Abstract
Renal dopamine 2 receptor dysfunction is associated with oxidative stress and high blood pressure. We have reported that DJ-1, an oxidative stress response protein, is positively regulated by dopamine 2 receptor in the kidney. The transcription factor Nrf2 regulates the expression of several antioxidant genes. We tested the hypothesis that Nrf2 is involved in the renal DJ-1-mediated inhibition of reactive oxygen species production. We have reported that silencing dopamine 2 receptor in mouse renal proximal tubule cells decreases the expression of DJ-1. We now report that silencing DJ-1 or dopamine 2 receptor in mouse proximal tubule cells and mouse kidneys, decreases Nrf2 expression and activity and increases reactive oxygen species production; blood pressure is also increased in mice in which renal DJ-1 or dopamine 2 receptor is silenced. DJ-1−/− mice have decreased renal Nrf2 expression and activity, and increased nitro-tyrosine levels an dopamine 2 receptor d blood pressure. Silencing Nrf2 in mouse proximal tubule cells does not alter the expression of DJ-1 or dopamine 2 receptor, indicating that Nrf2 is downstream of dopamine 2 receptor and DJ-1. A Nrf2 inducer, bardoxolone, normalizes the systolic blood pressure and renal malondialdehyde levels in DJ-1−/− mice without affecting them in their wild-type littermates. Because Nrf2 ubiquitination is increased in DJ-1−/− mice, we conclude that the protective effect of DJ-1 on renal oxidative stress is mediated, in part, by preventing Nrf2 degradation. Moreover, renal dopamine 2 receptor and DJ-1 are necessary for normal Nrf2 activity to keep a normal redox balance and blood pressure.
Keywords: Oxidative stress, Kidney, dopamine receptors, DJ-1, Nrf2
Introduction
Essential hypertension is associated with increased positive sodium balance and oxidative stress. Renal dopamine plays an important role in the normal regulation of sodium balance and systemic blood pressure (1–4). Indeed, hypertension is associated with decreased renal dopamine production and receptor function (1–4). Dopamine receptors (DR) are classified in two families: D1-like receptors (D1R and D5R) and D2-like receptors (D2R, D3R, and D4R). Deletion of any of the dopamine receptor genes in mice results in increased blood pressure by mechanisms that are dopamine receptor subtype-specific (4). In particular, D2−/− and D5−/− mice have hypertension that is associated with oxidative stress (5,6,7).
Dopamine and D2R agonists have antioxidant activity that may be responsible for their neuroprotective effects (8,9). We have reported that the antioxidant property of D2R is mediated, in part, by positive regulation of several antioxidant proteins, including heme oxygenase-2 (HO-2) (4,5), paraoxonase-2 (PON-2) (10), and DJ-1(11).
DJ-1(aka Park 7), initially identified as an oncogene and an autosomal recessive gene in Parkinson’s disease, is widely expressed in the body, including the brain, heart, kidney, and liver (12). DJ-1 is a peroxiredoxin that exerts a protective role against oxidative stress in several diseases (13–15). DJ-1 may be responsible for the neuroprotection afforded by D2R (16). We have reported that the antioxidant effect of D2R is dependent, in part, on DJ-1 expression/activity in the kidney (11). Renal silencing of DJ-1 in the mouse increases reactive oxygen species (ROS) production and blood pressure.
Nrf2 (nuclear factor erythroid 2-related factor 2) is a transcription factor that regulates the expression of several antioxidant genes. Nrf2, together with small Maf proteins, binds to antioxidant response element (ARE) in the regulatory regions of target genes (17). Nrf2 has also been reported to inhibit the development and progression of several diseases affecting the kidney (18). DJ-1 may be involved in the regulation of Nrf2 (19–23) but it is not known if the protective effect of DJ-1 on renal oxidative stress is dependent on Nrf2. Therefore, we tested the hypothesis that Nrf2 is involved in the antioxidant activity of DJ-1 in the kidney.
Materials and methods
The experimental procedures are detailed in the online data supplement.
Results
DJ-1 colocalizes with Nrf2 in the renal proximal tubule (RPT)
Immunofluorescence microscopy of mouse kidney sections showed that DJ-1 is expressed in the brush border and cytoplasm, while Nrf2 is expressed in the brush border, cytoplasm, and nucleus (Figure 1). Double immunofluorescence microscopy (Figure 1 merge) revealed that Nrf2 colocalized with DJ-1 in the cytoplasm and brush border of renal tubules.
Figure 1. Colocalization of DJ-1 and Nrf2 in mouse kidney.
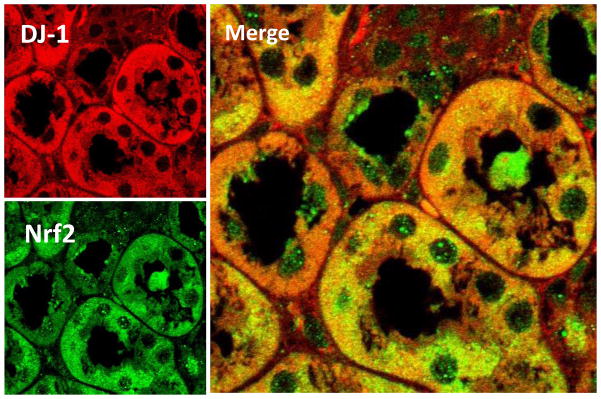
Formalin-fixed, paraffin-embedded mouse kidney sections were used to determine the colocalization of DJ-1 (pseudocolored red) and Nrf2 (pseudocolored green) by confocal microscopy. Colocalization of DJ-1 and Nrf2 (shown as yellow in merge images) was found in the cytoplasm and brush border of proximal tubules (Scale bar, 10 μm, ×600 magnification). Nrf2, but not DJ-1, was also localized in the nucleus.
Silencing Drd2 decreases DJ-1 expression, Nrf2 expression and activity, and increases ROS production
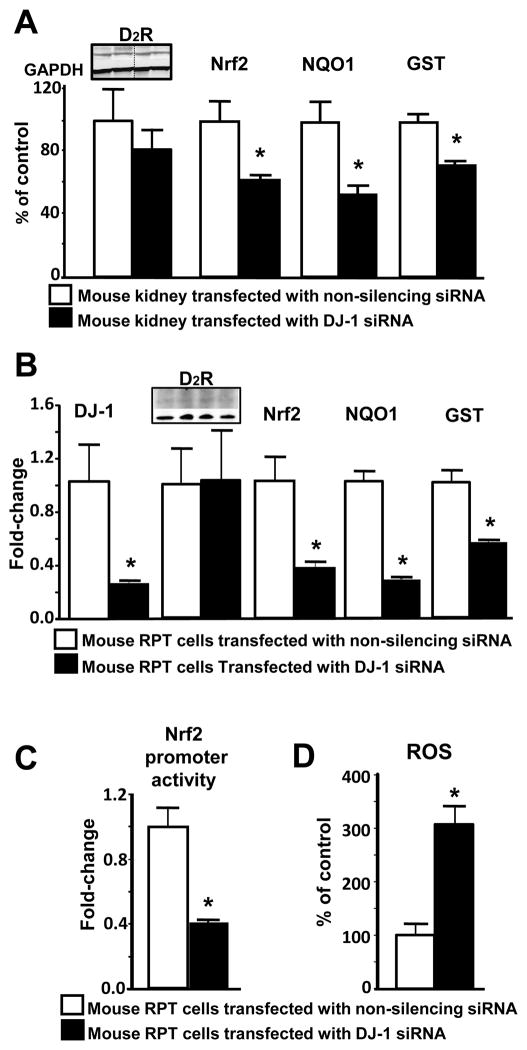
To determine if Nrf2 is involved in the antioxidant effect of DJ-1 and D2R in the kidney, we studied the kidneys of mice that underwent renal-selective silencing of Drd2. Renal-selective silencing of Drd2 decreased D2R expression (48±2%) and increased the systolic blood pressure (ΔSBP: 19±8%), in agreement with the increased blood pressure of D2−/− mice (5,11). Renal DJ-1 expression was decreased by -45±2%, Nrf2 by -22±2%, and glutathione S-transferase (GST) by -63±6%, while NADH quinone oxidoreductase (NQO1) expression was not affected (Figure 2A). Drd2 silencing in mouse RPT cells decreased Nrf2 promoter activity (-53±2%, Figure 2B) and increased ROS production (+146±43%, Figure 2C). These results confirm our report that DJ-1 expression and ROS production are regulated by the D2R in the kidney (11) and that D2R regulates renal Nrf2 expression activity.
Figure 2. Effect of renal-selective Drd2 silencing on renal DJ-1, Nrf2, NQO1, and GST expression, Nrf2 promoter activity, and ROS production.
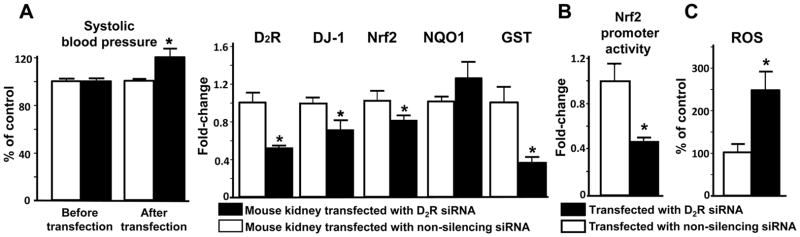
(A) Renal-selective Drd2 silencing in vivo. Renal cortical Drd2 was silenced as described in materials and methods. Systolic blood pressure was measured (Cardiomax II) from the aorta, via the femoral artery, under pentobarbital anesthesia. mRNA expressions of D2R, DJ-1, Nrf2, NQO1, and GST were quantified by qRT-PCR in mouse renal cortex. GAPDH was used for normalization of the data. Data are expressed as mean ± S.E. n=3–4/group. *P< 0.05 vs. non-silencing siRNA, t-test.
(B) RPT cell Nrf2 promoter activity. Mouse RPT cells were studied 72 hours after transfection with Drd2-siRNA or non-silencing siRNA. Nrf2 promoter activity was measured by a reporter assay (Qiagen). Data are expressed as mean ± S.E. n=6/group. *P< 0.05 vs. non-silencing siRNA, t-test.
(C) RPT cell ROS production. Mouse RPT cells were studied 72 hours after transfection with Drd2-siRNA or non-silencing siRNA. ROS production was measured in whole RPT cell homogenates using DCFDA, and corrected for protein concentration. Data are expressed as mean ± S.E. n=6/group. * P< 0.05 vs. non-silencing siRNA, t-test.
Renal silencing of DJ-1 decreases renal Nrf2 expression and activity and results in oxidative stress-dependent hypertension
We have reported that the renal silencing of DJ-1 decreased DJ-1 expression (30 ± 6%) and increased blood pressure (ΔBP 18±3%) (11). We now show in these mice that the renal silencing of DJ-1 also decreased the protein expression of Nrf2 (57±6%), and its target genes NQO1 (46±11%) and GST (28±7%), but not the protein expression of D2R (Figure 3A). We also determined if silencing DJ-1 with DJ-1 siRNA affects D2R protein expression in mouse RPT cells. We found that DJ-1 depletion decreased the expression of DJ-1 (-75±15%) but did not affect the protein expression of D2R (Figure 3B), which reflects our in vivo data. Moreover, DJ-1 silencing decreased the expression of Nrf2 (63±11%), its target genes, NQO1 (75±15%) and GST (61±8%), and Nrf2 promoter activity (58±1%) (Figure 3C), but increased ROS production (206±15%) (Figure 3D). These results suggest that DJ-1 maintains normal renal redox balance by positively regulating Nrf2 expression and function, and that D2R is upstream of DJ-1 in this pathway.
Figure 3. Effect of silencing DJ-1 on renal D2R, Nrf2, NQO1, and GST expression, Nrf2 promoter activity, and ROS production.
(A) Renal-selective DJ-1 silencing in vivo. Renal cortical DJ-1 was silenced as described in materials and methods. Renal cortical homogenates were immunoblotted using a D2R antibody. Expressions of Nrf2, NQO1, and GST were quantified by qRT-PCR. Data are expressed as mean ± S.E. n=3/group, *P< 0.05, vs. non-silencing siRNA, t-test.
(B) RPT cell DJ-1, Nrf2, NQO1, and GST expression. Mouse RPT cells were studied 72 hours after transfection with DJ-1-specific siRNA or non-silencing siRNA. Expressions (mRNA) of DJ-1, Nrf2, NQO1, and GST were quantified by qRT-PCR. GAPDH was used for normalization of the data. Data are expressed as mean ± S.E. n=3–4/group,*P< 0.05 vs. non-silencing siRNA, t-test.
(C) RPT cell Nrf2 promoter activity. Nrf2 promoter activity was measured as in figure 2. Data are expressed as mean ± S.E. n=5/group,*P< 0.05 vs. non-silencing siRNA, t-test
(D) RPT cell ROS production. Mouse RPT cells were studied 72 hours after transfection with DJ-1-specific siRNA or non-silencing siRNA. ROS production was measured as above. Data are expressed as mean ± S.E. n=4–5/group, *P< 0.05 vs. non-silencing siRNA, t-test.
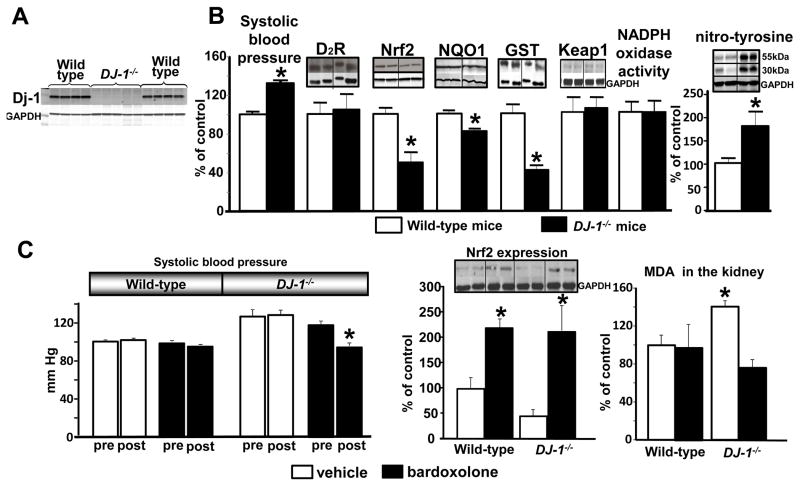
To determine if the antioxidant action of DJ-1 is exerted via Nrf2, we extended our studies in DJ-1−/− mice. The absence of DJ-1 expression in DJ-1−/− mice was confirmed by immunoblotting (Figure 4A). The systolic blood pressure was increased in DJ-1−/− mice by 31±8%, relative to their wild-type littermates (Figure 4B). Immunoblots showed that DJ-1−/− mice had decreased renal expression of Nrf2 (47±7%), NQO1 (19±3%), and GST (51±12%), but D2R and Keap1 expression were not affected (Figures 3A and 3B). NADPH oxidase activity was also not altered but nitro-tyrosine protein was increased by 77±31% in the kidney of DJ-1−/− mice, indicating that DJ-1 negatively regulates oxidative stress independent of NADPH oxidase activity (Figure 4B). The latter effect of DJ-1 is independent of D2R because germline deletion of D2R is associated with increased renal expression Nox1, Nox2, and Nox4 (5).
Figure 4. Effect of germ-line disruption of DJ-1 in mice on the expressions of renal D2R, Nrf2, NQO1, GST, and nitro-tyrosine, MDA levels, and blood pressure. Effect of bardoxolone treatment on renal MDA and blood pressure in DJ-1−/− mice.
(A) Renal DJ-1 expression in DJ-1−/− mice. Immunoblot analysis of DJ-1 in kidneys of DJ-1−/− mice and wild-type littermates.
(B) Systolic blood pressure in DJ-1−/− mice. Systolic blood pressure was measured as described in figure 2. Kidney homogenates were immunoblotted using antibodies against DJ-1, D2R, Nrf2, NQO1, GST, Keap1 and nitro-tyrosine. Data were corrected for GAPDH. NADPH oxidase activity (light units per milligram of protein) was determined by lucigenin (5 μmol/L). Data were expressed as mean ± S.E., n = 5–8/group, *P< 0.05 vs. wild-type littermates, t-test.
(C) Effect bardoxolone on systolic blood pressure in DJ-1−/− mice. The mice were treated with bardoxolone (150μl of 10μmol/L = 0.03 mg/kg) or vehicle (150μl) via intraperitoneal injection 3 times a week for 2 weeks. Blood pressure was measured as described in figure 2. Nrf2 expression was determined by immunoblotting and normalized by GAPDH. MDA concentration (corrected for protein concentration) in tissue homogenates was determined using a commercial kit (Cell Biolabs, Inc). Data are expressed as mean ± S.E. n = 3–5/group, *P< 0.05 vs. others, one-way factorial ANOVA.
To confirm the role of Nrf2 in the increased oxidative stress and blood pressure associated with renal DJ-1 depletion, DJ-1−/− mice were treated with bardoxolone, a Nrf2 inducer (Figure 4C). Bardoxolone increased renal Nrf2 expression in both wild-type (118±17%) and DJ-1−/− mice (112±49%), relative to their vehicle-treated controls, and normalized the increased blood pressure (before treatment118±3.9%: after treatment 95±3.97% vs WT) and renal malondialdehyde (MDA) production (before treatment 140±6%: after treatment 76±9% vs WT) in DJ-1−/− mice. Thus, the hypertension in DJ-1−/− mice is mediated by oxidative stress, in part, by the down-regulation of Nrf2 expression. The fact that bardoxolone increased Nrf2 expression in wild-type mice but did not decrease their normal MDA levels and blood pressure could be taken to indicate that under normal conditions, Nrf2 has no effect on oxygen radical formation or blood pressure.
Renal silencing of Nrf2 increases blood pressure and silencing Nrf2 decreases NQO1 and GST but not D2R and DJ-1 expression in mouse RPT cells
We, next, determined if Nrf2 directly participated in the DJ-1 and D2R-mediated regulation of renal ROS production and blood pressure. We found that renal-selective silencing of Nrf2 that decreased Nrf2 expression (-51±9%), increased blood pressure(+12±6.3 mg Hg) (Figure S1A). In mouse RPT cells, Nrf2 siRNA that decreased Nrf2 expression (34±4%), also decreased the expression of its target genes NQO1 (29±1%) and GST (25±7%). However, silencing of Nrf2 did not alter the expression of D2R and DJ-1, indicating that Nrf2 is downstream of D2R and DJ-1(Figure S1B). ROS production was not increased in Nrf2-deficient mouse RPT cells (Figure S1C).
D2R stimulation does not change Nrf2 expression and activity
We have reported that in mouse RPT cells, D2R stimulation with quinpirole, a D2R agonist, increased DJ-1 expression that was partially blocked by a selective D2R antagonist (11). To determine if the increase in DJ-1 expression with D2R stimulation was responsible for the increase in the expression of Nrf2 and its target genes, we quantified the mRNA expression of Nrf2 and target genes in mouse RPT cells treated with the D2R agonist quinpirole. We found that stimulation of D2R with quinpirole increased the expression of DJ-1 (22±5%), and decreased ROS production (18±3%), as expected (11), but did not modify the mRNA expression of Nrf2, NQO1, and GST, or Nrf2 promoter activity in mouse RPT cells (Figures S2A, S2B, and S2C), indicating that D2R and DJ-1 can decrease ROS production in mouse RPT cells independent of Nrf2.
Depletion of DJ-1 increases ubiquitination of Nrf2
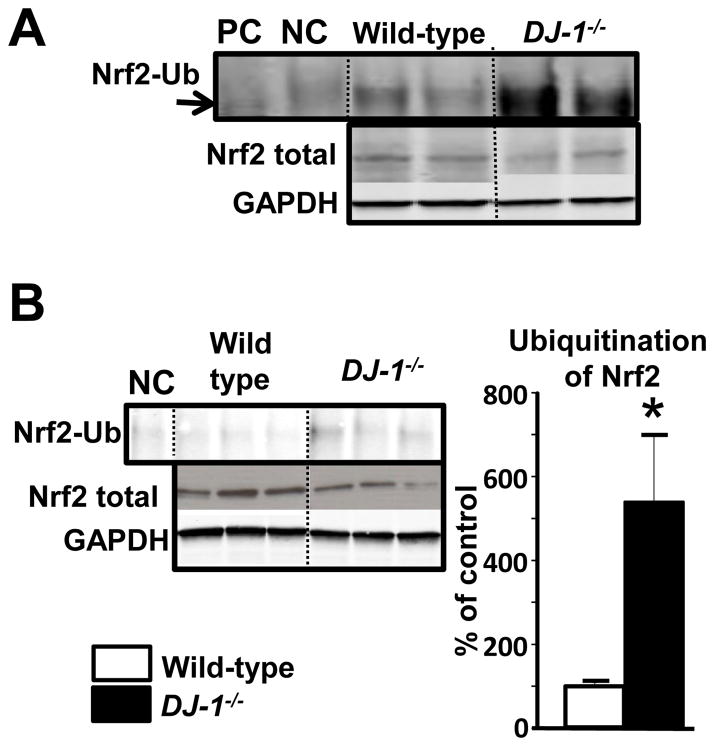
The preceding studies showed that silencing DJ-1 down-regulated Nrf2 expression and function in the kidney. Nrf2 degradation is regulated by Nrf2 ubiquitination (24). Therefore, we determined if renal DJ-1 could increase the stability of Nrf2 protein, by reducing its ubiquitination and presumably inhibiting its degradation, via the proteosomal pathway. We inmunoprecipitated Nrf2 from kidney homogenates of DJ-1−/− mice and wild-type littermates using rabbit Nrf2 antibody and inmunoblotted the immunoprecipitates with mouse monoclonal ubiquitin antibody. The amount of ubiquitinated Nrf2 was increased in DJ-1−/− mice compared to their wild-type littermates (Figure 5A). This result was corroborated by immunoprecipitating kidney homogenates with ubiquitin antibody and immunoblotting the immunoprecipitates with Nrf2 antibody (Figure 5B). In the kidneys of DJ-1−/− mice, Nrf2 ubiquitination was increased (438±161%), suggesting that the decrease in Nrf2 expression in DJ-1−/− mice may be the result of increased Nrf2 degradation of ubiquitinated Nrf2, via the proteasomal pathway (25).
Figure 5. Nrf2 ubiquitination in DJ-1−/− mice.
(A) Total kidney lysates from DJ-1−/− mice were immunoprecipitated with anti-ubiquitin antibody and immunoblotted with anti-Nrf2 antibody.
(B) Total kidney lysates from DJ-1−/− mice were immunoprecipitated with anti-Nrf2 antibody and immunoblotted with anti-mono ubiquitin antibody. Data are expressed as mean ± S.E. n = 3/group.*P< 0.05 vs. wild-type littermates, t-test, NC = negative control.
Discussion
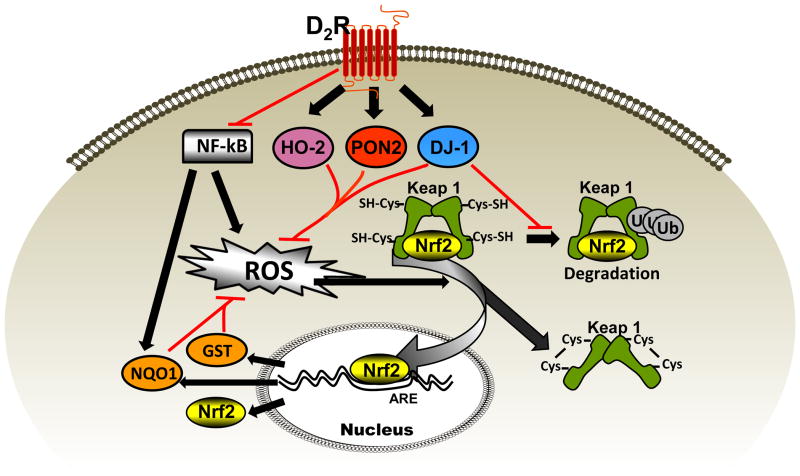
We have recently reported that disruption of the D2R gene Drd2 in mice results in hypertension that is associated with increased ROS production (5,10), and that the D2R-mediated negative regulation of ROS production is due, in part, by its positive regulation of DJ-1 expression (11). We now report that D2R and DJ-1 are necessary for the normal function of Nrf2 and that DJ-1 prevents its ubiquitination and presumably its proteasomal degradation. Because Drd2 silencing decreases both DJ-1 and Nrf2 expressions, while silencing DJ-1 decreases the expression of Nrf2 but not D2R, and silencing Nrf2 does not decrease the expression of either D2R or DJ-1, D2R is upstream of both DJ-1 and Nrf2, and DJ-1 is upstream of Nrf2, in the regulation of renal ROS production and blood pressure (Figure 6).
Figure 6. Pathway of D2R and DJ-1 regulation of Nrf2 and ROS.
D2R decreases reactive oxygen species (ROS) production in the kidney by increasing the expressions of HO-2, PON2, and DJ-1 and inhibiting NFκB activity (NFκB increases NQO1 expression). Nrf2 transcription and activity are increased by oxidative stress. Oxidation of cysteine SH groups of KEAP 1 by ROS releases Nrf2 allowing its translocation into the nucleus where it binds to antioxidant response element (ARE), increasing the expression of antioxidant proteins such as NQO1, GST, and its own (Nrf2) expression. DJ-1 prevents the ubiquitination of Nrf2 and therefore, its degradation.
We have reported that silencing of DJ-1 expression in mouse RPT cells and kidney increases Nox4 expression and NAPDH oxidase activity (11). However, NAPDH oxidase activity is not increased in DJ-1 −/− mice, indicating the activation of a compensatory mechanism with germline deletion of DJ-1 that remains to be identified. DJ-1−/− mice have a 2-fold increase in the mitochondrial H2O2 in the brain (14) and increased sensitivity to stroke-induced neural injury (15). Our data show that the renal production of nitro-tyrosine is increased in DJ-1−/− mice that may be the result of an increase in peroxynitrite production, because peroxynitrite is formed by the interaction between nitric oxide and superoxide (26).
DJ-1 positively regulates Nrf2 expression in hepatocyte-derived carcinoma, corneal endothelial cells (19,20), and human lung epithelial cells in conditions associated with oxidative stress (21). In addition, DJ-1 protects human neuroblastoma cells and astrocytes from oxidative stress via Nrf2 (22, 23). However, in neurons, Nrf2 can be activated independent of DJ-1 (27), suggesting that DJ-1-mediated regulation of Nrf2 could also be tissue-specific.
The potential role for Nrf2 on blood pressure regulation is not well defined. There were no differences in blood pressure between Nrf2−/− mice and wild-type littermates, in the basal state or even blood pressure was increased by angiotensin II infusion (28). In another study, bardoxolone improved renal function, vascular injury, and inflammation in patients with diabetes and chronic kidney disease but had no effect on blood pressure (29). In contrast, another inducer of Nrf2 activity, triterpenoid RTA dh404, improved the endothelial dysfunction and normalized blood pressure in rats with chronic kidney disease (30,31) and mitigated the pulmonary hypertension in mouse with emphysema induced by exposure to cigarette smoke (32). We have unreported studies showing that the renal selective silencing of Nrf2, via the renal subcapsular infusion of Nrf2-siRNA increases blood pressure. This in contrast to the normal blood pressure of mice with germ-line deletion of Nrf2 (28). It is possible that germline deletion of Nrf2 results in expression of genes, during development, or in non-renal tissues, that may compensate for the loss of Nrf2. This could also explain why ROS production was not increased in mouse RPT cells that had decreased Nrf2 expression following Nrf2-siRNA treatment, in spite of the ability of bardoxolone to normalize ROS production and blood pressure of DJ-1−/− mice.
The stimulation of the D2R in mouse RPT cells increased DJ-1expression and decreased ROS production, confirming our previous report (11). However, the stimulation of D2R with quinpirole did not affect Nrf2 expression and activity and the expression of its targets genes (Figure S2). These occurred in spite of the fact that silencing of Drd2 or DJ-1 increases ROS production (4,11) and decreases Nrf2 expression and Nrf2 promoter activity (current study). Nevertheless, part of the oxidative stress induced by D2R silencing may be due to the down-regulation of Nrf2 because D2R and DJ-1 are necessary for normal Nrf2 function. Antioxidants regulated by Nrf2, other than D2R and DJ-1, such as HO-1 could be involved in the antioxidant mechanism of DJ-1 and D2R. However, we have reported that the antioxidant effect of D2R is not related to HO-1 but rather due, in part, to HO-2 (5). Therefore, D2R stimulation increases the expression of some antioxidants such as HO-2 (7), PON2 (10), and DJ-1(11), independently of Nrf2 (Figure 6).
The inability of D2R to increase Nrf2 activity, in spite of the fact that both have antioxidant actions may at first glance contradictory. The apparently contradictory results stem from the fact that both D2R and DJ-1 decrease ROS production (5,7,11,19) and Nrf2 expression is stimulated by ROS (33). Therefore, when ROS production is decreased by D2R stimulation, Nrf2 expression could not be increased. Nrf2 can be specifically degraded by the ubiquitin-proteasome system (24); the ubiquitination of Nrf2 is increased in DJ-1−/− mice, suggesting that DJ-1 decreases the ubiquitination of Nrf2, and presumably its degradation (Figure 6). However, DJ-1 does not regulate renal Keap1 expression. Thus, DJ-1 may positively regulate Nrf2 protein stability that may be dependent on the state of oxidative stress, independent of Keap1. Moreover, the antioxidant response element (ARE) sequences in the promoter of the Nrf2 gene, indicate a capacity of Nrf2 to increase the synthesis of its own mRNA, in states of oxidative stress (34).
As indicated above, the hypertension of D2−/− mice is associated with oxidative stress (4, 10, 11) and the renal expression of DJ-1 in D2−/+ is decreased (11). The renal-selective silencing of Drd2 in mice increases blood pressure (35); renal-selective silencing of Drd2 in mice also increases ROS production and decreases DJ-1 and Nrf2 expression and Nrf2 promoter activity and the expression of one of the target genes of Nrf2, GST. However, in contrast to the decreased expression of NQO1, when DJ-1 or Nrf2 is silenced, NQO-1 expression is not down-regulated when Drd2 is silenced. The failure of NQO1 to be decreased when Drd2 is silenced may be related to NFκB which is upregulated when Drd2 is silenced (35); NFkB can increase NQO1 expression (36).
Banday and colleagues have reported that oxidative stress, via NFκB activation, impairs renal D1R expression and function (37). The activation of Nrf2-phase II enzyme pathway, directly and indirectly decreases NFκB activation, causing a decrease in oxidative stress and restoration of D1R expression and function (38). However, the renal D2R expression is not altered by the oxidative stress provoked by the down-regulation of DJ-1 and Nrf2 (current results). Thus, the effect of oxidative stress on the expression of dopamine receptors may be subtype-specific. D1R function could be impaired when D2R is silenced because of NFκB activation. However, renal D1R expression is not decreased in Drd2 knockout mice (unpublished studies). Reports of D1-like receptor binding in the striatum of Drd2 knockout mice are not consistent (39) but the concomitant absence of D1R and D2R signaling is lethal in mice (40).
In conclusion, Nrf2 mediates, in part, the antioxidant effects of D2R and DJ-1 in the kidney and the oxidative stress-dependent hypertension associated with renal-selective depletion of D2R or DJ-1. Germline deletion of D2R can result in salt-sensitive hypertension, that may be mouse strain dependent (41). The role of sodium balance in the hypertension associated with DJ-1 deficiency remains to be determined.
Perspectives
Our results show that D2R and DJ-1 are necessary for normal Nrf2 activity in the kidney. Renal selective silencing of Drd2 or DJ-1in the mouse increases ROS production and blood pressure that are mediated, in part, by increased Nrf2 degradation. Further studies are needed to determine whether or not modulation of renal DJ-1 and Nrf2 functions is a therapeutic approach in hypertension.
Supplementary Material
Novelty and Significance.
What Is New?
This is the first report providing evidence for a role of D2R and DJ-1 in the regulation of Nrf2 in the kidney, as related to oxidative stress.
What Is Relevant?
DJ-1, by preventing the ubiquitination of renal Nrf2, mitigates the development of oxidative stress-dependent hypertension. Modulation of renal D2R, DJ-1, and Nrf2 function could be a therapeutic approach in hypertension.
Summary.
The protective effect of renal D2R and DJ-1 on oxidative stress is dependent, in part, on Nrf2. DJ-1 prevents the ubiquitination of renal Nrf2; DJ-1 and Nrf2 have important roles in the pathogenesis of hypertension associated with oxidative stress.
Acknowledgments
Funding;
These studies were supported in part by grants from the NIH (HL068686) and National Kidney Foundation of Maryland (Mini-grant 2013–2014).
Footnotes
Conflict(s) of Interest/Disclosure(s)
None
References
- 1.Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med (Maywood) 2003;228:134–142. doi: 10.1177/153537020322800202. [DOI] [PubMed] [Google Scholar]
- 2.Zhang MZ, Yao B, Wang S, Fan X, Wu G, Yang H, Yin H, Yang S, Harris RC. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J Clin Invest. 2011;121:2845–2854. doi: 10.1172/JCI57324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aperia AC. Intrarenal dopamine: a key signal in the interactive regulation of sodium metabolism. Annu Rev Physiol. 2000;62:621–647. doi: 10.1146/annurev.physiol.62.1.621. [DOI] [PubMed] [Google Scholar]
- 4.Armando I, Villar VA, Jose PA. Dopamine and renal function and blood pressure regulation. Compr Physiol. 2011;1:1075–1117. doi: 10.1002/cphy.c100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armando I, Wang X, Villar VA, Jones JE, Asico LD, Escano C, Jose PA. Reactive oxygen species-dependent hypertension in dopamine D2 receptor-deficient mice. Hypertension. 2007;49:672–678. doi: 10.1161/01.HYP.0000254486.00883.3d. [DOI] [PubMed] [Google Scholar]
- 6.Asico L, Zhang X, Jiang J, Cabrera D, Escano CS, Sibley DR, Wang X, Yang Y, Mannon R, Jones JE, Armando I, Jose PA. Lack of renal dopamine D5 receptors promotes hypertension. J Am Soc Nephrol. 2011;22:82–89. doi: 10.1681/ASN.2010050533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuevas S, Villar VA, Jose PA, Armando I. Renal dopamine receptors, oxidative stress, and hypertension. Int J Mol Sci. 2013;14:17553–17572. doi: 10.3390/ijms140917553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogawa N, Tanaka K, Asanuma M, Kawai M, Masumizu T, Kohno M, Mori A. Bromocriptine protects mice against 6-hydroxydopamine and scavenges hydroxyl free radicals in vitro. Brain Res. 1994;657:207–213. doi: 10.1016/0006-8993(94)90969-5. [DOI] [PubMed] [Google Scholar]
- 9.Zou L, Xu J, Jankovic J, He Y, Appel SH, Le W. Pramipexole inhibits lipid peroxidation and reduces injury in the substantia nigra induced by the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57BL/6 mice. Neurosci Lett. 2000;281:167–170. doi: 10.1016/s0304-3940(00)00853-3. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Zhang Y, Cuevas S, Villar VA, Escano C, Asico DL, Yu P, Grandy DK, Felder RA, Armando I, Jose PA. Paraoxonase 2 decreases renal reactive oxygen species production, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of NADPH oxidase. Free Radic Biol Med. 2012;53:437–446. doi: 10.1016/j.freeradbiomed.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuevas S, Zhang Y, Yang Y, Escano C, Asico L, Jones JE, Armando I, Jose PA. Role of renal DJ-1 in the pathogenesis of hypertension associated with increased reactive oxygen species production. Hypertension. 2012;59:446–452. doi: 10.1161/HYPERTENSIONAHA.111.185744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SM, Ariga H. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem Biophys Res Commun. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- 13.Wilson MA. The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid Redox Signal. 2011;15:111–122. doi: 10.1089/ars.2010.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, Ko HS, Sasaki M, Ischiropoulos H, Przedborski S, Dawson TM, Dawson VL. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci USA. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aleyasin H, Rousseaux MW, Phillips M, Kim RH, Bland RJ, Callaghan S, Slack RS, During MJ, Mak TW, Park DS. The Parkinson’s disease gene DJ-1 is also a key regulator of stroke-induced damage. Proc Natl Acad Sci USA. 2007;104:18748–18753. doi: 10.1073/pnas.0709379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borrelli E. Without DJ-1, the D2 receptor doesn’t play. Neuron. 2005;45:479–481. doi: 10.1016/j.neuron.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Kansanen E, Kivela AM, Levonen AL. Regulation of Nrf2-dependentgene expression by 15-deoxy-delta 12, 14-prostaglandin J2. Free Radic Biol Med. 2009;47:1310–1317. doi: 10.1016/j.freeradbiomed.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 18.Shelton LM, Park BK, Copple IM. Role of Nrf2 in protection against acute kidney injury. Kidney Int. 2013;84:1090–1095. doi: 10.1038/ki.2013.248. [DOI] [PubMed] [Google Scholar]
- 19.Bitar MS, Liu C, Ziaei A, Chen Y, Schmedt T, Jurkunas UV. Decline in DJ-1 and decreased nuclear translocation of Nrf2 in Fuchs endothelial corneal dystrophy. Invest Ophthalmol Vis Sci. 2012;53:5806–5813. doi: 10.1167/iovs.12-10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci USA. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P, Tuder RM, Biswal S. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Im JY, Lee KW, Woo JM, Junn E, Mouradian MM. DJ-1 induces thioredoxin 1 expression through the Nrf2 pathway. Hum Mol Genet. 2012;21:3013–3024. doi: 10.1093/hmg/dds131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lev N, Barhum Y, Ben-Zur T, Melamed E, Steiner I, Offen D. Knocking out DJ-1 attenuates astrocytes neuroprotection against 6-hydroxydopamine toxicity. J Mol Neurosci. 2013;50:542–550. doi: 10.1007/s12031-013-9984-9. [DOI] [PubMed] [Google Scholar]
- 24.Stewart D, Killeen E, Naquin R, Alam S, Alam J. Degradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. J Biol Chem. 2003;278:2396–2402. doi: 10.1074/jbc.M209195200. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Armando I, Yu P, Escano C, Mueller SC, Asico L, Pascua A, Lu Q, Wang X, Villar VA, Jones JE, Wang Z, Periasamy A, Lau YS, Soares-da-Silva P, Creswell K, Guillemette G, Sibley DR, Eisner G, Gildea JJ, Felder RA, Jose PA. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J Clin Invest. 2008;118:2180–2189. doi: 10.1172/JCI33637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci USA. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gan L, Johnson DA, Johnson JA. Keap1-Nrf2 activation in the presence and absence of DJ-1. Eur J Neurosci. 2010;31:967–977. doi: 10.1111/j.1460-9568.2010.07138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Zhang C, Xing Y, Janicki JS, Yamamoto M, Wang XL, Tang DQ, Cui T. Up-regulation of p27(kip1) contributes to Nrf2-mediated protection against angiotensin II-induced cardiac hypertrophy. Cardiovasc Res. 2011;90:315–324. doi: 10.1093/cvr/cvr010. [DOI] [PubMed] [Google Scholar]
- 29.Pergola PE, Krauth M, Huff JW, Ferguson DA, Ruiz S, Meyer CJ, Warnock DG. Effect of bardoxolone methyl on kidney function in patients with T2D and Stage 3b-4 CKD. Am J Nephrol. 2011;33:469–476. doi: 10.1159/000327599. [DOI] [PubMed] [Google Scholar]
- 30.Aminzadeh MA, Reisman SA, Vaziri ND, Shelkovnikov S, Farzaneh SH, Khazaeli M, Meyer CJ. The synthetic triterpenoid RTA dh404 (CDDO-dhTFEA) restores endothelial function impaired by reduced Nrf2 activity in chronic kidney disease. Redox Biol. 2013;1:527–531. doi: 10.1016/j.redox.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aminzadeh MA, Reisman SA, Vaziri ND, Khazaeli M, Yuan J, Meyer CJ. The synthetic triterpenoid RTA dh404 (CDDO-dhTFEA) restores Nrf2 activity and attenuates oxidative stress, inflammation, and fibrosis in rats with chronic kidney disease. Xenobiotica. 2014;44:570–578. doi: 10.3109/00498254.2013.852705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sussan TE, Rangasamy T, Blake DJ, Malhotra D, El-Haddad H, Bedja D, Yates MS, Kombairaju P, Yamamoto M, Liby KT, Sporn MB, Gabrielson KL, Champion HC, Tuder RM, Kensler TW, Biswal S. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci USA. 2009;106:250–255. doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishii T, Mann GE. Redox status in mammalian cells and stem cells during culture in vitro: critical roles of Nrf2 and cystine transporter activity in the maintenance of redox balance. Redox Biol. 2014;2:786–794. doi: 10.1016/j.redox.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwak MK, Itoh K, Yamamoto M, Kensler TW. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol Cell Biol. 2002;22:2883–2892. doi: 10.1128/MCB.22.9.2883-2892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Cuevas S, Asico LD, Escano C, Yang Y, Pascua AM, Wang X, Jones JE, Grandy D, Eisner G, Jose PA, Armando I. Deficient dopamine D2 receptor function causes renal inflammation independently of high blood pressure. PLoS One. 2012;7:e38745. doi: 10.1371/journal.pone.0038745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao KS, O’Dwyer PJ. Involvement of NF-kappa B in the induction of NAD(P)H:quinone oxidoreductase (DT-diaphorase) by hypoxia, oltipraz and mitomycin C. Biochem Pharmacol. 1995;49:275–282. doi: 10.1016/0006-2952(94)00544-v. [DOI] [PubMed] [Google Scholar]
- 37.Banday AA, Lau YS, Lokhandwala MF. Oxidative stress causes renal dopamine D1 receptor dysfunction and salt-sensitive hypertension in Sprague-Dawley rats. Hypertension. 2008;51:367–375. doi: 10.1161/HYPERTENSIONAHA.107.102111. [DOI] [PubMed] [Google Scholar]
- 38.Banday AA, Lokhandwala MF. Transcription factor Nrf2 protects renal dopamine D1 receptor function during oxidative stress. Hypertension. 2013;62:512–517. doi: 10.1161/HYPERTENSIONAHA.113.01358. [DOI] [PubMed] [Google Scholar]
- 39.Holmes A, Lachowicz JE, Sibley DR. Phenotypic analysis of dopamine receptor knockout mice; recent insights into the functional specificity of dopamine receptor subtypes. Neuropharmacology. 2004:471117–1134. doi: 10.1016/j.neuropharm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi M, Iaccarino C, Saiardi A, Heidt V, Bozzi Y, Picetti R, Vitale C, Westphal H, Drago J, Borrelli E. Simultaneous absence of dopamine D1 and D2 receptor-mediated signaling is lethal in mice. Proc Natl Acad Sci USA. 2004;101:11465–11470. doi: 10.1073/pnas.0402028101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ozono R, Ueda A, Oishi Y, Yano A, Kambe M, Katsuki M, Oshima T. Dopamine D2 receptor modulates sodium handling via local production of dopamine in the kidney. J Cardiovasc Pharmacol. 2003;42:S75–S79. doi: 10.1097/00005344-200312001-00017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.