Abstract
Emerging evidence suggests that gut microbiota is critical in the maintenance of physiological homeostasis. The present study was designed to test the hypothesis that dysbiosis in gut microbiota is associated with hypertension since genetic, environmental, and dietary factors profoundly influence both gut microbiota and blood pressure. Bacterial DNA from fecal samples of two rat models of hypertension and a small cohort of patients was used for bacterial genomic analysis. We observed a significant decrease in microbial richness, diversity, and evenness in the spontaneously hypertensive rat, in addition to an increased Firmicutes to Bacteroidetes ratio. These changes were accompanied with decreases in acetate- and butyrate-producing bacteria. Additionally, the microbiota of a small cohort of human hypertension patients was found to follow a similar dysbiotic pattern, as it was less rich and diverse than that of control subjects. Similar changes in gut microbiota were observed in the chronic angiotensin II infusion rat model, most notably decreased microbial richness and an increased Firmicutes to Bacteroidetes ratio. In this model, we evaluated the efficacy of oral minocycline in restoring gut microbiota. In addition to attenuating high blood pressure, minocycline was able to rebalance the dysbiotic hypertension gut microbiota by reducing the Firmicutes to Bacteroidetes ratio. These observations demonstrate that high BP is associated with gut microbiota dysbiosis, both in animal and human hypertension. They suggest that dietary intervention to correct gut microbiota could be an innovative nutritional therapeutic strategy for hypertension.
Keywords: Hypertension, Microbiota, Dysbiosis, Minocycline, Butyrate
Introduction
Hypertension (HTN) is the most modifiable risk factor for cardiovascular disease (CVD) and stroke, but also a hallmark of obesity, diabetes and metabolic syndrome1. Despite recent advances in pharmacotherapy and widespread adoption of lifestyle changes, prevalence of HTN remains high. By some estimates the prevalence of resistant HTN is ~10–15% among the hypertensive patient population. Treatment-resistant hypertension (TRH) is characterized by high norepinephrine spillover, enhanced sympathetic outflow, and dampened parasympathetic activity, thus suggesting a neurogenic component that is essential in maintaining elevated blood pressure (BP) levels2, 3. Increasing evidence suggests that coupled to autonomic dysfunction, TRH is also accompanied by a chronic low-grade inflammatory profile that facilitates end-organ damage and perpetuates the hypertensive state4, 5. However, the intrinsic mechanisms that contribute to sustaining the systemic inflammatory profile found in these patients are yet to be fully understood. In the periphery, gut microbiota plays an important role in shaping a robust systemic and intestinal immune system6, 7. It is commonly referred to as an “essential” acquired organ, since its composition and richness are constantly adapting to the challenges presented by the environment and/or by the host, like age, diet, lifestyle modifications, and disease states8, 9. Typically, changes in gut microbiota have been related to chronic inflammatory diseases such as asthma, allergies, inflammatory bowel disease, and infectious diseases10. However, recent data suggest that it may also play a role in the development and maintenance of CVD and metabolic disorders such as obesity, diabetes and metabolic syndrome11–14.
Adult gut microbiota is very diverse; it is made up of trillions of microorganisms but mainly dominated by four phyla: (1) Firmicutes, (2) Bacteroidetes, (3) Actinobacteria and (4) Proteobacteria. A delicate balance in the gut microbiota composition is key in maintaining intestinal immunity and whole body homeostasis; any disruption of this balance could lead to devastating pathophysiological consequences. An imbalance in gut microbiota is commonly known as dysbiosis9. Although characterization of a “healthy” microbiota is at its initial stages15, increasing evidence suggest that changes in the ratio of the microbe communities Firmicutes (F) and Bacteroidetes (B), known as the F/B ratio, can be potentially used as a biomarker for pathological conditions8, 16. Several clinical trials have been conducted evaluating the use of probiotics and its effects on BP regulation. A meta-analysis of nine randomized trials showed a significant decrease in both systolic and diastolic BP in patients that consumed a daily dose of 109 CFU of probiotics17. This evidence indirectly suggests that gut microbiota may play a key role in the control of BP homeostasis and that any change in microbiota composition and/or imbalance may potentially result in HTN. Hence, we hypothesize that hypertensive risk factors such as genetic predisposition, diet, etc. induce changes in the gut microbiota that results in dysbiosis, and controlling this effect may prove as an alternative treatment for this disease. This study was designed to provide evidence for this hypothesis.
Methods
The online supplement includes detailed description of all the materials and methods. All animal procedures were approved by the University of Florida Institutional Animal Care and Use Committee. Human studies were approved by the University of Florida Institutional Review Board.
Results
HTN is associated with alteration in fecal microbiota composition in the SHR
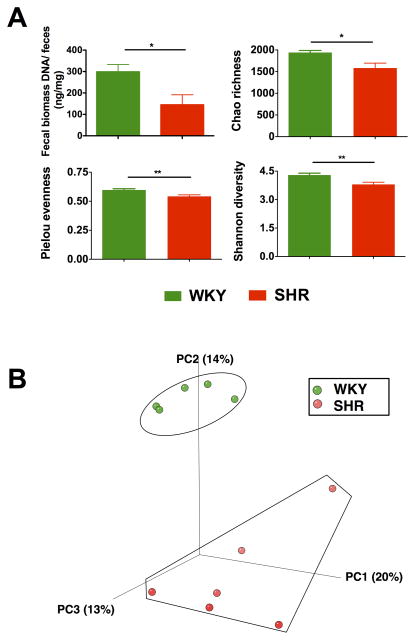
Fecal DNA was isolated from both Wistar Kyoto rats (WKY; MAP: 108 ± 2 mmHg) and spontaneously hypertensive rats (SHR; MAP: 148 ± 10 mmHg), and the bacterial loads were represented as fecal biomass DNA per mg of feces. A significant reduction of bacterial load was found in SHR when compared with WKY (Figure 1A). Subsequently, 16s ribosomal DNA sequencing and the bioinformatics alignment comparison against the Silva non-redundant database were performed. Significant fecal microbial alterations were found in the SHR when compared with the WKY. The compositions of bacterial communities were evaluated by calculating three major ecological parameters, including Chao richness (an estimate of a total number of Operational Taxonomic Units [OTU] present in the given community), Pielou evenness (to show how evenly the individuals in the community are distributed over different OTU) and Shannon diversity (the combined parameter of richness and evenness). Microbial richness, evenness, and diversity were all found to be drastically decreased in the SHR group when compared with the WKY control (Figure 1A). Furthermore, weighted UniFrac analyses were used to calculate distances between the fecal samples from the WKY and SHR, and three-dimensional scatterplots were generated by principal coordinates analysis (PCoA) to visualize whether the experimental groups in the input phylogenetic tree have significantly different microbial communities. This method allows us to present dissimilarities of the data in terms of distance18. Each PC axis percentage describes how much one dimension can account for. The composition of the fecal microbial communities of the WKY and SHR were found to be distinct, as presented in Figure 1B. A clear separation was observed in the PCoA between the two clusters, representing the microbial compositions of WKY and SHR, indicating two extremely different gut environments.
Figure 1. Gut microbiota communities in WKY rats and SHR.
A. Decreased micro-ecological parameters of the gut. Fecal samples were collected from WKY (n=5) and SHR (n=6) rats and bacterial 16S ribosomal DNA were amplified and sequenced to analyze the compositions of microbial communities. Fecal biomass and microbial richness, evenness, and diversity of WKY and SHR were evaluated. B. Principal coordinate analysis of WKY and SHR. Weighted uniFrac analyses were used to calculate the distances between fecal samples from WKY and SHR. Each axis percentage describes how much variation that one dimension accounts for. Darker colored circles indicate a point closer to the reader, while lighter colored circles indicate a point further away. By comparing the samples in a 3 dimensional figure, a clear separation was observed between the two clusters, representing WKY and SHR rats, respectively. Results were compared by student’s t-test; * p<0.05; **p<0.01.
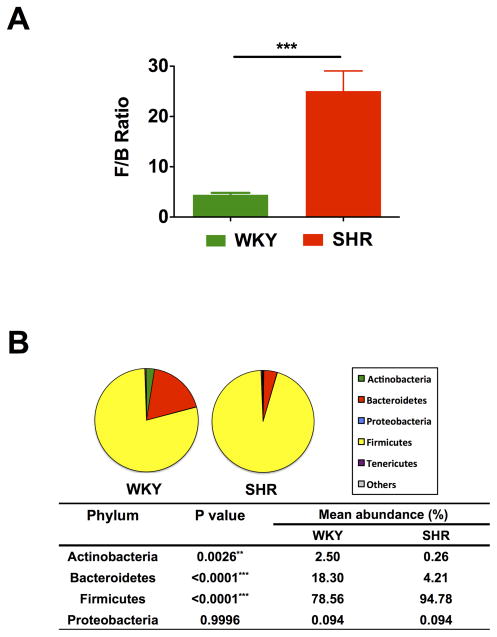
An increased F/B ratio, caused by an expansion of Firmicutes and/or a contraction of Bacteroidetes, has been widely considered a signature of gut dysbiosis. To demonstrate whether the hypertensive rat model also confers a similar gut microbiota pattern, we analyzed the proportions of 16S rDNA reads assigned to each phylum. The F/B ratio in the SHR was ~5-fold higher compared with WKY (Figure 2A). Fecal samples from both WKY and SHR were dominated by Firmicutes and Bacteroidetes, and with smaller proportions of Actinobacteria and Proteobacteria (Figure 2B). However, significant differences were observed between the groups in terms of the relative abundances of Actinobacteria, Bacteroidetes and Firmicutes. The diminished proportion of Actinobacteria indicates a less diverse microbiota in the SHR, which is consistent with the decrease in Chao richness and Shannon diversity.
Figure 2. Comparison of microbiota composition between WKY rats and SHR.
A. The Firmicutes to Bacteroidetes ratio (F/B ratio) was calculated as a biomarker of gut dysbiosis. B. Phylum breakdown of the four most abundant bacterial communities in the WKY (n=5) and SHR (n=6) fecal samples. A decrease of Bacteroidetes along with an increase of Firmicutes resulted in a dysbiosis signature of gut microbiota in SHR rats. A significant reduction of the Actinobacteria phylum correlated with a lower diversity value. Results were compared by student’s t-test; ** p<0.01; ***p<0.001.
Acetate and butyrate fermenters are decreased in the SHR
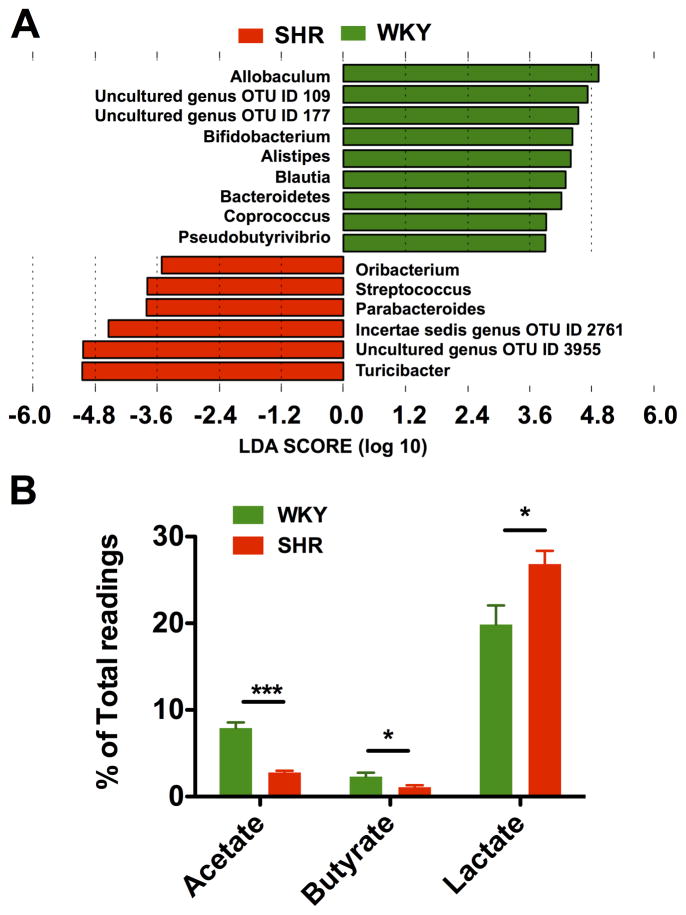
Given an imbalanced F/B ratio in the SHR, we asked what genera of bacteria contributed to the alteration of microbiota composition towards dysbiosis. LEfSe performed at the genus level demonstrated that butyrate-producing bacteria were found to be highly accumulated in WKY, including Coprococcus and Pseudobutyrivibrio (Figure 3A). In contrast, lactate-producing bacteria, Streptococcus and Turicibacter, were in higher quantities in the SHR. Two uncultured genera OTUs (109 and 177) that belonged to the Bacteroidetes phylum were more abundant in WKY. Interestingly, both of these two genera were completely depleted in the SHR. The abundance of other uncultured and incertae sedis bacteria genera OTUs (2761 and 3955) that belonged to Firmicutes were significantly enriched in the SHR. These changes were the major contributors to the increased F/B ratio in the SHR. Bifidobacterium, which belongs to the Actinobacteria phylum, is commonly considered a beneficial bacterial genus, and plays a critical role in the maturation and regulation of the immune system19, 20. The depletion of Bifidobacterium has been reported in multiple disease conditions21, 22 and is also a feature of dysbiosis. Remarkably, a significant depletion of Bifidobacterium was found in the SHR, which greatly contributed to the diminished proportion of Actinobacteria and therefore the decreased gut microbiota diversity.
Figure 3. Diminished acetate- and butyrate-producing bacteria in SHR.
A. Most enriched and depleted genera in the SHR versus WKY. Miseq reads of bacterial 16S ribosomal DNA were analyzed and assigned to specific genera based on the sequence similarities to Silva non-redundant 16S reference database. Linear discriminant analysis (LDA) along with effect size measurements was applied to present the enriched bacterial genera in SHR (red) and WKY (green). B. The relative proportions of acetate, butyrate and lactate producing bacteria in the gut microbiota in WKY (n=5) and SHR (n=6). Sequence reads were classified according to the primary end product of the assigned bacterial genera. Genera were classified into more than one group correspondingly if they were defined as producers of multiple metabolites. Genera that were defined as producing equol, histamine, hydrogen and propionate constituted a minor portion of the population and were therefore excluded from this analysis. Results were compared by student’s t-test; *p<0.05; ***p<0.001.
To illustrate the roles of other bacteria genera in the gut, we re-grouped all the bacterial 16s reads according to their major metabolic end-products as described in the methods. Butyrate, as one of the critical bacterial metabolic products, harbors multiple beneficial properties for the host23, 24. Some butyrate-producing bacteria can utilize acetate as an energy source to generate butyrate25. We observed ~3-fold decrease in acetate- and a 2-fold decrease in butyrate-producing bacteria in the SHR (Figure 3B). In contrast, the abundance of lactate-producing bacteria was significantly increased in the SHR. This indicates a dysfunction in both acetogenic and butyrogenic capabilities. Thus, HTN-associated dysbiosis is characterized as an accumulation of lactate-producing bacteria and a reduction of acetate and butyrate producers.
Lower richness and diversity of gut microbiota in HTN patients
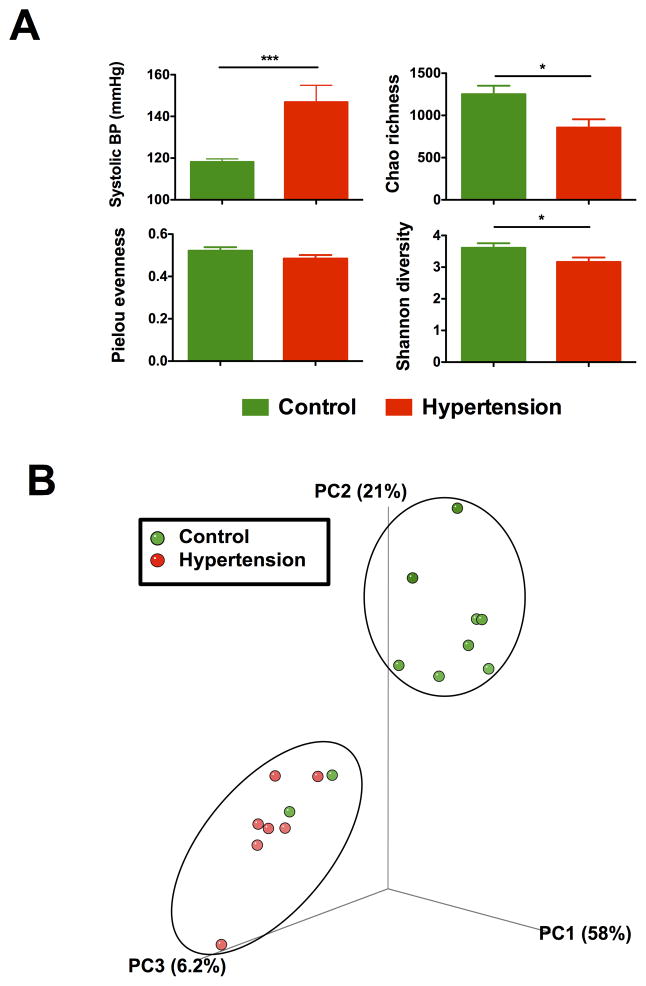
Based on these data, we speculated that the composition of the microbiota would also differ between patients with normal (119 ± 2 mmHg; n=10) and high (144 ± 9 mmHg; n=7) systolic blood pressure (SBP) (Figure 4A). Fecal samples were processed and analyzed via Illumina sequencing as described for rodent experiments. We observed a reduction in bacterial Chao richness and Shannon diversity in the patients with high SBP when compared with control patients with normal SBP (Figure 4A). There was a trend towards a decrease in Pielou evenness as well, but it did not reach statistical significance with this limited number of patients.
Figure 4. Reduced microbial richness and diversity in hypertension patients.
A. Lower microbial richness and diversity in hypertension patients. The hypertension patient demonstrated significantly higher ambient systolic blood pressure when compared with control. Fecal samples from hypertension patient were collected and 16S rDNA library was prepared and analyzed by illumina Miseq sequencer. Richness, evenness, and diversity were used to evaluate general differences of microbial composition in different groups. B. Principal coordinate analysis of control and hypertension groups. Weighted uniFrac analyses were performed to calculate the distances between fecal samples from control and hypertension patients. Each axis percentage describes how much variation that one dimension accounts for. By comparing the samples in a 3 dimensional PCoA figure, two separate clusters were formed. Results were compared by student’s t-test; * p<0.05, *** p<0.001. Control=10, Hypertension=7.
Next, weighted UniFrac analyses were employed to calculate distances, and three-dimensional scatterplots were generated using PCoA. As shown in Figure 4B, the two patient populations formed separate clusters. The analyzed human data clearly confirmed the observation made in our animal model where gut dysbiosis is observed between the high and normal SBP cohorts. Nonetheless, further studies to strengthen this observation need to be conducted using a larger cohort of HTN patients.
Gut microbial diversity is increased by treatment of minocycline
Since gut microbiota can be modified through the use of broad-spectrum antibiotics, we evaluated the efficacy of using minocycline in restoring gut microbiota. Minocycline is an anti-inflammatory antibiotic that freely crosses the blood-brain barrier and has been shown to produce beneficial effects in combating HTN26, 27. Additionally, minocycline is the drug approved in our patient studies (NCT#02133872 and 02133885) which has shown an impressive ability to decrease BP, improve HbA1C levels, and weight loss in our pilot study28. Therefore, understanding the effects of minocycline on microbiota will be greatly beneficial. For this purpose, we used the chronic Ang II infusion model, which is a well-validated and established model of HTN. As expected, oral minocycline for 4 weeks was able to significantly lower mean arterial pressure in Ang II-infused rats (24 hour MAP: 124 ± 2 mmHg vs 168 ± 2 mmHg) (Figure 5A). Consistent with the SHR, rats infused with Ang II showed a trend toward lower bacterial load, although this finding did not reach significance (Figure 5B). Minocycline treatment did not result in a further reduction of bacterial load. This is not surprising, as previous studies have indicated that the depletion of microbiota required a combination of at least of 3 antibiotics for 3–4 weeks29, 30, and single antibiotics may not be sufficient to reduce the total bacterial load31.
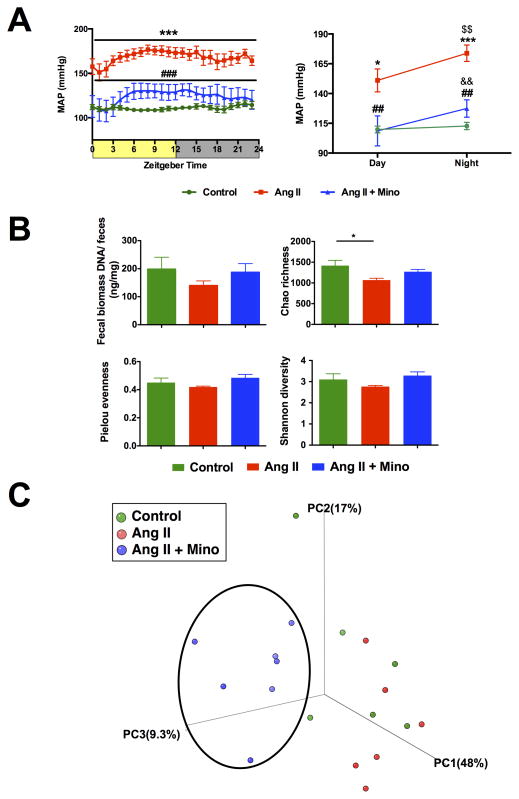
Figure 5. Minocycline attenuates Ang II infusion hypertension and causes a shift in the gut microbiota composition.
A. Mean arterial pressure at four weeks of Ang II infusion and oral minocycline (Mino) treatment presented as 3-hour moving averages over 24 hours and the lowest and highest point during day and night recording. B. Fecal samples were collected and bacterial 16S ribosomal DNA were amplified and sequenced to analyze the compositions of microbial communities. Fecal biomass, richness, evenness, and diversity were used to evaluate general differences of microbial composition in different groups. Ang II alone displayed a decrease in richness of gut microbiota when compared with control. C. Principal coordinate analysis of three groups. Weighted uniFrac analyses were used to calculate the distances among fecal samples from control, Ang II and Ang II + Mino. The fecal microbiota composition shifted upon minocycline treatment and differs from control and Ang II groups. Results were compared by one-way ANOVA and Newman-Keuls post-hoc test; *p<0.05, ***p<0.001 vs control; ##p<0.01, ###p<0.001 vs Ang II; $$p<0.01 vs Ang II day; &&p<0.01 vs Ang II + Mino day. Control n=6, Ang II n=6, Ang II+Mino n=7.
To test the effect of minocycline on microbial composition, 16S rDNA sequencing was performed and data were statistically analyzed as before. Rats infused with Ang II alone demonstrated a lower richness value when compared with control (Figure 5B). Despite this lower richness value, we did not observe a clear separation between the clusters of these two groups in PCoA analysis, due to the comparable values of evenness and diversity (Figure 5C). However, Ang II + minocycline rats displayed a significant separation from the other two groups, indicating a shift in the gut bacterial composition by the administration of minocycline.
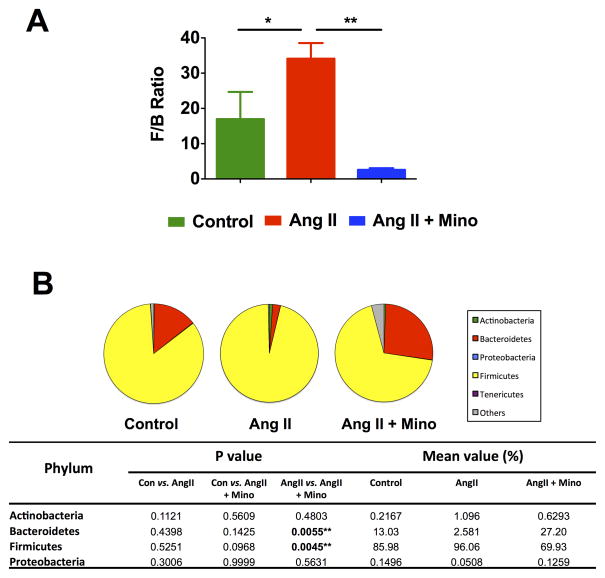
Most importantly, the F/B ratio was considerably increased in the Ang II-infused rats compared to control (Figure 6A). This was significantly reduced by minocycline treatment. Additionally, the pie charts that represent the composition of microbiota at the phylum level show a trend towards relative higher abundance of Firmicutes and a lower abundance of Bacteroidetes in Ang II-infused rats when compared with control (Figure 6B), though the differences between those two groups did not reach individual significance. This indicates that the dysbiosis in this model is characterized by both an increase in Firmicutes and a decrease in Bacteroidetes. This signature of gut microbiota dysbiosis is consistent with that previously shown in the SHR model. Interestingly, oral administration of minocycline was capable of increasing Bacteroidetes in the gut, which has been reported as a positive sign after successful fecal microbiota transplantation for the treatment of recurrent C. difficile infection induced dysbiosis32. In addition, we observed a striking bloom of other bacteria phyla (including Verrucomicrobia) beyond Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria and Tenericutes, indicating a significant shift of intestinal microbial composition towards diversity. In summary, we demonstrated that minocycline administration was able to restore the Bacteroidetes population and reshape the microbiota composition, by drastically increasing microbial diversity, and restoring the F/B ratio.
Figure 6. Comparison of microbiota composition in Ang II infusion model and minocycline treatment.
A. The Firmicutes to Bacteroidetes ratio (F/B ratio) was calculated as a biomarker of gut dysbiosis. B. Description of mean proportional values of indicated phyla by pie chart. Gut microbiota was altered by chronic induction of hypertension (Ang II) towards dysbiosis, but Ang II + Mino administration reshaped microbiota composition towards diversity. Results were compared by one-way ANOVA and Newman-Keuls post-hoc test; *p<0.05, **p<0.01. Control n=6, Ang II n=6, Ang II+Mino n=7.
Acetate- and butyrate-producing bacteria are expanded by minocycline treatment
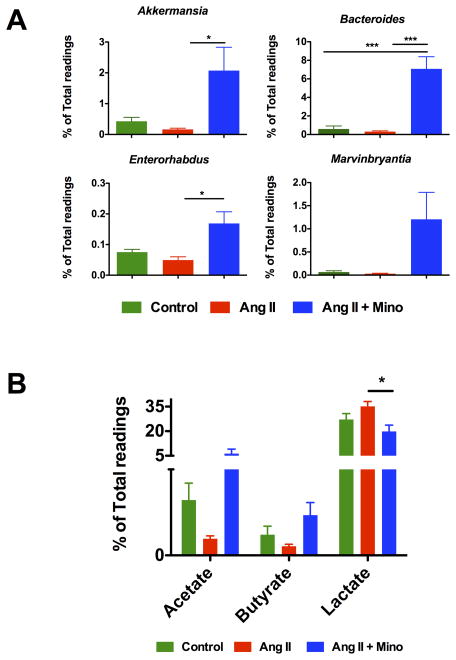
Since minocycline treatment caused a remarkable shift in the gut microbial environment, we investigated the specific bacteria with notable alteration of abundance that could be verified at the genus level by LEfSe analysis and identified the four most expanded genera in the Ang II + minocycline treated rats. Figure 7A shows the enrichment of Akkermansia, Bacteroides, Enterorhabdus and Marvinbryantia in Ang II + minocycline treated group. Functionally, both Bacteroides and Marvinbryantia produce acetate as their main fermentation product, and Akkermansia and Enterorhabdus have been found to be inversely associated with obesity and diabetes33.
Figure 7. Bloom of acetate and butyrate producing bacteria upon treatment of minocycline.
A. Expansion of several genera observed in the fecal samples from rats administrated by minocycline and angiotensin II. Miseq reads of bacterial 16S ribosomal DNA were analyzed and assigned to specific genera based on the sequence similarities to Silva non-redundant 16S reference database. The most accumulated genera of bacteria were identified by LEfSe as Akkermansia, Bacteroides, Enterorhabdus and Marvinbryantia. B. The relative proportions of acetate, butyrate and lactate producing bacteria in the gut microbiota in the rats either untreated or treated with Angiotensin II or Angiotensin II plus minocycline. Sequence reads were classified according to the primary end product of the assigned bacterial genera. Results were compared by one-way ANOVA and Newman-Keuls post-hoc test; *p<0.05, **p<0.01. Control n=6, Ang II n=6, Ang II+Mino n=7.
Similar to the SHR, the acetate- and butyrate-producing bacteria genera were also depleted in chronically infused Ang II rats, although this finding failed to reach significance (Figure 7B). However, oral administration of minocycline resulted in a remarkable increase of both the acetate- and butyrate-producing bacteria. This was associated with a decrease in the abundance of lactate-producing bacteria.
Discussion
This study provides the first evidence, to our knowledge, of an association of HTN with altered gut microbiota with the use of two different rat models of HTN and a small cohort of HTN patients. The major findings of this study are: (1) decreases in the microbial richness and marked increases in the F/B ratio in the animal models of HTN, implicating the existence of gut dysbiosis in HTN; (2) this dysbiosis was associated with decreases in acetate- and butyrate-producing bacteria, and an increase in the lactate-producing bacterial population; (3) gut microbiota dysbiosis was confirmed in a small cohort of human HTN patients, pointing to the potential clinical significance of this work; (4) oral minocycline could rebalance the gut microbiota in a rat model of HTN. These findings clearly implicate the role of gut microbiota in the pathophysiology of both animal and human HTN.
Gut microbial dysbiosis has been connected to many chronic diseases including pathophysiologies that are associated with the cardiovascular system such as diabetes, obesity, and cardiac dysfunctions12, 14, 16. In the present study, we employed three commonly used parameters to study the gut microbiota: Chao richness, Pielou evenness, and Shannon diversity. While all three of these ecological parameters were drastically decreased in the SHR model, only Chao richness was found to be lower in the Ang II infusion model of HTN. This discrepancy can be attributed to the differences in pathogenesis in both of these animal models, and may represent an important area of future research focus. However, the most important and recognized biomarker, the F/B ratio, was dramatically increased in both the SHR and Ang II infusion models. An increased F/B ratio, caused by an expansion of Firmicutes and/or a contraction of Bacteroidetes, has been widely considered a signature of gut dysbiosis and has been associated with obesity, diabetes, and CVD8, 13, 16. It is important to note that the F/B ratio is well validated in rodent and human samples; however its significance in pig models requires further studies33, 34. Finally, we found a reduction in the fecal biomass in the SHR, as well as a trend toward reduction in the Ang II infusion model. This is consistent with the previous reports that dysbiosis is associated with a decrease of bacterial loads35. Previous studies failed to identify differences in the gut microbiota between WKY and SHR36, and L-NAME hypertension37. However, the discrepancies may be accounted for by: (1) significant pathophysiological differences between WKY and SHR colonies depending on origin, breeding, and maintenance protocols; (2) lack of details regarding animal housing or pathogen-free facilities; (3) utilizing large enough number of rats necessary to detect a differences; (4) choosing the best models available for human HTN.
Increases in bacterial populations relevant to the production of acetate and butyrate and reduced microbial diversity are linked to diabetes, obesity, and cardiac dysfunctions38. Our observations are consistent with these reports and support the important role of microbial diversity in HTN. Of particular interest is the finding that lactate-producing bacteria were increased in both animal models of HTN, since plasma lactate levels have been associated with increased BP39, 40. However, we did not measure plasma lactate in any of our experiments, and this remains a future direction. Additionally, a greater microbial diversity of the gut is conducive to enrichment of bacterial colonies that are known to produce metabolites, such as short chain fatty acids (SCFA) that are beneficial in maintaining normal physiological homeostasis. Interestingly, decreases in the butyrate- and acetate-producing bacteria were found in both models of HTN. Some butyrate-producing bacteria can utilize acetate as an energy source to generate butyrate25. Butyrate, one of the most beneficial SCFA, harbors multiple effects for the host, such as reduction of inflammation in the intestine and adipose tissue41, improved insulin sensitivity in type 2 diabetes23, 42, protection against diet induced obesity43, and CVD44. This SCFA plays a regulatory role in the epithelial cell differentiation, barrier function, stimulation of regulatory T cells24, amelioration of mucosal inflammation, and suppression of colorectal cancer to name a few effects in the intestine45. Systematically, butyrate has been shown to prevent immune cell infiltration46 and improve renal function following ischemic damage47. Another SCFA, propionate, has been shown to induce vasodilation and acute hypertensive response in wild type mice, mediated by Gpr41 receptor48. Therefore, we can conclude that the HTN gut dysbiosis is characterized by an imbalance in specific microbial populations and their corresponding metabolites, specifically decreased acetate and butyrate-producing bacteria and increased lactate-producing bacteria. However, further experiments demonstrating the direct and indirect effects of SCFA on BP are necessary.
Our previous studies have indicated that minocycline influences microglial activation and neuroinflammation in autonomic brain regions27. Minocycline is an anti-inflammatory antibiotic that penetrates through the blood brain barrier and causes a reduction in high BP. Doxycycline belongs to the same antibiotic class but has limited ability to pass through the blood brain barrier, and its effects on BP are inconclusive as there are multiple conflicting reports25,55. In this study, we chose to focus on minocycline as it is the focus of our approved clinical studies, and therefore understanding the effects of this broad-spectrum antibiotic on the gut microbiota homeostasis is crucial. Oral minocycline treatment results in the attenuation of high BP induced by chronic Ang II infusion. The first important finding was that minocycline did not reduce fecal biomass, providing evidence that this antibiotic does not have negative effects on the gut microbiota. Additionally, our data show that this treatment resulted in a drastic shift in the microbial environment characterized by a bloom of diverse bacteria in the gut. A dramatic increase in Verrucomicrobia phylum bacteria was observed, indicating that minocycline produced an intestinal environment that was extremely conducive to nurturing the growth of bacteria of this phylum. Further analysis of Verrucomicrobia phylum revealed a significant increase in the mucin-degrading bacterium, Akkermansia. It is pertinent to point out that a decrease in Akkermansia has been associated with obesity and diabetes, and introduction of Akkermansia could reverse the metabolic disorder and improve inflammation in diabetic and obese patients49. In addition, preliminary data indicate that minocycline treatment was able to reverse the Ang II-induced decrease in cingulin in the colon. Cingulin is a tight junction protein whose decreased levels have been associated with gut barrier dysfunction leading to increased inflammation50. Thus, these gut specific effects of minocycline appear to be linked with minocycline’s beneficial effects on inflammation and HTN.
This raises an important question: is minocycline’s antihypertensive effect primarily on the gut, on the brain, or they are linked? There is no evidence to rule out either possibility at the present time, since minocycline can freely cross the blood-brain barrier26 and can directly influence both the gut and the brain. Minocycline has profound effects on the gut: (1) it inhibits expression of pro-inflammatory cytokines and intestinal damage in mouse model of colitis51, 52, (2) it attenuates gut damage and intestinal mucositis due to cancer chemotherapy53, and (3) our data show it improves gut microbial homeostasis during HTN dysbiosis and is able to rebalance the gut acetate-, butyrate-, and lactate-producing bacterial populations. Furthermore, evidence also exists of direct anti-inflammatory effects of minocycline in the brain in many neurodegenerative and neurobehavioral diseases54, 55.
We propose the following hypothesis: pro-hypertensive signals (e.g. diet, genetic predisposition, and obesity) impact gut microbial composition inducing dysbiosis, increase inflammatory cells in the bone marrow (BM) and blood56, as well as hematopoietic stem cells (HSCs). This increase in HSCs has been implicated in their extravasation into the brain and establishment of neuroinflammation in many neural diseases57,58. The combination of gut dysbiosis, peripheral inflammation, and neuroinflammation leads to the development, establishment, and maintenance of high BP. Further studies are needed to determine whether the gut dysbiosis precedes the high BP and is crucial for the development of HTN, or is rather important in the late stages of the disease. Additionally, we postulate the following sequence of events in mino-induced antihypertensive effects: (1) in addition to crossing the blood brain barrier and inhibiting microglial activation, minocycline treatment induces an anti-inflammatory environment in the gut which is conducive to enrichment of bacterial colonies that are known to produce metabolites, such as SCFAs that are beneficial in maintaining normal physiological homeostasis; (2) these metabolites directly or indirectly impact generation of hematopoietic stem cells from the BM, decreasing the levels of myeloid progenitors; (3) this would result in attenuation of peripheral inflammation and decrease in mobilization of myeloid cells to the brain. The latter would be associated with attenuation of neuroinflammation. Future experiments will be aimed to provide evidence for this hypothesis.
In summary, our study demonstrates that HTN is associated with gut microbiota dysbiosis, characterized by an increased F/B ratio, as well as a drastic decrease in acetate-, butyrate-, and an accumulation of lactate- producing microbial populations in two rat models of HTN. Treatment with oral minocycline dose that attenuates HTN also produces beneficial effects on dysbiosis. Most importantly, this study does indeed demonstrate a dysbiotic gut profile in patients with high BP. It is important to mention that the two control subjects observed within the HTN PCoA plot distribution pertain to subjects who were currently under anti-HTN treatment although optimum medication combination and dosage had not been met. Therefore, this suggests that reversing the gut dysbiosis might be affected by the stability of BP control and the time undergoing such treatment. This clinical trial continues to recruit patients to be able to further establish the profile of the HTN gut dysbiosis, in addition to provide enough patients to perform subgroup analyses. Nonetheless, these observations support our overall proposal of a microbial dysbiosis linked to HTN.
Perspectives.
The involvement of gut dysbiosis in the pathogenesis of many diseases including diabetes, obesity, cancer and mental disorders is rapidly emerging. However, little is known about the role of microbiota in HTN. In this study, we present evidence of gut dysbiosis in HTN for the first time. This includes: (1) significant depletion of bacterial richness and increased F/B ratio in two rat models of HTN, (2) decreases in SCFA-producing bacteria and increase in lactate-producing bacterial populations (3) treatment with minocycline which attenuates high BP and is able to rebalance gut microbiota, and (4) validation of this concept in a small cohort of HTN patients. These observations suggest that innovative dietary strategies that impact gut microbiota could be developed for control and treatment of HTN.
Novelty and Significance.
What is new?
This study provides evidence that gut microbiota dysbiosis is associated with hypertension (HTN). Animals with HTN exhibited decreased gut microbial richness and diversity with associated decreases in acetate- and butyrate-producing bacteria. This dysbiosis was rebalanced by minocycline treatment. Additionally, these findings were validated in the human HTN population.
What is Relevant?
Despite recent advances in pharmacotherapeutics prevalence of HTN remains high. We proposed that gut microbiota composition could be of major importance since both diet and environmental factors have significant impact on in the development and the establishment of HTN.
Summary
Gut microbiota dysbiosis has been linked to HTN in two widely utilized rodent models of HTN and human HTN patients. Treatment with minocycline which attenuates high blood pressure, rebalances the HTN-linked dysbiosis in the rodent model.
Acknowledgments
Sources of Funding
This work was supported by NIH grants HL33610, AI093370 and UL1 TR000064 Clinical and Translational Science Award, to the University of Florida, and a grant from Gatorade foundation. Monica M. Santisteban is a predoctoral fellow of the Greater Southeast Affiliate, American Heart Association (14PRE18590018).
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Mendizábal Y, Llorens S, Nava E. Hypertension in metabolic syndrome: Vascular pathophysiology. Int J Hypertens. 2013;2013:230868. doi: 10.1155/2013/230868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14:177–183. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- 3.Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, Hastings J, Aggarwal A, Esler MD. Sympathetic augmentation in hypertension: Role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension. 2004;43:169–175. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- 4.Harrison DG. The immune system in hypertension. Trans Am Clin Climatol Assoc. 2014;125:130–138. discussion 138–140. [PMC free article] [PubMed] [Google Scholar]
- 5.Singh MV, Chapleau MW, Harwani SC, Abboud FM. The immune system and hypertension. Immunol Res. 2014;59:243–253. doi: 10.1007/s12026-014-8548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow J, Lee SM, Shen Y, Khosravi A, Mazmanian SK. Host-bacterial symbiosis in health and disease. Adv Immunol. 2010;107:243–274. doi: 10.1016/B978-0-12-381300-8.00008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDermott AJ, Huffnagle GB. The microbiome and regulation of mucosal immunity. Immunology. 2014;142:24–31. doi: 10.1111/imm.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mariat D, Firmesse O, Levenez F, Guimaraes V, Sokol H, Dore J, Corthier G, Furet JP. The firmicutes/bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 10.Collado MC, Rautava S, Isolauri E, Salminen S. Gut microbiota: A source of novel tools to reduce the risk of human disease? Pediatr Res. 2015;77:182–188. doi: 10.1038/pr.2014.173. [DOI] [PubMed] [Google Scholar]
- 11.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121:2126–2132. doi: 10.1172/JCI58109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everard A, Cani PD. Diabetes, obesity and gut microbiota. Best Pract Res Clin Gastroenterol. 2013;27:73–83. doi: 10.1016/j.bpg.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Howitt MR, Garrett WS. A complex microworld in the gut: Gut microbiota and cardiovascular disease connectivity. Nat Med. 2012;18:1188–1189. doi: 10.1038/nm.2895. [DOI] [PubMed] [Google Scholar]
- 15.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanz Y, Moya-Perez A. Microbiota, inflammation and obesity. Adv Exp Med Biol. 2014;817:291–317. doi: 10.1007/978-1-4939-0897-4_14. [DOI] [PubMed] [Google Scholar]
- 17.Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: A systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64:897–903. doi: 10.1161/HYPERTENSIONAHA.114.03469. [DOI] [PubMed] [Google Scholar]
- 18.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. Qiime allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grönlund MM, Gueimonde M, Laitinen K, Kociubinski G, Grönroos T, Salminen S, Isolauri E. Maternal breast-milk and intestinal bifidobacteria guide the compositional development of the bifidobacterium microbiota in infants at risk of allergic disease. Clin Exp Allergy. 2007;37:1764–1772. doi: 10.1111/j.1365-2222.2007.02849.x. [DOI] [PubMed] [Google Scholar]
- 20.Grangette C. Bifidobacteria and subsets of dendritic cells: Friendly players in immune regulation! Gut. 2012;61:331–332. doi: 10.1136/gutjnl-2011-301476. [DOI] [PubMed] [Google Scholar]
- 21.Lê KA, Li Y, Xu X, Yang W, Liu T, Zhao X, Tang YG, Cai D, Go VL, Pandol S, Hui H. Alterations in fecal lactobacillus and bifidobacterium species in type 2 diabetic patients in southern china population. Front Physiol. 2012;3:496. doi: 10.3389/fphys.2012.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Million M, Maraninchi M, Henry M, Armougom F, Richet H, Carrieri P, Valero R, Raccah D, Vialettes B, Raoult D. Obesity-associated gut microbiota is enriched in lactobacillus reuteri and depleted in bifidobacterium animalis and methanobrevibacter smithii. Int J Obes (Lond) 2012;36:817–825. doi: 10.1038/ijo.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory t cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 25.Duncan SH, Holtrop G, Lobley GE, Calder AG, Stewart CS, Flint HJ. Contribution of acetate to butyrate formation by human faecal bacteria. Br J Nutr. 2004;91:915–923. doi: 10.1079/BJN20041150. [DOI] [PubMed] [Google Scholar]
- 26.Liao TV, Forehand CC, Hess DC, Fagan SC. Minocycline repurposing in critical illness: Focus on stroke. Curr Top Med Chem. 2013;13:2283–2290. doi: 10.2174/15680266113136660160. [DOI] [PubMed] [Google Scholar]
- 27.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu P, Thinschmidt JS, Yan Y, Hazra S, Bhatwadekar A, Caballero S, Salazar T, Miyan JA, Li W, Derbenev A, Zsombok A, Tikhonenko M, Dominguez JM, 2nd, McGorray SP, Saban DR, Boulton ME, Busik JV, Raizada MK, Chan-Ling T, Grant MB. Cns inflammation and bone marrow neuropathy in type 1 diabetes. Am J Pathol. 2013;183:1608–1620. doi: 10.1016/j.ajpath.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Reikvam DH, Erofeev A, Sandvik A, Grcic V, Jahnsen FL, Gaustad P, McCoy KD, Macpherson AJ, Meza-Zepeda LA, Johansen FE. Depletion of murine intestinal microbiota: Effects on gut mucosa and epithelial gene expression. PLoS One. 2011;6:e17996. doi: 10.1371/journal.pone.0017996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Million M, Lagier JC, Yahav D, Paul M. Gut bacterial microbiota and obesity. Clin Microbiol Infect. 2013;19:305–313. doi: 10.1111/1469-0691.12172. [DOI] [PubMed] [Google Scholar]
- 32.Seekatz AM, Aas J, Gessert CE, Rubin TA, Saman DM, Bakken JS, Young VB. Recovery of the gut microbiome following fecal microbiota transplantation. MBio. 2014;5:e00893–00814. doi: 10.1128/mBio.00893-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duca FA, Sakar Y, Lepage P, Devime F, Langelier B, Dore J, Covasa M. Replication of obesity and associated signaling pathways through transfer of microbiota from obese-prone rats. Diabetes. 2014;63:1624–1636. doi: 10.2337/db13-1526. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen R, Andersen AD, Molbak L, Stagsted J, Boye M. Changes in the gut microbiota of cloned and non-cloned control pigs during development of obesity: Gut microbiota during development of obesity in cloned pigs. BMC Microbiol. 2013;13:30. doi: 10.1186/1471-2180-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Starkel P, Windey K, Tremaroli V, Backhed F, Verbeke K, de Timary P, Delzenne NM. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111:E4485–4493. doi: 10.1073/pnas.1415174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petriz BA, Castro AP, Almeida JA, Gomes CP, Fernandes GR, Kruger RH, Pereira RW, Franco OL. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genomics. 2014;15:511. doi: 10.1186/1471-2164-15-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu J, Ahren IL, Prykhodko O, Olsson C, Ahrne S, Molin G. Intake of blueberry fermented by lactobacillus plantarum affects the gut microbiota of l-name treated rats. Evid Based Complement Alternat Med. 2013;2013:809128. doi: 10.1155/2013/809128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 39.Jansson PA, Larsson A, Lönnroth PN. Relationship between blood pressure, metabolic variables and blood flow in obese subjects with or without non-insulin-dependent diabetes mellitus. Eur J Clin Invest. 1998;28:813–818. doi: 10.1046/j.1365-2362.1998.00360.x. [DOI] [PubMed] [Google Scholar]
- 40.Juraschek SP, Bower JK, Selvin E, Subash Shantha GP, Hoogeveen RC, Ballantyne CM, Young JH. Plasma lactate and incident hypertension in the atherosclerosis risk in communities study. Am J Hypertens. 2015;28:216–24. doi: 10.1093/ajh/hpu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henagan TM, Stefanska B, Fang Z, Navard AM, Ye J, Lenard NR, Devarshi PP. Sodium butyrate epigenetically modulates high fat diet-induced skeletal muscle mitochondrial adaptation, obesity and insulin resistance through nucleosome positioning. Br J Pharmacol. 2015 Jan 5; doi: 10.1111/bph.13058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin HV, Frassetto A, Kowalik EJ, Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G, Marsh DJ. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7:e35240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berni Canani R, Di Costanzo M, Leone L. The epigenetic effects of butyrate: Potential therapeutic implications for clinical practice. Clin Epigenetics. 2012;4:4. doi: 10.1186/1868-7083-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17:1519–1528. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vieira EL, Leonel AJ, Sad AP, Beltrao NR, Costa TF, Ferreira TM, Gomes-Santos AC, Faria AM, Peluzio MC, Cara DC, Alvarez-Leite JI. Oral administration of sodium butyrate attenuates inflammation and mucosal lesion in experimental acute ulcerative colitis. J Nutr Biochem. 2012;23:430–436. doi: 10.1016/j.jnutbio.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJ, de Almeida DC, Bassi EJ, Moraes-Vieira PM, Hiyane MI, Rodas AC, Peron JP, Aguiar CF, Reis MA, Ribeiro WR, Valduga CJ, Curi R, Vinolo MA, Ferreira CM, Camara NO. Gut bacteria products prevent aki induced by ischemia-reperfusion. J Am Soc Nephrol. 2015 Jan 14; doi: 10.1681/ASN.2014030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bordin M, D’Atri F, Guillemot L, Citi S. Histone deacetylase inhibitors up-regulate the expression of tight junction proteins. Mol Cancer Res. 2004;2:692–701. [PubMed] [Google Scholar]
- 51.Garrido-Mesa N, Camuesco D, Arribas B, Comalada M, Bailón E, Cueto-Sola M, Utrilla P, Nieto A, Zarzuelo A, Rodríguez-Cabezas ME, Gálvez J. The intestinal anti-inflammatory effect of minocycline in experimental colitis involves both its immunomodulatory and antimicrobial properties. Pharmacol Res. 2011;63:308–319. doi: 10.1016/j.phrs.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 52.Garrido-Mesa N, Utrilla P, Comalada M, Zorrilla P, Garrido-Mesa J, Zarzuelo A, Rodríguez-Cabezas ME, Gálvez J. The association of minocycline and the probiotic escherichia coli nissle 1917 results in an additive beneficial effect in a dss model of reactivated colitis in mice. Biochem Pharmacol. 2011;82:1891–1900. doi: 10.1016/j.bcp.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 53.Huang TY, Chu HC, Lin YL, Ho WH, Hou HS, Chao YC, Liao CL. Minocycline attenuates 5-fluorouracil-induced small intestinal mucositis in mouse model. Biochem Biophys Res Commun. 2009;389:634–639. doi: 10.1016/j.bbrc.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 54.Galpern WR, Singhal AB. Neuroprotection: Lessons from a spectrum of neurological disorders. Int J Stroke. 2006;1:97–99. doi: 10.1111/j.1747-4949.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- 55.Garrido-Mesa N, Zarzuelo A, Gálvez J. Minocycline: Far beyond an antibiotic. Br J Pharmacol. 2013;169:337–352. doi: 10.1111/bph.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jun JY, Zubcevic J, Qi Y, Afzal A, Carvajal JM, Thinschmidt JS, Grant MB, Mocco J, Raizada MK. Brain-mediated dysregulation of the bone marrow activity in angiotensin ii-induced hypertension. Hypertension. 2012;60:1316–1323. doi: 10.1161/HYPERTENSIONAHA.112.199547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santisteban MM, Zubcevic J, Kim S, Marulanda-Carvajal J, Zingler M, Joseph J, Raizada MK. Iba1+ microglia/macrophages in the rat hypothalamic paraventricular nucleus are both resident and bone marrow-derived in ang ii induced hypertension. the faseb journal. 2014;28:1. [Google Scholar]
- 58.Katsumoto A, Lu H, Miranda AS, Ransohoff RM. Ontogeny and functions of central nervous system macrophages. J Immunol. 2014;193:2615–2621. doi: 10.4049/jimmunol.1400716. [DOI] [PMC free article] [PubMed] [Google Scholar]