Abstract
Virulence factors expressed by enteric bacteria are pivotal for pathogen colonization and induction of intestinal disease, but the mechanisms by which host immunity regulates pathogen virulence are largely unknown. Here we show that specific antibody responses are required for down-regulation of virulence gene expression in Citrobacter rodentium, an enteric pathogen that models human infections with attaching-and-effacing bacteria. In the absence of antibodies against the pathogen, phenotypically virulent C. rodentium, accumulated and infected the epithelium, and subsequently invaded the lamina propia causing host lethality. IgG induced after infection recognized virulence factors and bound virulent bacteria within the intestinal lumen leading to their engulfment by neutrophils, while phenotypically avirulent pathogens remained in the intestinal lumen and were eventually out-competed by the microbiota. Thus, the interplay of the innate and adaptive immune system selectively targets virulent C. rodentium in the intestinal lumen to promote pathogen eradication and host survival.
Introduction
Host innate and adaptive immune responses against invading pathogenic microorganisms are critical for pathogen eradication and host survival. To establish infection and successful replication, pathogens have evolved many strategies to acquire nutrients, circumvent host defenses and exploit the host cellular machinery (Roy and Mocarski, 2007). A key strategy is the expression of specific virulence factors that enable pathogens to colonize their host and replicate within its tissues by subverting host signaling pathways (Okumura and Nizet, 2014; Roy and Mocarski, 2007). While the virulence factors involved in pathogen colonization and invasion have been heavily studied, the immune mechanisms that regulate the expression of bacterial virulence during infection are largely unknown. Furthermore, it remains unknown whether the host immune system can recognize virulence factors to promote pathogen clearance. Enterohemorrhagic Escherichia coli (EHEC) and enteropathogenic E. coli (EPEC) are major causes of diarrheal disease and lethal infections worldwide (Kaper et al., 2004; Mundy et al., 2005). These Gram-negative bacteria are food- and waterborne non-invasive pathogens which attach to and colonize the intestinal tract by inducing characteristic attaching-and-effacing (A/E) lesions on the intestinal epithelium, leading to transient enteritis or colitis in humans (Kaper et al., 2004; Mundy et al., 2005). The genomes of EHEC, EPEC and the related natural mouse pathogen Citrobacter rodentium harbor the locus for enterocyte effacement (LEE) pathogenicity island which is critical for these pathogens to colonize hosts and cause pathology (Deng et al., 2001; Deng et al., 2004). The LEE virulence genes include those encoding several effector proteins, a type III secretion system (T3SS), proteins that mediate intimate epithelial attachment such as intimin and its translocated receptor as well as Ler, a global regulator that is required for expression of most, if not all, LEE genes (Deng et al., 2004). Notably, patients infected with EPEC develop IgG antibodies reactive to LEE virulence factors (Jenkins et al., 2000; Li et al., 2000; Martinez et al., 1999). However, the physiological relevance of such antibodies including their role in pathogen eradication is unclear.
C. rodentium is widely used to model human infections with EPEC and EHEC (Collins et al., 2014). In the early phase of the infection, C. rodentium expresses LEE virulence genes (Deng et al., 2001; Deng et al., 2004) that allow it to localize and replicate near the epithelium where competing commensals are largely absent (Kamada et al., 2012). By day 12 post-infection, the expression of LEE virulence is down-regulated and as a result, non-LEE expressing pathogens relocate to the lumen where they are out-competed by resident microbes (Kamada et al., 2012). Infection of germ-free (GF) mice with C. rodentium is also associated with down-regulation of LEE virulence at the late stages of infection, but unlike conventional mice, GF mice cannot eradicate C. rodentium but survive despite high pathogen loads in the intestine (Kamada et al., 2012). However, the mechanism that accounts for the down-regulation of LEE virulence during infection of conventional and GF mice remains unknown.
Several studies have revealed important roles for innate and adaptive immune responses in the control of C. rodentium infection (Collins et al., 2014). For example, deficiency of myeloid differentiation primary response protein 88 (Myd88), an adaptor molecule required for signaling through Toll-like receptor and interleukin-1 receptor superfamily is associated with impaired pathogen clearance and increased intestinal damage (Lebeis et al., 2007). IL-22, produced largely by intestinal Th17 cells and group 3 innate lymphoid cells, plays a critical role in the host defense against C. rodentium (Zheng et al., 2008). IL-22 is particularly critical early in infection by promoting epithelial integrity and preventing systemic spread of the bacteria, but has a marginal role in controlling pathogen colonization in the intestine (Basu et al., 2012). CD4+-dependent humoral immunity is essential for the clearance of C. rodentium and limiting systemic spread of the pathogen (Bry and Brenner, 2004; Simmons et al., 2003). Notably, pathogen-specific IgG antibodies, but not IgM or IgA, are required for pathogen clearance and host survival (Bry and Brenner, 2004; Maaser et al., 2004). However, the mechanism by which luminal IgG controls the eradication of C. rodentium and protects the host from lethality remains unclear. In this study, we show that specific antibody responses are required for elimination of LEE virulence in C. rodentium. In the absence of antibodies targeting the pathogen, phenotypically virulent C. rodentium accumulated and infected the epithelium, subsequently invading the lamina propia causing host lethality. Mechanistically, IgG induced after infection recognized LEE virulence factors within the intestinal lumen leading to selective eradication of virulent pathogens in vivo. IgG primarily bound virulent bacteria triggering their engulfment neutrophils within the lumen, whereas phenotypically avirulent C. rodentium remained in the intestinal lumen and were eventually out-competed by the microbiota.
Results
Adaptive immunity is required for down-regulation of LEE virulence during C. rodentium infection
The expression of LEE virulence is down-regulated by day 12 post-infection which coincides with the induction of pathogen-specific IgG responses (Kamada et al., 2012). To address the mechanism by which LEE virulence expression is regulated during infection, we first assessed the role of host immunity because several studies have shown that the adaptive immune system is critical for both C. rodentium eradication and host survival (Bry and Brenner, 2004; Maaser et al., 2004; Simmons et al., 2003). Consistent with previous reports, wildtype (WT) mice reared under conventional specific pathogen-free (SPF) conditions cleared C. rodentium, whereas Rag1-/- (lacking mature B and T lymphocytes) SPF mice did not and instead succumbed to infection (Fig. 1A). As expected, Rag1-/- mice raised under germ-free (GF) conditions were also unable to eradicate C. rodentium and ultimately succumbed to infection (Fig. 1B). In contrast, WT GF mice that can induce comparable IgG response to the pathogen (Kamada et al., 2012) were similarly impaired in the clearance of the pathogen, but remained alive (Fig. 1B). To determine whether adaptive immunity regulates LEE virulence, we monitored the expression of ler, the global regulator of LEE virulence, in the intestines of GF WT and Rag1-/- mice using a reporter ler-lux C. rodentium strain (Kamada et al., 2012). Expression of ler was detected at comparable levels in the intestine of WT and Rag1-/- mice on day 5 post-infection, but was down-regulated in WT GF mice, but not in Rag1-/- GF mice, by day 14 post-infection despite comparable pathogen loads (Fig. 1C, D).
Figure. 1. Host adaptive immunity is required for down-regulation of LEE virulence during C. rodentium infection.
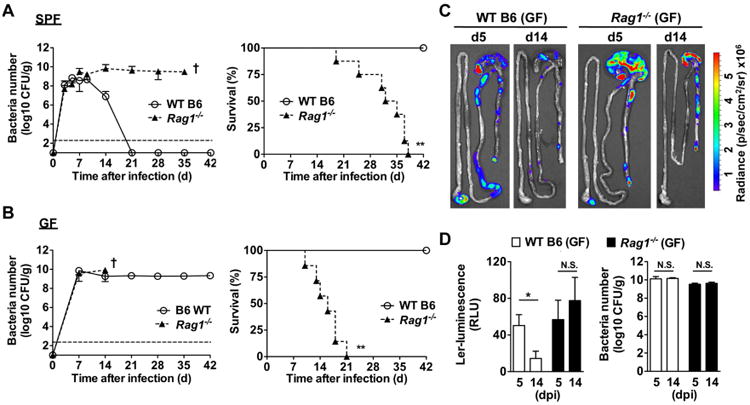
A, B, SPF (A) and GF (B) WT B6 and Rag1-/- mice (n= 7) were infected orally with 1×109 cfu of C. rodentium and pathogen load in feces (left) and mouse survival (right) were determined over the indicated time. Data points are given as mean ± SD. Results are representative of at least 3 independent experiments. † denotes bacterial loads could not be determined beyond this time due to mouse lethality. **; p<0.01 by Log-rank test. C, Bioluminescent imaging of ler expression in the intestines of GF WT and Rag1-/- mice infected with the ler-lux C. rodentium reporter strain. Imaging was performed on day 5 and 14 post-infection and the signal was quantified based on the color scale shown. Results are representative of 3 individual mice. D, Expression of ler (left) and bacterial burden (right) in fecal pellets of GF mice infected with the reporter ler-lux C. rodentium strain at the indicated day post-infection (dpi). Results show luminescence of ler-lux (relative light units) and C. rodentium cfu in the same samples. Data expressed as mean ± SD of individual mice (n=3). Results are representative of at least 2 experiments. *, p<0.05; N.S., not significant by Student's t test. See also Figure S1.
Lethality of Rag1-/- mice is associated with inappropriate retention of mucosa-associated LEE virulence and pathogen invasion
We examined histological analyses of the intestine of SPF and GF WT and Rag1-/- mice infected with C. rodentium to assess the cause of lethality. Transmission electron microscopy showed C. rodentium adherent to the intestinal epithelium on day 5, but not on day 21 post-infection in GF WT mice (Fig. 2a). In contrast, abundant C. rodentium were found infecting the epithelium and causing severe tissue damage on day 21 post-infection in Rag1-/- GF mice (Fig. 2A). Correspondingly, light microscopy revealed marked infiltration of acute inflammatory cells and extensive pathogen invasion into the intestinal tissues of both SPF and GF Rag1-/- mice, on day 14 post-infection, but not in WT GF and SPF mice correlating with down-regulation of ler in these mice (Fig. S1). To determine whether LEE virulence was required for the demise of Rag1-/- mice, we orally infected GF and SPF Rag1-/- mice with ler-deficient C. rodentium. The ler mutant strain efficiently colonized Rag1-/- GF mice, but not Rag1-/- SPF mice (Fig. 2B). Importantly, GF and SPF Rag1-/- mice infected with the ler deficient strain survived (Fig. 2B) and did not develop intestinal inflammation (Fig. S2). Furthermore, ler-deficient C. rodentium did not colonize the epithelium of Rag1-/- GF mice (Fig. 2A). These results indicate that the demise of Rag1-/-mice infected with C. rodentium requires LEE-dependent virulence. To determine whether ler-expressing C. rodentium were associated with the mucosa in the late stages of infection, we infected GF WT and Rag1-/- mice and assessed ler expression in mucosa-attached bacteria using the ler-lux reporter strain. On day 21 post-infection, while both mouse strains remained heavily colonized, ler expression was down-regulated in WT mice but enhanced in surviving Rag1-/- mice (Fig. 2C). As previously reported (Kamada et al., 2012), C. rodentium was effectively out-competed at the late phase of infection by transfer of the microbiota into WT GF mice by co-housing them with conventional SPF WT mice (Fig. 2D). In contrast, only the ler-deficient, but not WT C. rodentium, were out-competed by the microbiota in Rag1-/- GF mice which is consistent with their localization within the intestinal lumen rather than to the epithelium (Fig. 2D). Collectively, these results indicate that host adaptive immunity is required for down-regulation of LEE virulence and inappropriate retention of LEE virulence by C. rodentium results in pathogen invasion of the mucosa and host lethality.
Figure. 2. Inappropriate retention of mucosa-associated LEE virulence and pathogen invasion in mice lacking adaptive immunity.
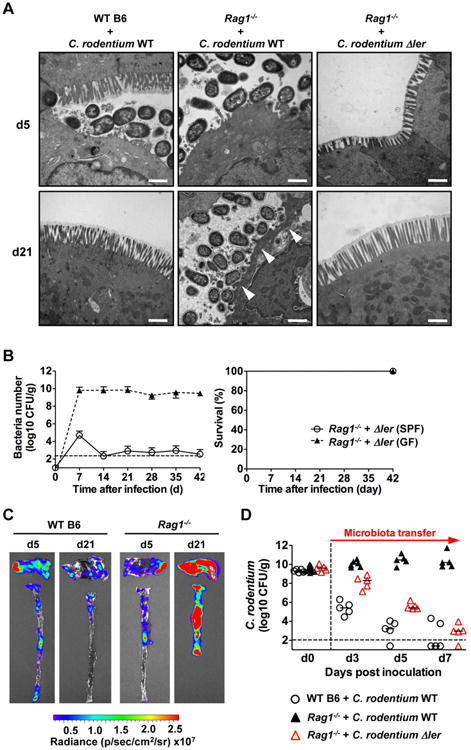
A, GF WT and Rag1-/- deficient mice were infected with WT and Δler mutant C. rodentium. On day 5 (top) or day 21 (bottom) post-infection, mucosa-associated bacteria in ceca were assessed by Transmission Electron Microscopy. Original magnification: 13,500×. Scale bar: 1 μm. Results are representative of 2 experiments. B, SPF and GF Rag1-/- mice (n= 5) were infected orally with 1×109 cfu of C. rodentium and pathogen load in feces (left) and mouse survival (right) were determined over the indicated time. Data points are given as mean ± SD. Results are representative of at least 2 independent experiments. C, GF WT and Rag1-/- mice were infected with the ler-lux C. rodentium strain for 5 or 21 days. Cecum and colonic tissues were collected at the indicated day and then washed with PBS to remove non-adherent bacteria. Bioluminescent imaging of ler expression of C. rodentium attached to the cecum (top) and colon (bottom). Results are representative of 2 experiments using 3-4 different mice. D, GF WT and Rag1-/- mice were infected with WT and Δler mutant C. rodentium. On day 10 post infection, mice were co-housed with SPF mice to transfer microbiota (1:1). Pathogen load was determined in feces on indicated days after co-housing. Dots represent individual mice. See also Figure S2.
Pathogen-specific IgG is required for downregulation of LEE virulence expression
We next addressed the mechanism by which host adaptive immunity regulates LEE virulence. Consistent with previous reports (Maaser et al., 2004), B-cell deficient μMT mice were unable to control C. rodentium infection much like Rag1-/- mice, suggesting that B cells play a key role in controlling pathogen eradication (data not shown). To begin to address the role of antibodies in the regulation of LEE virulence, we assessed the presence of pathogen-specific antibodies in the intestinal lumen of infected mice. C. rodentium-specific IgG and IgA, but not IgM, were detected in the luminal content on day 12 and 22 post-infection in WT mice, whereas no pathogen-reactive antibodies were present before infection (Fig. S3). Analysis of IgA-deficient mice revealed that IgA was dispensable for the intestinal eradication of C. rodentium (data not shown) in agreement with previous studies (Maaser et al., 2004). Because B cell deficient mice may have multiple defects in addition to impaired antibody production, we used quasi-monoclonal (QM) mice that contain B cells, but their primary repertoire is largely monospecific for the hapten 4-hydroxy-3-nitrophenylacetate (Cascalho et al., 1996). Consistently, IgG and IgA responses against C. rodentium infection in the serum and luminal contents of QM mice were greatly impaired when compared to WT mice (Fig. 3A). Notably, QM mice displayed impaired pathogen clearance and showed greater pathogen burden in the intestine even in the late phases of infection (day 18 and 21) when WT mice had already eradicated the pathogen (Fig. 3B). Furthermore, ler-expressing C. rodentium were found to colonize the mucosal surface at 14 days post-infection in QM mice, but not WT mice (Fig. 3C).
Figure. 3. Pathogen-specific IgG responses are required for downregulation of LEE virulence expression.
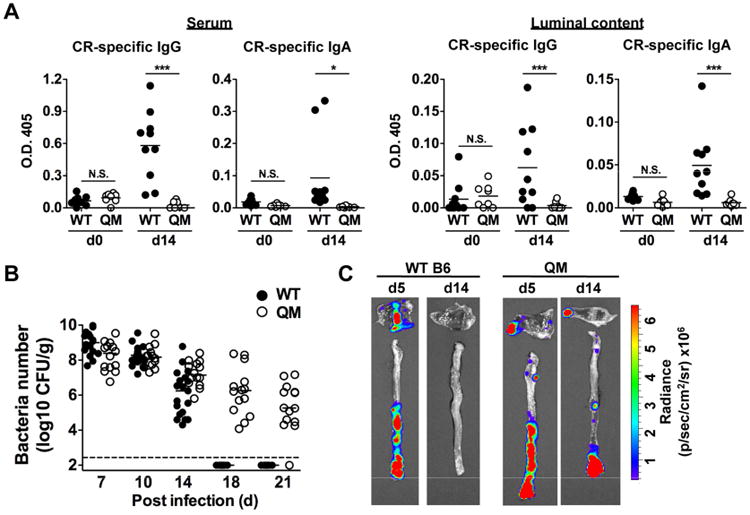
A, Production of total IgG and IgA against C. rodentium in the serum and luminal content of QM mice before (d0) and after (day 14) oral infection with C. rodentium. Dots represent individual mice. *, p<0.05; ***, p<0.001; N.S., not significant by Dunn's test. B, WT and QM mice were infected with C. rodentium and pathogen load in feces were determined over the indicated time. Dots represent individual mice and pooled 3 independent experiments. C, WT and QM mice were infected with the ler-lux C. rodentium strain for 5 or 14 days. Cecum and colonic tissues were collected at the indicated day and then washed with PBS to remove non-adherent bacteria. Bioluminescent imaging of ler expression of C. rodentium attached to the cecum (top) and colon (bottom). Results are representative of 3 independent experiments. See also Figure S3.
Virulence factor-specific IgG selectively binds virulent bacteria
Next, we asked whether the elicited luminal IgG bind to C. rodentium during infection. To test this, GF WT mice were mono-associated with green-fluorescence protein (GFP)-expressing C. rodentium, and antibody-bound bacteria were quantitated by flow cytometry. On day 3 post-infection, IgG-bound C. rodentium were not detected in the intestinal lumen (Fig. 4A). On day 14 post-infection, however, >80% of the C. rodentium in the intestinal lumen were bound with IgG, but not IgA (Fig. 4A). Further analysis showed that IgG binding to luminal virulent bacteria was observed at day 7 day and peaked at 10 day post infection (Fig. S4A). These results suggest that pathogen-specific IgG is involved in the down-regulation of LEE virulence and pathogen eradication in the intestine. We next tested whether IgG reactive with C. rodentium can directly regulate LEE virulence. To assess this, we incubated C. rodentium with IgG purified from the sera of infected GF mice or control IgG. Culture of C. rodentium in DMEM, but not LB medium, triggered robust ler expression in the ler-lux reported strain as reported (Barba et al., 2005; Deng et al., 2004). Under these conditions, pathogen-specific IgG did not inhibit ler expression or bacterial growth (Fig. S4B). Thus, IgG does not directly regulate LEE virulence in vitro. We therefore hypothesized that distinct populations of C. rodentium may be present during infection and ler down-regulation may be explained by selective elimination of ler-expressing C. rodentium by specific IgG. Thus, we assessed whether IgG induced in the intestinal lumen after infection differentially recognizes ler-expressing C. rodentium. Immunoblotting analysis revealed reactivity of the serum and luminal IgG against multiple proteins in the extracts of WT C. rodentium, whereas the reactivity was greatly reduced in extracts of ler-deficient bacterium (Fig. 4B). Increased IgG reactivity against WT compared to Δler mutant C. rodentium was observed in both SPF and GF mice that were infected with C. rodentium (Fig. 4B and Fig. S4C). Consistently, luminal IgG from infected animals reacted against the extracellular domain of intimin (Fig. S4D), a Ler-dependent virulence factor that is expressed on the surface of the bacterium and mediates intimate attachment to the epithelium (Schauer and Falkow, 1993). IgG against commensal bacteria was not induced after C. rodentium infection, further supporting the notion that induced IgG responses during infection are directed primarily against virulence factors (Fig. S4B, C). To further assess whether luminal IgG selectively targets ler-expressing C. rodentium, sera from infected mice and naïve mice were incubated with a 1:1 mixture of WT and ler-deficient C. rodentium and the binding of IgG to the surface of the bacteria was assessed by flow cytometry. Notably, IgG from infected mice recognized >90% of WT C. rodentium, but less than 2% of the ler-deficient bacterium (Fig. 4C). Collectively, these results that IgG induced by C. rodentium infection selectively recognizes virulent bacteria.
Figure. 4. Virulence factor-specific IgG is induced in the late phase of C. rodentium infection.
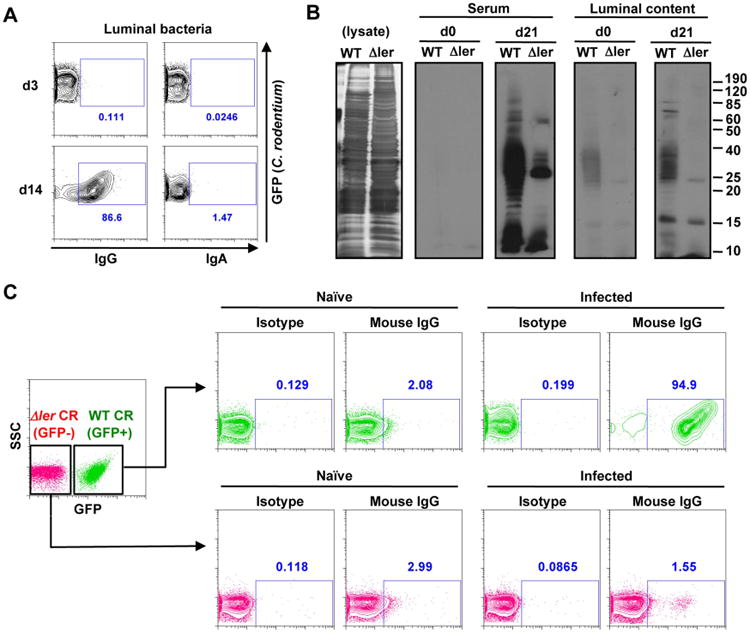
A, GF WT mice were infected with GFP-expressing C. rodentium. Cecal bacteria were harvested at indicated days post-infection and binding of IgG and IgA was analyzed by flow cytometry. Results are representative of 3 experiments. B, Bacterial lysates of WT and Δler mutant C. rodentium were loaded with SDS-PAGE. For confirmation of amount of loaded protein, gels were stained with silver staining reagent (left panel). Serum or luminal content were obtained from naïve (d0) and C. rodentium-infected (d21) GF mice, and used as primary antibodies. C. rodentium-specific IgG was detected by anti-mouse IgG secondary Ab. C, WT (GFP+) and Δler mutant (GFP-) C. rodentium were cultured in DMEM for 6 hrs. Cultured bacteria were then washed with PBS and mixed at 1:1 ratio. Mixed bacteria were then incubated with 5% of serum from naïve and C. rodentium pre-infected (day 21 post-infection) GF mice for 30 min on ice. After incubation, bacteria were washed and IgG-binding to the bacteria was detected by biotin-conjugated anti-mouse IgG antibody and streptavidin-APC. Rat IgG was used as a control staining. IgG binding to each bacterial strain was analyzed by flow cytometry. Results are representative of 2 independent experiments. See also Figure S4.
IgG promotes selective elimination of phenotypically virulent C. rodentium in vivo
To determine whether phenotypically different subpopulations of C. rodentium could be detected in vivo, GF WT mice were infected with C. rodentium and the expression of ler was assessed on day 7 post-infection in mucosa-associated and luminal bacterial populations by quantitative PCR. Notably, ler was detected primarily in mucosa-associated C. rodentium, and less so in the pathogen found in the luminal population (Fig. 5A). In contrast, comparable expression of rpoB, a gene encoding DNA-directed RNA polymerase beta chain, was found in both bacterial populations (Fig. 5A). To confirm the selective recognition of virulent C. rodentium by IgG in vivo, GF mice were orally co-infected with GFP-WT and unlabeled ler mutant C. rodentium, and IgG-bound bacteria was assessed in the luminal content on day 21 post-infection by flow cytometry. Consistent with the in vitro results, IgG bound > 50% of WT C. rodentium but less than 4% of ler-deficient bacteria (Fig. 5B). To determine whether WT C. rodentium is selectively phagocytosed in vivo, naïve mice as well as mice infected with C. rodentium for 21 days were injected with thioglycollate into the peritoneal cavity to elicit neutrophil infiltration and the mice infected 30 min later i. p. with the same number of WT and ler-deficient C. rodentium. Notably, there was marked phagocytosis of WT C. rodentium, but not ler mutant bacteria by neutrophils in the peritoneal cavity of pre-infected mice, but not naïve mice (Fig. 5C). To determine whether the serum of infected mice enhances pathogen phagocytosis, WT and ler-deficient C. rodentium were incubated with serum from naïve and infected mice and the bacteria were injected into the neutrophil-rich peritoneal cavity of thioglycollate-treated mice. Incubation with serum from infected mice, but not naïve mice enhanced phagocytosis of WT C. rodentium, but not of the ler-deficient bacterium (Fig. 5D). To determine whether ler-expressing C. rodentium is selectively eliminated in the intestine, GF WT and Rag1-/- mice were co-infected with GFP-WT and ler mutant C. rodentium and the colonization index (CI; ratio of WT/mutant) was assessed on day 3 and day 21 post-infection. On day 3, there was 100-fold more colonization by the ler-deficient strain than WT C. rodentium in WT GF mice (Fig. 5E). Thus, there is a fitness cost associated with virulence that can be revealed in GF mice which is consistent with that found in Salmonella (Sturm et al., 2011). Notably, the CI decreased 10-fold further on day 21, suggesting that WT strain is preferentially eliminated after induction of specific IgG (Fig. 5E). In contrast, the CI was comparable to WT mice on day 3, but it reversed ∼10,000-fold on day 21 in Rag1-/- mice, indicating that the WT C. rodentium accumulates preferentially over the ler-deficient strain in the absence of adaptive immunity (Fig. 5E). Collectively, these results indicate that luminal IgG generated against Ler-dependent virulence factors promotes selective elimination of virulent C. rodentium.
Figure 5. Targeting of LEE virulence by IgG promotes selective elimination of phenotypically virulent pathogens.
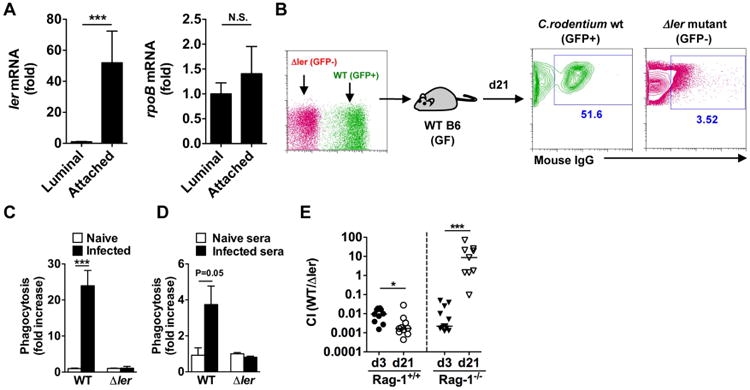
A, GF WT mice were infected with C. rodentium. At day 7 post-infection, mucosal-associated and luminal bacteria were harvested from the cecum (n=10) and ler and rpoB mRNA levels were determined by qPCR. Expression was normalized to that of the 16S rRNA gene rrsA. Results are given as mean ± SD. ***; p<0.001; N.S., not significant by Mann-Whitney U test. B, GF WT mice were infected with 1:1 ratio of WT (GFP+) and Δler mutant (GFP-) C. rodentium. After 21 days, cecal bacteria were harvested and binding of IgG was analyzed by flow cytometry. Results are representative of 3 experiments. C, WT (ChlR) and Δler (KanR) C. rodentium were mixed at a 1:1 ratio (1×107 cfu in 200 ml PBS), and injected into the peritoneal cavity of naïve and C. rodentium infected (d21 post infection) mice that had been pre-injected with thioglycollate to induce neutrophil recruitment into the peritoneal cavity. Neutrophils were harvested from the peritoneal cavity 30 min after injection of bacteria, and numbers of WT and Δler bacteria engulfed by neutrophils were assessed. Data are given as mean ± SD (n=3). Results are representative of 2 experiments. D, WT (ChlR) and Δler (KanR) C. rodentium were mixed at a 1:1 ratio, and then incubated with serum from naïve or C. rodentium infected (d21 post infection) GF WT mice for 30 min on ice. After washing, serum-treated bacteria were injected into the peritoneal cavity of naïve WT mice pre-injected with thioglycollate. The numbers of engulfed bacteria by neutrophils were assessed as described in (C). Data are given as mean ± SD (n=3). Results are representative of 2 experiments. ***; p<0.001 by Student's t test. E, GF WT and Rag1-/- mice were infected with 1:1 ratio of WT (ChlR) and Δler mutant (KanR) C. rodentium. After 3 and 21 days, intestinal burden of WT and mutant bacteria was measured. Dots represent colonization index (CI; ratio of WT/Δler) of individual mice and pooled 3 independent experiments with 3 mice. *; p<0.05, ***; p<0.001 by Mann-Whitney U test.
Neutrophils are required for eradication of phenotypically virulent C. rodentium in the intestine
IgG enhances the engulfment and killing of IgG-bound bacteria by phagocytes via opsonization (Van Oss and Gillman, 1972). Histological analysis of C. rodentium-infected mice revealed the presence of numerous polymorphonuclear neutrophils in the intestinal lumen (Fig. S5A). Furthermore, marked accumulation of CD45+CD11b+Ly6G+ neutrophils was detected in the intestinal lumen on day 11 post-infection by flow cytometry (Fig. 6A). Notably, Ly6G+ neutrophils, but not Ly6G- macrophages, were found to preferentially contain GFP-expressing C. rodentium in the intestinal lumen (Fig. 6A). These results suggest that after infection, neutrophils transmigrate from the lamina propia into the intestinal lumen and engulf C. rodentium near the epithelium. To address the role of neutrophils in the eradication of WT C. rodentium in the intestine, we generated mouse chimeras by transplanting lethally irradiated WT recipient mice with bone marrow from LysMcreMcl1fl/fl mice that are deficient in neutrophils, but contain normal numbers of macrophages, due to the deletion of the essential anti-apoptotic molecule Mcl-1 for neutrophils (Dzhagalov et al., 2007). Consistently, chimeric mice reconstituted with the bone marrow of LysMcreMcl1fl/fl mice exhibited ∼ 90% reduction in the number of CD45+CD11b+Ly6Ghi neutrophils, but still harbored normal numbers of CD45+CD11b+Ly6Glo monocytes in the peripheral blood when compared to control chimeric mice (Fig. S5B). After infection with C. rodentium, LysMcreMcl1fl/fl chimeric animals displayed marked susceptibility to the pathogen and unlike control LysMcreMcl1wt/wt chimeric mice, they succumbed by day 14 post-infection (Fig. 6B). Notably, the percentage of neutrophils that transmigrated to the intestinal lumen was diminished in LysMcreMcl1fl/fl chimeric mice (Fig. 6C). Importantly, while the expression of ler was down-regulated in the intestinal mucosa of LysMcreMcl1wt/wt chimeric mice by day 8 post-infection, it was greatly enhanced in LysMcreMcl-1fl/fl chimeric mice despite comparable pathogen load in the feces on days 5, 7 and 10 (Fig. 6D and Fig. S5C). The induction of C. rodentium-specific IgG in the serum and intestinal lumen was not impaired in mice deficient in neutrophils (Fig. S6D). Collectively, these findings indicate that neutrophils are also critical for the removal of LEE virulent C. rodentium and host survival.
Figure. 6. Neutrophils elicit selective elimination of opsonized virulent bacteria.
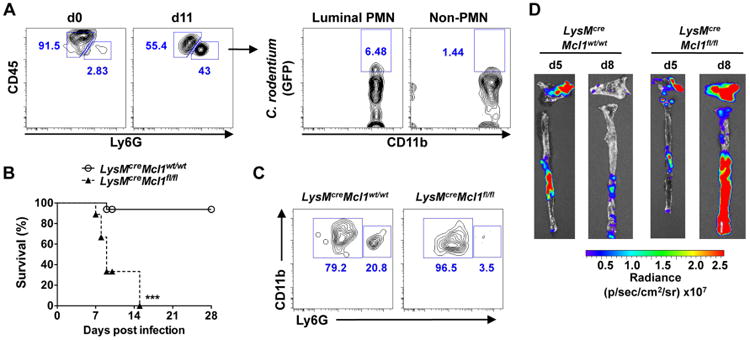
A, SPF WT mice were infected with GFP-expressing C. rodentium. At day 11 post infection, cecal content was harvested. Luminal debris and bacteria were removed by filtering and centrifugation, and then stained with antibodies for CD45, CD11b, Ly6G, and 7-AAD and analyzed by flow cytometry. Data are representative of 3 independent experiments. B, LysMCreMcl1wt/wt (control) and LysMCreMcl1fl/fl (neutrophil deficient) chimeric mice (n= 16; Mcl1wt/wt, n=9; Mcl1fl/fl) were infected with C. rodentium and mouse mortality was determined over the indicated time. ***; p<0.001 by Log-rank test. C, LysMCreMcl1wt/wt and LysMCreMcl1fl/fl chimeric mice were infected with C. rodentium and luminal emigration of neutrophils was analyzed on day 5 post-infection. 7-AAD-CD45+CD11b+ cells in luminal content were gated and further analyzed for Ly6G expression. Results are representative of 2 individual mice. D, LysMCreMcl1wt/wt and LysMCreMcl1fl/fl chimeric mice were infected ler-lux C. rodentium strain for 5 or 8 days. Cecum and colonic tissues were collected at the indicated day and then washed with PBS to remove non-adherent bacteria. Bioluminescent imaging of ler expression in C. rodentium attached to the cecum (top) and colon (bottom). Results are representative of 3 independent experiments. See also Figure S5.
Discussion
Our results suggest that the eradication of C. rodentium involves at least two major steps: First, antibodies are generated against Ler-regulated virulence factors that selectively target phenotypically virulent bacteria for killing in the intestinal lumen by neutrophils that transmigrate from the lamina propia. The second step involves the removal of the phenotypically avirulent pathogen by competing commensals (Kamada et al., 2012). Unlike GF and SPF Rag1-/- mice lacking adaptive immunity, GF animals survive infection despite their inability to eradicate C. rodentium because they can mount appropriate adaptive immune responses and virulent bacteria are selectively eliminated by the immune system. Our results strongly suggest that this selective elimination of virulent pathogens in the intestinal lumen is, at least in part, mediated by virulence factor-specific IgG responses. The presence of phenotypically virulent and avirulent pathogen subpopulations generated during infection has been previously reported in Salmonella and may contribute to the stability of pathogen virulence (Diard et al., 2013). The development of avirulent and virulent pathogen populations during intestinal infection may reflect the induction of LEE virulence in a subset of the overall population (e. g. in newly replicating bacteria) based on either host or environmental factors or just the fact that it is an unsynchronized community and virulence may provide an adaptive advantage. The observation that C. rodentium expresses LEE virulence in GF mice, which is critical for epithelial localization, indicates that its regulation is independent of the microbiota. Bicarbonate ions which are found at high levels in the small intestine can activate RegA, an AraC-like transcription factor, and the Ler-GrlA regulatory loop (Yang et al., 2008). However, there is no evidence that bicarbonate ions play any role in the regulation of ler during infection in vivo. It is possible that the induction of LEE virulence is stochastic in a subset of bacteria and Ler-positive pathogens selectively accumulate near the epithelium or that LEE virulence is induced upon initial contact with epithelial cells. Regardless of the mechanism involved, our results indicate that inappropriate accumulation of virulent C. rodentium after intraluminal pathogen replication is highly deleterious to the host. In the absence of pathogen-specific antibodies, there is marked accumulation of mucosa-associated virulent C. rodentium leading to exaggerated intestinal damage and pathogen invasion.
These studies suggest that IgG, but not IgA, are generated against Ler-regulated factors such as intimin that are expressed on the pathogen surface in the intestinal lumen. Much like in mice infected with C. rodentium, antibodies reactive to LEE virulence factors develop in patients infected with EPEC, although the relevance of such antibodies in human disease remains to be determined (Jenkins et al., 2000; Li et al., 2000; Martinez et al., 1999). It is noteworthy that C. rodentium infection preferentially induced IgG against LEE virulence factors but not other surface antigens that shared with the avirulent C. rodentium strain or other commensal Gram-negative microbes (Fig. 4B and Fig. S4C). The precise mechanisms by which C. rodentium infection induces IgG mainly against virulence factors, it is conceivable that virulence factors exhibit stronger immunoreactivity than other surface antigens. Although some surface antigens expressed on Gram-negative bacteria, such as flagellin, harbor strong immunoreactivity, the C. rodentium strain used in this study is not flagellated (Khan et al., 2008; Petty et al., 2011). This may explain why C. rodentium infection did not induce IgG against non-virulence related, high immunoreactive antigens, such as flagellin. The mechanism underlying the preferential development of IgG against LEE virulence factors during infection is unclear. One possibility is that it reflects, at least in part, the large amount of LEE virulence proteins expressed at or near the epithelial surface where they can be captured and processed by phagocytic cells. Alternatively, it may be explained by high antigenic activity of LEE virulence proteins, some of which like intimin can be recognized on the surface of the pathogen by induced IgG. In animal models, vaccination against intimin elicit robust antibody responses which are effective in reducing C. rodentium colonization (Ghaem-Maghami et al., 2001). Our results suggest that antibodies against surface virulence factors may act, at least in part, by targeting the virulent pathogen for removal by intraluminal neutrophils. The mechanism by which IgG enters the lumen is unclear, but it may leak passively through a damaged and leaky epithelium or via FcRn-mediated epithelial transport (Bry et al., 2006) (Spiekermann et al., 2002). Notably, mice deficient in FcRn are more susceptible to C. rodentium and exhibit impaired pathogen clearance. However, the phenotype of FcRn-/- mice is modest compared to that of Rag1-/- or B-cell deficient mice (Yoshida et al., 2006), suggesting that the transfer of IgG into the intestinal lumen may involve multiple mechanisms. Consistent with the current work, previous studies revealed that infection of C57BL/6 mice with C. rodentium induces high amounts of IgG2b and IgG2c and IgG2b in particular enters the intestinal lumen (Bry et al., 2006). This IgG profile is consistent with IgG responses that bind neutrophil Fcγ receptors for pathogen opsonization. Because bacteria coated with IgG can trigger complement activation, pathogen engulfment by neutrophils may also be mediated by complement receptors which can synergize with Fc receptors for enhanced phagocytosis (Scribner and Fahrney, 1976). Although mice deficient in pathogen-specific IgG (QM mice) exhibited greatly impaired eradication of C. rodentium compared to WT mice, the phenotype was less severe than that observed in Rag1-/- mice. This suggests that host adaptive immunity responses other than IgG production may contribute to the control of pathogen virulence and host protection. Because specific Ig responses are not totally absent in QM mice (Cascalho et al., 1996), it is also possible that QM mice can mount weak but significant pathogen-specific IgG responses that could account for the observed phenotype when compared to Rag1-/- mice. Likewise, neutrophil deficient mice displayed more severe phenotype than Rag1-/- mice, suggesting that neutrophils play additional protective roles in addition to the elimination of IgG-bound virulent bacteria in the intestinal lumen. Collectively, these studies reveal a dynamic interplay between the expression of bacterial virulence, the microbiota and both innate and adaptive immunity in the regulation of C. rodentium colonization. Further understanding these interactions may lead to the development of novel strategies to reduce or eliminate colonization of pathogenic E. coli species in animals and humans.
Methods
Mice
C57BL/6, Rag1-/- mice and quasi-monoclonal mice (QM) both in the C57BL/6 background (Cascalho et al., 1996) were bred and kept under specific pathogen-free (SPF) conditions in the University of Michigan. Germ-free (GF) C57BL/6 and Rag1-/- mice in the C57BL/6 background were housed in the Germ-free Animal Facility at University of Michigan. GF mice were maintained in flexible film isolators and were checked weekly for GF status by aerobic and anaerobic culture. The absence of microbiota was verified by microscopic analysis of stained cecal contents to detect unculturable contamination. LysMCreMcl1fl/fl and Mcl1fl/fl mice in the C57BL/6 background were kindly provided from Dr. Attila Mocsai, Semmelweis University, Budapest. All animal studies were performed under protocols approved by the University of Committee on Use and Care of Animals (UCUCA) at University of Michigan.
C. rodentium infection
The kanamycin-resistant (KanR) wild-type Citrobacter rodentium strain DBS 120 (pCRP1∷Tn5) was a gift of Dr. David Schauer, Massachusetts Institute of Technology. The isogenic C. rodentium Δler mutant (KanR), C. rodentium ler-lux reporter strain (KanR), and GFP-expressing C. rodentium strain (Chloramphenicol resistant; ChlR) have been described (Bergstrom et al., 2010; Deng et al., 2004; Kamada et al., 2012). For inoculations, bacteria were grown overnight in Luria-Bertani (LB) broth supplemented with Kan (50 μg/ml) with shaking at 37°C. Mice were infected by oral gavage with 0.2 ml of PBS containing approximately 1 × 109 CFU of C. rodentium. To determine bacterial numbers in the feces, fecal pellets were collected from individual mice, homogenized in cold PBS and plated at serial dilutions onto MacConkey agar containing 50 μg/ml Kan, and the number of CFU was determined after overnight incubation at 37°C. Mice were sacrificed at various time points post-infection (p.i.), and colons were flushed with PBS and used for colonic cell isolation or fixed in Carnoy's solution and then processed for H&E staining.
Measurement of ler expression
For in vivo bioluminescence imaging (BLI), the entire gastrointestinal tract was immediately removed and placed into the light-tight chamber of the CCD camera system (IVIS200, Xenogen). Luminescence emitted from lux-expressing bacteria in the tissue was quantified using the software program living image (Xenogen) (Kamada et al., 2012). For in vitro detection of ler expression, C. rodentium ler-lux strain was cultured in LB (negative control) or Dulbecco's modified eagle's medium (DMEM) (positive control). ler-lux-expressing bacteria was measured using a LMax luminometer (Molecular Device) (Kamada et al., 2012). Quantitative real time RT-PCR (qPCR) for ler was performed using a SYBR green PCR master mix and the StepOne Real-time PCR system (Applied Biosystems) and normalized to the expression of the 16S rRNA gene (rrsA). The following primer sets were used: ler; 5′-AAT ATA CCT GAT GGT GCT CTT G-3′ and 5′-TTC TTC CAT TCA ATA ATG CTT CTT-3′. rpoB; 5′-GTG TAC GCG CAG ACT AAC GA-3′ and 5′-ATC AAC CAC GCG ACG ATA C-3′. rrsA; 5′-AGG CCT TCG GGT TGT AAA GT-3′ and 5′-ATT CCG ATT AAC GCT TGC AC-3′.
Purification of C. rodentium intimin protein
The extracellular carboxyl-terminal 385 amino acids of Intimin from C. rodentium was purified from E. coli transformed with a plasmid expressing histidine-tagged Intimin385 by nickel-affinity chromatography (Sinclair and O'Brien, 2004).
Detection of binding of antibodies to luminal bacteria
For in vitro experiments, bacteria were cultured in standing DMEM for 6 hours to allow the expression of virulence factors. Cultured bacteria were then washed with ice-cold PBS and incubated with diluted serum or luminal content for 30 min. After washing, the bacteria were then incubated with biotin-conjugated anti-mouse IgG, IgM, or IgA antibodies. For in vivo experiments, the luminal content was harvested from cecal or fecal samples of GF or SPF mice infected with C. rodentium and resuspended in ice-cold PBS, and then filtrated sequentially through 100μm, 70μm, and 40μm strainers. The filtrated luminal content was then centrifuged at 1,000 rpm for 15 sec to remove debris. Luminal bacteria were then pelleted down, and fixed with 4% paraformaldehyde. Fixed bacteria were then washed with FACS staining buffer and stained with biotin-conjugated anti-mouse IgG, IgM, or IgA antibodies (eBiosciences). Rat IgG antibody was used as an isotype control. Immunoglobulin bound bacteria were then stained with streptavidin-APC, and analyzed by FACSCalibur or FACSCanto II (BD Biosciences).
Measurement of C. rodentium-reactive immunoglobulins
For measurement of C. rodentium-specific Ig by ELISA, 96-well ELISA plates were coated with heat-killed C. rodentium. Diluted serum or luminal content were then added to the coated plate, and the presence of C. rodentium-specific Igs was detected by alkaline phosphatase-conjugated polyclonal goat anti-mouse IgG, IgM or IgA Abs (Southern Biotechnology Associates, Birmingham, AL). Plates were developed using p-nitrophenyl phosphate substrate (Southern Biotechnology Associates) and OD405 values determined. For detection of C. rodentium-specific Ig by immunoblotting, heat-killed WT C. rodentium, Δler mutant strain or purified C. rodentium Intimin were loaded with 12% SDS-PAGE, and proteins detected with anti-mouse IgG antibody and enhanced chemiluminescent substrate (Thermo Scientific).
Detection of luminal neutrophils
Intraluminal cells were isolated from cecal samples of C. rodentium infected and uninfected mice. The Luminal content was filtrated sequentially through 100μm, 70μm, and 40μm cell strainer and then centrifuged at 1,000 rpm for 15 sec to remove debris. Cells were then used for flow cytometry. Cell surface fluorescence was assessed using a FACSCanto II and analyzed using FlowJo software (TreeStar). Dead cells were excluded with 7-AAD staining. Fluorescence-conjugated mAb against CD11b (M1/70), Ly6G (1A8), Ly6C (AL-21), CD45 (30-F11) were from eBioscience. Isotype-matched antibodies (eBioscience) were used for control staining.
Neutrophil phagocytosis assay
To elicit neutrophils in the peritoneal cavity, naïve or C. rodentium pre-infected mice (day 28 post-infection) were injected i. p. with thioglycollate 4 hours prior to bacterial challenge. C. rodentium wild-type (ChlR) and Δler mutant (KanR) were cultured in DMEM with standing culture for 6 hours to allow the expression of virulence factors. Bacteria were then washed with ice-cold PBS and injected (1×107 CFU/mouse) into neutrophil-rich peritoneal cavity of mice. In some experiments, bacteria were incubated with serum from naïve or C. rodentium-infected mice before i. p. injection. After 30 min, intracellular bacterial numbers in neutrophils harvested from the peritoneal cavity were assessed by plating lysates at serial dilutions onto MacConkey agar plates containing 50 μg/ml Kan or 30 μg/ml Chl.
Transmission Electron Microscopy
GF wild-type mice were orally infected with C. rodentium and Δler mutant. At indicated days post infection, ceca were collected and fixed with 2.5 % glutaraldehyde in 0.1 M Sorensen's buffer (pH 7.4). Fixed tissues were then 1 % osmium tetroxide in 0.1 M Sorensen's buffer, sequentially dehydrated through graded alcohols and propylene oxide, and then infiltrated in Spurrs or Epon. Ultrathin sections were cut with a diamond knife, stained, and examined with Philips CM-100 transmission electron microscope.
Statistical analyses
Statistical analyses were performed using GraphPad Prism software version 5.0 (GraphPad Software Inc.). Differences between two groups were evaluated using Student's t test (parametric) or Mann-Whitney U test (non-parametric). For the comparison of more than 3 groups, one-way ANOVA (parametric) or Kruscal-Wallis test (non-parametric) were used, and then the Dunnett's or Bonferroni test for parametric samples, or Dunn's test for non-parametric samples were performed as a post-hoc test. Survival between groups of mice was compared using Log-rank (Mantel-Cox) test. Differences at P<0.05 were considered significant.
Supplementary Material
Highlights.
Enteric pathogens reside as phenotypically virulent and avirulent subpopulations.
Enteric pathogen infection induces virulence factor-specific IgG.
Virulent pathogens, but not commensals or avirulent pathogens, are recognized by IgG.
IgG-bound virulent bacteria are eliminated intraluminally by migrated neutrophils.
Acknowledgments
The authors thank the University of Michigan Germ-free Animal Core, Microscopy and Image Analysis Laboratory, the Center for Molecular Imaging, and Flow Cytometry Core for support. Grace Chen for review of the manuscript, Jenna Rousseau for technical assistance and Alison O'Brien for providing E. coli expressing Intimin. B. A. V. is the CH. I. L. D. Foundation Chair in Pediatrics IBD Research. This work was supported by a Career Development Award from the Crohn's and Colitis Foundation of America (to N. K. and Y-G. K.), Michigan Gastrointestinal Peptide Research Center NIDDK 5P30DK034933 (N. K.), Grant-in-Aid for Japan Society for the Promotion of Science Fellows, Kanae Foundation For The Promotion of Medical Science, and Mishima Kaiun Memorial Foundation (K. S.), NIH Training Grant T32DK094775 (to M. Y. Z), grants from the NIH DK095782 and the Bill & Melinda Gates Foundation (G.N.).
Footnotes
Author Contributions: N.K. and G.N. conceived and designed experiments. N.K. conducted most of the experiments with technical help by K.S., S.-U.S, M.Y.Z, and Y.-G.K. M.C., B.A., and J.L.P provided materials and assisted with data interpretation. N.K. and G.N. analyzed all the data and wrote the manuscript. N.K. and G.N. wrote the manuscript with contributions from all authors.
Competing Financial Interests: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barba J, Bustamante VH, Flores-Valdez MA, Deng W, Finlay BB, Puente JL. A positive regulatory loop controls expression of the locus of enterocyte effacement-encoded regulators Ler and GrlA. J Bacteriol. 2005;187:7918–7930. doi: 10.1128/JB.187.23.7918-7930.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, O'Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, Hatton RD, Weaver CT. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 2012;37:1061–1075. doi: 10.1016/j.immuni.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6:e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bry L, Brenner MB. Critical role of T cell-dependent serum antibody, but not the gut-associated lymphoid tissue, for surviving acute mucosal infection with Citrobacter rodentium, an attaching and effacing pathogen. J Immunol. 2004;172:433–441. doi: 10.4049/jimmunol.172.1.433. [DOI] [PubMed] [Google Scholar]
- Bry L, Brigl M, Brenner MB. CD4+-T-cell effector functions and costimulatory requirements essential for surviving mucosal infection with Citrobacter rodentium. Infect Immun. 2006;74:673–681. doi: 10.1128/IAI.74.1.673-681.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascalho M, Ma A, Lee S, Masat L, Wabl M. A quasi-monoclonal mouse. Science. 1996;272:1649–1652. doi: 10.1126/science.272.5268.1649. [DOI] [PubMed] [Google Scholar]
- Collins JW, Keeney KM, Crepin VF, Rathinam VA, Fitzgerald KA, Finlay BB, Frankel G. Citrobacter rodentium: infection, inflammation and the microbiota. Nat Rev Microbiol. 2014;12:612–623. doi: 10.1038/nrmicro3315. [DOI] [PubMed] [Google Scholar]
- Deng W, Li Y, Vallance BA, Finlay BB. Locus of enterocyte effacement from Citrobacter rodentium: sequence analysis and evidence for horizontal transfer among attaching and effacing pathogens. Infect Immun. 2001;69:6323–6335. doi: 10.1128/IAI.69.10.6323-6335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Puente JL, Gruenheid S, Li Y, Vallance BA, Vazquez A, Barba J, Ibarra JA, O'Donnell P, Metalnikov P, et al. Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc Natl Acad Sci U S A. 2004;101:3597–3602. doi: 10.1073/pnas.0400326101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diard M, Garcia V, Maier L, Remus-Emsermann MN, Regoes RR, Ackermann M, Hardt WD. Stabilization of cooperative virulence by the expression of an avirulent phenotype. Nature. 2013;494:353–356. doi: 10.1038/nature11913. [DOI] [PubMed] [Google Scholar]
- Dzhagalov I, St John A, He YW. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2007;109:1620–1626. doi: 10.1182/blood-2006-03-013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaem-Maghami M, Simmons CP, Daniell S, Pizza M, Lewis D, Frankel G, Dougan G. Intimin-specific immune responses prevent bacterial colonization by the attaching-effacing pathogen Citrobacter rodentium. Infect Immun. 2001;69:5597–5605. doi: 10.1128/IAI.69.9.5597-5605.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins C, Chart H, Smith HR, Hartland EL, Batchelor M, Delahay RM, Dougan G, Frankel G. Antibody response of patients infected with verocytotoxin-producing Escherichia coli to protein antigens encoded on the LEE locus. J Med Microbiol. 2000;49:97–101. doi: 10.1099/0022-1317-49-1-97. [DOI] [PubMed] [Google Scholar]
- Kamada N, Kim YG, Sham HP, Vallance BA, Puente JL, Martens EC, Nunez G. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science. 2012;336:1325–1329. doi: 10.1126/science.1222195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- Khan MA, Bouzari S, Ma C, Rosenberger CM, Bergstrom KS, Gibson DL, Steiner TS, Vallance BA. Flagellin-dependent and -independent inflammatory responses following infection by enteropathogenic Escherichia coli and Citrobacter rodentium. Infect Immun. 2008;76:1410–1422. doi: 10.1128/IAI.01141-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeis SL, Bommarius B, Parkos CA, Sherman MA, Kalman D. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J Immunol. 2007;179:566–577. doi: 10.4049/jimmunol.179.1.566. [DOI] [PubMed] [Google Scholar]
- Li Y, Frey E, Mackenzie AM, Finlay BB. Human response to Escherichia coli O157:H7 infection: antibodies to secreted virulence factors. Infect Immun. 2000;68:5090–5095. doi: 10.1128/iai.68.9.5090-5095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaser C, Housley MP, Iimura M, Smith JR, Vallance BA, Finlay BB, Schreiber JR, Varki NM, Kagnoff MF, Eckmann L. Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect Immun. 2004;72:3315–3324. doi: 10.1128/IAI.72.6.3315-3324.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez MB, Taddei CR, Ruiz-Tagle A, Trabulsi LR, Giron JA. Antibody response of children with enteropathogenic Escherichia coli infection to the bundle-forming pilus and locus of enterocyte effacement-encoded virulence determinants. J Infect Dis. 1999;179:269–274. doi: 10.1086/314549. [DOI] [PubMed] [Google Scholar]
- Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. Citrobacter rodentium of mice and man. Cell Microbiol. 2005;7:1697–1706. doi: 10.1111/j.1462-5822.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- Okumura CY, Nizet V. Subterfuge and sabotage: evasion of host innate defenses by invasive gram-positive bacterial pathogens. Annu Rev Microbiol. 2014;68:439–458. doi: 10.1146/annurev-micro-092412-155711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty NK, Feltwell T, Pickard D, Clare S, Toribio AL, Fookes M, Roberts K, Monson R, Nair S, Kingsley RA, et al. Citrobacter rodentium is an unstable pathogen showing evidence of significant genomic flux. PLoS Pathog. 2011;7:e1002018. doi: 10.1371/journal.ppat.1002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy CR, Mocarski ES. Pathogen subversion of cell-intrinsic innate immunity. Nat Immunol. 2007;8:1179–1187. doi: 10.1038/ni1528. [DOI] [PubMed] [Google Scholar]
- Schauer DB, Falkow S. Attaching and effacing locus of a Citrobacter freundii biotype that causes transmissible murine colonic hyperplasia. Infect Immun. 1993;61:2486–2492. doi: 10.1128/iai.61.6.2486-2492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scribner DJ, Fahrney D. Neutrophil receptors for IgG and complement: their roles in the attachment and ingestion phases of phagocytosis. J Immunol. 1976;116:892–897. [PubMed] [Google Scholar]
- Simmons CP, Clare S, Ghaem-Maghami M, Uren TK, Rankin J, Huett A, Goldin R, Lewis DJ, MacDonald TT, Strugnell RA, et al. Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect Immun. 2003;71:5077–5086. doi: 10.1128/IAI.71.9.5077-5086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JF, O'Brien AD. Intimin types alpha, beta, and gamma bind to nucleolin with equivalent affinity but lower avidity than to the translocated intimin receptor. J Biol Chem. 2004;279:33751–33758. doi: 10.1074/jbc.M401616200. [DOI] [PubMed] [Google Scholar]
- Spiekermann GM, Finn PW, Ward ES, Dumont J, Dickinson BL, Blumberg RS, Lencer WI. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung. J Exp Med. 2002;196:303–310. doi: 10.1084/jem.20020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A, Heinemann M, Arnoldini M, Benecke A, Ackermann M, Benz M, Dormann J, Hardt WD. The cost of virulence: retarded growth of Salmonella Typhimurium cells expressing type III secretion system 1. PLoS Pathog. 2011;7:e1002143. doi: 10.1371/journal.ppat.1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oss CJ, Gillman CF. Phagocytosis as a surface phenomenon. II. Contact angles and phagocytosis of encapsulated bacteria before and after opsonization by specific antiserum and complement. J Reticuloendothel Soc. 1972;12:497–502. [PubMed] [Google Scholar]
- Yang J, Hart E, Tauschek M, Price GD, Hartland EL, Strugnell RA, Robins-Browne RM. Bicarbonate-mediated transcriptional activation of divergent operons by the virulence regulatory protein, RegA, from Citrobacter rodentium. Mol Microbiol. 2008;68:314–327. doi: 10.1111/j.1365-2958.2008.06171.x. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, Claypool SM, Kaser A, Nagaishi T, Higgins DE, Mizoguchi E, et al. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest. 2006;116:2142–2151. doi: 10.1172/JCI27821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


