Abstract
Previously, we showed that Cyp1b1 gene disruption minimizes angiotensin II-induced hypertension and associated pathophysiological changes in male mice. This study was conducted to test the hypothesis that cytochrome P450 1B1-generated metabolites of testosterone, 6β-hydroxytestoterone and 16α-hydroxytestosterone, contribute to angiotensin II-induced hypertension and its pathogenesis. Angiotensin II infusion for 2 weeks increased cardiac cytochrome P450 1B1 activity and plasma levels of 6β-hydroxytestosterone, but not 16α-hydroxytestosterone, in Cyp1b1+/+ mice without altering Cyp1b1 gene expression; these effects of angiotensin II were not observed in Cyp1b1−/− mice. Angiotensin II-induced increase in systolic blood pressure and associated cardiac hypertrophy, and fibrosis, measured by intracardiac accumulation of α-smooth muscle actin, collagen and transforming growth factor-β, and increased nicotinamide adenine dinucleotide phosphate oxidase activity and production of reactive oxygen species; these changes were minimized in Cyp1b1−/− or castrated Cyp1b1+/+ mice, and restored by treatment with 6β-hydroxytestoterone. In Cyp1b1+/+ mice, 6β-hydroxytestosterone did not alter the angiotensin II-induced increase in systolic blood pressure; the basal systolic blood pressure was also not affected by this agent in either genotype. Angiotensin II or castration did not alter cardiac, angiotensin II type 1 receptor, angiotensin converting enzyme, Mas receptor, or androgen receptor mRNA levels in Cyp1b1+/+ or in Cyp1b1−/− mice. These data suggest that the testosterone metabolite, 6β-hydroxytestosterone, contributes to angiotensin II-induced hypertension and associated cardiac pathogenesis in male mice, most likely by acting as a permissive factor. Moreover, cytochrome P450 1B1 could serve as a novel target for developing agents for treating renin-angiotensin and testosterone-dependent hypertension and associated pathogenesis in males.
Keywords: 6β-hydroxytestosterone, CYP1B1, hypertension, castration, oxidative stress
Introduction
Hypertension is a major cause of cardiovascular disease, and studies in several animal models of hypertension, and epidemiological and clinical studies, have demonstrated a sexual dimorphism in the development of hypertension and cardiovascular disease.1–4 Sex differences that appear at puberty are maintained throughout adulthood.5 Men have a higher risk of developing hypertension than do premenopausal women of the same age.6 These sex differences in blood pressure (BP) control have been attributed to sex chromosomes and gonadal hormones.7 Angiotensin (Ang) II also increases BP to a greater degree in males than in females, and castration protects males, whereas ovariectomy prevents protection in female mice against Ang II-induced hypertension.8 Castration or testosterone receptor antagonists also reduce blood pressure in spontaneously hypertensive rats (SHR) or Dahl-salt sensitive rats.9–11 However, the mechanism by which testosterone contributes to Ang II-induced hypertension is not known. In male SHR or Dahl-salt sensitive rats, the rise in BP has been attributed to the effect of testosterone to increase plasma renin levels and hepatic and/or renal angiotensinogen mRNA expression12 and oxidative stress.13–15 Sex-specific differences in BP have also been observed in Cyp4a14−/− mice (an increase in males but not females).16 Hypertension in male Cyp4a14−/− mice that was attributed to increased expression of CYP4A12A and associated increase in testosterone and its metabolite dihydrotestosterone (DHT), and production of 20-hydroxyeicosatetraenoic acid (20-HETE), a prohypertensive eicosanoid, was abolished by castration.16 Administration of DHT to rats increases renal expression of CYP4A2, a mouse homologue of CYP4A14, and conversion of arachidonic acid to 20-HETE that increases vascular tone, response to vasoconstrictor agents, and inhibition of nitric oxide synthesis, oxidative stress, endothelial dysfunction, and BP.17
Previous studies revealed that CYP1B1, which is highly expressed in various extra-hepatic tissues including the cardiovascular system,18 contributes to Ang II and deoxycorticosterone acetate salt-induced hypertension in male mice or rats19–22 and in SHR23 and associated cardiovascular and renal pathogenesis, most likely via increased oxidative stress. However, in female mice where Ang II-induced increase in BP and associated pathophysiological changes are minimized as compared to male mice, inhibition of CYP1B1 activity with 2,3′,4,5′-tetramethoxystilbene (TMS) or Cyp1b1 gene disruption (Cyp1b1−/−) produced opposite effects and increased BP and associated pathophysiological changes that were not different from those observed in male mice.24 The protective effect of CYP1B1 against Ang II-induced hypertension and its pathogenesis in female mice was minimized by 2-methoxyestradiol that is generated from an estradiol metabolite of Cyp1b1, 2-OH estradiol, by catechol-O-methyltransferase.25,26 Since testosterone can also be metabolized by CYP1B1 into 6β-hydroxytestosterone (6β-OHT) and 16α-hydroxytestosterone (16α-OHT),27,28 it led us to hypothesize that these testosterone metabolites contribute to Ang II-induced hypertension and associated cardiovascular pathophysiological changes in male mice. To test this hypothesis, we examined the effect of Ang II on plasma levels of testosterone and its metabolites and effect of the testosterone metabolites of CYP1B1, 6β-OHT and 16α-OHT, on Ang II-induced hypertension and associated cardiovascular pathological changes in intact and castrated Cyp1b1+/+ and Cyp1b1−/− male mice. The results show that Ang II selectively increased plasma levels of 6β-OHT in Cyp1b1+/+ mice but not in Cyp1b1−/− or castrated mice. Moreover, Ang II-induced increase in systolic blood pressure (SBP) and associated cardiac pathophysiological changes, including fibrosis and oxidative stress that were minimized in Cyp1b1−/− and castrated Cyp1b1+/+ mice, were reversed by 6β-OHT.
Methods
For detailed methods, see the online-only data supplement http://hyper.ahajournals.org.
Results
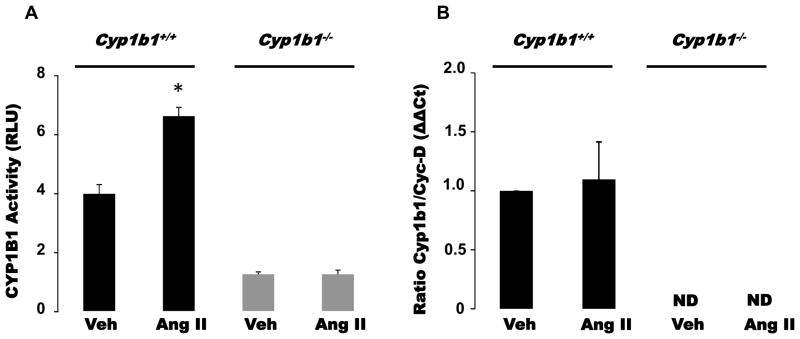
Ang II Infusion Increased Cardiac CYP1B1 Activity and Plasma Levels of its Testosterone Metabolite, 6β-OHT, in Cyp1b1+/+ but Not Cyp1b1−/− Mice
Ang II infusion increased cardiac CYP1B1 activity without altering Cyp1b1 mRNA expression in Cyp1b1+/+ mice (Figure 1A, 1B). Ang II infusion also increased plasma levels of 6β-OHT but did not alter that of testosterone, DHT, or 16α-OHT in Cyp1b1+/+ mice as measured by ultra-performance liquid chromatography-coupled with-quadrupole time of flight-mass spectrometer (UPLC-qTOFMS) (Table 1). The coefficient correlation of standard curves prepared from different concentrations of testosterone and its metabolites, and the representative ion chromatograms of authentic testosterone and its metabolites and their plasma levels from Cyp1b1+/+ mice infused with Ang II, analyzed by UPLC-qTOFMS are shown in Table S1 and Figure S1, respectively. In Cyp1b1−/− mice, the basal plasma levels of testosterone, DHT, and 16α-OHT were not significantly different compared to the corresponding levels of these steroids observed in Cyp1b1+/+ mice; 6β-OHT was not detected. During infusion of Ang II in Cyp1b1−/− mice, the plasma levels of testosterone, but not DHT or 16α-OHT, was reduced compared to corresponding values obtained in Cyp1b1+/+ mice; 6β-OHT was not detected.
Figure 1. Angiotensin II (Ang II) increased cardiac cytochrome P450 (CYP) 1B1 activity in Cyp1b1+/+ mice without altering Cyp1b1 gene expression.
Cyp1b1+/+ and Cyp1b1−/− mice were infused with vehicle or Ang II for 2 weeks. At the end of Ang II or its vehicle (Veh) infusion, cardiac tissue was collected to measure CYP1B1 activity using the P450-Glo assay kit and expressed as relative luminescence units (RLU) (A). Cyp1b1 mRNA expression was determined by real time PCR. Cyp1b1 mRNA expression was normalized against cyclophilin-D (Cyc-D) (B). *p<0.05 vehicle vs. corresponding value from Ang II-infused animals (n=3–5 for all experiments; data are expressed as mean±SEM).
Table 1.
Plasma levels (ng/ml) of testosterone and its metabolites in Cyp1b1+/+ and Cyp1b1−/− mice.
| Cyp1b1+/+ | Cyp1b1−/− | |||
|---|---|---|---|---|
|
| ||||
| Compound | Vehicle | Ang II | Vehicle | Ang II |
| Testosterone | 1.45±0.27 | 1.43±0.19 | 0.75±0.06 | 0.01±0.00† |
| Dihydrotestosterone | 0.72±0.23 | 1.05±0.27 | 0.56±0.14 | 0.33±0.05† |
| 6β-hydroxytestosterone | Undetected | 0.50±0.0* | Undetected | Undetected |
| 16α-hydroxytestosterone | 0.20±0.14 | 0.24±0.12 | 0.10±0.05 | 0.12±0.08 |
At the termination of Ang II infusion, plasma was collected and analyzed for levels of testosterone and its metabolites by ultra-performance liquid chromatography-coupled with quadrupole time of flight-mass spectrometer (UPLC-QTOFMS) (Table 1).
P<0.05 vehicle vs. corresponding value from Ang II-infused animals;
p < 0.05, Cyp1b1+/+ Ang II vs. Cyp1b1−/− Ang II (n=3–5 for all experiments; data are expressed as mean±SEM).
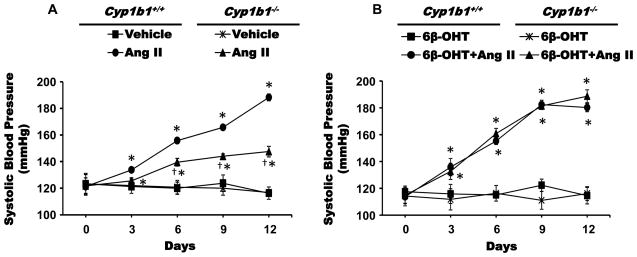
Cyp1b1 Gene Disruption Minimized Ang II-Induced Increase in Systolic Blood Pressure (SBP)
Infusion of Ang II (700 ng/kg/min) for 2 weeks increased SBP, measured by tail cuff every 3rd day, in Cyp1b1+/+ and Cyp1b1−/− mice, but the increase was significantly less in Cyp1b1−/− than in Cyp1b1+/+ mice (Figure 2A). The difference in SBP increase observed between these two groups was consistent, reproducible, and similar to that reported previously.19
Figure 2. Cyp1b1 gene disruption reduced hypertensive effect of angiotensin (Ang II), which was restored by 6β-hydroxytestosterone (6β-OHT).
Cyp1b1+/+ and Cyp1b1−/− mice were infused with Ang II or vehicle for 2 weeks and given intraperitoneal injections of the Cyp1b1-derived metabolite of testosterone, 6β-OHT (15 μg/g, i.p.) every 3rd day for 2 weeks. (A) and (B) systolic blood pressure was measured by tail cuff method twice weekly. *p<0.05 Ang II vs vehicle, Ang II+ 6β-OHT vs. vehicle+ 6β-OHT; †p<0.05, Cyp1b1+/+ Ang II vs. Cyp1b1−/− Ang II (n=4–5 for all experiments; data are expressed as mean±SEM).
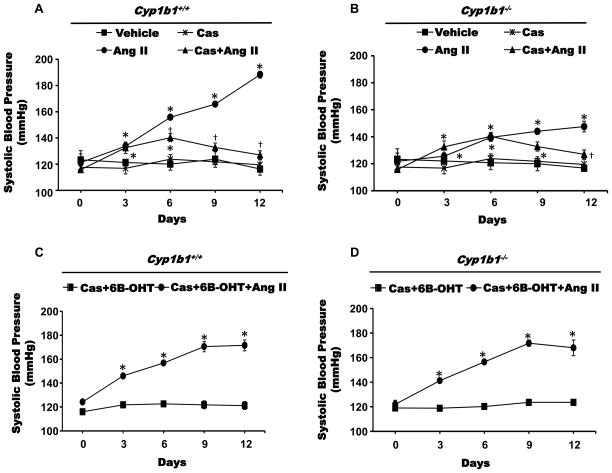
6β-OHT Treatment Restored Ang II-Induced Hypertension in Cyp1b1−/− and Castrated Cyp1b1+/+ Mice
To investigate whether testosterone metabolites generated by CYP1B1 contribute to Ang II-induced increase in SBP in male mice, we examined the effect of 6β-OHT and 16α-OHT on the actions of Ang II on SBP in intact and castrated Cyp1b1+/+ and Cyp1b1−/− mice. The ability of Ang II to increase SBP is significantly reduced in Cyp1b1−/− mice compared to that observed in Cyp1b1+/+ mice,19 which we confirmed in this study. Administration of 6β-OHT (Figure 2B), but not 16α-OHT (Figure S2), in Cyp1b1−/− mice restored the ability of Ang II to increase SBP to levels not different from those obtained in Cyp1b1+/+ mice. It is well established that the ability of Ang II to increase SBP in male mice is diminished by castration.8 Ang II infusion in castrated Cyp1b1+/+ mice produced a small increase in SBP on 3rd and 6th day which was diminished by the 9th and 12th day of infusion to levels not different from those observed in intact or castrated non-Ang II-infused Cyp1b1+/+ mice. The Ang II-induced increase in SBP that was reduced in Cyp1b1−/− mice (Figure 3A), was further diminished and reached levels at the 12th day not different from those of intact or castrated Cyp1b1−/− mice (Figure 3B). Castration did not alter basal BP in either Cyp1b1+/+ or Cyp1b1−/− mice. In castrated Cyp1b1+/+ and Cyp1b1−/− mice, administration of 6β-OHT (Figure 3C and 3D) restored the hypertensive effect of Ang II to levels similar to those observed in intact Cyp1b1+/+ mice without any change in SBP in animals treated with only 6β-OHT. Since Ang II did not alter plasma levels of 16α-OHT in Cyp1b1+/+ or Cyp1b1−/− mice, and 16α-OHT did not restore the effect of Ang II on SBP in Cyp1b1−/− mice (Figure S2), we did not investigate its effect on cardiac remodeling or in castrated mice.
Figure 3. Castration (Cas) reduced the hypertensive effect of angiotensin II (Ang II) in Cyp1b1+/+, which was restored by 6β-hydroxytestosterone (6β-OHT).
Cyp1b1+/+ and Cyp1b1−/− mice were sham operated or castrated and subsequently infused with Ang II or vehicle for 2 weeks and given 6β-OHT, as described in Figure 2. (A) and (B) systolic blood pressure was measured by tail cuff method twice weekly. *p<0.05 Ang II vs. vehicle, Cas+Ang II vs. Cas, and Cas+6β-OHT+Ang II vs. Cas+6β-OHT; †p<0.05, Cas+Ang II vs. Ang II (n=4–5 for all experiments; data are expressed as mean±SEM).
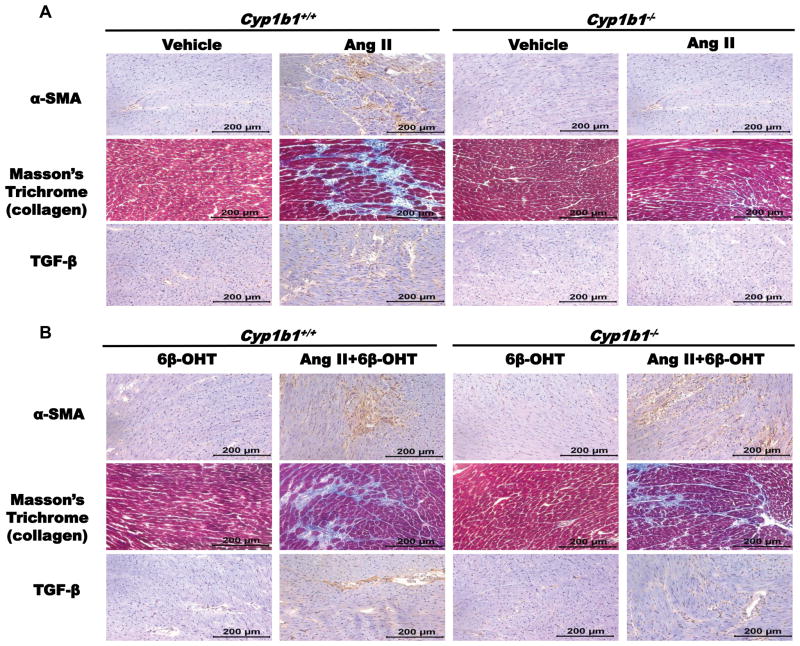
Cyp1b1 Gene Disruption Attenuated Cardiac Hypertrophy and Fibrosis Associated with Ang II-Induced Hypertension, which was Reversed by 6β-OHT
Infusion of Ang II increased heart:body weight ratio (HW/BW), an indicator of cardiac hypertrophy, in Cyp1b1+/+, which was diminished in Cyp1b1−/− mice (Table S2). Hearts from Ang II-infused Cyp1b1+/+ mice, but not from Cyp1b1−/− mice, also displayed fibrosis as indicated by α-smooth muscle actin (α-SMA) positive myofibroblasts and collagen deposition in the myocardium and increased transforming growth factor (TGF)-β staining cells (Figure 4A). Treatment with 6β-OHT did not alter these effects of Ang II in Cyp1b1+/+ mice but restored them in Cyp1b1−/− mice (Table S2 and Figure 4B).
Figure 4. Cyp1b1 gene disruption minimizes angiotensin II (Ang II)-induced cardiac fibrosis which is restored by 6β-hydroxytestosterone (6β-OHT).
Mice infused with vehicle or Ang II for 2 weeks and injected with 6β-OHT as described in methods. After the end of Ang II infusion, heart was removed and processed for immunohistochemistry. (A) α-smooth muscle actin (α-SMA) positive myofibroblasts, Masson’s trichrome staining revealed intracardiac collagen deposition (intense blue staining) and intracardiac transforming growth factor-β (TGF-β). Ang II infusion in Cyp1b1−/− mice treated with 6β-OHT but not in mice treated with 6β-OHT alone, produced cardiac fibrosis in Cyp1b1−/− mice as evident by (B) α-smooth muscle actin (α-SMA) positive myofibroblasts, collagen deposition and intracardiac transforming growth factor-β (TGF-β). Photomicrographs are representative images of at least three animals from each experimental group.
Castration Reduced Cardiac Hypertrophy and Fibrosis Associated with Ang II-Induced Hypertension, which was Prevented by 6β-OHT Treatment
Eight week old mice were castrated, and after a 7-day washout period for the depletion of residual testosterone, the mice were divided into two groups and infused with vehicle or Ang II as described in methods. Castration reduced Ang II-induced increase in HW/BW ratio (Table S2), α-SMA, collagen deposition and TGF-β staining in Cyp1b1+/+ mice; these effects of Ang II that were minimized in Cyp1b1−/− mice were abolished by castration (Figure S3). Concurrent treatment with 6β-OHT restored the ability of Ang II to cause cardiac hypertrophy (Table S2) and fibrosis in castrated Cyp1b1+/+ and Cyp1b1−/− mice (Figure S4). 6β-OHT alone did not cause cardiac hypertrophy or fibrosis in either intact or castrated mice of these genotypes (Figure 4B and Figure S4).
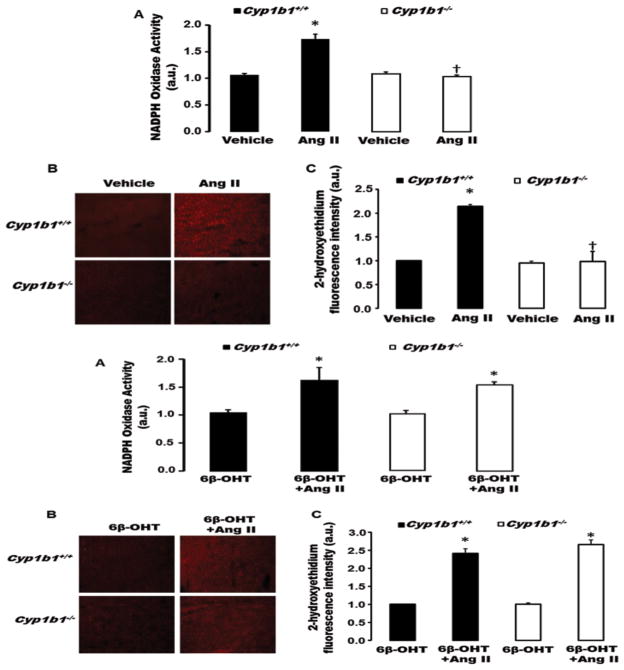
6β-OHT Restored the Ability of Ang II to Increase Cardiac Nicotinamide Adenine Dinucleotide Phosphate Oxidase (NADPH) Activity and Production of Reactive Oxygen Species (ROS), which was Diminished in Male Cyp1b1−/− Mice
ROS have been implicated in hypertension and cardiovascular dysfunction in male models of experimental hypertension.14 Ang II infusion increased cardiac NADPH oxidase activity, and ROS production as indicated by increased cardiac 2-hydroxyethidium fluorescence, in Cyp1b1+/+, but not Cyp1b1−/− mice (Figure 5 upper panel A–C). Treatment with 6β-OHT did not alter Ang II-induced increase in cardiac NADPH oxidase activity or ROS production in Cyp1b1+/+ mice, but restored the loss of this action of Ang II in Cyp1b1−/− mice (Figure 5 lower panel A–C). 6β-OHT did not alter basal levels of NADPH oxidase activity or ROS production in Cyp1b1+/+ or Cyp1b1−/− mice (Figure 5 lower panel A–C).
Figure 5. Cyp1b1 gene disruption minimizes angiotensin II (Ang II)-induced cardiac nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity and superoxide production in Cyp1b1−/− mice which is reversed by 6β-hydroxytestosterone (6β-OHT).
Cyp1b1+/+ and Cyp1b1−/− mice were infused with vehicle or Ang II (upper panel) and treated with 6β-OHT or 6β-OHT+Ang II (lower panel) for 2 weeks. (A) NADPH oxidase activity was measured in cardiac homogenates. (B) Cardiac superoxide production was determined by the fluorescence intensity of 2-hydoxyethidium. Photomicrographs are representative of hearts from mice in each of the different treatment groups following incubation with dihydroethidium. (C) Graph depicts quantified data. *p<0.05 Ang II vs. vehicle; †p<0.05 Cyp1b1−/− Ang II vs. Cyp1b1+/+ Ang II (n=4 for all experiments and data are expressed as mean±SEM).
6β-OHT Restored Loss of Effect of Ang II-Induced Increase in Cardiac NADPH Oxidase Activity and ROS Production in Castrated Male Cyp1b1+/+ and Cyp1b1−/− Mice
Castration is known to reduce oxidative stress in SHR rats. 14 The effect of Ang II to increase cardiac NADPH oxidase activity or ROS production was diminished in, as indicated by decrease in 2-hydroxyethidium fluorescence in Cyp1b1+/+ mice (Figure S5 A–C). In intact Cyp1b1−/− mice where Ang II did not increase cardiac NADPH oxidase activity or ROS production (Figure 5 upper panel A–C), were not altered by castration (Figure S5 A–C). Treatment with 6β-OHT restored the ability of Ang II to increase cardiac NADPH oxidase activity and ROS production in castrated Cyp1b1+/+ and Cyp1b1−/− mice (Figure S6 A–C). 6β-OHT did not alter the basal NADPH oxidase activity or ROS production in castrated Cyp1b1+/+ or Cyp1b1−/− mice (Figure S6 A–C).
Cyp1b1 Gene Disruption or Castration Did Not Alter Cardiac Angiotensin-Converting Enzyme (ACE), Ang II Type 1 Receptor (AT1a), and Mas Receptors and Androgen Receptor (AR) or CYP4A12A mRNA Expression in Ang II-Infused Male Mice
To determine whether alterations in Ang II actions in Cyp1b1−/− or castrated Cyp1b1+/+ mice are due to changes in At1a receptor, Ace, Mas receptor, Ar, or Cyp4a12a mRNA expression, we measured their respective mRNA expression in cardiac tissue. Cardiac At1a receptor, Ace, Mas and Ar receptor mRNA expression were not altered in intact (Figure S7 A–D) or castrated (Figure S8 A–D) Cyp1b1−/− and Cyp1b1+/+ mice during infusion of Ang II or its vehicle. Cyp4a12a expression was not detected in cardiac tissues of Cyp1b1−/− or Cyp1b1+/+ mice (data not shown).
Discussion
This study demonstrates for the first time that CYP1B1 maintains testosterone levels during Ang II infusion, and Ang II selectively stimulates production of 6β-OHT, which contributes to its hypertensive effect and associated cardiac hypertrophy, fibrosis, and oxidative stress in male mice. Previously, we showed that Cyp1b1 gene disruption or inhibition of CYP1B1 activity with TMS minimized Ang II-induced hypertension and associated cardiac hypertrophy, fibrosis, and oxidative stress.19 However, the mechanism by which CYP1B1 contributes to Ang II-induced hypertension was not known. Since CYP1B1 is constitutively active, it needs a substrate to produce a product that mediates and/or modulates the effect of Ang II. CYP1B1 can metabolize several substrates including retinoids, fatty acids, and steroids.29–31 Therefore, more than one product generated from these substrates could contribute to the CYP1B1-dependent hypertensive effect of Ang II and associated pathogenesis. Testosterone is known to promote cardiac hypertrophy and fibrosis32 and to contribute to Ang II-induced hypertension.8 In hypertrophic human and SHR left ventricles, the expression of AR and the metabolism of testosterone into more than one metabolite including 6β-OHT, but not 16α-OHT, is increased, and is diminished in humans with a left ventricle-assisted device.33 Testosterone is also metabolized into 6β-OHT in adult rat cultured myocytes.34 In the present study in male Cyp1b1+/+ mice, Ang II infusion selectively increased plasma levels of testosterone metabolite of CYP1B1 6β-OHT, but not testosterone, DHT, or 16α-OHT. The lack of reduction in plasma levels of testosterone despite an increase in 6β-OHT levels in Cyp1b1+/+ mice, suggests that testosterone levels are maintained most likely by its increased production by Ang II. Supporting this view was our observation that in Cyp1b1−/− mice, the plasma level of testosterone during Ang II infusion was markedly diminished and 6β-OHT was undetectable. The plasma levels of DHT were also reduced by Ang II but it did not reach statistical significance; the basal plasma levels of testosterone, DHT, and 16α-OHT levels were not altered, and 6β-OHT was undetectable in Cyp1b1−/− mice. The mechanism by which CYP1B1 contributes to testosterone production by Ang II that could involve hydroxylation of one or more of its precursors remains to be determined. Moreover, further studies of the measurement of protein bound and tissue levels of testosterone are required to address an insignificant effect of Ang II on plasma levels of DHT despite significant decrease in testosterone levels in Cyp1b1−/− mice. Our demonstration that the ability of Ang II to selectively stimulate production of 6β-OHT, but not 16α-OHT, in Cyp1b1+/+ mice that was abolished by Cyp1b1 gene disruption raises the possibility that it might contribute to Ang II-induced hypertension and associated cardiac hypertrophy, fibrosis, and oxidative stress. Therefore, we examined the effect of 6β-OHT on Ang II-induced hypertension and associated cardiac effects. Administration of 6β-OHT did not alter the effect of Ang II to increase SBP and associated cardiac hypertrophy as determined by increased HW/BW ratio in male Cyp1b1+/+ mice. It also failed to alter the effect of Ang II to cause cardiac fibrosis as assessed by increased intracardiac accumulation of α-SMA, TGF-β, or collagen and oxidative stress as determined by increased cardiac NADPH oxidase activity and ROS production in Cyp1b1+/+ mice. However, these effects of Ang II that were minimized in male Cyp1b1−/− mice were restored by concurrent treatment with 6β-OHT, indicating that this CYP1B1 testosterone metabolite contributes to Ang II-induced hypertension and associated cardiac hypertrophy, fibrosis, and oxidative stress in male Cyp1b1+/+ mice. The effect of 6β-OHT in restoring the effects of Ang II to increase SBP and associated cardiac effects in Cyp1b1−/− mice were selective, because the CYP1B1-generated testosterone metabolite 16α-OHT failed to restore these effects of Ang II in male Cyp1b1−/− mice. Although recombinant CYP1B1 in vitro produces 16α-OHT,28 Cyp1b1 gene disruption did not alter plasma levels of 16α-OHT during infusion of Ang II or its vehicle, suggesting that it is most likely produced by ≥ 1 CYPs other than CYP1B1 in male mice.
Supporting evidence for the role of the testosterone metabolite, 6β-OHT, in Ang II-induced hypertension and its associated cardiac effects was obtained in castrated mice. In castrated Cyp1b1+/+ mice, Ang II-induced increase in SBP was minimized at days 3 and 6 and thereafter abolished at 12th day of Ang II infusion. In Cyp1b1−/− mice, a component of Ang II-induced increase in SBP that was independent of CYP1B1 was also abolished by castration at 12th day of Ang II infusion. However, concurrent administration of 6β-OHT restored Ang II-induced increase in SBP and associated cardiac hypertrophy, fibrosis, and oxidative stress in both castrated Cyp1b1+/+ and Cyp1b1−/− mice. Since testosterone can be also metabolized into 6β-OHT by CYP3A4,35 the Cyp1b1-independent, but gonad-dependent, component of Ang II-induced increase in SBP in mice could be due to formation of 6β-OHT by Cyp3a4. However, it is unlikely, because Ang II-induced production of 6β-OHT was abolished in Cyp1b1−/− mice. Therefore, the CYP1B1-independent component of Ang II-induced increase in SBP could be due to the direct effect of the fraction of testosterone not metabolized by CYP1B1 and of DHT on AR to modulate the effect of Ang II. The effect of testosterone is unlikely to be due to its conversion to DHT, because Ang II did not stimulate production of DHT in Cyp1b1+/+ mice or decrease its levels in Cyp1b1−/− mice. Also, AR antagonist, flutamide, but not finasteride, an inhibitor of 5α reductase that converts testosterone into DHT, lowers BP in SHR.10 Exogenous DHT that increases renal vascular expression in male rats of CYP4A8, and its orthologue CYP4A12 in male mice, stimulates 20-HETE production and increases the activity of the renin-angiotensin system and BP.36–38 Moreover, DHT-induced hypertension is minimized in angiotensinogen deficient mice.39 However, Cyp1b1 gene disruption in male mice does not alter renal expression of CYP4A1/A2/A3 (rat orthologues of mouse CYP4A10/12/14).20 In the present study, expression of Cyp4a12a mRNA was also not detected in the heart after infusion of Ang II or its vehicle in male Cyp1b1+/+ or Cyp1b1−/− mice. Therefore, DHT does not appear to contribute to Ang II-induced hypertension in male mice.
The ability of Cyp1b1 gene disruption and castration to inhibit Ang II-induced hypertension and associated cardiac effects and their restoration by 6β-OHT could be due to alterations in expression of the pressor (AT1a receptor, ACE) or depressor (Mas receptor) components of the renin-angiotensin system. However, it is unlikely, because neither Cyp1b1 gene disruption nor castration altered cardiac expression of mRNA of AT1a receptor and ACE or Mas receptor expression in mice.
The restoration by 6β-OHT of cardiac remodeling by Ang II in Cyp1b1−/− mice and castrated Cyp1b1+/+ mice could be due to restoration of the effect of Ang II on BP. Although we cannot exclude this possibility, Ang II also produces cardiac remodeling independent of an increase in BP.40 Although in our study 6β-OHT restored the hypertensive effects of Ang II cardiac hypertrophy, fibrosis, and oxidative stress in Cyp1b1−/− mice and in castrated Cyp1b1+/+ mice, it did not alter the basal BP, suggesting that 6β-OHT acts as a permissive factor in the development of Ang II-induced hypertension and associated cardiac effects. The mechanism by which 6β-OHT modulates the effects of Ang II is not known. Whether the effect of 6β-OHT to restore Ang II actions is mediated via genomic or non-genomic AR41,42 remains to be determined. Restoration by 6β-OHT of Ang II actions in Cyp1b1 gene-disrupted or castrated mice was not due to alterations in expression of AR, because mRNA expression of AR was not altered in these mice. Testosterone-dependent enhanced pressor response to Ang II in growth-restricted43 and increased uterine arterial contraction to Ang II in testosterone-treated pregnant rats has also been observed.44 Ang II produces vascular contraction and hypertension by increasing RhoA activity via Jak2-induced phosphorylation of Rho exchange factor Arhgef1 in mice.45 Moreover, testosterone-dependent renal vascular response to Ang II in New Zealand genetic hypertensive male rats has been attributed to upregulation of Rho kinase signaling pathway.46 Therefore, further studies are required to determine a) if 6β-OHT contributes to the actions of elevated testosterone levels on vascular effects of Ang II, b) role of 6β-OHT in the actions of Ang II including endothelial and renal dysfunction and end-organ damage and the possible role of Rho kinase signaling pathway in male mice, c) contribution of 6β-OHT to the vascular actions of other vasoconstrictor agents, norepinephrine, endothelin-1 and vasopressin, and to the other models of experimental hypertension and SHR. An androgen-dependent increased sensitivity to Ang II-induced renal damage and hypertension has also been demonstrated by early life stress caused by maternal-pup separation in male rats.47 This androgen-dependent increased sensitivity to Ang II-induced hypertension by early life stress that could be mediated by 6β-OHT remains to be determined. Moreover, the ability of peripherally administered Ang II to increase SBP is mediated by its action in subfornical organ via generation of superoxides,48 in the kidney,49 and activation of immune cells.50 Therefore, it is possible that the effect of 6β-OHT to restore Ang II-induced hypertension in Cyp1b1−/− and castrated male mice could be mediated by its action to modulate the effect of Ang II in the brain, immune cells, and the kidney. Since CYP1B151 and AR are present in the brain,52 6β-OHT could also be generated locally in the brain and modulate the central actions of peripheral Ang II.
In conclusion, this study provides evidence that the testosterone metabolite of Cyp1b1 6β-OHT formed by Ang II acts as a permissive factor and contributes to the hypertensive effect of this peptide and associated cardiac hypertrophy, fibrosis, increased NADPH oxidase activity, and oxidative stress in male mice. In contrast, we have shown that, in female mice, CYP1B1 protects against Ang II-induced hypertension and its pathogenesis via metabolism of estradiol into 2-hydroxyestradiol and subsequently its metabolism by catechol-O-methyltransferase into 2-methoxyestradiol that acts as a permissive factor to suppress Ang II actions.24 However, recently the effect of sex chromosome complement has been implicated in Ang II-induced hypertension,7 Therefore, it appears that, in addition to sex chromosomes, 17β-estradiol and testosterone metabolites of CYP1B1 contribute to sex differences in Ang II-induced hypertension and associated pathogenesis in male and female mice, respectively. Finally, CYP1B1 could serve as a novel target for developing agents that inhibit CYP1B1 for treating hypertension and associated pathogenesis in males, but inhibitors of CYP1B1 could be detrimental in treating hypertension in females.
Perspectives
Sex differences in BP in various models of experimental hypertension have been attributed to sex chromosome complement and female and male sex hormones. The present study provides the first evidence that the CYP1B1 metabolite of testosterone, 6β-OHT, in male mice contributes to Ang II-induced hypertension and associated cardiac hypertrophy, fibrosis, and oxidative stress, most likely by acting as a permissive factor. Therefore, characterization of the interaction of 6β-OHT with testosterone receptor and underlying mechanism of its action to promote Ang II-induced hypertension and development of selective inhibitors of the effects of 6β-OHT and CYP1B1 activity could be useful for treating hypertension and associated pathogenesis in males.
Supplementary Material
Novelty and Significance.
What is New?
Demonstration for the first time that CYP1B1 participates in maintaining testosterone plasma levels during Ang II infusion in male mice.
Provides first evidence that Ang II selectively stimulates production of CYP1B1-generated metabolite of testosterone, 6β-OHT, which contributes to hypertension and associated cardiac hypertrophy, fibrosis, and oxidative stress caused by Ang II in male mice.
6β-OHT contributes to Ang II-induced hypertension by acting as a permissive factor in male mice.
What is Relevant?
The results of this study advance our knowledge of the mechanism by which CYP1B1, through metabolism of testosterone to 6β-OHT, contributes to Ang II-induced hypertension and associated cardiac hypertrophy, fibrosis, and oxidative stress in males.
Furthermore, it has an important pathophysiological significance in hypertension and cardiac disease such as heart failure associated with increased activity of the renin-angiotensin system and increased use of testosterone, whereby production of 6β-OHT would promote cardiac hypertrophy, fibrosis, and oxidative stress in males.
Finally, our study also has translational significance in developing selective CYP1B1 inhibitors for treating renin-angiotensin and testosterone-dependent hypertension in men.
Summary
Cyp1b1 gene disruption or castration in Cyp1b1+/+mice minimized Ang II-induced hypertension and associated cardiac hypertrophy, fibrosis, and oxidative stress. Ang II increased cardiac CYP1B1 activity and stimulated production of CYP1B1-generated testosterone metabolite, 6β-OHT, and not 16α-OHT. Administration of 6β-OHT to Cyp1b1−/− or castrated Cyp1b1+/+mice restored Ang II-induced hypertension and associated cardiac hypertrophy, fibrosis, and oxidative stress. Since 6β-OHT did not alter basal BP in Cyp1b1+/+ or Cyp1b1−/− mice, it appears that 6β-OHT acts as a permissive factor in Ang II actions to cause hypertension and associated cardiac pathogenesis in male mice. Therefore, developing selective inhibitors of CYP1B1 could be useful for treating renin-angiotensin and testosterone-dependent hypertension in males.
Acknowledgments
We thank Dr. David Armbruster for editorial assistance.
Sources of Funding
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute grants R01-19134–39 and R0-R01HL079109-08 (K.U.M). M.K. was supported by a fellowship from Department of Pharmacology, Faculty of Medicine, Erciyes University, Kayseri, Turkey-38039. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute.
Footnotes
Conflicts of Interest/Disclosures
None.
References
- 1.Messerli FH, Garavaglia GE, Schmieder RE, Sundgaard-Riise K, Nunez BD, Amodeo C. Disparate cardiovascular findings in men and women with essential hypertension. Ann Intern Med. 1987;107:158–161. doi: 10.7326/0003-4819-107-2-158. [DOI] [PubMed] [Google Scholar]
- 2.Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med. 1991;325:756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- 3.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 4.Staessen J, Bulpitt CJ, Fagard R, Lijnen P, Amery A. The influence of menopause on blood pressure. J Hum Hypertens. 1989;3:427–433. [PubMed] [Google Scholar]
- 5.Yong LC, Kuller LH, Rutan G, Bunker C. Longitudinal study of blood pressure changes and determinants from adolescence to middle age. The Dormont High School Follow-Up Study, 1957–1963 to 1989–1990. Am J Epidemiol. 1993;138:973–983. doi: 10.1093/oxfordjournals.aje.a116817. [DOI] [PubMed] [Google Scholar]
- 6.Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res. 2002;53:688–708. doi: 10.1016/s0008-6363(01)00527-2. [DOI] [PubMed] [Google Scholar]
- 7.Ji H, Zheng W, Wu X, Liu J, Ecelbarger CM, Watkins R, Arnold AP, Sandberg K. Sex chromosome effects unmasked in angiotensin II-induced hypertension. Hypertension. 2010;55:1275–1282. doi: 10.1161/HYPERTENSIONAHA.109.144949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol. 2005;288:2177–2184. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- 9.Masubuchi Y, Kumai T, Uematsu A, Komoriyama K, Hirai M. Gonadectomy-induced reduction of blood pressure in adult spontaneously hypertensive rats. Acta Endocrinol (Copenh) 1982;101:154–160. doi: 10.1530/acta.0.1010154. [DOI] [PubMed] [Google Scholar]
- 10.Reckelhoff JF, Zhang H, Srivastava K, Granger JP. Gender differences in hypertension in spontaneously hypertensive rats role of androgens and androgen receptor. Hypertension. 1999;34:920–923. doi: 10.1161/01.hyp.34.4.920. [DOI] [PubMed] [Google Scholar]
- 11.Rowland NE, Fregly MJ. Role of gonadal hormones in hypertension in the Dahl salt-sensitive rat. Clin Exp Hypertens A. 1992;14:367–375. doi: 10.3109/10641969209036195. [DOI] [PubMed] [Google Scholar]
- 12.Chen YF, Naftilan AJ, Oparil S. Androgen dependent angiotensinogen and renin messenger RNA in hypertensive rats. Hypertension. 1992;19:456–463. doi: 10.1161/01.hyp.19.5.456. [DOI] [PubMed] [Google Scholar]
- 13.Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol. 2009;296:771–779. doi: 10.1152/ajprenal.90389.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iliescu R, Cucchiarelli VE, Yanes LL, Iles JW, Reckelhoff JF. Impact of androgen-induced oxidative stress on hypertension in male SHR. Am J Physiol Regul Integr Comp Physiol. 2007;292:731–735. doi: 10.1152/ajpregu.00353.2006. [DOI] [PubMed] [Google Scholar]
- 15.Meng S, Cason GW, Gannon AW, Racusen LC, Manning RD., Jr Oxidative stress in Dahl salt-sensitive hypertension. Hypertension. 2003;41:1346–1352. doi: 10.1161/01.HYP.0000070028.99408.E8. [DOI] [PubMed] [Google Scholar]
- 16.Holla VR, Adas F, Imig JD, Zhao X, Price E, Jr, Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci U S A. 2001;98:5211–5216. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu CC, Schwartzman ML. The role of 20-HETE in androgen-mediated hypertension. Prostaglandins Other Lipid Mediat. 2011;96:45–53. doi: 10.1016/j.prostaglandins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korashy HM, El-Kadi AO. The role of aryl hydrocarbon receptor in the pathogenesis of cardiovascular diseases. Drug Metab Rev. 2006;38:411–450. doi: 10.1080/03602530600632063. [DOI] [PubMed] [Google Scholar]
- 19.Jennings BL, Sahan-Firat S, Estes AM, Das K, Farjana N, Fang XR, Gonzalez FJ, Malik KU. Cytochrome P450 1B1 contributes to angiotensin II-induced hypertension and associated pathophysiology. Hypertension. 2010;56:667–674. doi: 10.1161/HYPERTENSIONAHA.110.154518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jennings BL, Anderson LJ, Estes AM, Yaghini FA, Fang XR, Porter J, Gonzalez FJ, Campbell WB, Malik KU. Cytochrome P450 1B1 contributes to renal dysfunction and damage caused by angiotensin II in mice. Hypertension. 2012;59:348–354. doi: 10.1161/HYPERTENSIONAHA.111.183301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jennings BL, Estes AM, Anderson LJ, Fang XR, Yaghini FA, Fan Z, Gonzalez FJ, Campbell WB, Malik KU. Cytochrome P450 1B1 gene disruption minimizes deoxycorticosterone acetate-salt-induced hypertension and associated cardiac dysfunction and renal damage in mice. Hypertension. 2012;60:1510–1516. doi: 10.1161/HYPERTENSIONAHA.112.202606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahan-Firat S, Jennings BL, Yaghini FA, Song CY, Estes AM, Fang XR, Farjana N, Khan AI, Malik KU. 2,3′, 4,5-Tetramethoxystilbene prevents deoxycorticosterone-salt-induced hypertension: contribution of cytochrome P450 1B1. Am J Physiol Heart Circ Physiol. 2010;299:1891–1901. doi: 10.1152/ajpheart.00655.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jennings BL, Montanez DE, May ME, Jr, Estes AM, Fang XR, Yaghini FA, Kanu A, Malik KU. Cytochrome P450 1B1 contributes to increased blood pressure and cardiovascular and renal dysfunction in spontaneously hypertensive rats. Cardiovasc Drugs Ther. 2014;28:145–161. doi: 10.1007/s10557-014-6510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jennings BL, George LW, Pingili AK, Khan NS, Estes AM, Fang XR, Gonzalez FJ, Malik KU. Estrogen metabolism by cytochrome P450 1B1 modulates the hypertensive effect of angiotensin II in female mice. Hypertension. 2014;64:134–140. doi: 10.1161/HYPERTENSIONAHA.114.03275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahman M, Sutter CH, Emmert GL, Sutter TR. Regioselective 2-hydroxylation of 17β-estradiol by rat cytochrome P4501B1. Toxicol Appl Pharmacol. 2006;216:469–478. doi: 10.1016/j.taap.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Zacharia LC, Dubey RK, Mi Z, Jackson EK. Methylation of 2-hydroxyestradiol in isolated organs. Hypertension. 2003;42:82–87. doi: 10.1161/01.HYP.0000074702.06466.27. [DOI] [PubMed] [Google Scholar]
- 27.Crespi CL, Penman BW, Steimel DT, Smith T, Yang CS, Sutter TR. Development of a human lymphoblastoid cell line constitutively expressing human CYP1B1 cDNA: substrate specificity with model substrates and promutagens. Mutagenesis. 1997;12:83–89. doi: 10.1093/mutage/12.2.83. [DOI] [PubMed] [Google Scholar]
- 28.Song J, Wadhwa L, Bejjani BA, O’Brien WE. Determination of 3-keto-4-ene steroids and their hydroxylated metabolites catalyzed by recombinant human cytochrome P450 1B1 enzyme using gas chromatography-mass spectrometry with trimethylsilyl derivatization. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;791:127–135. doi: 10.1016/s1570-0232(03)00228-9. [DOI] [PubMed] [Google Scholar]
- 29.Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. Metabolism of retinoids and arachidonic acid by human and mouse cytochrome P450 1b1. Drug Metab Dispos. 2004;32:840–847. doi: 10.1124/dmd.32.8.840. [DOI] [PubMed] [Google Scholar]
- 30.Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. 17 beta- estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci U S A. 1996;93:9776–9781. doi: 10.1073/pnas.93.18.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sissung TM, Price DK, Sparreboom L, Figg WD. Pharmacogenetics and regulation of human cytochrome p450 1b1: implications in hormone-mediated tumor metabolism and a novel target for therapeutic intervention. Mol Cancer Res. 2006;4:135–150. doi: 10.1158/1541-7786.MCR-05-0101. [DOI] [PubMed] [Google Scholar]
- 32.Papamitsou T, Barlagiannis D, Papaliagkas V, Kotanidou E, Dermentzopoulou-Theodoridou M. Testosterone-induced hypertrophy, fibrosis and apoptosis of cardiac cells – an ultrastructural and immunohistochemical study. Med Sci Monit. 2011;17:266–273. doi: 10.12659/MSM.881930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thum T, Borlak J. Testosterone, cytochrome P450, and cardiac hypertrophy. FASEB J. 2002;16:1537–1549. doi: 10.1096/fj.02-0138com. [DOI] [PubMed] [Google Scholar]
- 34.Thum T, Borlak J. Cytochrome P450 mono-oxygenase gene expression and protein activity in cultures of adult cardiomyocytes of the rat. Br J Pharmacol. 2000;130:1745–1752. doi: 10.1038/sj.bjp.0703465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krauser JA, Voehler M, Tseng L-H, Schefer AB, Godejohann M, Guengerich FP. Testosterone 1β-hydroxylation by human cytochrome P450 3A4. Eur J Biochem. 2004;271:3962–3969. doi: 10.1111/j.1432-1033.2004.04339.x. [DOI] [PubMed] [Google Scholar]
- 36.Sodhi K, Wu C-C, Cheng J, Gotlinger K, Inoue K, Goli M, Falck JR, Abraham NG, Schwartzman ML. CYP4A2-induced hypertension is 20-hydroxyeicosatetraenoic acid–and angiotensin II-dependent. Hypertension. 2010;56:871–878. doi: 10.1161/HYPERTENSIONAHA.110.154559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh H, Cheng J, Deng H, Kemp R, Ishizuka T, Nasjletti A, Schwartzman ML. Vascular cytochorme p450 4a expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension. 2007;50:123–129. doi: 10.1161/HYPERTENSIONAHA.107.089599. [DOI] [PubMed] [Google Scholar]
- 38.Wu CC, Mei S, Cheng J, Ding Y, Weidenhammer A, Garcia V, Zhang F, Gotlinger K, Manthati VL, Falck JR, Capdevila JH, Schwartzman ML. Androgen-sensitive hypertension associates with upregulated vascular CYP4A12-20-HETE synthase. J Am Soc Nephrol. 2013;24:1288–1296. doi: 10.1681/ASN.2012070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia V, Cheng J, Weidenhammer A, Ding Y, Wu CC, Zhang F, Gotlinger K, Falck JR, Schwartzman ML. Androgen-induced hypertension in angiotensinogen deficient mice: Role of 20-HETE and EETS. Prostaglandins Other Lipid Mediat. 2014 doi: 10.1016/j.prostaglandins.2014.12.001. S1098-8823:00076-00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Letavernier E, Perez J, Bellocq A, Mesnard L, de Castro Keller A, Haymann JP, Baud L. Targeting the calpain/calpastatin system as a new strategy to prevent cardiovascular remodeling in angiotensin II-induced hypertension. Circ res. 2008;102:720–728. doi: 10.1161/CIRCRESAHA.107.160077. [DOI] [PubMed] [Google Scholar]
- 41.Magee J, Chang LW, Stormo GA, Milbrandt J. Direct androgen receptor-mediated regulation of the FKBP5 gene via a distal enhancer element. Endocrinology. 2006;147:590–598. doi: 10.1210/en.2005-1001. [DOI] [PubMed] [Google Scholar]
- 42.Mendelsohm ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 43.Ojeda NB, Royals TP, Black JT, Dasinger JH, Johnson JM, Alexander BT. Enhanced sensitivity to acute angiotensin II is testosterone dependent in adult male growth-restricted offspring. Am J Physiol Regul Integr Comp Physiol. 2010;298:1421–1427. doi: 10.1152/ajpregu.00096.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chinnathambi V, Blesson CS, Vincent KL, Saade GR, Hankins GD, Yallampalli C, Sathishkumar K. Elevated testosterone levels during rat pregnancy cause hypersensitivity to angiotensin II and attenuation of endothelium-dependent vasodilation in uterine arteries. Hypertension. 2014;64:405–414. doi: 10.1161/HYPERTENSIONAHA.114.03283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guilluy C, Brégeon J, Toumaniantz G, Rolli-Derkinderen M, Retailleau K, Loufrani L, Henrion D, Scalbert E, Bril A, Torres RM, Offermanns S, Pacaud P, Loirand G. The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nat Med. 2010;16:183–190. doi: 10.1038/nm.2079. [DOI] [PubMed] [Google Scholar]
- 46.Song J, Kost CK, Jr, Martin DS. Androgens potentiate renal vascular responses to angiotensin II via amplification of the Rho kinase signaling pathway. Cardiovasc Res. 2006;72:456–463. doi: 10.1016/j.cardiores.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Loria AS, Yamamoto T, Pollock DM, Pollock JS. Early life stress induces renal dysfunction in adult male rats but not female rats. Am J Physiol Regul Integr Comp Physiol. 2013;304:R121–R129. doi: 10.1152/ajpregu.00364.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002;91:1038–1045. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]
- 49.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim H-S, Smithies O, Le TH, Coffman TM. Distinct roles for the kidney and systemic tissues in blood pressure regulation. Proc Natl Acad Sci. 2006;103:7985–7990. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II–induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muskhelishvili L, Thompson PA, Kusewitt DF, Wang C, Kadlubar FF. In situ hybridization and immunohistochemical analysis of cytochrome P450 1B1 expression in human normal tissues. J Histochem Cytochem. 2001;49:229–236. doi: 10.1177/002215540104900210. [DOI] [PubMed] [Google Scholar]
- 52.Heritage AS, Stumpf WE, Sar M, Grant LD. (3-H)-dihydrotestosterone in catecholamine neurons of rat brain stem: combined localization by autoradiography and formaldehyde-induced fluorescence. J Comp Neurol. 1981;200:289–330. doi: 10.1002/cne.902000208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.