SUMMARY
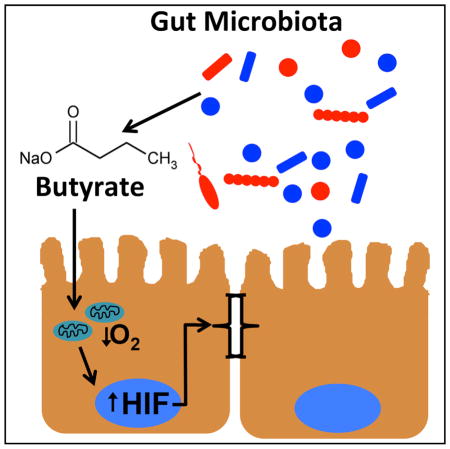
Interactions between the microbiota and distal gut are fundamental determinants of human health. Such interactions are concentrated at the colonic mucosa and provide energy for the host epithelium through the production of the short-chain fatty acid butyrate. We sought to determine the role of epithelial butyrate metabolism in establishing the austere oxygenation profile of the distal gut. Bacteria-derived butyrate affects epithelial O2 consumption and results in stabilization of hypoxia-inducible factor (HIF), a transcription factor coordinating barrier protection. Antibiotic-mediated depletion of the microbiota reduces colonic butyrate and HIF expression, both of which are restored by butyrate supplementation. Additionally, germ-free mice exhibit diminished retention of O2-sensitive dyes and decreased stabilized HIF. Furthermore, the effects of butyrate are lost in cells lacking HIF, thus linking butyrate metabolism to stabilized HIF and barrier function. This work highlights a mechanism where host-microbe interactions augment barrier function in the distal gut.
Graphical Abstract
INTRODUCTION
The mammalian GI tract harbors trillions of bacteria. A finely balanced mutualism exists within the intestinal mucosa, where microbes, essential for host health, can also initiate and perpetuate mucosal disease (Lozupone et al., 2012). Nutrient provision by microbes is one benefit enjoyed by the host. In addition to aiding in digestion, microbes produce a number of vitamins and benefit the host through the local synthesis of short-chain fatty acids (SCFAs), including butyrate, propionate, and acetate. SCFAs can reach luminal concentrations of 130 mM in the proximal colon, and butyrate in particular serves as the preferred metabolic substrate for intestinal epithelial cells (Hamer et al., 2008). A decrease of butyrate-producing microbial species has been inversely associated with colonic disease, including inflammatory bowel disease, supporting the view that butyrate is a critical determinant of disease resistance (Machiels et al., 2013; Eeckhaut et al., 2013; Sokol et al., 2009).
The low-O2 conditions that enable SCFA production also place unusual metabolic demands on the colonic epithelium. A steep O2 gradient traverses the surface of the colonic mucosa between the O2-rich lamina propria and the gut lumen, which is dominated by anaerobic organisms (Albenberg et al., 2014). Situated at this interface, the colonic epithelium functions at a pO2 well below that of other tissues (He et al., 1999). Not surprisingly, the colonic epithelium is uniquely adapted to this austere environment, and cellular programming by this “physiologic hypoxia” has been shown to tonally regulate intestinal barrier function (Colgan and Taylor, 2010). HIF is the major cellular mechanism coordinating the transcriptional response to low-O2 environments (Semenza, 2011). In the intestine, HIF target genes are basally regulated by normal physiology to maintain tissue barrier and include genes critical for microbial defense (Kelly et al., 2013), xenobiotic clearance (Wartenberg et al., 2003), barrier function (Furuta et al., 2001), mucin production (Louis et al., 2006), and cellular energetics (Glover et al., 2013). Furthermore, epithelial HIF has emerged a therapeutic target in colitis. In animal models, both genetic (Tambuwala et al., 2010; Karhausen et al., 2004) and pharmacologic (Keely et al., 2014; Robinson et al., 2008; Hindryckx et al., 2010) stabilization of HIF are disease protective, while loss of epithelial HIF-1α increases susceptibility to colitis (Karhausen et al., 2004). Despite the central role of HIF in maintenance of epithelial barrier function and the critical role of O2 metabolism during inflammation (Campbell et al., 2014), the determinants of such “physiologic hypoxia” have not been characterized.
Butyrate has long been recognized as a favored metabolic substrate for epithelial energy homeostasis (Donohoe et al., 2011). Despite O2 scarcity, high concentrations of butyrate direct metabolism away from glycolysis and toward further butyrate utilization (Blouin et al., 2011). These observations lead us to hypothesize that epithelial metabolism of SCFA are a primary determinant of “physiologic hypoxia” in the mucosa. We show that SCFA promote intestinal epithelial O2 consumption to the extent that HIF is stabilized and the expression of barrier-protective HIF target genes, linking microbe, metabolism, and mucosal innate immunity.
RESULTS
Analysis of PMDZ Dye Retention in Healthy Mouse Colon
Initially, we examined “physiologic hypoxia” using the O2-sensitive dye pimonidazole (PMDZ), which is retained in tissues at pO2 levels ≤ 10 mm Hg (Pogue et al., 2001). As shown in Figure 1 (additional images in Figure S1S), analysis of mouse colon sections (n = 12 mice) for retention of PMDZ along the crypt-villous axis (Figure 1A) revealed a prominent lumen-to-serosa gradient of dye retention. Dye retention diminished exponentially from the most luminal aspect (R2 = 0.89, p < 0.01 by ANOVA, Figure 1B), implicating a luminally derived signal that drives epithelial metabolism.
Figure 1. Microbial-Derived Short-Chain Fatty Acids Promote Localized O2 Depletion.
(A) Mice (n = 12) were administered PMDZ 30 min prior to sacrifice. Colon samples were paraffin-embedded and stained according to the manufacturer’s instructions and counterstained with DAPI.
(B) For analysis of “physiologic hypoxia” along the length of the lumen-to-serosa axis, samples (n = 3 per animal) were photographed and imported into ImageJ, where 100 μm sections were divided into equivalent 10 μm and quantified for PMDZ retention. Results are pooled and plotted as fluorescence intensity per μm2.
(C–E) Real-time O2 content in responses to 10 mM NaB (C), acetate (D), and propionate (E) (blue) were compared to vehicle control (black). Inhibition of oxidative phosphorylation by the addition of oligomycin to each SCFA treatment increased O2 content in media (red). Error bars represent SEM.
(F and G) The rate of O2 consumption 30 min after treatment with NaB (F) and acetate (G) was increased (p < 0.05).
(H) Sodium propionate did not significantly influence the rate of O2 consumption.
(I) O2 content of media was inversely related to concentration of NaB treatment with the greatest decrease at the physiologic concentration of 10 mM (p < 0.01).
(J) Rate of O2 consumption 30 min after treatment was also greatest with 10 mM NaB (p < 0.01).
(K) Validation of the method for O2 consumption using the XF Analyzer. Shown here is the influence of buffer (closed circles) and 10mM NaB (closed squares) on Caco2 cell O2 consumption over a period of 100 min (p < 0.01).
(L and M) Mitochondria-depleted Caco2 cells (L) consumed less O2 compared with control after exposure to NaB (M). Data represent mean ± SEM from three ([I]–[M]) or four ([A]–[H]) independent experiments. Significance was determined using one-way ANOVA ([F]–[K]) and Student’s t test ([L] and [M]).
SCFAs Enhance O2 Consumption in Intestinal Epithelial Cell Lines
Microbiota-derived butyrate is recognized as the preferred metabolic substrate for colonic epithelial cells (Roediger, 1980). We determined whether this preference for butyrate extends to O2 metabolism. We subjected cultured Caco2 cells to glucose (5.55 mM) in the presence and absence of sodium butyrate (NaB), acetate, or propionate and monitored rates of O2 consumption. These experiments utilized a recently described real-time O2 monitoring system integrating a fluorescent O2 sensor spatially separated from the cell monolayer (Campbell et al., 2014). NaB (blue lines in Figure 1C) produced a significant and sustained increase in O2 consumption compared with control (black lines, p < 0.05). Likewise, sodium acetate (Figure 1D, p < 0.05) and to a lesser extent sodium propionate (Figure 1E, p > 0.05) treatments also increased O2 consumption. Such changes in O2 consumption were abolished by co-treatment with oligomycin, an inhibitor of mitochondrial respiration (red lines). Likewise, the rate of O2 consumption at 30 min was increased with NaB (4.6- ± 1.7-fold, p < 0.05) and acetate treatment (2.9- ± 1.4-fold, p < 0.05) and was abolished by co-treatment with oligomycin (Figures 1F–1H). Physiologic concentrations of NaB (10 mM) provided the greatest reduction in media O2 content (20.1% ± 5.7%, p < 0.01) compared to media alone (Figure 1I). The rate of O2 consumption, calculated after 30 min, was also greatest with 10 mM NaB (p < 0.01), as shown in Figure 2J. To validate this system (Campbell et al., 2014), we employed the XF analyzer to quantify epithelial metabolic responses to NaB. As shown in Figure 1K, treatment of Caco2 cell monolayers with NaB (10 mM) resulted in a profound increase in the O2 consumption rate over a 100 min period of monitoring (p < 0.01).
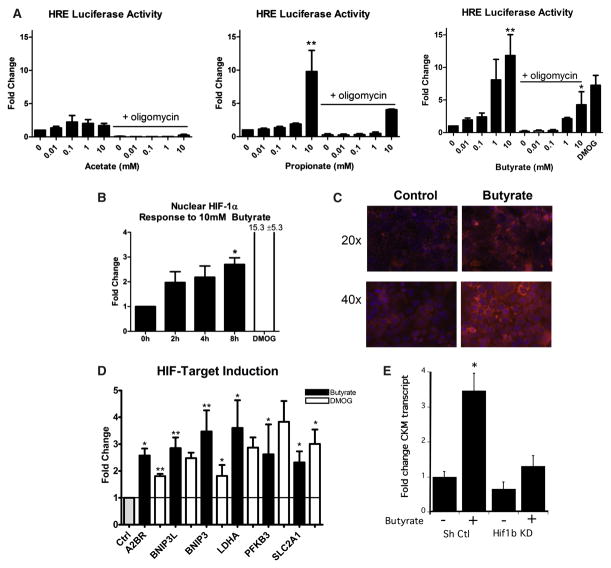
Figure 2. Buytrate Stabilizes HIF-1α and Transactivates HIF Target Genes.
(A) The influence of acetate, propionate or NaB at indicated concentrations on the HIF HRE response (p < 0.01 for propionate and NaB). The response was abolished with oligomycin co-treatment in Caco2 cells transfected with a HIF reporter plasmid. DMOG is shown as a positive control.
(B) NaB treatment for 8 hr increased nuclear HIF-1α in Caco2 cells (p < 0.05). This treatment increased retention of the O2-sensitive probe in Caco2 cells.
(C and D) Exposure of Caco2 cells to vehicle (gray bar), NaB (10 mM, 8 hr, black bars), or positive control DMOG (1 mM, 8 hr, white bars) induced indicated HIF target gene expression (D).
(E) Influence of NaB on the expression of the HIF target gene CKM in T84 cells expressing lentiviral shRNA against Hif1b (Hif1b KD) relative to non-targeting control lentivirus (Sh Ctl). Data represent average ± SEM of three independent experiments. Significance determined using one-way ANOVA. *p < 0.05; **p < 0.01.
To confirm that O2 depletion was dependent on respiration, we generated Rho-0 mitochondrial–depleted Caco2 cells (83% ± 10% loss of mDNA compared with control, p < 0.05; Figure 1L). Rho-0 Caco2 cells exhibited reduced O2 consumption (−4.7% ± 2.2% Rho-0 versus −15.4% ± 4.8% control) in response to 10 mM NaB treatment (Figure 1M), confirming mitochondria-dependent O2 consumption. Such findings implicate SCFAs in the enhancement of mitochondrial-dependent O2 consumption by cultured epithelial cell lines.
NaB Stabilizes HIF
We next sought to determine whether changes in local O2 levels by NaB were sufficient to stabilize HIF. Herein, Caco2 cells were transfected with a canonical HIF reporter plasmid and assessed for HIF stabilization. Acetate did not significantly increase luciferase activity (Figure 2A). By contrast, 10 mM sodium propionate and NaB increased luciferase activity (9.8- ± 3.2-fold and 11.8- ± 3.2- fold, respectively; p < 0.01). The HIF-stabilizing prolyl hydroxylase domain (PHD) inhibitor DMOG was used as a positive control and significantly increased HIF reporter activity (Figure 2A, p < 0.05). Consistent with our observations in O2 consumption responses (see Figure 1), the addition of oligomycin in combination with SCFAs significantly blunted this luciferase reporter response (Figure 2A).
We next sought to quantify HIF-1α protein in response to NaB. As depicted in Figure 2B, 8 hr of NaB (10 mM) significantly increased nuclear HIF-1α (2.7- ± 0.26-fold, p < 0.05). By comparison, the PHD inhibitor DMOG increased nuclear HIF-1α by 15.3- ± 5.3-fold (p < 0.05). In parallel, we visually assessed the influence of NaB on O2 tension within the cell monolayer using PMDZ. As shown in Figure 2C, PMDZ retention provided visual confirmation that NaB reduced local pO2 levels in cultured Caco2 cells.
We next determined whether NaB-induced stabilization of HIF extended to the transcriptional regulation of target genes. Here, we utilized real-time PCR to profile the influence of NaB on a number of established HIF target genes. As shown in Figure 2D, exposure of intestinal epithelia to a physiologic concentration of NaB (10 mM) for 0 and 8 hr resulted in variable induction of genes involved in adenosine metabolism/signaling (ADORA2B), cell survival (BNIP3 and BNIP3L), and glycolytic metabolism (PFKB3, LDH, and SLC2A1). For each of these HIF target genes, NaB was as potent, and in some cases more so, than the positive control DMOG.
As an additional line of evidence to interrogate NaB-induced HIF responses, we quantified the induction of the HIF target gene CKM (Glover et al., 2013) by NaB (10 mM, 8 hr) in lentiviral shRNA-mediated knockdown of Hif1b relative to non-targeting shRNA controls. As shown in Figure 2E, this analysis revealed a prominent loss of CKM induction by NaB in cells lacking HIF-1β. These findings indicate that SCFAs, particularly NaB, influences epithelial O2 metabolism to the extent that HIF is stabilized and transcriptionally active.
Broad-Spectrum Antibiotics diminish “Physiologic Hypoxia”
We next used broad-spectrum antibiotics (Abx) to deplete bacteria in vivo, with the goal of diminishing colonic SCFAs. 3 days of Abx administered by oral gavage resulted in a nearly complete loss of bacterial groups detected by 16S denaturing gradient gel electrophoresis (Figure 3A). Despite outgrowth of several resistant organisms (Figure 3A), cecal contents from Abx-treated mice lost the ability to produce SCFAs from inulin (Figure 3B), which was reflected by inability to change the color of pH-sensitive (bromocresol purple) agar plates cultured in reduced-O2 conditions (Figure 3B; yellow indicates pH < 5.2). Stool cultures of fecal pellets plated on brain-heart infusion medium revealed a complete loss of culturable anaerobes following 3 days of oral Abx treatment (Figure 3C, p < 0.025). In parallel, we measured cecal SCFA concentrations. Abx treatment reduced cecal content of acetate, propionate, and NaB by 87% ± 2%, 91% ± 4%, and 92% ± 4%, respectively (Figure 3D, all p < 0.001).
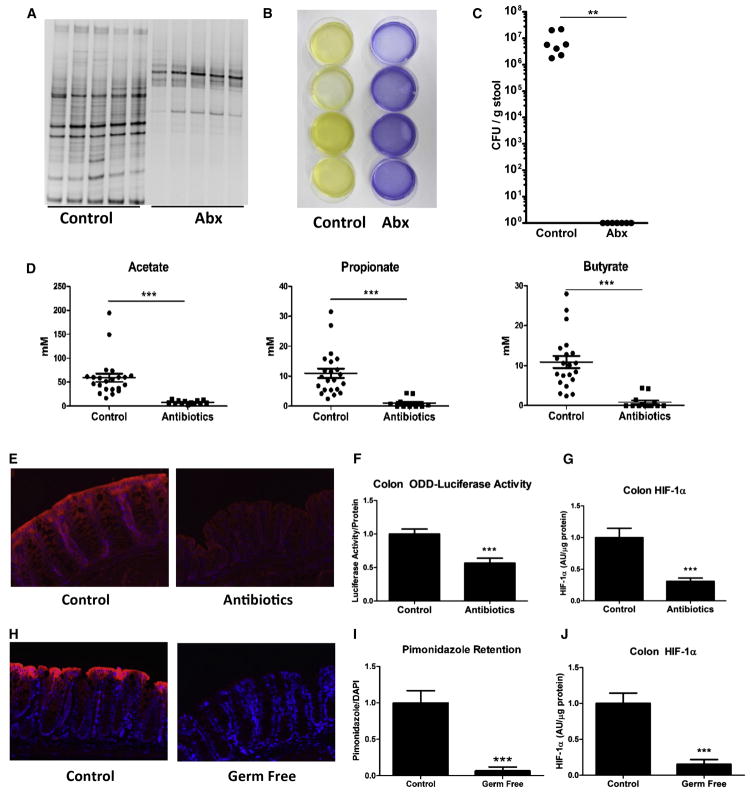
Figure 3. Antibiotics Reduce SCFA Concentration and HIF in the Colon.
(A) Denaturing gradient gel electrophoresis, where each lane represents an individual animal and each band represents a distinct bacterial population, revealed depletion of most bacterial populations in the cecum of ODD-Luc mice with Abx treatment.
(A and B) Despite appearance of resistant organisms (A), cecal contents from Abx-treated mice lost their ability to ferment inulin to SCFA. Each plate contained cecal contents from an individual animal, and yellow color indicates pH < 5.2 (B).
(C) Analysis of culturable stool microbiota from vehicle and Abx-treated mice (n = 7 per group, where ** indicates p < 0.025).
(D) Accordingly, cecal acetate, propionate, and NaB were reduced with Abx treatment (p < 0.001).
(A–C) Data represent average ± SEM from three independent experiments ([A] and [B]). Each lane (A), dish (B), and symbol (C) represent an individual animal.
(E) Significance was determined using Student’s t test. Abx treatment reduced retention of PMDZ in the colon indicating loss of “physiologic hypoxia.”
(F and G) This influence reflected reduced luciferase activity ([F], p < 0.001) and HIF-1α protein ([G], p < 0.001) in the colon of ODD-Luc animals.
(H) PMDZ localization in control and germ-free mouse colon (n = 6 per group). PMDZ was quantified (H) where *** indicates p < 0.001.
(J) HIF-1α protein in control and germ-free mouse colon (n = 6 per group). Data represent average ± SEM from three independent experiments where *** indicates p < 0.001.
To determine whether such conditions translated to a change in epithelial metabolism in vivo, we localized the O2-sensitive PMDZ dye in the colons of Abx-treated mice. This analysis confirmed previous reports of basal “physiologic hypoxia” (Colgan and Taylor, 2010) in a luminal-to-serosal gradient across the epithelium of control animals (Figure 3E and analysis from Figure 1). Abx treatment resulted in a striking reduction of probe retention (Figure 3E), thereby indicating that microbial-derived luminal signals determine basal tissue oxygenation.
To further quantify the influence of microbial signals to HIF stabilization, we utilized a HIF reporter mouse (ODD-Luc) with ubiquitous expression of firefly luciferase linked to the same O2-dependent degradation domain contained in the HIF-1α sub-unit (Safran et al., 2006). Abx treatment in these mice reduced colon luciferase by 43.4% ± 7.4% (Figure 3F). Direct measurement of HIF-1α protein revealed a 69.2% ± 5.3% reduction compared with untreated animals (Figure 3G, p < 0.001).
Since broad-spectrum Abx can have independent influences on a number of functional endpoints, we extended these findings of PMDZ retention and basal epithelial HIF expression to germ-free mice. As shown in Figure 3H (additional images included as Figure S2S), a comparison of germ-free and conventionally reared animals revealed that tissue pO2 (as reflected by PMDZ retention) revealed a nearly complete absence of the state of “physiologic hypoxia” (Figures 3H and 3I, p < 0.001) as well as a significant decrease in the expression of epithelial HIF-1α (Figure 3J, p < 0.001). These findings in germ-free mice confirm our in vitro findings in vivo and strongly implicate luminal signals, including SCFAs, as major determinants of “physiologic hypoxia” in the healthy colon.
Direct Actions of NaB on Epithelial O2 Metabolism and Barrier Function
We next sought to define whether NaB influenced epithelial metabolism and function. Initially, we determined whether the addition of NaB could restore “physiologic hypoxia” in Abx-treated mice (see Figure 3). Gross organ imaging in Abx-treated ODD-Luc animals 4 hr after oral gavage of NaB (5 g/kg) (Egorin et al., 1999) revealed intense HIF stabilization (reflected as tissue luciferase) in the cecum (Figure 4A). Given that butyrate can be absorbed in the proximal gut (Egorin et al., 1999), in subsequent studies we gavaged tributyrin (1,2,3-tributyrylglycerol [TBN]) as an analog of butyrate. TBN is a concentrated form of butyrate that is sodium free and exhibits delayed absorption with greater likelihood of increasing luminal butyrate in the distal gut. In Abx-treated animals, oral gavage of TBN increased cecal butyrate from undetectable levels to 19.2 ± 7.9 μM/g (p < 0.05; Figure 4B). Interestingly, acetate also increased after bolus administration of TBN by 1.98- ± 0.48-fold (p < 0.05; Figure 4B). Propionate was undetectable in these samples. Using Abx-treated ODD-Luc animals, we quantified luciferase activity in the cecum at 4, 8, 12, and 24 hr following TBN administration. As shown in Figure 4C, oral gavage of TBN elicited a 3.6- ± 1.1-fold and 4.5- ± 1.5-fold increase in cecal luciferase at 8 and 12 hr, respectively (both p < 0.05). Similar results were obtained by probing for HIF-1α protein directly using western blot (Figure 4D), which revealed a 2.4- ± 0.04-fold increase in HIF-1α by 8 hr (Figure 4D). Likewise, we found that oral administration of TBN subsequent to Abx treatment increased epithelial retention of an O2-sensitive probe pimodinazole in a patchy distribution (Figure 4E).
Figure 4. Butyrate Rescues “Physiologic Hypoxia” and Protects Barrier via HIF.
(A) 4 hr following oral gavage of butyrate, tissue luciferase activity increased in the distal gut of Abx-treated ODD-Luc mice.
(B–D) Oral gavage of TBN increased cecal acetate and butyrate (B), luciferase activity (C), and HIF-1α protein (D) in Abx-treated ODD-Luc mice. Daily TBN gavage for 3 days increased retention of O2-sensitive probe in the colon of Abx-treated mice (n = 6 per group).
(H) Validation of lentiviral knockdown of HIF-1β in high-resistance T84 monolayers is shown by western blot.
(I) Role of HIF-1β in mediating influences of NaB treatment is shown by the lower flux ratio in NaB/control-treated control cells compared with HIF-1 β knockdown cells (p < 0.05). Data represent average ± SEM from three independent experiments.
(C, F, and G) Significance was determined using Kruskal-Wallis (C) and Student’s t test ([F] and [G]).
Finally, we sought to determine the contribution of HIF to maintenance of barrier integrity in the context of butyrate. We confirmed the barrier protective influences of NaB (10 mM for 24 hr.) on the rate of flux of 3-kD FITC-dextran through Caco2 monolayers, which was reduced by 30% ± 7% compared with control (p < 0.05; Figure 4G). To specifically test the role of HIF in NaB treatment, we generated lentiviral HIF-1β knockdown in T84 cells. This achieved 69% knockdown compared to short hairpin (Sh) control cells as evaluated by western blot (Figure 4F). We then compared the flux ratio of NaB/control treatment using 3-kD FITC-dextran in these high-resistance HIF-1β knockdown and Sh control monolayers. This revealed 6.3- ± 1.8-fold greater NaB/control flux ratio in HIF-1β knockdown compared with Sh control (p < 0.05), indicating a fundamental role for HIF in maintaining barrier integrity (Figure 4G). These studies reveal that microbial-derived SCFAs play a central role in O2 metabolism in the colon in vivo and implicate a protective influence of butyrate on HIF-regulated barrier function.
DISCUSSION
There is currently significant interest in understanding how the host communicates with the microbial world (Lozupone et al., 2012). Epithelial cells are uniquely positioned to function as the primary interface for host-microbial communication. We report here that epithelial metabolism of SCFAs may serve as an important yet underappreciated conduit for communication between the host and luminal microbes.
Given the range of available substrates, it is notable that a predominant energy source is butyrate, an end product of bacterial metabolism. Butyrate typically constitutes 15%–20% of SCFA in the human colon with absolute concentrations above 10 mM in human feces (Hamer et al., 2008). Butyrate is efficiently absorbed and metabolized by the epithelium, and in contrast to other SCFAs, little butyrate is released into portal circulation (Hamer et al., 2008). One factor contributing to the preference of the colonic epithelium for butyrate is that butyrate stimulates expression of pyruvate dehydrogenase kinases, which inhibit the pyruvate dehydrogenase complex (Blouin et al., 2011). In this report, we demonstrate that metabolism of butyrate, and to a lesser extent acetate and propionate, by epithelial cell lines depletes local O2 to the extent that HIF is stabilized. Given the multiple levels of protection provided by basal HIF expression within the mucosa (Colgan and Taylor, 2010), these findings reveal a host-microbe crosstalk pathway wherein SCFAs may signal protective distal gut functions.
Accumulated literature supports butyrate as anti-carcinogenic, anti-inflammatory, and barrier protective in the distal gut (Plöger et al., 2012; Hamer et al., 2008). The protection afforded by fiber and resistant starch in experimental colitis are thought to be dependent on SCFA production (Ito et al., 2009; Morita et al., 2004; Videla et al., 2001), and administration of exogenous butyrate promotes resistance to experimental colitis (Cresci et al., 2013; Leonel et al., 2013). Studies investigating dysbiosis in inflammatory bowel disease identified reduced abundance of butyrate-producing organisms (e.g., certain Roseburia and Faecalibacterium genera) and lower concentrations of butyrate associated with disease (Machiels et al., 2013; Eeckhaut et al., 2013; Sokol et al., 2009). The importance of butyrate as the preferred epithelial substrate has been highlighted by demonstration that pharmacologic inhibition of β-oxidation induces colitis (Roediger and Nance, 1986) and that mice with mitochondrial polymorphisms that maintain increased oxidative phosphorylation activity are resistant to colitis (Bar et al., 2013). Several trials have evaluated the efficacy of butyrate in the treatment of human disease, primarily ulcerative colitis, with mixed results (Hamer et al., 2008). This variability might be explained by impaired uptake and metabolism of butyrate associated with inflammation, implying that maintenance of remission may be a more rational therapeutic goal (De Preter et al., 2012). The physiologic role of SCFAs in mucosal protection is perhaps best illustrated in colitis caused by surgical diversion of the fecal stream, where restoration of luminal SCFAs is a first-line therapy (Harig et al., 1989).
The pleiotropic influences of butyrate in the mucosa make it difficult to isolate influences of the HIF pathway from previously established mechanisms of action, including HDAC inhibition, stimulation of GPR43 and GPR109a, and metabolic fuel. It is likely that the mechanisms involved are synergistic with HIF stabilization. For example, we show that butyrate, a classic HDAC inhibitor, increases HIF in epithelial cell lines. This could enable HIF to alter transcription of genes that would otherwise be inaccessible by chromatin structure. A caveat to our studies here is recent work that demonstrated that the primary energy source for cancerous colonocytes is glucose (i.e., the Warburg effect) and that, in high concentrations of butyrate, functions primarily as an HDAC inhibitor (Donohoe et al., 2012). It remains unclear to what extent butyrate might influence glucose utilization by colonic cell lines. A recent report indicated that activated AMPK is an endogenous inhibitor of the Warburg effect (Faubert et al., 2013), and that butyrate is a potent activator of AMPK in colonic cell lines (Elamin et al., 2013). Donohoe et al. (2011) showed that compared with conventionalized mice, the colonocytes of germ-free mice are ATP deficient and the provision of butyrate can reverse this energy deficit by restoring oxidative respiration. Likewise, we report here that germ-free animals lack the classical “physiologic hypoxia.” This observation is unlikely to be an artifact of PMDZ biochemistry in germ-free animals, since a strong correlation exists between hypoxia and PMDZ binding (Arteel et al., 1995). One caveat is the presence of nitroreductases in some inflammatory cells that can reduce PMDZs in an O2-insensitive manner (Kizaka-Kondoh and Konse-Nagasawa, 2009). Our analysis also indicated that germ-free animals express less epithelial HIF-1α. Given the intrinsic link between oxidative phosphorylation and ATP generation with O2 consumption, it is difficult to separate ATP generation from O2 consumption and, in this regard, HIF pathway activation.
For a number of reasons, it is likely that HIF stabilization by butyrate augments epithelial barrier in the healthy colon and in underlying disease. First, epithelial HIF stabilization is linked at many levels to enhanced epithelial barrier in vitro and in vivo (Glover and Colgan, 2011). Recent studies have, for example, identified important roles for localized O2 metabolism (Campbell et al., 2014) and alternative energy sources (e.g., creatine; Glover et al., 2013) as important components to intestinal homeostasis. Second, the enhanced barrier function we report in butyrate-treated HIF-1β knockdown cells demonstrates a role for HIF, independent of ATP generation. In this context, we demonstrate that CKM is a differentiating target in response to butyrate in the Hif1b KD cell line. CK’s were recently shown to function as a fundamental link between cellular energy and junctional integrity in colonic epithelial cells (Glover et al., 2013). This observation is supported by a growing literature that has established the role of HIF-1α in mucosal protection in vivo (Hindryckx et al., 2010; Karhausen et al., 2004; Keely et al., 2014, Robinson et al., 2008; Tambuwala et al., 2010).
This work highlights the importance of HIF stabilization through SCFA metabolism as an independent mechanism to promote homeostasis in the distal intestine. This host-microbial crosstalk axis provides important insight into how microbial-derived products ultimately influence tissue function.
EXPERIMENTAL PROCEDURES
Additional details can be found in Supplemental Information
Analysis of PMDZ Dye Retention in Colon
PMDZ (Hypoxyprobe, Inc.) was administered by intraperitoneal injection 30 min prior to sacrifice and samples were analyzed as before (Campbell et al., 2014). For analysis of “physiologic hypoxia” along the length of the lumen-to-serosa axis, samples were photographed and imported into ImageJ, where 100 μm sections were divided into equivalent 10 μm and quantified for PMDZ retention.
Cell Culture, shRNA Knockdown, and Mitochondrial Depletion
Caco2 and T84 cells were cultured as described previously (Glover et al., 2013). The generation of Hif1b KD cell lines using lentiviral particles encoding shRNA were performed as described before (Glover et al., 2013). Mitochondrial depletion was accomplished by passaging Caco2 cells in 50 ng/ml ethidium bromide for a minimum of 2 weeks (King and Attardi, 1996).
Real-Time O2 Consumption
The SensorDish Reader from Applikon Biotechnology was used as described previously (Campbell et al., 2014).
Protein Analysis and Immunofluorescence
HIF-1α protein was quantified after nuclear and cytoplasmic extraction as described previously (Glover et al., 2013).
Gene Expression
Real-time PCR analysis was performed as described elsewhere (Glover et al., 2013) using primer sets described in Supplemental Information.
FITC-Dextran Flux
Flux of 3kD FITC-dextran was utilized to quantify barrier function as described previously (Sanders et al., 1995).
Animal Studies
Studies in HIF-luciferase reporter mice (Safran et al., 2006) were performed as described elsewhere (Campbell et al., 2014) and detailed in Supplemental Information. SCFA quantification was performed with gas chromatography-mass spectrometry (GC-MS) following extraction of SCFAs from cecal contents in acidified water as described elsewhere (Weir et al., 2013). Additional details in Supplemental Information.
Germ-Free Mice
Mice were bred and maintained in flexible vinyl positive pressure isolators within the Gnotobiotic Facility at the University of Colorado as described in Supplemental Information.
Supplementary Material
Highlights.
The mammalian colon exists in a state of relative hypoxia
Hypoxic regions of the normal colon provide a signaling axis through HIF-1
Microbial-derived butyrate depletes O2 and activates HIF-1
Microbiota-derived butyrate is barrier-protective in the mucosa
Acknowledgments
The authors thank Chris Mulligan for assistance with GC-MS as well as Cathy Lozupone and Jody Donnelly for assistance with anaerobic culture. This work was funded by NIH grants F30DK096709, TL1RR025778, DK50189, DK104713 and DK95491 as well as grants from the Crohn’s and Colitis Foundation of America.
Footnotes
Supplemental Information includes two figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.chom.2015.03.005.
AUTHOR CONTRIBUTIONS
Author contributions are detailed in the Supplement to this paper.
References
- Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, Laughlin A, Grunberg S, Baldassano RN, Lewis JD, Li H, et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147:1055–1063.e8. doi: 10.1053/j.gastro.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteel GE, Thurman RG, Yates JM, Raleigh JA. Evidence that hypoxia markers detect oxygen gradients in liver: pimonidazole and retrograde perfusion of rat liver. Br J Cancer. 1995;72:889–895. doi: 10.1038/bjc.1995.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar F, Bochmann W, Widok A, Von MEDEM K, Pagel R, Hirose M, Yu X, Kalies K, Konig P, Bohm R, Herdegen T, Reinicke AT, Buning J, Lehnert H, Fellermann K, Ibrahim S, Sina C. Mitochondrial gene polymorphisms that protect mice from colitis. Gastroenterology. 2013;145:1055–1063.e3. doi: 10.1053/j.gastro.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Blouin JM, Penot G, Collinet M, Nacfer M, Forest C, Laurent-Puig P, Coumoul X, Barouki R, Benelli C, Bortoli S. Butyrate elicits a metabolic switch in human colon cancer cells by targeting the pyruvate dehydrogenase complex. Int J Cancer. 2011;128:2591–2601. doi: 10.1002/ijc.25599. [DOI] [PubMed] [Google Scholar]
- Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, Bowers BE, Bayless AJ, Scully M, Saeedi BJ, Golden-Mason L, et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40:66–77. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresci G, Nagy LE, Ganapathy V. Lactobacillus GG and tributyrin supplementation reduce antibiotic-induced intestinal injury. JPEN J Parenter Enteral Nutr. 2013;37:763–774. doi: 10.1177/0148607113486809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Preter V, Arijs I, Windey K, Vanhove W, Vermeire S, Schuit F, Rutgeerts P, Verbeke K. Impaired butyrate oxidation in ulcerative colitis is due to decreased butyrate uptake and a defect in the oxidation pathway. Inflamm Bowel Dis. 2012;18:1127–1136. doi: 10.1002/ibd.21894. [DOI] [PubMed] [Google Scholar]
- Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe DR, Collins LB, Wali A, Bigler R, Sun W, Bultman SJ. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol Cell. 2012;48:612–626. doi: 10.1016/j.molcel.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhaut V, Machiels K, Perrier C, Romero C, Maes S, Flahou B, Steppe M, Haesebrouck F, Sas B, Ducatelle R, et al. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut. 2013;62:1745–1752. doi: 10.1136/gutjnl-2012-303611. [DOI] [PubMed] [Google Scholar]
- Egorin MJ, Yuan ZM, Sentz DL, Plaisance K, Eiseman JL. Plasma pharmacokinetics of butyrate after intravenous administration of sodium butyrate or oral administration of tributyrin or sodium butyrate to mice and rats. Cancer Chemother Pharmacol. 1999;43:445–453. doi: 10.1007/s002800050922. [DOI] [PubMed] [Google Scholar]
- Elamin EE, Masclee AA, Dekker J, Pieters HJ, Jonkers DM. Short-chain fatty acids activate AMP-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in Caco-2 cell monolayers. J Nutr. 2013;143:1872–1881. doi: 10.3945/jn.113.179549. [DOI] [PubMed] [Google Scholar]
- Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, Dupuy F, Chambers C, Fuerth BJ, Viollet B, et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17:113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover LE, Colgan SP. Hypoxia and metabolic factors that influence inflammatory bowel disease pathogenesis. Gastroenterology. 2011;140:1748–1755. doi: 10.1053/j.gastro.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover LE, Bowers BE, Saeedi B, Ehrentraut SF, Campbell EL, Bayless AJ, Dobrinskikh E, Kendrick AA, Kelly CJ, Burgess A, et al. Control of creatine metabolism by HIF is an endogenous mechanism of barrier regulation in colitis. Proc Natl Acad Sci USA. 2013;110:19820–19825. doi: 10.1073/pnas.1302840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- Harig JM, Soergel KH, Komorowski RA, Wood CM. Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med. 1989;320:23–28. doi: 10.1056/NEJM198901053200105. [DOI] [PubMed] [Google Scholar]
- He G, Shankar RA, Chzhan M, Samouilov A, Kuppusamy P, Zweier JL. Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc Natl Acad Sci USA. 1999;96:4586–4591. doi: 10.1073/pnas.96.8.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindryckx P, De Vos M, Jacques P, Ferdinande L, Peeters H, Olievier K, Bogaert S, Brinkman B, Vandenabeele P, Elewaut D, Laukens D. Hydroxylase inhibition abrogates TNF-alpha-induced intestinal epithelial damage by hypoxia-inducible factor-1-dependent repression of FADD. J Immunol. 2010;185:6306–6316. doi: 10.4049/jimmunol.1002541. [DOI] [PubMed] [Google Scholar]
- Ito H, Tanabe H, Kawagishi H, Tadashi W, Yasuhiko T, Sugiyama K, Kiriyama S, Morita T. Short-chain inulin-like fructans reduce endotoxin and bacterial translocations and attenuate development of TNBS-induced colitis in rats. Dig Dis Sci. 2009;54:2100–2108. doi: 10.1007/s10620-008-0599-x. [DOI] [PubMed] [Google Scholar]
- Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely S, Campbell EL, Baird AW, Hansbro PM, Shalwitz RA, Kotsakis A, McNamee EN, Eltzschig HK, Kominsky DJ, Colgan SP. Contribution of epithelial innate immunity to systemic protection afforded by prolyl hydroxylase inhibition in murine colitis. Mucosal Immunol. 2014;7:114–123. doi: 10.1038/mi.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CJ, Glover LE, Campbell EL, Kominsky DJ, Ehrentraut SF, Bowers BE, Bayless AJ, Saeedi BJ, Colgan SP. Fundamental role for HIF-1α in constitutive expression of human β defensin-1. Mucosal Immunol. 2013;6:1110–1118. doi: 10.1038/mi.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MP, Attardi G. Isolation of human cell lines lacking mitochondrial DNA. Methods Enzymol. 1996;264:304–313. doi: 10.1016/s0076-6879(96)64029-4. [DOI] [PubMed] [Google Scholar]
- Kizaka-Kondoh S, Konse-Nagasawa H. Significance of nitroimidazole compounds and hypoxia-inducible factor-1 for imaging tumor hypoxia. Cancer Sci. 2009;100:1366–1373. doi: 10.1111/j.1349-7006.2009.01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonel AJ, Teixeira LG, Oliveira RP, Santiago AF, Batista NV, Ferreira TR, Santos RC, Cardoso VN, Cara DC, Faria AM, Alvarez-Leite J. Antioxidative and immunomodulatory effects of tributyrin supplementation on experimental colitis. Br J Nutr. 2013;109:1396–1407. doi: 10.1017/S000711451200342X. [DOI] [PubMed] [Google Scholar]
- Louis NA, Hamilton KE, Canny G, Shekels LL, Ho SB, Colgan SP. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J Cell Biochem. 2006;99:1616–1627. doi: 10.1002/jcb.20947. [DOI] [PubMed] [Google Scholar]
- Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2013;63:1275–1283. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- Morita T, Tanabe H, Sugiyama K, Kasaoka S, Kiriyama S. Dietary resistant starch alters the characteristics of colonic mucosa and exerts a protective effect on trinitrobenzene sulfonic acid-induced colitis in rats. Biosci Biotechnol Biochem. 2004;68:2155–2164. doi: 10.1271/bbb.68.2155. [DOI] [PubMed] [Google Scholar]
- Plöger S, Stumpff F, Penner GB, Schulzke JD, Gäbel G, Martens H, Shen Z, Günzel D, Aschenbach JR. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci. 2012;1258:52–59. doi: 10.1111/j.1749-6632.2012.06553.x. [DOI] [PubMed] [Google Scholar]
- Pogue BW, Paulsen KD, O’Hara JA, Wilmot CM, Swartz HM. Estimation of oxygen distribution in RIF-1 tumors by diffusion model-based interpretation of pimonidazole hypoxia and eppendorf measurements. Radiat Res. 2001;155:15–25. doi: 10.1667/0033-7587(2001)155[0015:eoodir]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–155. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21:793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger WE, Nance S. Metabolic induction of experimental ulcerative colitis by inhibition of fatty acid oxidation. Br J Exp Pathol. 1986;67:773–782. [PMC free article] [PubMed] [Google Scholar]
- Safran M, Kim WY, O’Connell F, Flippin L, Günzler V, Horner JW, Depinho RA, Kaelin WG., Jr Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production. Proc Natl Acad Sci USA. 2006;103:105–110. doi: 10.1073/pnas.0509459103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SE, Madara JL, Mcguirk DK, Gelman DS, Colgan SP. Assessment of infammatory events on epithelial permeability: A rapid screening method using fluorescein dextrans. Epithelial Cell Biol. 1995;4:25–34. [PubMed] [Google Scholar]
- Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, Cosnes J, Corthier G, Marteau P, Doré J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, Scholz CC, Fraisl P, Lasitschka F, Mollenhauer M, Saunders SP, et al. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology. 2010;139:2093–2101. doi: 10.1053/j.gastro.2010.06.068. [DOI] [PubMed] [Google Scholar]
- Videla S, Vilaseca J, Antolín M, García-Lafuente A, Guarner F, Crespo E, Casalots J, Salas A, Malagelada JR. Dietary inulin improves distal colitis induced by dextran sodium sulfate in the rat. Am J Gastroenterol. 2001;96:1486–1493. doi: 10.1111/j.1572-0241.2001.03802.x. [DOI] [PubMed] [Google Scholar]
- Wartenberg M, Ling FC, Müschen M, Klein F, Acker H, Gassmann M, Petrat K, Pütz V, Hescheler J, Sauer H. Regulation of the multi-drug resistance transporter P-glycoprotein in multicellular tumor spheroids by hypoxia-inducible factor (HIF-1) and reactive oxygen species. FASEB J. 2003;17:503–505. doi: 10.1096/fj.02-0358fje. [DOI] [PubMed] [Google Scholar]
- Weir TL, Manter DK, Sheflin AM, Barnett BA, Heuberger AL, Ryan EP. Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PLoS One. 2013;8:e70803. doi: 10.1371/journal.pone.0070803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.