Abstract
Objective
Dystonia is a disabling motor disorder often without effective therapies. To better understand the genesis of dystonia after childhood stroke, we analyzed electroencephalographic (EEG) recordings in this population.
Methods
Resting spectral power of EEG signals over bilateral sensorimotor cortices (Powrest), resting inter-hemispheric sensorimotor coherence (Cohrest), and task-related changes in power (TRPow) and coherence (TRCoh) during wrist extension were analyzed in individuals with dystonia (age 20±3 years) and healthy volunteers (age 17±5 years).
Results
Ipsilesional TRPow decrease was significantly lower in patients than controls during the more affected wrist task. Force deficits of the affected wrist correlated with reduced alpha TRPow decrease on the ipsilesional and not the contralesional hemisphere. Cohrest was significantly lower in patients than controls, and correlated with more severe dystonia and poorer hand function. Powrest and TRCoh were similar between groups.
Conclusions
The association between weakness and cortical activation during wrist extension highlights the importance of ipsilesional sensorimotor activation on function. Reduction of Cohrest in patients reflects a loss of inter-hemispheric connectivity that may result from structural changes and neuroplasticity, potentially contributing to the development of dystonia.
Significance
Cortical and motor dysfunction are correlated in patients with childhood stroke and may in part explain the genesis of dystonia.
Keywords: Childhood stroke, dystonia, electroencephalography, cortical activation, coherence, cerebral palsy
Introduction
Motor disorders following childhood stroke are common (Lynch and Nelson, 2001), yet are insufficiently studied (Bejot et al., 2012). Of the observed post-stroke motor sequelae, childhood dystonia is the most frequently occurring disorder (Bejot et al., 2012). Dystonia, as it occurs in children, is defined as a movement disorder in which involuntary sustained or intermittent muscle contractions cause twisting and repetitive movements, abnormal postures, or both (Sanger et al., 2010). Post-stroke dystonia is most commonly observed on the side of the body contralateral to the stroke, and is referred to as hemidystonia. Unilateral impairments in children with hemidystonia usually affect the arm (Chuang et al., 2002), prevent the development of typical arm function, and can lead to substantial disability persisting into adulthood. However, there are limited effective treatments for children with dystonia.
Some of the proposed contributing mechanisms of dystonia include deficits of inhibition (at the muscular, spinal, and cortical levels), abnormal sensorimotor integration, and maladaptive plasticity (Lin and Hallett, 2009). Dystonia is often attributed to abnormalities of the basal ganglia, however damage to the basal ganglia is not always present in children with dystonia (Sanger, 2003). Due to the common observation that symptoms of dystonia have a delayed onset relative to the causative brain injury, it has been suggested that dystonia arises as a result of functional changes in the basal ganglia secondary to overt structural changes due to injury in other brain regions (Breakefield et al., 2008). Others hypothesize that there is a network of brain regions that, in addition to the basal ganglia, include cerebral cortex, cerebellum, thalamus, midbrain, and brainstem, within which abnormalities can be associated with the presence of dystonia (Neychev et al., 2011). Irrespective of which brain areas are involved, abnormal neural activity finally converges on the alpha motor neurons of the spinal cord leading to involuntary muscle contractions and dystonic postures. Since the sensorimotor cortex contributes to a large portion of the corticospinal tract neurons (Seo and Jang, 2013), this study focuses on abnormalities of sensorimotor cortical activation in individuals with dystonia due to childhood stroke.
Analysis of the functional roles of various brain regions can be accomplished using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). However, the necessity for an injection of a radioactive tracer in PET and the requirement to remain still in an fMRI scanner render these methods challenging to use in some individuals, particularly for children with dystonia. Studies which have been done in adults with idiopathic dystonia using PET (Ceballos-Baumann et al., 1995; Odergren et al., 1998; Ibanez et al., 1999; Lerner et al., 2004) and fMRI (Pujol et al., 2000; Preibisch et al., 2001) indicate involvement of the sensorimotor cortices in addition to other cortical regions, cerebellum, and thalamus during tasks that trigger dystonia. However, the direction of sensorimotor cortical activation (over- or under-activation) was inconsistent and remains to be studied in childhood-onset dystonia.
In order to explore the nature of sensorimotor cortical activation in dystonia due to childhood stroke, we used electroencephalography (EEG). EEG signals can be recorded through electrodes placed non-invasively on the subject’s scalp while maintaining their freedom of movement, unlike PET and fMRI scans. When the electrodes are placed over the sensorimotor cortices, the observed EEG signal is generated primarily by the excitatory and inhibitory post-synaptic potentials of pyramidal neurons in the motor and somatosensory cortices. Recorded signals typically have a rhythmic nature and the prominent rhythms vary in different cortical regions and during different tasks. Spectral power of sensorimotor cortical EEG signals indicates the prominence of oscillations at particular frequencies, and decreases in spectral power due to voluntary movement reflect task-related cortical activation (Chatrian et al., 1959). The functional coupling between two cortical regions can also be assessed using EEG through coherence measurement, and changes in coherence can be measured during voluntary movement to assess task-related alterations in inter-regional coupling (Andrew and Pfurtscheller, 1996; Manganotti et al., 1998).
The purpose of this study was to use EEG to explore sensorimotor cortical EEG power and coherence between hemispheres at rest and during a task that activates the motor system in individuals with dystonia due to childhood stroke. Due to the high prevalence of wrist impairments in dystonia, and the known variability in force production by children with dystonia due to early brain injuries (Chu and Sanger, 2009), an isometric constant-force wrist extension task was used. The chosen task also reflects a movement component vital to functional hand use that is a common target for rehabilitation. Results from this exploratory study should provide a deeper understanding of the neurophysiological basis of dystonia in the specific context of childhood stroke, which is critical to developing and selecting effective treatments for this population.
Method
Design and participants
This observational case-control study presents neurophysiological outcomes in a group of children and young adults with wrist dystonia secondary to childhood stroke (DYS group), and a group of healthy volunteers (HV group). Participants were included if they met the following criteria: (1) 7 to 40 years of age, (2) good general health, (3) ability to understand and comply with instructions, (4) ability to actively extend both wrists, and (5) agreement to refrain from caffeine or alcohol for at least 24 hours before testing. In patients, diagnosis of dystonia was made using the Hypertonia Assessment Tool (by K.E.A.). The onset of hemidystonia was required to be before the age of 13 years. All patients had the diagnosis of unilateral cerebral palsy (CP, by K.E.A.), which is reported as a common outcome of perinatal stroke with similar risk factors and associated motor control challenges (Kirton and deVeber, 2006; Golomb et al., 2008).
HV were excluded if they exhibited any neurological disorders on an examination (by K.E.A.). Patients were excluded if they had received botulinum toxin injections in the flexor carpi radialis or extensor carpi radialis muscles in the previous 6 months, or were concurrently using any medicines for muscle tone (e.g., baclofen, trihexyphenedyl, dantrolene sodium, tizanidine, or carbidopa/levodopa).
Eleven patients were recruited from local outpatient movement disorders clinics, and 10 HV were recruited from the community. All 11 patients (18 ± 5 years, 3 female) and 9 HV (17 ± 5 years, 8 female) were eligible to participate and enrolled in the study. One HV was excluded due to the discovery of an abnormal magnetic resonance imaging (MRI) scan after enrollment. Patient characteristics are shown in Table 1. One HV did not undergo testing on the non-dominant side due to an inability to remain relaxed throughout the 2-hour session. Four patients were excluded from the analysis of the more affected wrist, and five from the analysis of the less affected wrist for one of the following three reasons: (1) they did not complete the task correctly, (2) they exhibited more muscle activity during intended rest compared to intended activation, or (3) they were not able to rest completely during the rest trial. For the remaining participants, artifact-free EEG signals were analyzed. Table 1 indicates which patients were included in the analyses for each wrist. Table 2 indicates the presence or absence of damage to various brain regions in the subset of patients whose data were analyzed. Brain injury type and location were identified by a neuroradiologist (N.P.) on structural MRI scans.
Table 1.
Characteristics of individuals with unilateral dystonia due to childhood stroke
| Subject | Age (yrs) | Age at injury | Etiology | Dom Side | MACS | BFM arm score | Task completiona | ||
|---|---|---|---|---|---|---|---|---|---|
| LA | MA | D/LA | ND/MA | ||||||
| 1 | 8.1 | prenatal | MCA stroke (L) | L | II | 2 | 9 | unable to complete | unable to complete |
| 2 | 11.2 | birth | MCA stroke (L) | L | I | 1 | 12 | unable to complete | unable to complete |
| 3 | 13.6 | prenatal | MCA stroke (L) | L | II | 2 | 16 | unable to rest | Rest > Task EMG |
| 4 | 16.8 | prenatal | ACA/MCA stroke (L) | L | I | 1 | 6 | analyzed | analyzed |
| 5 | 17.1 | birth | MCA stroke (L) | L | III | 1 | 16 | analyzed | analyzed |
| 6 | 19.0 | birth | Internal carotid artery stroke (L>R) | L | II | 0 | 16 | analyzed | analyzed |
| 7 | 19.1 | prenatal | MCA stroke (L) | L | II | 2 | 12 | analyzed | Rest > Task EMG |
| 8 | 19.3 | prenatal | MCA stroke (L) | L | II | 1 | 16 | analyzed | analyzed |
| 9 | 20.2 | 4 years | MCA stroke (R) | R | II | 1 | 16 | analyzed | analyzed |
| 10 | 23.5 | 10 months | bilateral frontal contusion, bilateral ACA/MCA stroke | L | II | 1 | 16 | unable to rest | unable to rest |
| 11 | 24.9 | birth | Basilar artery stroke (R>L) | R | I | 1 | 9 | analyzed | analyzed |
| 9 HV | 9.0 – 24.0 | N/A | N/A | 1L | N/A | N/A | N/A | 9 analyzed | 8 analyzed |
Task completion refers to the general outcome of the experiment. Only a subset of patients (indicated by bold font) who could complete the task was included in the analysis. Other patients were unable to rest or complete the task.
MACS = Manual Ability Classification System, BFM = Burke-Fahn-Marsden dystonia rating scale, LA = Less affected, MA = More affected, D = Dominant, ND = Non-dominant, MCA = Middle cerebral artery, ACA = Anterior cerebral artery, R = Right, L = Left, EMG = Electromyogram, HV = Healthy volunteers
Table 2.
Brain injury MRI findings of individuals with unilateral dystonia due to childhood stroke. Abnormalities in each brain region are indicated by 1; 0 is normal. For the cerebellum, brainstem, basal ganglia, thalamus, and internal capsule, entries indicate results for both hemispheres (CL/IL).
| Subject | Ventricles | Cerebellum | Corpus Callosum | Brainstem | BG: Lenticular | BG: Caudate | Thalamus | PLIC | ALIC |
|---|---|---|---|---|---|---|---|---|---|
| 4 | 1 | 0/0 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| 5 | 1 | 0/0 | 1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 |
| 6a,b | 0 | 1/1 | 1 | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 | 0/0 |
| 7 | 1 | 0/0 | 1 | 0/1 | 0/1 | 0/0 | 0/1 | 0/1 | 0/0 |
| 8 | 1 | 0/0 | 1 | 0/1 | 0/1 | 0/0 | 0/1 | 0/1 | 0/0 |
| 9a | 1 | 0/0 | 1 | 0/1 | 0/1 | 1/0 | 0/1 | 0/1 | 0/1 |
| 11a | 0 | 0/0 | 0 | 1/1 | 0/1 | 0/0 | 0/0 | 0/0 | 0/0 |
Bilateral brain imaging findings
Evidence of peri-ventricular leukomalacia
CL = Contralesional, IL = Ipsilesional, BG = Basal ganglia, PLIC = Posterior limb of the internal capsule, ALIC = Anterior limb of the internal capsule
All adult participants gave written consent for participation. Participants under 18 years of age gave written assent and were accompanied by a parent who provided written consent for the child’s participation. All procedures adhered to the ethical principles for research with human subjects (Declaration of Helsinki) and were approved by the NIH Institutional Review Board (ClinicalTrials.gov Identifier: NCT01432899). Data collection spanned a period of 15 months. This study was a part of a larger protocol which includes brain imaging, sensory testing, arm kinematic assessments, and posturography.
Apparatus and recordings
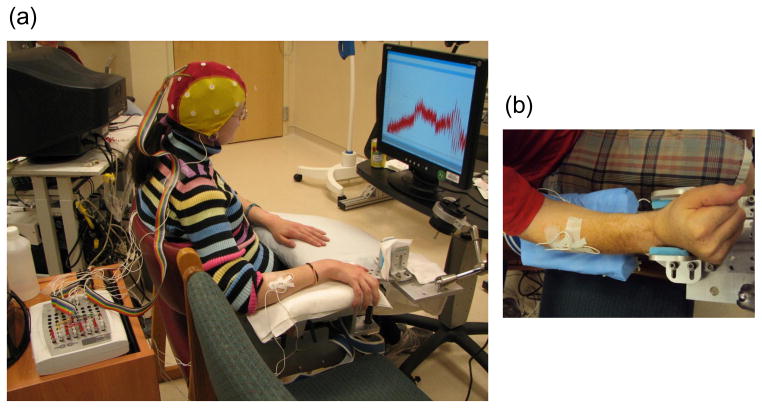
Participants were seated comfortably with one forearm on a stabilizing armrest designed to allow isometric wrist flexion and extension against a force sensor (Honeywell International Inc, Columbus, OH). The posture of the arm was approximately 30° shoulder flexion, 120° elbow extension, and a neutral wrist (Figure 1). The armrest included forearm straps and hand straps for stable positioning.
Figure 1.
Experimental apparatus. In (a), a participant is shown seated with an EEG electrode cap on the head, EMG sensors on the forearms, and looking forward at a feedback display of the isometric wrist extension force. The wrist force sensor is housed under the wrist positioning device. In (b), a different participant’s right wrist is shown positioned appropriately in the device. To test the left wrist, the subject was seated in the adjacent chair so the left arm could be positioned in the device, and the display was moved to be in front of the participant.
Cortical brain activity was recorded using EEG (Compumedics Neuroscan, Charlotte, NC). Nineteen Ag/AgCl electrodes were placed on the scalp according to the International 10–20 system, and one electrode was placed on each earlobe. EEG signals (bandpass DC-100Hz, sampling rate 1 kHz) were referenced to the right earlobe electrode during data collection and later re-referenced to linked-earlobes before analysis.
Surface electromyography (EMG) was used to record muscle activity bilaterally from a wrist extensor muscle (extensor carpi radialis, ECR) and a wrist flexor muscle (flexor carpi radialis, FCR). EMG signals (bandpass 5–200 Hz, sampling rate 1 kHz, Compumedics Neuroscan, Charlotte, NC) were recorded using two Ag/AgCl electrodes (2cm spacing) on the belly of each muscle. EEG and EMG impedances were kept below 5 kΩ.
Task description
At the beginning of the testing session, participants performed maximal isometric wrist extension with real-time feedback of their force displayed as a line on a computer monitor placed at eye-level 1 m in front of the head. The investigator encouraged participants to try to increase the height of the line by giving more effort. Force recordings from three 5-second trials were collected. The mean force achieved during the middle 3 seconds of each trial was computed and the maximum mean force (Fmax) was used to scale subsequent wrist extension tasks for that wrist.
EEG and EMG signals were recorded under two conditions: rest and task. In the rest condition, participants sat with their testing arm in the arm apparatus for 60 seconds while attempting to rest. They fixated their vision on a cursor in the center of the computer monitor. In the task condition, participants extended their wrist to 15% Fmax with visual feedback of their force level. The feedback display was scaled such that the target force was in the center of the monitor. Each of 20 seven-second trials was cued by an auditory signal and separated by a 4-second rest break. Numerous short trials were favored over fewer longer trials because motor control deficits in the DYS group were anticipated to impair the ability to sustain muscle contractions for long periods of time.
Before recording data, all trials were explained and demonstrated by the investigator, and participants were shown EEG signals in real-time to train them on minimizing facial and neck movements during subsequent data collection. Silence was maintained during testing. Both wrists were tested and were categorized as dominant (D) or non-dominant (ND) in the HV group, and as less affected (LA) or more affected (MA) in the DYS group. Similarly, the cortical hemisphere contralateral to the D/LA wrist was categorized as the dominant (D) or contralesional (CL) hemisphere, and the hemisphere contralateral to the ND/MA wrist was categorized as the non-dominant (ND) or ipsilesional (IL) hemisphere.
Signal processing
To study cortical activity over the sensorimotor cortex, the C3 and C4 EEG signals (recorded above the left and right sensorimotor areas) were analyzed. Raw EEG signals were band-pass filtered (1–50 Hz) with a finite impulse response filter. Each 60-second rest trial was divided into 12 5-second segments. Each task trial was segmented by extracting the middle 5 seconds of data (1 – 6 seconds after task onset). The mean EEG value of each rest and task segment was subtracted from that segment. Independent component analysis was performed to identify and remove artifacts associated with eye-blinks and muscle activation. Rest and task power spectra were computed for each rest and task segment using the Fast Fourier Transform with a 10% Tukey window (4096 data points). Magnitude squared coherence was estimated between C3 and C4 for each rest and task segment using Welch’s averaged periodogram method with non-overlapping Hann windows (1024 data points). Mean spectral power and mean coherence estimates were computed over all trials within one condition (rest or task) in the delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–15 Hz) and beta (15–30 Hz) frequency ranges.
To confirm that participants were resting the wrist during rest trials and activating the ECR muscle during task trials, EMG signals were processed. EEG data from participants who did not rest or activate the ECR as intended were eliminated from analysis, as mentioned above. EMG signals from the middle 5 seconds of the task trials without EEG artifacts were de-meaned and concatenated. The mean EMG value was also removed from each 60-second rest EMG segment. Data from each rest EMG segment and the concatenated task EMG segment were rectified and averaged over the whole segment. All data processing was done using MATLAB (MathWorks, Natick, Massachusetts) and the EEGLAB toolbox (Delorme and Makeig, 2004), an open source environment for electrophysiological signal processing.
Outcome measures
The primary outcome measures were the resting EEG spectral power at C3 and C4 (Powrest), resting inter-hemispheric (C3–C4) coherence (Cohrest), mean task-related spectral power change at C3 and C4 (TRPow), and mean task-related inter-hemispheric (C3–C4) coherence change (TRCoh), in four frequency bands (delta, theta, alpha, and beta).
Task-related power loss (TRPow, Equation 1) was computed as the difference in EEG spectral power between the task condition (Powtask) and the rest condition (Powrest) normalized by the power in the rest condition (Hummel et al., 2002). Similarly, task-related coherence (TRCoh, Equation 2) was computed as the difference between inter-hemispheric coherence in the task condition (Cohtask) and the rest condition (Cohrest) normalized by resting coherence. Both TRPow and TRCoh were expressed as a percentage. In the alpha frequency band, partial coherence with respect to the O1 electrode was used in computing Cohtask and Cohrest to account for the confounding effects of occipital alpha oscillations on sensorimotor alpha rhythms through volume conduction (Mima et al., 2000). O1 signals were pre-processed the same way as the C3 and C4 signals described above.
| Equation 1 |
| Equation 2 |
In order to evaluate wrist strength, Fmax was considered as a secondary outcome measure.
Statistical analysis
Full-factorial analysis of variance (ANOVA) was used to model Powrest, Cohrest, TRPow, and TRCoh. Task-related measures (TRPow and TRCoh) from the D/LA and ND/MA wrist tasks were modeled separately. Fixed factors in the models for Powrest and TRPow included group (HV, DYS), frequency (delta, theta, alpha, beta), and hemisphere (D/CL, ND/IL). Fixed factors in the models for Cohrest and TRCoh included group (HV, DYS), and frequency (delta, theta, alpha, beta). Prior to statistical analysis, Powrest was logarithm-transformed and Cohrest was logit-transformed. Full-factorial ANOVA was used to assess the effects of group (HV, DYS), and wrist (D/LA, ND/MA) on Fmax.
EEG spectral power and EEG coherence are known to be independent measures (Shaw, 1981), however the multiple (3) analyses of power (Powrest, TRPow for D/LA, TRPow for ND/MA) and coherence (Cohrest, TRCoh for D/LA, TRCoh for ND/MA) may increase the likelihood of detecting significant effects. To address this potential issue of multiple comparisons, the Bonferroni correction was used to adjust p-values in the models for power and coherence. In the results below, unadjusted p-values are presented with the adjusted p-values immediately following in brackets. Figures include unadjusted p-values.
A significance level of 0.05 was used for all statistical tests. Statistical analysis was done using SPSS 22 (IBM Corporation, Armonk, NY). Post-hoc analysis for ANOVA models was done using Tukey’s multiple comparison analysis method. Normality of all model residuals was tested and confirmed using the Shapiro-Wilk test.
Results
Resting EEG spectral power (Powrest, Figure 2)
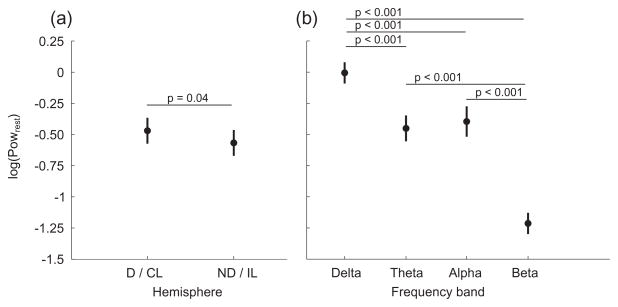
Figure 2.
Mean resting EEG spectral power (Powrest) in each hemisphere (a) and in each frequency band (b). Data from both subject groups are combined since there was no statistical difference between groups. D/CL = Dominant/Contralesional hemisphere, ND/IL = Non-dominant/Ipsilesional hemisphere.
There was an effect of hemisphere (F(1,15) = 4.2, p = 0.04 [0.12]) and frequency (F(3,15) = 101.4, p < 0.001 [0.003]) on Powrest, but no effect of group. The hemispheric difference was slightly more power in the D/CL compared to the ND/IL hemisphere (Figure 2a). Examination of the effect of frequency indicated Powrest was smallest in the beta range and largest in the delta range (Figure 2b).
Resting inter-hemispheric coherence (Cohrest, Figure 3)
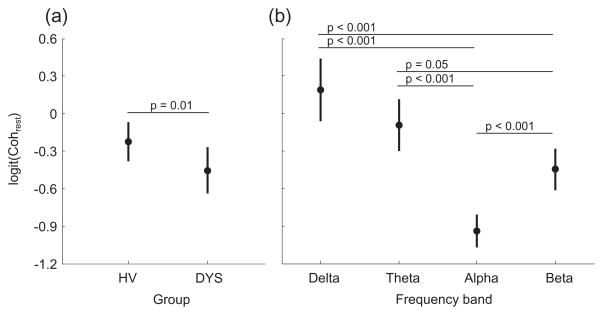
Figure 3.
Mean resting inter-hemispheric coherence (Cohrest) for each group (a) and in the each frequency band (b). Bars represent the 95% confidence interval. HV = Healthy volunteers, DYS = Dystonia group.
Cohrest was significantly less in the DYS group compared to the HV group (Figure 3a, F(1,7) = 6.2, p = 0.01 [0.03]) and varied in different frequency bands (F(3,7) = 26.6, p < 0.001 [0.003]). The effect of frequency on Cohrest can be appreciated in Figure 3b and indicates the lowest coherence in the alpha range, and the greatest in the delta range. Within patients, to explore the relation between the abnormally low Cohrest and clinical characteristics, Spearman’s rank-order correlation was computed between Cohrest and the MACS score, and between Cohrest and the MA BFM arm score. There was a significant negative correlation between Cohrest and MACS scores (rs(52) = −0.54, p < 0.001), and between Cohrest and BFM arm scores (rs(52) = −0.51, p < 0.001) indicating that the patients with poorer function (higher MACS scores) and greater dystonia severity (higher BFM arm scores) had lower values of Cohrest.
Furthermore, to investigate the effect of inter-hemispheric structural MRI findings on inter-hemispheric EEG connectivity, a post hoc Kruskal-Wallis H Test was used. A significant effect of thinning of the corpus callosum was found on Cohrest (χ2(2) = 22.0, p < 0.001). The DYS subgroup with callosal abnormalities (Table 2) had significantly lower mean rank of Cohrest (39.3; n = 36) than the DYS subgroup without callosal abnormalities (83.1; n = 16) and HV (66.4; n = 68).
Task-related EEG spectral power percent change (TRPow, Figure 4)
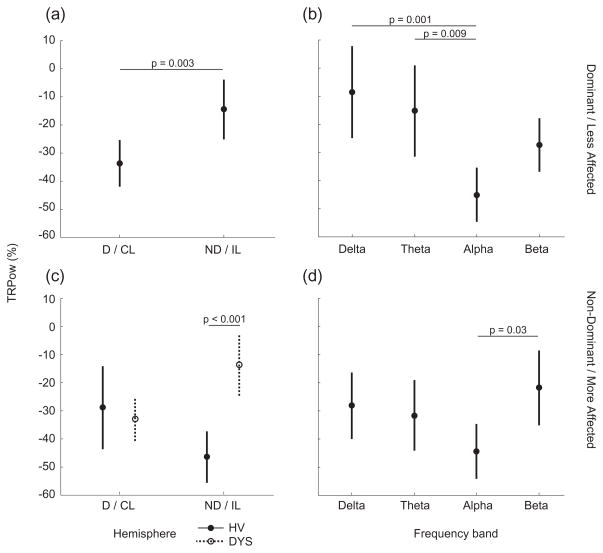
Figure 4.
Mean task-related EEG spectral power change (TRPow) in each hemisphere (a and c) and in each frequency band (b and d). Data from the dominant/less affected wrist are in the top row (a and b); data from the non-dominant/more affected wrist are in the bottom row (c and d). P values for all statistical differences between factors are indicated. Bars represent the 95% confidence interval. Closed circles represent HV and open circles represent DYS in order to show the group-hemisphere interaction on TRPow from the Non-dominant/more affected wrist task. D/CL = Dominant/Contralesional hemisphere, ND/IL = Non-dominant/Ipsilesional hemisphere, HV = Healthy volunteers, DYS = Dystonia group.
In the D/LA wrist task (Figure 4a and 4b), TRPow decrease was significantly greater in the D/CL than ND/IL hemisphere (F(1,15) = 9.3, p = 0.003 [0.009]), and varied by frequency (F(3,15) = 5.7, p = 0.001 [0.003]). TRPow was not different between subject groups. Figure 4b depicts the effect of frequency on TRPow decrease, which was greatest in the alpha and least in the delta frequency range.
In the ND/MA task (Figure 4c and 4d), there was a significant interaction between group and hemisphere on TRPow (F(1,15) = 10.4, p = 0.002 [0.006]). This interaction indicates that the lateralization of TRPow decrease to the hemisphere contralateral to the task muscle seen in the HV group (ND hemisphere during ND task) is not observed in the DYS group. Rather, patients display more activation of the hemisphere ipsilateral to the more affected wrist (CL hemisphere during MA task). In addition, there was a significant effect of frequency on TRPow (F(3,15) = 2.9, p = 0.04 [0.12]). Figure 4d depicts the effect of frequency on TRPow decrease, which was greatest in the alpha and least in the beta frequency range. To explore the relation between TRPow decrease during the MA wrist task and motor function in the DYS group, Spearman’s rank-order correlation was computed between TRPow decrease in the alpha range (the frequency band with the largest change) and each patient’s Fmax. There was a significant correlation between IL alpha TRPow and Fmax (rs(6) = 0.83, p = 0.04) indicating individuals with greater IL TRPow decrease could generate larger forces with the MA wrist. On the other hand, there was no correlation between CL alpha TRPow and Fmax (p = 0.87) suggesting that wrist strength was not associated with the task-related changes in CL alpha EEG power.
Task-related inter-hemispheric coherence percent change (TRCoh, Table 3)
Table 3.
Mean TRCoh (95% confidence interval) during wrist extension for both wrists and both subject groups in each frequency band.
| Frequency band | Dominant/Less Affected | Non-Dominant/More Affected | ||
|---|---|---|---|---|
|
| ||||
| HV | DYS | HV | DYS | |
| Delta | 1.03 (−19.21 to 21.27) | 8.20 (−5.59 to 21.99) | −15.25 (−46.74 to 16.24) | 5.01 (−15.26 to 25.28) |
| Theta | 2.89 (−18.81 to 24.59) | 6.85 (−8.54 to 22.24) | −5.97 (−39.46 to 27.52) | 4.21 (−18 to 26.68) |
| Alpha | −1.33 (−22.23 to 19.57) | 1.51 (−20.24 to 23.25) | −2.83 (−29.26 to 23.61) | 18 (−19.99 to 55.98) |
| Beta | −5.36 (−20.76 to 10.03) | 6.00 (−10.67 to 22.67) | −0.48 (−27.71 to 26.75) | 3.42 (−6.86 to 13.69) |
HV = Healthy volunteers, DYS = Dystonia group
TRCoh was minimal at all 4 frequency bands in both wrists, indicating little change in inter-hemispheric coherence due to the task (Table 3). There was no group difference in any of the frequency bands for the D/LA or ND/MA wrist tasks.
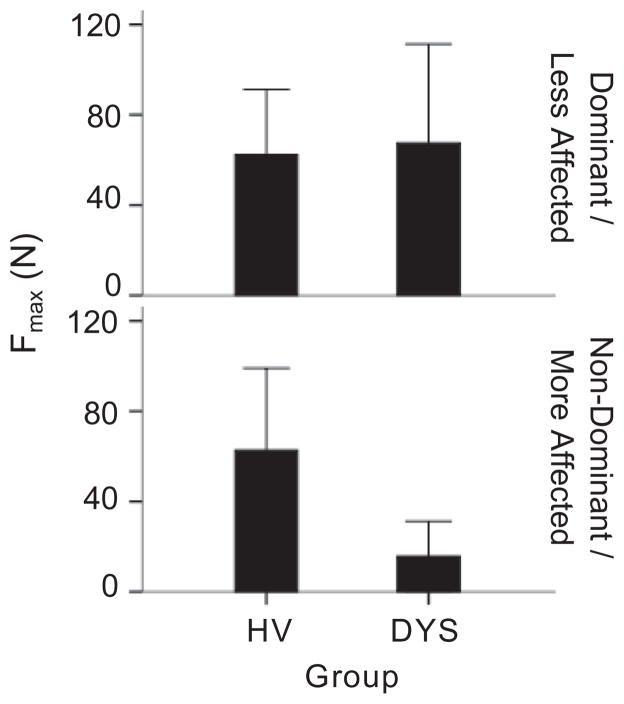
Wrist strength (Fmax, Figure 5)
Figure 5.
Maximum wrist force (Fmax) for the D/LA wrist (top row) and the ND/MA wrist (bottom row). Bars represent the mean value of force and error bars represent 1 standard deviation. D/LA = Dominant/Less affected, ND/MA = Non-dominant/More affected, HV = Healthy volunteers, DYS = Dystonia group.
There was a significant interaction between subject group and wrist on Fmax (F(1,28) = 5.0, y0.034) such that the DYS group produced less force with the ND/MA wrist than the HV group (p = 0.008), whereas the groups produced similar forces with the D/LA wrist (p = 0.76).
Discussion
In this study, quantitative EEG features were found to differentiate a sample group of individuals with dystonia due to childhood stroke from a comparison group of HV at rest and during isometric unilateral wrist extension. At rest, reduced inter-hemispheric sensorimotor connectivity (Cohrest) in the DYS compared to the HV group was associated with more severe dystonia in the MA arm and reduced hand function. During wrist extension, changes in EEG spectral power in the DYS group were found to be reduced compared to HV in the IL hemisphere during extension of the more affected wrist. The reduced IL task response, correlated with reduced MA wrist strength within the DYS group.
Sensorimotor cortical activation
The blocking of alpha and beta EEG rhythms (as indicated by decreased TRPow) is known to be related to cortical activation during voluntary movement (Chatrian et al., 1959; Pfurtscheller, 1989). Therefore, reduced IL EEG power loss in patients compared to HV during the ND/MA wrist extension task is likely due to under-activation of IL sensorimotor cortical areas. Our methods cannot expose the cause of this under-activation. Potential mechanisms include a primary injury to the IL sensorimotor area, and indirect effects from injury to other cortical and subcortical areas that influence the IL sensorimotor area. The significant loss of IL sensorimotor activation may be an important target for rehabilitation. In support of this, two studies in individuals with perinatal stroke showed improvements in MA hand function after constraint-induced movement therapy that were associated with concurrent increases in IL corticospinal excitability (Juenger et al., 2007; Walther et al., 2009). Another technique to increase IL corticospinal excitability is intermittent theta burst transcranial magnetic stimulation (TMS), which was shown to be related to improvements in clinical scales of neurologic deficits and function in a pilot study in adults with subacute MCA stroke (Hsu et al., 2013). Consequently, future rehabilitative efforts in childhood stroke would likely benefit from methods that can improve IL sensorimotor cortical activation during hand movement.
The positive correlation between IL alpha power loss and wrist weakness suggests that in this group of individuals, the MA wrist was largely associated with activity in the IL hemisphere rather than the CL hemisphere, which was not correlated with wrist force. In perinatal unilateral brain injury, disruption of the contralateral/crossed corticospinal projection originating from the IL hemisphere has been shown to lead to a strengthening of the ipsilateral/uncrossed corticospinal projection from the CL hemisphere to the paretic extremities (Eyre et al., 2001; Staudt et al., 2002; Eyre et al., 2007). By this mechanism, we may expect CL cortical activation to be significantly related to a motor task of the paretic limb. However, this result was not seen in this study and may be related to the timing of brain injury in the sample groups studied. Whereas brain lesions acquired in the first and second trimesters have been associated with the presence of functional ipsilateral/uncrossed corticospinal projections from the CL hemisphere, brain lesions due to MCA infarctions acquired later in the third trimester have not (Staudt et al., 2004). This effect of injury timing, obtained using TMS and fMRI, was suggested by Staudt and colleagues to reflect a reduction in the reorganization potential of the CL hemisphere in the late stages of gestation, which may account for the lack of correlation between CL cortical activation and MA wrist strength in our sample stroke group.
Central cortical oscillations in the alpha and beta frequency ranges are associated with voluntary movement; however, there is evidence that the neural circuits involved in each rhythm are distinct (Pfurtscheller et al., 1994; Schnitzler et al., 2000). Oscillations around 10 Hz (alpha range) are likely to originate from the postcentral gyrus, indicating more somatosensory activation. On the other hand, 20 Hz oscillations (beta range) are thought to originate from the precentral gyrus, suggesting more motor activation. Our observation that the decrease in TRPow was greatest in the alpha rhythm may indicate that sensory integration is faulty in these patients. This finding is consistent with other evidence of sensory abnormalities in dystonia of different causes (Hallett, 1995; Sanger and Kukke, 2007). Another reason our measurement of TRPow decrease was less in the beta compared to alpha frequencies could be the particular task we studied. We explored the state of active isometric muscle contraction rather than discrete finger movements, as has been commonly done in previous studies of event-related desynchronization (Pfurtscheller and Berghold, 1989; Toro et al., 1994). In the active muscle contraction state, there is likely more somatosensory cortical involvement (alpha range) to maintain the target wrist extension force compared to discrete tasks, which may require more motor cortical involvement (beta range).
We did not detect a difference between subject groups in EEG spectral power of the sensorimotor cortex (C3 and C4) during the rest condition. In contrast, other studies reported a significant C3/C4 alpha power decrease and delta power increase during attempted rest in children with similar diagnoses, including hemiplegic CP (Kulak and Sobaniec, 2005) and spastic CP (Sajedi et al., 2013), compared to controls. These two studies had contradictory results in the theta frequency range; there was an increase in resting power described by Kulak and Sobaniec, and a decrease in resting power in the study by Sajedi and colleagues. These discrepancies in resting EEG power could be explained partly by any unreported muscle activation during attempted rest in the other studies since depression in alpha power is related to muscle activation. Variability of brain injury location in patient groups, absence of dystonia in some participants in the studies referenced, and small sample sizes could also be factors contributing to differences between studies.
Other EEG studies in children with CP have indicated distributed cortical alpha activation in patients compared to control subjects during a grasp task (Lee et al., 2012; Shin et al., 2012). Whereas control subjects exhibited alpha EEG power loss primarily in the sensorimotor cortex, patients showed spectral changes in the sensorimotor cortex, supplementary motor area, posterior parietal cortex, and parieto-occipital area. Since only the sensorimotor cortex was examined in our study, it is not clear whether spectral changes could have been seen over a larger cortical area. However, distributed activation may be less likely since we studied an isolated isometric wrist extension task rather than the more complex functional grasp task used in the two aforementioned studies.
Inter-hemispheric connectivity
The pattern of decreasing resting inter-hemispheric sensorimotor coherence with increasing frequency observed in HV in this study matches results from a previous study in typically developing children (Gasser et al., 1987). However, the decrease in resting inter-hemispheric coherence we found in individuals with secondary dystonia compared to controls has not been previously reported and may be related to decreased connectivity of the hemispheres through the corpus callosum. There are common neuroanatomical signs of white matter damage in individuals with early brain injury (Korzeniewski et al., 2008), and white matter damage has been related to thinning of the corpus callosum (Panigrahy et al., 2005). Based on a theory of the excitatory function of the corpus callosum, decreased size would reflect more laterality of brain function (Bloom and Hynd, 2005). Considering the laterality of brain function following asymmetric childhood stroke, structural changes related to the corpus callosum may be partly responsible for the reduction in inter-hemispheric sensorimotor coherence in patients. Correspondingly, in our study, the presence of thinning of the corpus callosum had a significant negative effect on the amount of resting inter-hemispheric coherence. This idea is also supported by the observation that children with agenesis of the corpus callosum have reduced inter-hemispheric EEG coherence (Koeda et al., 1995).
The association of reduced C3–C4 EEG coherence with greater dystonia severity (BFM arm score) suggests alterations in inter-hemispheric connectivity are involved in the development of dystonia. One potential explanation of this relation is that reduced excitatory drive from the CL hemisphere to inhibitory interneurons in the IL hemisphere can lead to disinhibition of the IL motor cortex causing excessive cortical activation and increased dystonia severity. Supporting this notion, there has been research using single-pulse TMS of the motor cortex indicating increased cortical activation (Ikoma et al., 1996) and decreased cortical inhibition (Ridding et al., 1995; Filipovic et al., 1997) in patients with primary (no identifiable cause) dystonia. More recently, there has been a study of adult patients with secondary (due to focal lesions of caudate and putamen) hemidystonia (Trompetto et al., 2012) similarly indicating increased cortical excitability, and decreased inhibition compared to controls. Further, in patients with mirror dystonia (movement of the unaffected hand triggers dystonia in the affected hand), double-pulse TMS studies have shown reduced inhibition of the IL hemisphere from the CL hemisphere (Beck et al., 2009; Sattler et al., 2014). However, whether a reduction in inter-hemispheric connectivity causes dystonia, is a result of dystonia, or is related to an injury unassociated with dystonia is not clear from this study and is an important topic for future investigations. For example, asymmetry in arm movements and/or decreased frequency of bilateral arm movements due to unilateral dystonia may increase asymmetry in cortical activation through use-dependent plasticity and reduce inter-hemispheric connectivity.
The correlation between reduced inter-hemispheric sensorimotor connectivity and the MACS score suggest that the coupling between homologous cortical regions at rest may have functional significance. Correspondingly, an fMRI study in adults with ischemic stroke showed a correlation between reduced inter-hemispheric connectivity at rest and reduced hand function (Carter et al., 2010). Another fMRI study in adults with brain tumors indicated a reduction in resting inter-hemispheric connectivity in patients with weakness that was not present in patients without weakness (Otten et al., 2012). Similarly, our results indicate that all the individuals in the DYS group had low inter-hemispheric connectivity at rest and wrist weakness. The mechanism by which spontaneous neural activity at rest impacts task performance is not known. However, one hypothesis is that performing a motor task from an altered baseline level of neural connectivity can affect how neurons communicate to initiate the desired motor output (Wu et al., 2011). In future longitudinal studies of development or interventional trials, we propose the use of Cohrest as a potential neurophysiological outcome measure to explore the link between resting inter-hemispheric connectivity and functional changes within an individual.
In a study investigating EEG outcomes in children with hemiplegic CP (Kulak and Sobaniec, 2005), the authors also described a decrease in resting inter-hemispheric sensorimotor coherence in all but the delta frequency range. This discrepancy in the delta frequencies could be due to differences in the etiologies of the patients assessed since only 2 out of 12 patients in their study shared a history of ischemic stroke with the patients in our study.
Our observed lack of change in inter-hemispheric coherence related to the isometric wrist extension task (TRCoh) may indicate that the simple task did not depend on coordination of both brain hemispheres. It may also be related to the small sample size tested in this study. If TRCoh for each wrist task in each group is used to estimate the sample size required for detecting a group difference, 62 subjects per group would be required for the D/LA wrist, and 24 subjects per group would be required for the ND/MA wrist (with alpha = 0.05, beta = 0.8). It is therefore possible that with more subjects, group differences may be found such that there is a slight increase in TRCoh in the DYS group and a slight decrease in the HV group during extension of the ND/MA wrist. However, this remains to be tested in a larger group.
Limitations
The patient group in this study was selected on the basis of dystonia in one arm resulting from childhood stroke. However, the heterogeneity of brain injury location, mechanism, and timing in the small patient group and the similarity of our results with other studies of adult and pediatric stroke suggest that the EEG features that distinguish the DYS and HV groups may not be specific to dystonia. Therefore, it will be crucial to incorporate larger and more homogeneous groups with additional experimental controls in future studies of dystonia (e.g., a patient group with early brain injury and without dystonia). Similarly, although all patients in this study were diagnosed with hemiplegic CP, it is not clear to what extent the results in this study can be expanded to the CP population at large since stroke is not typically the primary cause of CP.
The electrode positions on the IL hemisphere may have been above slightly different parts of the sensorimotor area than the CL brain hemisphere in patients due to atrophy of the IL hemisphere (Piovesana et al., 2001). In the future, this may be addressed through MRI-guided electrode placement. Future work should build upon the results from this study to address outstanding questions, including assessing a task that is affected by dystonia but is achievable by the patient group, expanding the EEG analysis spatially to determine where the maximum power change and maximum inter-hemispheric coherence originates, and incorporating a larger sample group.
Highlights.
In individuals with dystonia after childhood stroke, functionally relevant EEG abnormalities can be identified.
Ipsilesional sensorimotor cortical activation in the 8–12 Hz range is abnormally reduced in patients and correlates with weakness of the more affected wrist.
Resting inter-hemispheric sensorimotor connectivity in patients is abnormally reduced and correlates with increased dystonia severity and reduced hand function.
Acknowledgments
The authors acknowledge the assistance of Sherry Vorbach in all data collection sessions, and Sophia Francis and Francesca Gajofatto in preliminary data analysis. This work was supported by the Intramural Research Programs of the NIH, National Institute of Neurological Disorders and Stroke, and the NIH Clinical Center.
Footnotes
Conflict of Interest
None of the authors have potential conflicts of interest to be disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrew C, Pfurtscheller G. Event-related coherence as a tool for studying dynamic interaction of brain regions. Electroencephalogr Clin Neurophysiol. 1996;98:144–8. doi: 10.1016/0013-4694(95)00228-6. [DOI] [PubMed] [Google Scholar]
- Beck S, Shamim EA, Richardson SP, Schubert M, Hallett M. Inter-hemispheric inhibition is impaired in mirror dystonia. Eur J Neurosci. 2009;29:1634–40. doi: 10.1111/j.1460-9568.2009.06710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejot Y, Giroud M, Moreau T, Benatru I. Clinical spectrum of movement disorders after stroke in childhood and adulthood. Eur Neurol. 2012;68:59–64. doi: 10.1159/000336740. [DOI] [PubMed] [Google Scholar]
- Bloom JS, Hynd GW. The role of the corpus callosum in interhemispheric transfer of information: excitation or inhibition? Neuropsychol Rev. 2005;15:59–71. doi: 10.1007/s11065-005-6252-y. [DOI] [PubMed] [Google Scholar]
- Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–34. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, et al. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67:365–75. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos-Baumann AO, Passingham RE, Warner T, Playford ED, Marsden CD, Brooks DJ. Overactive prefrontal and underactive motor cortical areas in idiopathic dystonia. Ann Neurol. 1995;37:363–72. doi: 10.1002/ana.410370313. [DOI] [PubMed] [Google Scholar]
- Chatrian GE, Petersen MC, Lazarte JA. The blocking of the rolandic wicket rhythm and some central changes related to movement. Electroencephalogr Clin Neurophysiol. 1959;11:497–510. doi: 10.1016/0013-4694(59)90048-3. [DOI] [PubMed] [Google Scholar]
- Chu WT, Sanger TD. Force variability during isometric biceps contraction in children with secondary dystonia due to cerebral palsy. Mov Disord. 2009;24:1299–305. doi: 10.1002/mds.22573. [DOI] [PubMed] [Google Scholar]
- Chuang C, Fahn S, Frucht SJ. The natural history and treatment of acquired hemidystonia: report of 33 cases and review of the literature. J Neurol Neurosurg Psychiatry. 2002;72:59–67. doi: 10.1136/jnnp.72.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Eyre JA, Smith M, Dabydeen L, Clowry GJ, Petacchi E, Battini R, et al. Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system? Ann Neurol. 2007;62:493–503. doi: 10.1002/ana.21108. [DOI] [PubMed] [Google Scholar]
- Eyre JA, Taylor JP, Villagra F, Smith M, Miller S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57:1543–54. doi: 10.1212/wnl.57.9.1543. [DOI] [PubMed] [Google Scholar]
- Filipovic SR, Ljubisavljevic M, Svetel M, Milanovic S, Kacar A, Kostic VS. Impairment of cortical inhibition in writer’s cramp as revealed by changes in electromyographic silent period after transcranial magnetic stimulation. Neurosci Lett. 1997;222:167–70. doi: 10.1016/s0304-3940(97)13370-5. [DOI] [PubMed] [Google Scholar]
- Gasser T, Jennen-Steinmetz C, Verleger R. EEG coherence at rest and during a visual task in two groups of children. Electroencephalogr Clin Neurophysiol. 1987;67:151–8. doi: 10.1016/0013-4694(87)90038-1. [DOI] [PubMed] [Google Scholar]
- Golomb MR, Garg BP, Saha C, Azzouz F, Williams LS. Cerebral palsy after perinatal arterial ischemic stroke. J Child Neurol. 2008;23:279–86. doi: 10.1177/0883073807309246. [DOI] [PubMed] [Google Scholar]
- Hallett M. Is dystonia a sensory disorder? Ann Neurol. 1995;38:139–40. doi: 10.1002/ana.410380203. [DOI] [PubMed] [Google Scholar]
- Hsu YF, Huang YZ, Lin YY, Tang CW, Liao KK, Lee PL, et al. Intermittent theta burst stimulation over ipsilesional primary motor cortex of subacute ischemic stroke patients: a pilot study. Brain Stimul. 2013;6:166–74. doi: 10.1016/j.brs.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Hummel F, Andres F, Altenmuller E, Dichgans J, Gerloff C. Inhibitory control of acquired motor programmes in the human brain. Brain. 2002;125:404–20. doi: 10.1093/brain/awf030. [DOI] [PubMed] [Google Scholar]
- Ibanez V, Sadato N, Karp B, Deiber MP, Hallett M. Deficient activation of the motor cortical network in patients with writer’s cramp. Neurology. 1999;53:96–105. doi: 10.1212/wnl.53.1.96. [DOI] [PubMed] [Google Scholar]
- Ikoma K, Samii A, Mercuri B, Wassermann EM, Hallett M. Abnormal cortical motor excitability in dystonia. Neurology. 1996;46:1371–6. doi: 10.1212/wnl.46.5.1371. [DOI] [PubMed] [Google Scholar]
- Juenger H, Linder-Lucht M, Walther M, Berweck S, Mall V, Staudt M. Cortical neuromodulation by constraint-induced movement therapy in congenital hemiparesis: an FMRI study. Neuropediatrics. 2007;38:130–6. doi: 10.1055/s-2007-985904. [DOI] [PubMed] [Google Scholar]
- Kirton A, deVeber G. Cerebral palsy secondary to perinatal ischemic stroke. Clin Perinatol. 2006;33:367–86. doi: 10.1016/j.clp.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Koeda T, Knyazeva M, Njiokiktjien C, Jonkman EJ, De Sonneville L, Vildavsky V. The EEG in acallosal children. Coherence values in the resting state: left hemisphere compensatory mechanism? Electroencephalogr Clin Neurophysiol. 1995;95:397–407. doi: 10.1016/0013-4694(95)00171-9. [DOI] [PubMed] [Google Scholar]
- Korzeniewski SJ, Birbeck G, DeLano MC, Potchen MJ, Paneth N. A systematic review of neuroimaging for cerebral palsy. J Child Neurol. 2008;23:216–27. doi: 10.1177/0883073807307983. [DOI] [PubMed] [Google Scholar]
- Kulak W, Sobaniec W. Quantitative EEG analysis in children with hemiparetic cerebral palsy. NeuroRehabilitation. 2005;20:75–84. [PubMed] [Google Scholar]
- Lee NG, Kang SK, Lee DR, Hwang HJ, Jung JH, You JS, et al. Feasibility and test-retest reliability of an electroencephalography-based brain mapping system in children with cerebral palsy: a preliminary investigation. Arch Phys Med Rehabil. 2012;93:882–8. doi: 10.1016/j.apmr.2011.10.028. [DOI] [PubMed] [Google Scholar]
- Lerner A, Shill H, Hanakawa T, Bushara K, Goldfine A, Hallett M. Regional cerebral blood flow correlates of the severity of writer’s cramp symptoms. Neuroimage. 2004;21:904–13. doi: 10.1016/j.neuroimage.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Lin PT, Hallett M. The pathophysiology of focal hand dystonia. J Hand Ther. 2009;22:109–13. doi: 10.1016/j.jht.2008.10.008. quiz 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JK, Nelson KB. Epidemiology of perinatal stroke. Curr Opin Pediatr. 2001;13:499–505. doi: 10.1097/00008480-200112000-00002. [DOI] [PubMed] [Google Scholar]
- Manganotti P, Gerloff C, Toro C, Katsuta H, Sadato N, Zhuang P, et al. Task-related coherence and task-related spectral power changes during sequential finger movements. Electroencephalogr Clin Neurophysiol. 1998;109:50–62. doi: 10.1016/s0924-980x(97)00074-x. [DOI] [PubMed] [Google Scholar]
- Mima T, Matsuoka T, Hallett M. Functional coupling of human right and left cortical motor areas demonstrated with partial coherence analysis. Neurosci Lett. 2000;287:93–6. doi: 10.1016/s0304-3940(00)01165-4. [DOI] [PubMed] [Google Scholar]
- Neychev VK, Gross RE, Lehericy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiol Dis. 2011;42:185–201. doi: 10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odergren T, Stone-Elander S, Ingvar M. Cerebral and cerebellar activation in correlation to the action-induced dystonia in writer’s cramp. Mov Disord. 1998;13:497–508. doi: 10.1002/mds.870130321. [DOI] [PubMed] [Google Scholar]
- Otten ML, Mikell CB, Youngerman BE, Liston C, Sisti MB, Bruce JN, et al. Motor deficits correlate with resting state motor network connectivity in patients with brain tumours. Brain. 2012;135:1017–26. doi: 10.1093/brain/aws041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahy A, Barnes PD, Robertson RL, Sleeper LA, Sayre JW. Quantitative analysis of the corpus callosum in children with cerebral palsy and developmental delay: correlation with cerebral white matter volume. Pediatr Radiol. 2005;35:1199–207. doi: 10.1007/s00247-005-1577-5. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G. Functional topography during sensorimotor activation studied with event-related desynchronization mapping. J Clin Neurophysiol. 1989;6:75–84. doi: 10.1097/00004691-198901000-00003. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Berghold A. Patterns of cortical activation during planning of voluntary movement. Electroencephalogr Clin Neurophysiol. 1989;72:250–8. doi: 10.1016/0013-4694(89)90250-2. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Pregenzer M, Neuper C. Visualization of sensorimotor areas involved in preparation for hand movement based on classification of mu and central beta rhythms in single EEG trials in man. Neurosci Lett. 1994;181:43–6. doi: 10.1016/0304-3940(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Piovesana AM, Moura-Ribeiro MV, Zanardi V, Goncalves VM. Hemiparetic cerebral palsy: etiological risk factors and neuroimaging. Arq Neuropsiquiatr. 2001;59:29–34. doi: 10.1590/s0004-282x2001000100007. [DOI] [PubMed] [Google Scholar]
- Preibisch C, Berg D, Hofmann E, Solymosi L, Naumann M. Cerebral activation patterns in patients with writer’s cramp: a functional magnetic resonance imaging study. J Neurol. 2001;248:10–7. doi: 10.1007/s004150170263. [DOI] [PubMed] [Google Scholar]
- Pujol J, Roset-Llobet J, Rosines-Cubells D, Deus J, Narberhaus B, Valls-Sole J, et al. Brain cortical activation during guitar-induced hand dystonia studied by functional MRI. Neuroimage. 2000;12:257–67. doi: 10.1006/nimg.2000.0615. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Sheean G, Rothwell JC, Inzelberg R, Kujirai T. Changes in the balance between motor cortical excitation and inhibition in focal, task specific dystonia. J Neurol Neurosurg Psychiatry. 1995;59:493–8. doi: 10.1136/jnnp.59.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajedi F, Ahmadlou M, Vameghi R, Gharib M, Hemmati S. Linear and nonlinear analysis of brain dynamics in children with cerebral palsy. Res Dev Disabil. 2013;34:1388–96. doi: 10.1016/j.ridd.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Sanger TD. Pathophysiology of pediatric movement disorders. J Child Neurol. 2003;18 (Suppl 1):S9–24. doi: 10.1177/0883073803018001S0401. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Chen D, Fehlings DL, Hallett M, Lang AE, Mink JW, et al. Definition and classification of hyperkinetic movements in childhood. Mov Disord. 2010;25:1538–49. doi: 10.1002/mds.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger TD, Kukke SN. Abnormalities of tactile sensory function in children with dystonic and diplegic cerebral palsy. J Child Neurol. 2007;22:289–93. doi: 10.1177/0883073807300530. [DOI] [PubMed] [Google Scholar]
- Sattler V, Dickler M, Michaud M, Meunier S, Simonetta-Moreau M. Does abnormal interhemispheric inhibition play a role in mirror dystonia? Mov Disord. 2014;29:787–96. doi: 10.1002/mds.25768. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Gross J, Timmermann L. Synchronised oscillations of the human sensorimotor cortex. Acta Neurobiol Exp (Wars) 2000;60:271–87. doi: 10.55782/ane-2000-1346. [DOI] [PubMed] [Google Scholar]
- Seo JP, Jang SH. Different Characteristics of the Corticospinal Tract According to the Cerebral Origin: DTI Study. AJNR Am J Neuroradiol. 2013;34:1359–1363. doi: 10.3174/ajnr.A3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JC. An introduction to the coherence function and its use in EEG signal analysis. J Med Eng Technol. 1981;5:279–88. doi: 10.3109/03091908109009362. [DOI] [PubMed] [Google Scholar]
- Shin YK, Lee DR, Hwang HJ, You SJ, Im CH. A novel EEG-based brain mapping to determine cortical activation patterns in normal children and children with cerebral palsy during motor imagery tasks. NeuroRehabilitation. 2012;31:349–55. doi: 10.3233/NRE-2012-00803. [DOI] [PubMed] [Google Scholar]
- Staudt M, Gerloff C, Grodd W, Holthausen H, Niemann G, Krageloh-Mann I. Reorganization in congenital hemiparesis acquired at different gestational ages. Ann Neurol. 2004;56:854–63. doi: 10.1002/ana.20297. [DOI] [PubMed] [Google Scholar]
- Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krageloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis: a TMS and fMRI study. Brain. 2002;125:2222–37. doi: 10.1093/brain/awf227. [DOI] [PubMed] [Google Scholar]
- Toro C, Deuschl G, Thatcher R, Sato S, Kufta C, Hallett M. Event-related desynchronization and movement-related cortical potentials on the ECoG and EEG. Electroencephalogr Clin Neurophysiol. 1994;93:380–9. doi: 10.1016/0168-5597(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Trompetto C, Avanzino L, Marinelli L, Mori L, Pelosin E, Roccatagliata L, et al. Corticospinal excitability in patients with secondary dystonia due to focal lesions of the basal ganglia and thalamus. Clin Neurophysiol. 2012;123:808–14. doi: 10.1016/j.clinph.2011.06.033. [DOI] [PubMed] [Google Scholar]
- Walther M, Juenger H, Kuhnke N, Wilke M, Brodbeck V, Berweck S, Staudt M, Mall V. Motor cortex plasticity in ischemic perinatal stroke: a transcranial magnetic stimulation and functional MRI study. Pediatr Neurol. 2009;41:171–8. doi: 10.1016/j.pediatrneurol.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Wu T, Long X, Wang L, Hallett M, Zang Y, Li K, Chan P. Functional connectivity of cortical motor areas in the resting state in Parkinson’s disease. Hum Brain Mapp. 2011;32:1443–57. doi: 10.1002/hbm.21118. [DOI] [PMC free article] [PubMed] [Google Scholar]