Kong CK, Mak KF, Leung MY, Lau LK. Gastrointestinal stromal tumors in a cohort of Chinese patients in Hong Kong. World J Gastroenterol. 2006;12:2223–2228. doi: 10.3748/wjg.v12.i14.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiche J, Rouleau M, Gounon P, Brahimi-Horn MC, Pouyssegur J, Mazure NM. Hypoxic enlarged mitochondria protect cancer cells from apoptotic stimuli. J Cell Physiol. 2010;222:648–657. doi: 10.1002/jcp.21984. [DOI] [PubMed] [Google Scholar]
- Corless CL, Heinrich MC. Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol. 2008;3:557–586. doi: 10.1146/annurev.pathmechdis.3.121806.151538. [DOI] [PubMed] [Google Scholar]
- Doyle LA, Nelson D, Heinrich MC, Corless CL, Hornick JL. Loss of succinate dehydrogenase subunit B (SDHB) expression is limited to a distinctive subset of gastric wild-type gastrointestinal stromal tumours: a comprehensive genotype-phenotype correlation study. Histopathology. 2012;61:801–809. doi: 10.1111/j.1365-2559.2012.04300.x. [DOI] [PubMed] [Google Scholar]
- Durham MM, Gow KW, Shehata BM, Katzenstein HM, Lorenzo RL, Ricketts RR. Gastrointestinal stromal tumors arising from the stomach: a report of three children. J Pediatr Surg. 2004;39:1495–1499. doi: 10.1016/j.jpedsurg.2004.06.014. [DOI] [PubMed] [Google Scholar]
- El-Rifai W, Sarlomo-Rikala M, Andersson LC, Knuutila S, Miettinen M. DNA sequence copy number changes in gastrointestinal stromal tumors: tumor progression and prognostic significance. Cancer research. 2000;60:3899–3903. [PubMed] [Google Scholar]
- Gaal J, Stratakis CA, Carney JA, Ball ER, Korpershoek E, Lodish MB, Levy I, Xekouki P, van Nederveen FH, den Bakker MA, O’Sullivan M, Dinjens WN, de Krijger RR. SDHB immunohistochemistry: a useful tool in the diagnosis of Carney-Stratakis and Carney triad gastrointestinal stromal tumors. Mod Pathol. 2011;24:147–151. doi: 10.1038/modpathol.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill AJ, Pachter NS, Chou A, Young B, Clarkson A, Tucker KM, Winship IM, Earls P, Benn DE, Robinson BG, Fleming S, Clifton-Bligh RJ. Renal tumors associated with germline SDHB mutation show distinctive morphology. The American journal of surgical pathology. 2011;35:1578–1585. doi: 10.1097/PAS.0b013e318227e7f4. [DOI] [PubMed] [Google Scholar]
- Goettsch WG, Bos SD, Breekveldt-Postma N, Casparie M, Herings RM, Hogendoorn PC. Incidence of gastrointestinal stromal tumours is underestimated: results of a nation-wide study. Eur J Cancer. 2005;41:2868–2872. doi: 10.1016/j.ejca.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Gouveia AM, Pimenta AP, Lopes JM, Capelinha AF, Ferreira SS, Valbuena C, Oliveira MC. Esophageal GIST: therapeutic implications of an uncommon presentation of a rare tumor. Dis Esophagus. 2005;18:70–73. doi: 10.1111/j.1442-2050.2005.00446.x. [DOI] [PubMed] [Google Scholar]
- Hagg M, Wennstrom S. Activation of hypoxia-induced transcription in normoxia. Exp Cell Res. 2005;306:180–191. doi: 10.1016/j.yexcr.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Haller F, Moskalev EA, Faucz FR, Barthelmess S, Wiemann S, Bieg M, Assie G, Bertherat J, Schaefer IM, Otto C, Rattenberry E, Maher ER, Strobel P, Werner M, Carney JA, Hartmann A, Stratakis CA, Agaimy A. Aberrant DNA hypermethylation of SDHC: a novel mechanism of tumor development in Carney triad. Endocr Relat Cancer. 2014;21:567–577. doi: 10.1530/ERC-14-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CD, Fletcher JA. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- Hirota S, Isozaki K. Pathology of gastrointestinal stromal tumors. Pathology international. 2006;56:1–9. doi: 10.1111/j.1440-1827.2006.01924.x. [DOI] [PubMed] [Google Scholar]
- Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- Huang YC, Wang JY, Lin PY, Chin CC, Chen CS. Synchronous prostate stromal sarcoma and gastrointestinal stromal tumor of rectum: case report and review of the literature. Urology. 2006;68:672, e611–673. doi: 10.1016/j.urology.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Imperiale A, Moussallieh F, Roche P, Battini S, Cicek AE, Sebag F, Brunaud L, Barlier A, Elbayed K, Loundou K, Bachellier P, Goichot B, Stratakis CA, Pacak K, Namer I, Taïeb D. Metabolome Profiling by HRMAS NMR Spectroscopy of Pheochromocytomas and Paragangliomas Detects SDH Deficiency: Clinical and Pathophysiological Implications. Neoplasia. 2015;17:55–65. doi: 10.1016/j.neo.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway KA, Kim SY, Lodish M, Nose V, Rustin P, Gaal J, Dahia PL, Liegl B, Ball ER, Raygada M, Lai AH, Kelly L, Hornick JL, O’Sullivan M, de Krijger RR, Dinjens WN, Demetri GD, Antonescu CR, Fletcher JA, Helman L, Stratakis CA. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:314–318. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway KA, Pappo A. Treatment guidelines for gastrointestinal stromal tumors in children and young adults. Journal of pediatric hematology/oncology. 2012;34(Suppl 2):S69–72. doi: 10.1097/MPH.0b013e31824e3899. [DOI] [PubMed] [Google Scholar]
- Kaemmer DA, Otto J, Lassay L, Steinau G, Klink C, Junge K, Klinge U, Schumpelick V. The Gist of literature on pediatric GIST: review of clinical presentation. Journal of pediatric hematology/oncology. 2009;31:108–112. doi: 10.1097/MPH.0b013e3181923cd8. [DOI] [PubMed] [Google Scholar]
- Killian JK, Kim SY, Miettinen M, Smith C, Merino M, Tsokos M, Quezado M, Smith WI, Jr, Jahromi MS, Xekouki P, Szarek E, Walker RL, Lasota J, Raffeld M, Klotzle B, Wang Z, Jones L, Zhu Y, Wang Y, Waterfall JJ, O’Sullivan MJ, Bibikova M, Pacak K, Stratakis C, Janeway KA, Schiffman JD, Fan JB, Helman L, Meltzer PS. Succinate dehydrogenase mutation underlies global epigenomic divergence in gastrointestinal stromal tumor. Cancer Discov. 2013;3:648–657. doi: 10.1158/2159-8290.CD-13-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259–1269. [PMC free article] [PubMed] [Google Scholar]
- Lancaster CR. Succinate:quinone oxidoreductases: an overview. Biochim Biophys Acta. 2002;1553:1–6. doi: 10.1016/s0005-2728(01)00240-7. [DOI] [PubMed] [Google Scholar]
- Lasota J, Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology. 2008;53:245–266. doi: 10.1111/j.1365-2559.2008.02977.x. [DOI] [PubMed] [Google Scholar]
- Maher Louis J, III, EHS, Rueter Emily M, Becker Nicole A, Bida John Paul, Nelson-Holte Molly, Palomo José Ignacio Piruat, García-Flores Paula, López-Barneo José, van Deursen Jan. Chapter 3-Mouse Models of Human Familial Paraganglioma. In: Martin JF, editor. Pheochromocytoma - A New View of the Old Problem. InTech; Rijeka, Croatia: 2011. [Google Scholar]
- Maher LJ, III, Smith EH, Rueter EM, Becker NA, Bida JP, Nelson-Holte M, Piruat Palomo JI, García-Flores P, López-Barneo J, van Deursen J. In: Mouse Models of Human Familial Paraganglioma, Pheochromocytoma - A New View of the Old Problem. Martin DJF, editor. 2011. [Google Scholar]
- Matsumoto K, Min W, Yamada N, Asano G. Gastrointestinal autonomic nerve tumors: immunohistochemical and ultrastructural studies in cases of gastrointestinal stromal tumor. Pathology international. 1997;47:308–314. doi: 10.1111/j.1440-1827.1997.tb04498.x. [DOI] [PubMed] [Google Scholar]
- Matyakhina L, Bei TA, McWhinney SR, Pasini B, Cameron S, Gunawan B, Stergiopoulos SG, Boikos S, Muchow M, Dutra A, Pak E, Campo E, Cid MC, Gomez F, Gaillard RC, Assie G, Fuzesi L, Baysal BE, Eng C, Carney JA, Stratakis CA. Genetics of carney triad: recurrent losses at chromosome 1 but lack of germline mutations in genes associated with paragangliomas and gastrointestinal stromal tumors. J Clin Endocrinol Metab. 2007;92:2938–2943. doi: 10.1210/jc.2007-0797. [DOI] [PubMed] [Google Scholar]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Seminars in diagnostic pathology. 2006a;23:70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006b;130:1466–1478. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. The American journal of surgical pathology. 2006;30:477–489. doi: 10.1097/00000478-200604000-00008. [DOI] [PubMed] [Google Scholar]
- Min KW, Leabu M. Interstitial cells of Cajal (ICC) and gastrointestinal stromal tumor (GIST): facts, speculations, and myths. J Cell Mol Med. 2006;10:995–1013. doi: 10.1111/j.1582-4934.2006.tb00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll UM, Schramm LM. p53--an acrobat in tumorigenesis. Crit Rev Oral Biol Med. 1998;9:23–37. doi: 10.1177/10454411980090010101. [DOI] [PubMed] [Google Scholar]
- Newmeyer DD, Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell. 2003;112:481–490. doi: 10.1016/s0092-8674(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Niemann S, Muller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nature genetics. 2000;26:268–270. doi: 10.1038/81551. [DOI] [PubMed] [Google Scholar]
- Nilsson B, Bumming P, Meis-Kindblom JM, Oden A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- Paral J, Slaninka I, Kalabova H, Hadzi-Nikolov D. Gastrointestinal stromal tumors: review on morphology, molecular pathology, diagnostics, prognosis and treatment options. Acta Gastroenterol Belg. 2010;73:349–359. [PubMed] [Google Scholar]
- Park SH, Kim MK, Kim H, Song BJ, Chi JG. Ultrastructural studies of gastrointestinal stromal tumors. J Korean Med Sci. 2004;19:234–244. doi: 10.3346/jkms.2004.19.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin B, Chugh R. Molecular pathology of gastrointestinal stromal tumors and implications for treatment and prognosis. Curr Probl Cancer. 2011;35:245–254. doi: 10.1016/j.currproblcancer.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Segal A, Carello S, Caterina P, Papadimitriou JM, Spagnolo DV. Gastrointestinal autonomic nerve tumors: a clinicopathological, immunohistochemical and ultrastructural study of 10 cases. Pathology. 1994;26:439–447. doi: 10.1080/00313029400169162. [DOI] [PubMed] [Google Scholar]
- Stratakis CA, Carney JA. The triad of paragangliomas, gastric stromal tumours and pulmonary chondromas (Carney triad), and the dyad of paragangliomas and gastric stromal sarcomas (Carney-Stratakis syndrome): molecular genetics and clinical implications. Journal of internal medicine. 2009;266:43–52. doi: 10.1111/j.1365-2796.2009.02110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taieb D, Timmers HJ, Hindie E, Guillet BA, Neumann HP, Walz MK, Opocher G, de Herder WW, Boedeker CC, de Krijger RR, Chiti A, Al-Nahhas A, Pacak K, Rubello D. EANM 2012 guidelines for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2012;39:1977–1995. doi: 10.1007/s00259-012-2215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco A, Palomar-Asenjo V, Ganan L, Catasus L, Llecha N, Panizo A, Palomar-Garcia V, Quer M, Matias-Guiu X. Mutation analysis of the SDHD gene in four kindreds with familial paraganglioma: description of one novel germline mutation. Diagn Mol Pathol. 2005;14:109–114. doi: 10.1097/01.pas.0000158987.07907.7e. [DOI] [PubMed] [Google Scholar]
- Vukobrat-Bijedic Z, Husic-Selimovic A, Bijedic N, Saray A, Djuran A, Gogov B, Bjelogrlic I. Gastrointestinal stromal tumors and its frequency in our clinical samples. Med Arh. 2012;66:369–371. doi: 10.5455/medarh.2012.66.369-371. [DOI] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xekouki P, Pacak K, Almeida M, Wassif CA, Rustin P, Nesterova M, de la Luz Sierra M, Matro J, Ball E, Azevedo M, Horvath A, Lyssikatos C, Quezado M, Patronas N, Ferrando B, Pasini B, Lytras A, Tolis G, Stratakis CA. Succinate dehydrogenase (SDH) D subunit (SDHD) inactivation in a growth-hormone-producing pituitary tumor: a new association for SDH? The Journal of clinical endocrinology and metabolism. 2012;97:E357–366. doi: 10.1210/jc.2011-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xekouki P, Stratakis CA. Succinate dehydrogenase (SDHx) mutations in pituitary tumors: could this be a new role for mitochondrial complex II and/or Krebs cycle defects? Endocrine-related cancer. 2012;19:C33–40. doi: 10.1530/ERC-12-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xekouki P, Szarek E, Bullova P, Giubellino A, Quezado M, Mastroyannis SA, Mastorakos P, Wassif CA, Raygada M, Rentia N, Dye L, Cougnoux A, Koziol D, de La Luz Sierra M, Lyssikatos C, Belyavskaya E, Malchoff C, Moline J, Eng C, Maher LJT, Pacak K, Lodish M, Stratakis CA. Pituitary adenoma with paraganglioma/pheochromocytoma (3PAs) and succinate dehydrogenase defects in human and mice. J Clin Endocrinol Metab. 2015:jc20144297. doi: 10.1210/jc.2014-4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantiss RK, Rosenberg AE, Selig MK, Nielsen GP. Gastrointestinal stromal tumors: an ultrastructural study. Int J Surg Pathol. 2002;10:101–113. doi: 10.1177/106689690201000203. [DOI] [PubMed] [Google Scholar]


