Summary
The differentiation and protective capacity of Plasmodium-specific T cells are regulated by both positive and negative signals during malaria, but the molecular and cellular details remain poorly defined. Here we show that malaria patients and Plasmodium-infected rodents exhibit atypical expression of the co-stimulatory receptor OX40 on CD4 T cells and that therapeutic enhancement of OX40 signaling enhances helper CD4 T cell activity, humoral immunity and parasite clearance in rodents. However, these beneficial effects of OX40 signaling are abrogated following coordinate blockade of PD-1 co-inhibitory pathways, which are also up-regulated during malaria and associated with elevated parasitemia. Co-administration of biologics blocking PD-1 and promoting OX40 signaling induces excessive interferon-gamma that directly limits helper T cell-mediated support of humoral immunity and decreases parasite control. Our results show that targeting OX40 can enhance Plasmodium control and that crosstalk between co-inhibitory and co-stimulatory pathways in pathogen-specific CD4 T cells can impact pathogen clearance.
Introduction
Plasmodium infections and the disease malaria remain global health emergencies (W.H.O., 2013). Although clinical immunity against malaria is documented following repeated exposure to Plasmodium parasites, sterilizing immunity against the parasite rarely if ever develops (Tran et al., 2013). Many studies show that immune-mediated resistance against blood stage Plasmodium infection requires the activity of T and B cells (Amante and Good, 1997, 2001; Butler et al., 2012; Elliott et al., 2005; Kumar and Miller, 1990; Langhorne, 1989; Pombo et al., 2002; Riley et al., 2006). However, the extent to which T cell-expressed immunoregulatory receptors either promote or constrain the generation of potent anti-Plasmodium T and B cell-meditated immunity remains poorly defined.
Previous work showed that P. falciparum infection is associated with the expression of inhibitory receptors that are known to limit the activity of parasite-specific lymphocytes (Illingworth et al., 2013). We and others have shown that the receptors programmed cell death 1 (PD-1) and/or lymphocyte-activation gene 3 (LAG-3) are aberrantly expressed during rodent malaria, and that they contribute to dysfunctional parasite-specific T cell responses and limit parasite clearance (Butler et al., 2012; Horne-Debets et al., 2013). In contrast to negative regulatory circuits, whether co-stimulatory pathways additionally regulate an established T cell response during prolonged or chronic Plasmodium infection is not known. Moreover, whether negative co-inhibitory circuits are functionally counterbalanced by co-stimulatory networks to maintain T cell immunity during blood stage Plasmodium infection has not been examined.
One co-stimulatory molecule that could play an important role during Plasmodium infection is the OX40 receptor. OX40 is a member of the tumor necrosis factor receptor (TNFR) superfamily and is reported to be transiently expressed on T cells following cognate interactions between T cell receptors (TCRs) and antigen-major histocompatibility (MHC) complexes on antigen presenting cells (APCs) (Croft, 2010). OX40 signaling promotes T cell proliferation and survival, influences CD4 T cell differentiation into T helper Type I (Th1), Type 2 (Th2) and T follicular helper (Tfh) cell subsets (Croft, 2010; Walker et al., 1999) and is reported to reverse CD4 T cell hypo-responsiveness (Bansal-Pakala et al., 2001). For these reasons we hypothesized that therapeutic ligation of OX40 during Plasmodium blood stage infection would enhance parasite-specific CD4 T cell activity, limit the degree of CD4 T cell exhaustion, and promote parasite clearance from the host.
Here we report marked upregulation of OX40 on CD4 T cells during human and rodent malaria, with atypical patterns of sustained OX40 expression in rodents. Therapeutic enhancement of OX40 signaling during established rodent malaria promoted the accumulation of multiple functionally distinct CD4 T cell subsets, enhanced T-dependent humoral immunity and limited parasite growth. Strikingly, co-administration of biologics to block PD-1 and promote OX40 signaling obstructed Tfh and germinal center (GC) reactions in an interferon-gamma (IFN-γ-dependent manner, resulting in loss of antibody-mediated parasite control. Collectively, our results demonstrate that excess IFN-γ can block the differentiation or survival of Plasmodium-specific Tfh cells during blood stage infection. Moreover, our data reveal that crosstalk between T cell co-stimulatory and co-inhibitory signaling pathways during malaria modulates cellular and humoral immunity, which has broad implications for immunotherapeutic strategies designed to impact the function of CD4 T cells during persistent infection or cancer.
Results
Blood stage Plasmodium infection induces OX40 expression on human and rodent CD4 T cells
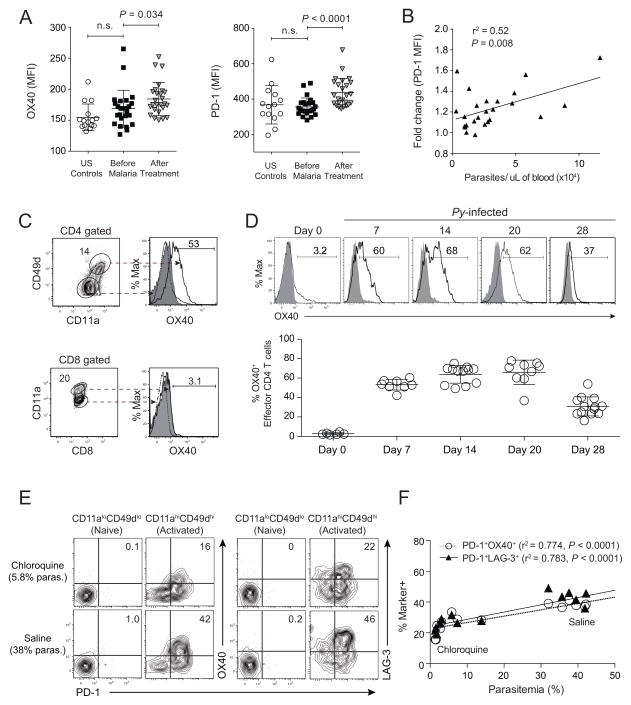
We first examined whether P. falciparum infection was associated with changes in OX40 and PD-1 expression in a longitudinal cohort of children in Mali whose circulating CD4 T cells were examined at the healthy baseline before febrile malaria, and 7 days after anti-malarial treatment. The mean fluorescence intensities (MFI) of OX40 and PD-1 were significantly elevated on CD45RO+CD45RA− CD4 T cells (Fig S1A) 7 days after treatment (Fig 1A) and the upregulation of PD-1 expression on CD4 T cells also positively correlated with parasite burden in the blood during febrile malaria (Fig 1B). To determine whether these patterns were paralleled during rodent malaria, we examined their expression on parasite-specific splenic CD4+ (CD11ahiCD49dhi) and CD8+ (CD11ahiCD8αlo) T cells (Butler et al., 2012) at various times after P. yoelii infection. On day 7 p.i. OX40 was expressed by a large fraction (>50%) of parasite-specific CD4 T cells, but not CD8 T cells (Fig 1C). Strikingly, OX40 expression was sustained on parasite-specific CD4 T cells through day 28 p.i. (Fig 1D). OX40 was also expressed by >70% of CXCR5+PD-1hi T follicular helper (Tfh) cells (Fig S1B) and both resting (CD11aloCD44lo) and activated (CD11ahiCD44hi) Foxp3+ T regulatory cells (Tregs) on day 14 p.i. (Fig S1C). Notably, Tregs comprised ~15% of all OX40+ CD4 T cells following P. yoelii infection (Fig S1D), supporting that the majority (~85%) of OX40+ cells represent other functionally distinct, parasite-specific effector and memory CD4 T cell populations. We also assayed several other cell types (not shown) and found that only a subset of NK cells expressed OX40 after blood stage P. yoelii infection (Fig S1E). Consistent with our previous report (Butler et al., 2012), we found that higher parasite burden was associated with sustained, coordinate expression of co-inhibitory receptors PD-1 and LAG-3 (Fig 1E,F). Moreover, OX40 was coordinately expressed with PD-1 and LAG-3, with highest PD-1, LAG-3 and OX40 expression on CD4 T cells in mice with highest parasite burdens (Figs 1E,F and S1F). These data show that human malaria is associated with the upregulation of both co-inhibitory (PD-1) and co-stimulatory (OX40) receptors on CD4 T cells. Moreover, coordinate expression of PD-1, LAG-3 and OX40 on rodent CD4 T cells is consistent with the notion that co-inhibitory receptor signaling may be modified or counterbalanced by co-stimulatory signaling pathways in CD4 T cells responding to Plasmodium infection.
Figure 1. OX40 expression on CD4 T cells during clinical and experimental malaria.
(A) Cumulative data displaying the MFI of OX40 (left) and PD-1 (right) expression on CD45RA−CD45RO+ (activated) CD4+ T cells in the peripheral blood of U.S. controls (n=15) and children in Mali (n=26) at the healthy baseline (Before Malaria) and 7 days after diagnosis and treatment of acute P. falciparum malaria (After Treatment). Data (mean ± SD) for P. falciparum-infected children in (A) were analyzed using paired student’s t tests. (B) Cumulative data depicting the association between the fold change in PD-1 MFI (Before Malaria to After Treatment) and parasite densities in the blood of children at first diagnosis of acute malaria. Data in (B) were analyzed using linear regression. (C–F) Mice were infected with 106 P. yoelii-infected RBCs and splenocytes were isolated at the indicated time points. (C) Representative plots showing the fraction of splenic P. yoelii-specific (CD11ahiCD49dhi) CD4 T cells and (CD11ahiCD8αlo) CD8 T cells expressing OX40 on day 7 p.i. (D) Representative histograms (top) and summary kinetics (bottom) of OX40 expression on splenic CD11ahiCD49dhi CD4 T cells from naïve and P. yoelii-infected mice on days 7, 14, 20, and 28 p.i. Dashed lines and shaded histograms represent naïve T cells and isotype staining, respectively. Symbols represent individual mice. (E,F) Representative plots (E) and summary data (F) showing coordinate OX40 and PD-1 expression or LAG-3 and PD-1 expression among naïve (CD11aloCD49dlo) and activated (CD11ahiCD49dhi) splenic CD4 T cells on day 14 p.i. in chloroquine- versus saline-treated mice. See also Figure S1.
The OX40-OX40L pathway regulates Plasmodium-specific CD4 T cell responses and control of blood stage P. yoelii infection
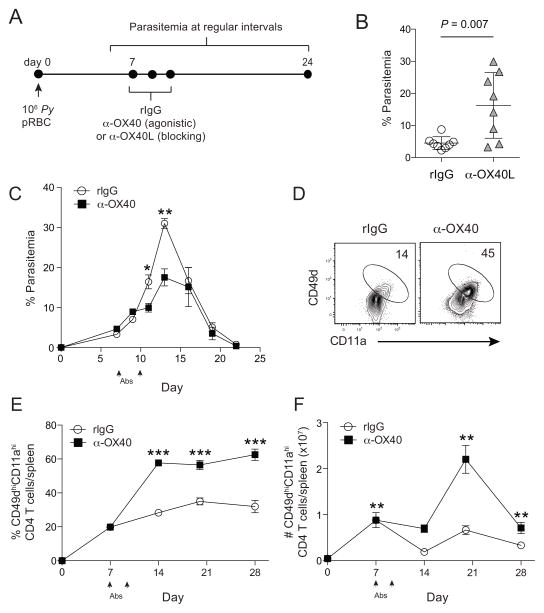
To directly test whether the OX40 co-stimulatory pathway is biologically relevant during an established Plasmodium infection, we manipulated this pathway in vivo by administering one of two biologics to P. yoelli-infected mice (Fig 2A). We used either an antagonistic (blocking) monoclonal antibody (mAb) directed against OX40L (Akiba et al., 1999) or an agonistic (stimulating) mAb that triggers OX40 signaling pathways in cells expressing OX40 (Gramaglia et al., 2000). Daily administration of the α-OX40L blocking mAb from days 7 to 10 p.i. resulted in significantly elevated parasite burdens, compared to mice receiving control rIgG mAb (Fig 2B). Conversely, P. yoelii-infected mice injected with agonistic α-OX40 mAb on days 7 and 10 p.i. exhibited significantly reduced peak parasitemia compared to mice administered control rIgG (Fig 2C).
Figure 2. OX40L/OX40 pathway promotes parasite control during experimental malaria.
(A) Experimental design. Mice were infected with 106 P. yoelii-infected RBCs and treated with control rat IgG, α-OX40L, or α-OX40 at the indicated time points. Parasite burden (% Parasitemia) was measured at regular intervals. (B) Parasitemia on day 17 p.i. in mice administered rat IgG (n=8) or α-OX40L (n=8). Symbols represent individual mice. Data (mean ± SD) in (B) were pooled from two independent experiments and analyzed using a Student’s t test. (C) Parasite growth kinetics in mice (n=5/group) treated with rat IgG or α-OX40 on d7 and 10 p.i. (arrows). (*P < 0.05, **P < 0.01). Data in C (mean ± SEM) were analyzed using multiple t tests correcting for multiple comparisons using the Holm-Sidak method and are representative of 8 independent experiments. Representative plots (D) and cumulative data showing the frequency (E) and total numbers (F) of CD11ahiCD49dhi CD4+ T cells on day 14 p.i. in mice receiving rat IgG or α-OX40 on days 7 and 10 p.i. Data (mean ± SEM) in (E–F) are from 5–6 mice per group per time point and were analyzed using multiple unpaired Student’s t tests and are representative of 3 independent experiments (***P < 0.0001 **P < 0.01 *P < 0.05). See also Figure S2.
Given that OX40 expression was primarily restricted to CD4 T cells and NK cells during rodent malaria, we examined specific attributes of these cells following α-OX40 treatment. Consistent with the notion that co-stimulatory pathways counterbalance co-inhibitory signaling, α-OX40 agonistic mAb resulted in a 25% increase in the proportion of parasite-specific CD4 T cells expressing Ki67, a marker of recent proliferation (Fig S2A,B). However, α-OX40 treatment did not increase splenic NK cell numbers, degranulation or cytokine secreting capacity (Fig S2C–E). In contrast, we found that OX40 agonistic mAb treatment resulted in 3–4-fold expansions in the frequency and number of parasite-specific CD4 T cells by day 14 p.i., an effect that was sustained through day 28 p.i. (Fig 2E,F). Collectively, these data show that sustained OX40 signaling regulates the magnitude of the CD4 T cell response and parasite control following blood stage Plasmodium infection.
The OX40-OX40L pathway modulates the differentiation and accumulation of T helper Type I Plasmodium-specific CD4 T cells
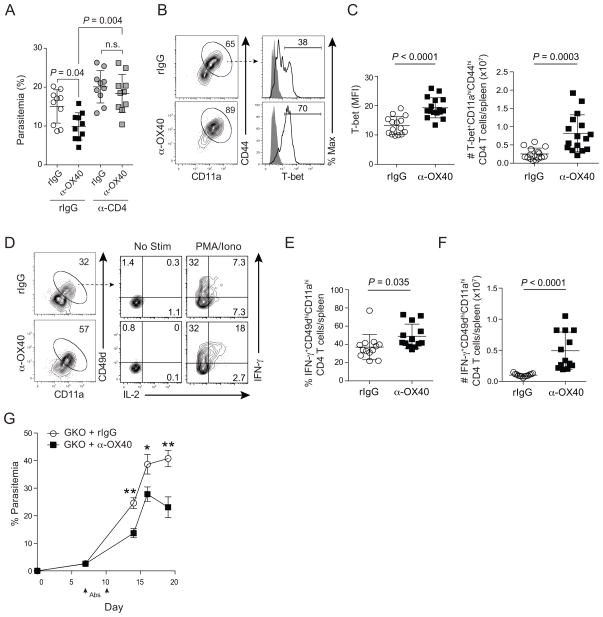
To formally test whether CD4 T cells are necessary for the in vivo protective effects of αOX40 during experimental malaria, we repeated our studies in CD4 T cell-depleted mice. Anti-OX40 treatment had no effect on parasite control in the absence of CD4 T cells (Fig 3A), supporting that CD4 T cells are functional targets of agonistic α-OX40 mAb in vivo. Thus, we next examined whether α-OX40 treatment expanded one or more functionally distinct CD4 T cell subsets. We first focused on T-bet+ Th1 cells, as this subset is widely linked to protection against blood stage Plasmodium infection (Amante and Good, 1997; De Souza et al., 1997; Langhorne et al., 1990; Oakley et al., 2014). We found the fraction of effector CD4 T cells expressing T-bet, the MFI of T-bet, and the total number of T-bet+ parasite-specific CD4 T cells were significantly elevated in mice treated with α-OX40 mAb (Figs 3B,C). The effects on T-bet expression correlated with increases in the proportion of CD4 T cells competent to express IFN-γ and interleukin (IL)-2 in α-OX40-treated mice (Fig 3D,E). As a consequence of the 3–4-fold expansion of parasite-specific effector CD4 T cells (Fig 2D), the total number of IFNγ expressing CD4 T cells was elevated approximately 5-fold in α-OX40-treated P. yoelii-infected mice (Fig 3F). Unexpectedly, we also observed protective effects of agonistic α-OX40 mAb when it was administered to P. yoelii-infected IFN-γ knockout (GKO) mice on days 7 and 10 p.i. (Fig 3G). Enhanced parasite control in GKO mice treated with α-OX40 was associated with larger effector CD4 T cell responses (Fig S3A). Notably, despite numerically equivalent GC B cell responses (Fig S3B), α-OX40-treated GKO mice exhibited significantly larger T-dependent, parasite-specific antibody responses, compared to rIgG-treated GKO mice (Fig S3C). Collectively, these data show that therapeutic OX40 stimulation following P. yoelii malaria expands the number of IFN-γ expressing Th1 CD4 T cells that may contribute to, but are not essential for, the protective effects of agonistic α-OX40 mAb treatment.
Figure 3. Agonistic α-OX40 enhances P. yoelii-specific T helper Type I responses.
(A–G) Mice were infected with 106 P. yoelii-infected RBCs and treated with rat IgG or α-OX40 on days 7 and 10 p.i. (A) Parasitemia on day 9 p.i. in control (IgG) or CD4 T cell-depleted (α-CD4) mice. Data (mean ± SD) were analyzed using ANOVA. n=10 mice per group pooled from 2 independent experiments. (B) Representative plots depicting T-bet expression in CD11ahiCD44hi CD4+ T cells on d14 p.i. (C) Cumulative data showing the MFI (left) and total number (right) of T-bet+CD11ahiCD44hi CD4+ T cells. Data (mean ± SD) in (C) were pooled from 3 independent experiments and were analyzed using unpaired Student’s t tests. (D) Representative plots showing IFN-γ and IL-2 expression on day 14 p.i. in CD11ahiCD49dhi CD4 T cells after ex vivo stimulation with PMA and ionomycin. (E,F) Cumulative data showing the percent (E) and number (F) of IFN-γ CD11ahiCD49dhiCD4+ T cells in rIgG (n=13) and α-OX40-treated mice (n=13). Data (mean ± SD) in (E,F) were pooled from 3 independent experiments and were analyzed using unpaired Student’s t tests. (G) Parasitemia in IFN-γ knockout (GKO) mice treated with either rIgG (n=11) or α-OX40 (n=11) on days 7 and 10 p.i. Data (mean ± SEM) in (G) are pooled from 3 independent experiments. Symbols in (3C,E–F) represent individual mice. See also Figure S3.
Anti-OX40 agonist treatment is associated with expansion of multiple CD4 T cell subsets and enhanced parasite-specific antibody responses
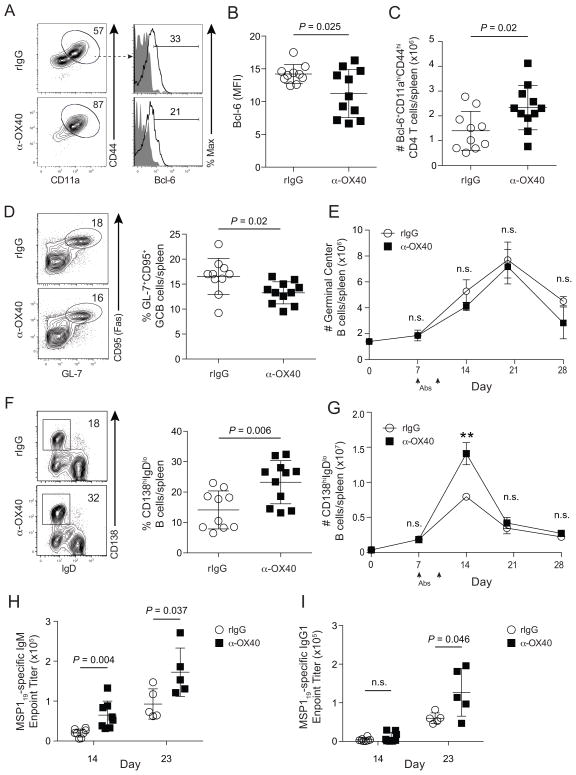
Because OX40 signaling is reported to promote the differentiation and accumulation of CXCR5+CD4+ T cells (Walker et al., 2000; Walker et al., 1999), we next examined features of splenic parasite-specific Tfh cells in control- and α-OX40-treated mice. Despite modest reductions in the proportion of Bcl-6+ effector CD4 T cells and the MFI of Bcl-6 on day 14 p.i. (Fig 4A,B), the total number of Bcl-6+ CD4 T cells was, on average, 2-fold higher in mice treated with α-OX40 agonistic mAb (Fig 4C). When we examined Tfh cells using canonical cell surface markers (CXCR5, PD-1, and ICOS), we found 3-fold larger Tfh responses following α-OX40 agonist treatment (Fig S4A-C). Notably, sort-purified CD4 effector T cells from control- and α-OX40-treated P. yoelii-infected mice exhibited equivalent expression of bcl6 and il21 mRNA (not shown). We also found significant increases in the fraction and total number of GATA-3+ effector CD4 T cells in α-OX40-treated mice (Fig S4D,E), which correlated with elevated levels of serum IL-4 through day 23 p.i. (Fig S4F). Finally, consistent with OX40 expression on Tregs, we also observed a 35% expansion in the total number of Foxp3+ Treg cells following α-OX40 agonist treatment (Fig S4G,H). Collectively, these data demonstrate that therapeutic OX40 stimulation expands and maintains not only parasite-specific Th1 cells in P. yoelii-infected mice, but also other functionally distinct CD4 T cell subsets, including Tfh cells.
Figure 4. Agonistic α-OX40 promotes humoral immunity during experimental malaria.
(A–I) Mice were infected with 106 P. yoelii-infected RBCs and treated with rat IgG or α-OX40 on days 7 and 10 p.i. (A) Representative plots showing Bcl-6 expression among splenic effector CD4+ T cells on day 14 p.i. (B,C) Cumulative data (mean ± SD) showing the MFI of Bcl-6 expression and total number of Bcl-6+ parasite-specific CD4 T cells in rat IgG and α-OX40-treated mice on day 14 p.i. (D,E) Representative plots (D) and cumulative data (E) showing the kinetics of CD95+GL-7+CD19+ B cell responses in rat IgG and α-OX40-treated mice. (F) Flow cytometric plots (left) and cumulative data (right) depicting the fraction of CD138hiIgDloCD19+B220+ plasmablasts on d14 p.i. (G) Kinetics of the plasmablast response (**P < 0.01). (H,I) Cumulative data showing MSP119-specific IgM (H) and MSP119-specific IgG1 (I) endpoint titers for rat IgG and α-OX40-treated mice on days 14 and 23 p.i. Cumulative data (mean ± SD) in (B–D,F) were pooled from 2 independent experiments and were analyzed using unpaired Student’s t tests. Data in (E,G) are from at least 5 mice per group per time point and were analyzed using multiple t tests and correcting for multiple comparisons using the Holm-Sidak method. Data in (E,G–I) are representative of 3 independent experiments. Symbols in (B–D, F, H–I) represent individual mice. See also Figure S4.
Although Tfh cell numbers were expanded after α-OX40 treatment, compared to rIgG treatment, the total numbers of splenic GC B cells were not statistically different between days 14 and 28 p.i. (Fig 4D,E). However, α-OX40-treated mice harbored significantly higher numbers of splenic CD138+IgDlo/− plasmablasts on day 14 p.i. (Fig 4F,G), a larger fraction of which retained IgM expression (Fig S4I). Further analyses suggested these non-switched plasmablasts derived from extra-follicular responses, as the vast majority (>85%) of CD138+IgDlo/− cells were also GL-7lo/− (Fig S4J). Consistent with numerically expanded populations of IgM+ plasmablasts and elevated serum IL-4, α-OX40-treated mice displayed significantly elevated merozoite surface protein 1 (MSP119)-specific IgM (Fig 4H) and IgG1 (Fig 4I) titers, respectively. As a composite, these data show that, despite expanded Treg populations, enhanced parasite control following therapeutic OX40 stimulation during P. yoelii malaria is associated with enhanced extra-follicular plasmablast formation, secretion of parasite-specific IgM, sustained Tfh responses and GC reactions, and T-dependent parasite-specific antibody class-switching to IgG1.
Simultaneous blockade of PD-1 and stimulation of OX40 during P. yoelii malaria results in dysregulated T follicular helper and germinal center B cell reactions and loss of parasite control
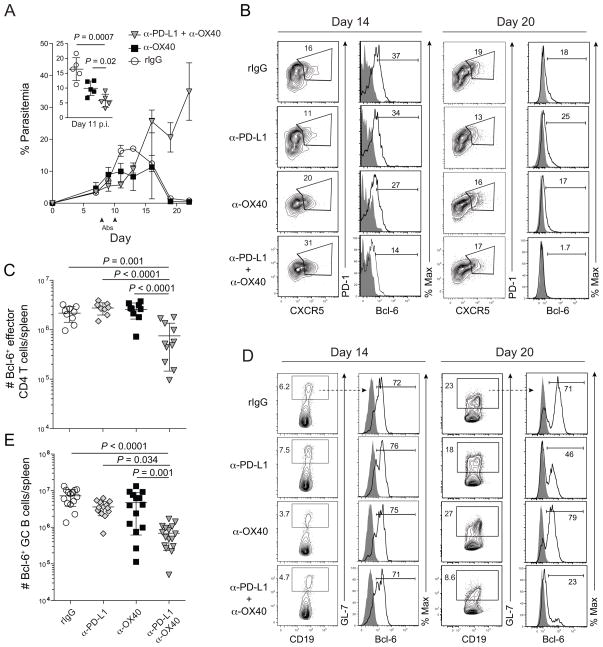
Given that exogenous α-OX40 stimulation can quantitatively enhance parasite-specific CD4 T cell and antibody responses and promote parasite control, and OX40 and PD-1 are coordinately expressed on P. yoelli-specific CD4 T cells (Fig 1E), we reasoned that simultaneously blocking the PD-1 co-inhibitory pathway and stimulating the OX40 co-stimulatory pathway would synergistically enhance CD4 T cell immunity and promote resolution of blood stage P. yoelii infection. To directly test this, we co-administered α-PD-L1 blocking and α-OX40 agonistic mAbs to P. yoelii-infected mice on days 7 and 10 p.i. PD-L1 is a major ligand for the PD-1 co-inhibitory receptor and mAbs targeting PD-L1 have been used extensively to reverse PD-1-mediated T cell exhaustion (Barber et al., 2006; Blackburn et al., 2009; Butler et al., 2012). As predicted, combined PD-L1 blockade and OX40 stimulation (α-PD-L1 + α-OX40) markedly suppressed parasite replication from days 8–11 p.i., even more potently than α-OX40 alone (Fig 5A, inset). For clarity, α-PD-L1 alone was omitted from the graph, but consistent with our previous observations (Butler et al., 2012) this treatment modestly restricted parasite growth (Fig S5A). Despite the early enhanced control of parasite growth in α-PD-L1 + α-OX40 treated mice, parasite burdens subsequently spiked and mice succumbed to hyper-parasitemia by day 24 p.i. (Fig 5A). Importantly, both early suppression and eventual loss of parasite control were also observed when using an α-PD-1 blocking mAb (Fig S5B), supporting that the effects of α-PD-L1 were not due to disruption of interactions between PD-L1 and a second documented PD-L1 binding partner, CD80 (Butte et al., 2007). Notably, the relative degree of parasite control in α-OX40- versus α-PD-L1 + α-OX40-treated mice was not associated with marked changes in total RBC counts (Fig S5C). However, on average, weight loss, anemia and liver damage were most impacted in mice treated with both α-PD-L1 + α-OX40 (Fig S5D–G), consistent with a modest exacerbation of immunopathology.
Figure 5. Coordinate α-OX40 ligation and PD-1 blockade during experimental malaria abrogates T follicular helper and germinal center B cell responses.
(A–E) Mice were infected with 106 P. yoelii-infected RBCs and treated with rat IgG, α-PD-L1, α-OX40, or α-PD-L1 and α-OX40 on days 7 and 10 p.i. (A) Parasitemia kinetics (n=5 mice/group). Data (mean ± SEM) in (A) are representative of 5 independent experiments. (B) Representative dot plots showing PD1+CXCR5+ Tfh cells and histograms showing Bcl-6 expression on days 14 (left) and 20 (right) p.i. (C) Cumulative data (mean ± SD) showing the total number of Bcl-6+ effector CD4 T cells on day 20 p.i. pooled from 2 independent experiments. (D) Representative dot plots showing CD19+GL-7+ B cells and histograms showing Bcl-6 expression on days 14 (left) and 20 (right) p.i. (E) Cumulative data (mean ± SD) showing the total number of Bcl-6+CD19+GL-7+ B cells on 20 p.i. pooled from 3 independent experiments were analyzed using one-way ANOVA while correcting for multiple comparisons via the Tukey method. Data in (B–E) are representative of 3 independent experiments. Symbols in (C,E) represent individual mice. See also Figure S5.
Despite the subtle immunopathologic changes, the eventual loss of parasite control following α-PD-L1 + α-OX40 treatment was unexpected and suggests that functional crosstalk between pathways of OX40 co-stimulatory and PD-1 co-inhibitory signaling may modulate the differentiation and/or survival of one or more CD4 T cell subsets required for Plasmodium resistance. Thus, we next examined whether Tfh cells were affected by the coordinate blockade of PD-1 and stimulation of OX40 signaling during P. yoelii malaria. Although the frequency and total number of CD4 T cells exhibiting a CXCR5+PD-1+ Tfh cell surface phenotype on day 14 p.i. were significantly elevated in both α-OX40- and α-PD-L1 + α-OX40-treated mice (Fig S5H), the number of Bcl-6+ effector CD4 T cells was reduced by >65% in mice treated with α-PD-L1 + α-OX40 (Fig 5B,C), with Bcl-6 expression in CD4 T cells approaching undetectable levels by day 20 p.i. (Fig 5B, right). Consistent with the aborted Bcl-6+ Tfh response, we also found that parasite-specific, T-dependent serum antibody responses (Fig S5I) and the frequency of GL-7+ GC B cells (Fig 5D) were markedly diminished by day 20 p.i. in α-PD-L1 + α-OX40-treated mice. Notably, of the GC B cells that could be detected on day 20 p.i. in α-PD-L1 + α-OX40-treated mice, fewer than 25% expressed the memory marker Bcl-6 (Fig 5D, right). Furthermore, by day 20 p.i. the total number of Bcl-6+ GC B cells was reduced by >95% in P. yoelii-infected mice treated with α-PD-L1 + α-OX40 (Fig 5E). Collectively, these data suggest that pathways of OX40 co-stimulatory receptor signaling may be modified by PD-1 co-inhibitory signaling in CD4 T cells during rodent malaria, which markedly alters the differentiation or maintenance of parasite-specific Tfh cells.
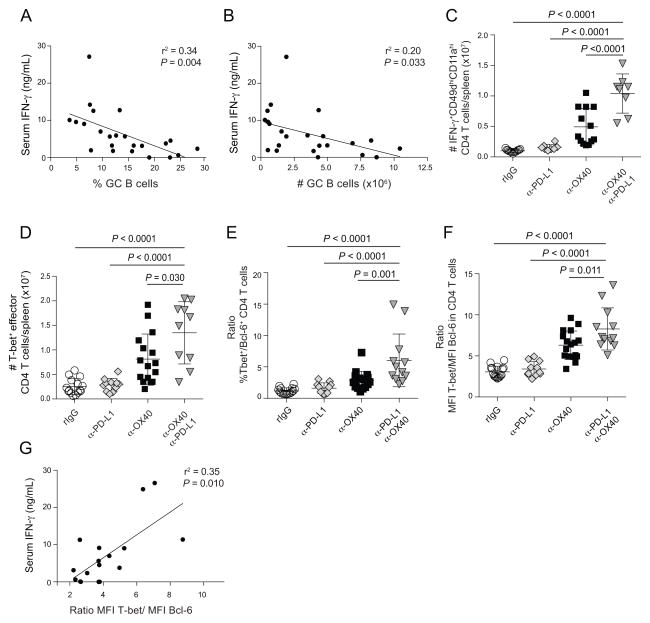
Serum IFN-γ levels negatively correlate with germinal center B cell numbers and are associated with relative amounts of T-bet and Bcl-6 in CD4 effector T cells
Exogenous OX40 stimulation in models of acute bacterial or chronic viral infection abrogates Bcl-6-dependent Tfh cell differentiation (Boettler et al., 2013; Marriott et al., 2014); Tfh loss was linked to enhanced STAT5-dependent, IL-2/CD25 signaling and subsequent expression of Blimp-1 (Boettler et al., 2013), a transcriptional repressor of Bcl-6 (Johnston et al., 2009). During malaria, α-PD-L1 + α-OX40 treatment, but not α-OX40 alone, also profoundly diminished Tfh responses. Thus, we examined these circuits in our control and experimental P. yoelii-infected mice. Notably, we found no increases in CD25 expression on effector CD4 T cells in α-PD-L1 + α-OX40-treated mice (Fig S6A–C) and no relationship between the levels of circulating IL-2 and the magnitude of the GC B cell response in any group (Fig S6D,E). Remarkably, of all the factors we examined, only IFN-γ correlated with loss of GC B cell activity (Fig 6A,B), humoral immunity and pathogen control. Indeed, we observed 10-fold increases in the number of splenic IFN-γ+ (Fig 6C) and T-bet+ (Fig 6D) Th1 effector CD4 T cells on day 14 p.i. in α-PD-L1 + α-OX40-treated mice, relative to control mice treated with rIgG. IFN-γ can act in a ‘feed-forward’ mechanism to promote T-bet expression in Th1 cells (Djuretic et al., 2007; Schulz et al., 2009) and T-bet can directly associate with Bcl-6, thereby limiting the activity of this transcriptional repressor (Oestreich et al., 2012). Morover, Blimp-1 expression is elevated and Tfh gene expression profiles are repressed in CD4 T cells ectopically expressing T-bet in vitro (Oestreich et al., 2012; Oestreich and Weinmann, 2012). Thus, we examined in vivo the relative ratios of T-bet versus Bcl-6 expression in effector CD4 T cells in our experiments. Strikingly, ratios of T-bet/Bcl-6 expression in CD4 T cells (Fig 6E) and T-bet/Bcl-6 MFI (Fig 6F) were markedly elevated in mice administered both α-PD-L1 + α-OX40, which also positively correlated with elevated Blimp-1 expression (Figs S6F–H) and levels of serum IFN-γ (Fig 6G). These data suggest that α-PD-L1 + α-OX40 treatment induces excessive IFN-γ production, which may skew T-bet/Bcl-6 ratios in effector CD4 T cells and limit the development of Tfh and GC B cell responses and humoral immunity during Plasmodium infection.
Figure 6. Circulating interferon-gamma levels negatively correlate with T-dependent humoral immunity during experimental malaria.
Mice were infected with 106 P. yoelii-infected RBCs and treated with rat IgG, α-PD-L1, α-OX40, or α-PD-L1 and α-OX40. (A–B) Negative correlations between serum IFN-γ and the frequency (A) and total number (B) of GC B cells. Data are pooled from 3 independent experiments spanning days 14 to 20 p.i. and were analyzed using linear regression. (C–D) Number of IFN-γ+ (C) and T-bet+ (D) effector CD11ahiCD49dhi CD4 T cells on day 14 p.i. (E–F) Ratios of T-bet+ versus Bcl-6+ (E) and MFI of T-bet versus Bcl-6 (F) in effector CD4 T cells on day 14 p.i. (G) Positive correlation between circulating IFN-γ and T-bet/Bcl-6 ratios in effector CD4 T cells on day 20 p.i. Data in (G) are pooled from 2 independent experiments. Data (mean ± SD) in (C–F) were analyzed using one-way ANOVAs while correcting for multiple comparisons via the Tukey method. All data are representative of 3 independent experiments. Symbols in represent individual mice. See also Figure S6.
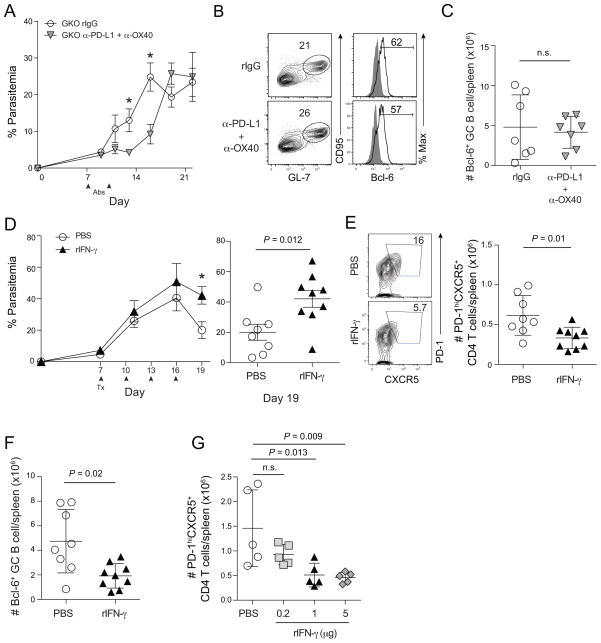
Excessive IFN-γ is necessary and sufficient to dysregulate T follicular helper cell and germinal center B cell responses during P. yoelii malaria
To formally examine a ‘feed-forward’ role for IFN-γ in limiting Tfh activity and GC B cell reactions, we repeated our experiments in P. yoelii-infected GKO mice. As we observed for α-OX40 alone (Fig 3G), the transient protective effects of α-PD-L1 + α-OX40 were independent of IFN-γ expression (Fig 7A). Although mice lacking IFN-γ eventually succumb to chronic parasitemia (data not shown), we identified that Bcl-6+ Tfh cell populations (data no shown) and GC B cell responses. Fig 7B,C were numerically identical in rIgG- and α-PD-L1 + α-OX40-treated GKO mice. Strikingly, despite equivalent cell numbers, both the quantity and quality of T-dependent secreted antibody were enhanced in GKO mice after administration of both α-PD-L1 + α-OX40 mAbs (Fig S7A). Consistent with our studies in GKO mice, neutralization of IFN-γ in P. yoelii-infected WT mice treated with α-PD-L1 + α-OX40 prolonged survival (not shown) and was associated with reduced parasitemia on average (Fig S7B). To determine whether excess IFN-γ can be sufficient to impede T-dependent humoral immunity during Plasmodium infection, we administered 1 μg of recombinant IFN-γ to P. yoelii-infected mice every 3 days from day 7 to 16 p.i. Consistent with the apparent deleterious effect of IFN-γ on parasite control following α-PD-L1 + α-OX40 treatment, P. yoelii-infected mice administered recombinant IFN-γ also failed to fully control parasite replication (Fig 7D). By day 20 p.i., mice receiving exogenous IFN-γ harbored 2-fold fewer Tfh and GC B cells (Fig 7E,F), resulting in markedly reduced T-dependent parasite-specific antibody titers (Fig S7C). Importantly, we also observed dose-dependent effects of rIFN-γ on the number and frequency of Tfh cells (Fig 7G), GC B cells (Fig S7D) and parasite burdens (Fig S7E). Collectively, these data show that IFN-γ can function as a negative regulator of Tfh differentiation or maintenance, which impairs parasite-specific antibody responses during malaria. Although IFN-γ is necessary for eventual resolution of blood stage Plasmodium infection, these data illustrate that in excess IFN-γ sharply limits the survival or differentiation of Tfh cells that are required to sustain GC B cell reactions and the secretion of protective anti-Plasmodium antibodies.
Figure 7. Interferon-gamma constrains T follicular helper responses and humoral immunity during experimental malaria.
Mice were infected with 106 P. yoelii-infected RBCs and treated with rat IgG or α-PD-L1 and α-OX40 (A–C), or with PBS or rIFN-γ (D–G) at the indicated time (arrows). (A) Parasitemia (mean ± SEM) in IFN-γ knockout (GKO) mice treated with rat IgG (n=5) or α-PD-L1 + α-OX40 (n=5). (B) Representative plots (left) and histograms (right) depicting the frequency of GC B cells expressing Bcl-6 on day 20 p.i in P. yoelii-infected GKO mice treated with rat IgG or α-PD-L1 and α-OX40. (C) Number of GC B cells on day 20 p.i. Data (mean ± SD) in (C) were analyzed using an unpaired Student’s t test. (D) Parasitemia kinetics (left) and parasitemia on day 19 p.i. (right) in P. yoelii-infected mice treated with PBS or 1 μg rIFN-γ. Data (mean ± SEM) in (D) are from 9 mice per group. (E) Representative plots (left) and summary data (right) showing the fraction and total number of CXCR5+PD-1+ effector CD4 T cells on day 20 p.i. in mice treated with PBS or 1 μg rIFN-γ. (F) Total number of CD95+GL-7+ GC B cells on day 20 p.i. in mice treated with PBS or 1 μg rIFN-γ. Data (mean ± SD) in (F) were analyzed using unpaired Student’s t tests. (G) Dose-dependent effects of rIFN-γ on the magnitude of the PD-1hiCXCR5+ CD4 T cell responses on day 20 p.i. Data (mean ± SD) in (G) were analyzed using one-way ANOVA while correcting for multiple comparisons via the Tukey method. Data (mean ± SEM) in (A,D) were analyzed using multiple t tests while correcting for multiple comparisons using the Holm-Sidak method. (*P < 0.05). Data in (A–G) are representative of 3 independent experiments. See also Figure S7.
Discussion
Here we provide insight into how Plasmodium parasites impact host immunity and identify that a specific host-factor, OX40, can be therapeutically targeted to enhance immune-mediated resistance to Plasmodium infection. Moreover, our experiments reveal a previously unknown mechanism of how biological crosstalk between co-inhibitory and co-stimulatory pathways can affect CD4 Tfh cell activity during experimental malaria.
OX40 is classically regarded as a transiently expressed co-stimulatory receptor that regulates the proliferation and survival of T cells via activation of NFκB, Akt, survivins, and Bcl-2- and Bcl-xL-dependent anti-apoptotic pathways (Song et al., 2007; Song et al., 2005; Song et al., 2008). Following acute infections, OX40 is down-regulated 5–6 days after initial T cell activation (Croft, 2010). However, during chronic infections, such as with LCMV clone 13, OX40 expression is reportedly sustained for weeks (Boettler et al., 2013; Boettler et al., 2012; Salek-Ardakani et al., 2008). Our data show that OX40 and PD-1 are upregulated on activated CD4 T cells during human P. falciparum infection, with parasite burden closely linked to PD-1 expression. We also show that OX40 is coordinately expressed with PD-1 and LAG-3 co-inhibitory receptors on parasite-specific CD4 T cells in rodents.
We tested the biological relevance of these observations by therapeutically administering agonistic α-OX40 biologics to P. yoelii-infected mice at the start of the second week of infection, which resulted in the substantial accumulation of multiple subsets of parasite-specific effector CD4 T cells. Ligation of OX40 also resulted in enhanced parasite control that correlated with a profound expansion of CD138+IgD− plasmablasts and elevated serum titers of MSP119-specific IgM and IgG1. Notably, the protective effects of α-OX40 were also independent of IFN-γ, suggesting that parasite control was independent of Th1 effector activity. Although our data show correlations between enhanced humoral immunity following α-OX40 stimulation, additional CD4 T cell-intrinsic, IFN-γ-independent pathways that potentially contribute to protection warrant further investigation.
Notably, our results contrast sharply with the LCMV cl13 model, wherein exogenous α-OX40 administered on day 7 p.i. had no effect on the phenotype, number or function of virus-specific CD4 T cells. Thus, compared to CD4 T cells responding to persistent LCMV infection, Plasmodium-specific CD4 T cells remain receptive to co-stimulatory signals well after their initial activation. Regarding therapeutic strategies, a wider window to manipulate effector CD4 T cell subset differentiation or activity may therefore exist during malaria compared to chronic viral infection.
Our data also reveal clear differences in the effects of α-OX40 ligation on Plasmodium-specific versus bacterial- or virus-specific CD4 T cells. For example, administration of α-OX40 mAb 1–2 days after either Listeria monocytogenes (Lm) or LCMV infection prevented Tfh cell differentiation and abrogated humoral immunity (Boettler et al., 2013; Marriott et al., 2014), whereas exogenous α-OX40 on days 7 and 10 post-P. yoelii infection expanded parasite-specific Tfh cells. This discrepancy likely reflects the time point at which the agonist was administered. Most parasite-specific CD4 T cells may have already committed to various pathways of differentiation after the first week of P. yoelii infection. Thus, rather than skewing CD4 T cell differentiation, α-OX40 administered on days 7 and 10 p.i. promoted the expansion of multiple parasite-specific T helper subsets, including Tfh cells, which in turn promoted T-dependent humoral immunity. Consistent with our results, agonistic α-OX40 delivered to simian immunodeficiency virus (SIV)-infected macaques also triggered marked increases in pathogen-specific antibody titers. Furthermore, in a phase 1 clinical trial, patients receiving α-OX40 mAb as an experimental therapy for metastatic solid malignancy exhibited significantly elevated antibody responses against KLH and tetanus reporter antigens (Curti et al., 2013). Multiple studies have reported impaired T-dependent humoral immunity in OX40-deficient rodents (Boettler et al., 2012; Gaspal et al., 2005), which is also consistent with the positive role of OX40 signaling in regulating the induction humoral immunity. Collectively, these data suggest that OX40 co-stimulation can either promote or constrain humoral immunity depending on when OX40 signaling is triggered relative to initial T cell activation and the nature of the infection or immunization. Therefore, strategies designed to modulate OX40 signaling warrant consideration of these relationships.
The extent to which pathways of co-stimulation and co-inhibition integrate to shape T cell function is an area that has only recently received attention, even though biologics targeting both types of pathways are already in independent clinical trials (Chen and Flies, 2013). Thus, we additionally explored whether simultaneously blocking PD-1 and activating OX40 signaling during P. yoelii malaria would synergistically enhance CD4 T cell activity and parasite control. Strikingly, combined administration of biologics to manipulate these pathways resulted in the complete loss of parasite-specific Tfh cell activity and GC B cell reactions, which rendered mice profoundly susceptible to Plasmodium. The α-OX40-induced block in LCMV-specific Tfh development noted above was linked to enhanced IL-2 signaling and STAT5-mediated transactivation of Blimp-1 (Boettler et al., 2013). Although Blimp-1 was elevated in CD4 T cells in α-OX40 + α-PD-L1-treated mice, we found that serum IFN-γ levels, but not IL-2, most strongly correlated with loss of GC B cells.
T-bet is required for Th1 activity and expression of IFN-γ by Th1 T cells (Szabo et al., 2000). Although T-bet activity is commonly associated with IL-12 signaling, IFN-γ signaling through the IFN-γ receptor (IFNGγ)-STAT1 pathway also induces T-bet expression (Afkarian et al., 2002). Thus, IFN-γ can further potentiate T-bet expression through a feed-forward mechanism (Djuretic et al., 2007; Schulz et al., 2009). Notably, T-bet can directly complex with Bcl-6 and thereby block Bcl-6-mediated suppression of Blimp-1 (Oestreich et al., 2012; Oestreich and Weinmann, 2012). As a consequence, when present in excess, T-bet indirectly represses Tfh gene signatures. Given this, we examined the relative ratios of T-bet versus Bcl-6 expression in effector CD4 T cells in vivo. Mice treated with both α-PD-L1 + α-OX40 exhibited the highest ratios of T-bet to Bcl-6 MFI and also expressed highest levels of Blimp-1, indicative of reduced Bcl-6 activity. Remarkably, in mice lacking IFN-γ, α-PD-L1 + α-OX40 treatment triggered sustained GC reactions, increased MSP119-specific antibody titers and enhanced parasite control. Furthermore, administration of rIFN-γ was sufficient to decrease the frequencies and total numbers of Tfh and GC B cells and reduce parasite-specific antibody titers. Of note, excessive IFN-γ is reported to drive pathologically large Tfh responses that contribute to autoimmunity (Lee et al., 2012). While the reasons for these discrepancies are unclear, it is possible that IFN-γ may restrict the formation or maintenance of Tfh during systemic infection compared to the genetic model of lupus. Indeed, our data support that excessive IFN-γ (and excess T-bet) can be detrimental to T-dependent humoral immunity during prolonged P. yoelii infection, which is consistent with a clinical study of P. falciparum-infected patients that showed that high concentrations of serum IFN-γ were associated with low parasite-specific IgM and IgG titers (Fernandes et al., 2008). Dissecting the relative contribution of these pathways during human malaria, in which P. falciparum-specific antibody responses are relatively short-lived, particularly in children, (Portugal et al., 2013), remains an important goal.
Collectively, our results reveal the profound impact that crosstalk between PD-1 co-inhibitory and OX40 co-stimulatory pathways has on immune modulation during experimental malaria. Although α-OX40 treatment alone promoted T-bet expression in effector CD4 T cells, numbers of Bcl-6+ Tfh cells were expanded and humoral immunity were also enhanced. Strikingly, an uncoupling of PD-1 co-inhibitory signaling during concurrent exogenous OX40 stimulation led to a significant increase in T-bet and a dramatic reduction in Bcl-6 expression and activity in parasite-specific CD4 T cells. These data demonstrate that PD-1 signaling can function as a potent negative regulator of Th1 effector differentiation, which in turn facilitates the generation of a more effective Tfh response. Furthermore, we provide evidence that co-stimulatory pathways may directly counterbalance co-inhibitory pathways, as exogenous OX40 stimulation markedly enhanced numerical and functional attributes of P. yoelii-specific CD4 T cells. A greater dissection of the crosstalk and counterbalance between co-stimulatory and co-inhibitory pathways will inform future immunotherapeutic strategies focused on eliciting distinct pathways of CD4 T cell differentiation during infection or cancer.
Experimental Procedures
Ethical approval
The Ethics Committee of the Faculty of Medicine, Pharmacy and Dentistry at the University of Sciences, Technique and Technology of Bamako, and the IRB of the NIAID, NIH approved the human components of this study. Written informed consent was obtained from adult participants and from the parents or guardians of participating children.
Mali study site and design
The field study was conducted in the rural village of Kalifabougou, Mali where intense P. falciparum transmission occurs from June through December each year. The cohort study has been described in detail previously (Tran et al., 2013). Briefly, 695 healthy children and adults aged 3 months to 25 years were enrolled in an ongoing cohort study in May 2011. Exclusion criteria at enrollment included a hemoglobin level <7 g/dL, axillary temperature ≥37.5°C, acute systemic illness, underlying chronic disease, or use of antimalarial or immunosuppressive medications in the past 30 days. For this study we identified 26 children aged 6–12 years who were asymptomatic and not infected with P. falciparum (by PCR) in May 2013, and who also had peripheral blood mononuclear cells (PBMCs) available at the end of the 6-month dry season (May 2013) and 7 days after treatment of their first febrile malaria episode of the ensuing 6-month malaria season. Clinical malaria was defined as ≥2,500 asexual parasites/μL, an axillary temperature of ≥37.5°C and no other cause of fever discernible by physical exam. Malaria episodes were detected prospectively by self-referral to the study clinic and through weekly active clinical surveillance. All individuals with signs and symptoms of malaria and any level of parasitemia detected by light microscopy were treated according to the Malian National Malaria Control Program guidelines. PBMCs from healthy adult donors (n = 15) and a blood bank in the USA were analyzed. Demographic and travel history data were not available from these anonymous donors, but prior P. falciparum exposure was unlikely. Blood samples were obtained for research use after written informed consent was obtained from all study participants enrolled in a protocol approved by the IRB noted above (protocol 99-CC-0168).
Human and mouse T cells
For human studies, at each of the aforementioned time points, blood samples were obtained by venipuncture into sodium citrate–containing tubes (Vacutainer CPT Tubes; BD), PBMCs were isolated according to the manufacturer’s instructions and were frozen in FBS containing 7.5% (vol/vol) dimethyl sulfoxide (DMSO; Sigma-Aldrich), kept at −80 °C for 24 h, and then stored at −196 °C in liquid nitrogen until use. Human and mouse cells were analyzed with flow cytometry as detailed in Supplementary Methods.
Mice, parasites and biologics
C57BL/6 mice (8 weeks, 18–22 g) were purchased from Jackson Laboratories and housed in the Biomedical Sciences Building at OUHSC. The OUHSC IACUC approved all experiments. Plasmodium yoelii (clone 17XNL, obtained from MR4, ATCC) was routinely passaged through mosquitoes and mouse infections were initiated by serial transfer of 106 parasite-infected red blood cells via tail vein injection. Parasitemia was measured using flow cytometry as described (Malleret et al., 2011). Giemsa staining of thin blood smears was done in parallel. At the indicated times, mice were injected i.p. with 200 μg α-CD4 (GK1.5), 500 μg of α-IFN-γ (XMG1.2), 200 μg α-PD-L1 (10F.9G2), 50 μg of α-OX40 Ab (OX86), 200 μg α-PD-L1 and 50 μg α-OX40, or 200 μg α-PD-1 (RMP1-14) and 50 μg α-OX40, or equivalent amounts of rat IgG. All biologics were acquired from BioXcell. Recombinant IFN-γ was acquired from Tonbo.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism 6 software (GrapPad). Specific tests of statistical significance are detailed in figure legends.
Supplementary Material
Acknowledgments
We thank Lauren Zenewicz, Mark Lang and Linda Thompson for critical review, Chaonan Hsu for technical assistance, and the Flow Cytometry Laboratory at OUHSC. This work was supported by grants from the NIH (T32AI007633 to R.A.Z.; 1K22AI099070 to N.S.B.), the American Heart Association (13BGIA17140002 to N.S.B.) and the Presbyterian Health Foundation of Oklahoma City (PHF Seed Grant to N.S.B.). N.S.B. is also an OK-INBRE scholar supported by a grant from the NIH/NIGMS (8P20GM103447). The study in Mali was supported by the Division of Intramural Research, NIH/NIAID.
Footnotes
Author Contributions
R.A.Z., N.O-A., P.D.C. and N.S.B designed and performed the experiments; R.A.Z., N.O-A., J.J.G., D.I.K., P.D.C. and N.S.B performed the experiments and analyzed the data; A.O., B.T. and P.D.C. coordinated the field studies and study site participants; J.L. maintained parasite passage through Anopheles mosquitoes and analyzed data; and R.A.Z. and N.S.B wrote the manuscript.
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nature immunology. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- Akiba H, Oshima H, Takeda K, Atsuta M, Nakano H, Nakajima A, Nohara C, Yagita H, Okumura K. CD28-independent costimulation of T cells by OX40 ligand and CD70 on activated B cells. J Immunol. 1999;162:7058–7066. [PubMed] [Google Scholar]
- Amante FH, Good MF. Prolonged Th1-like response generated by a Plasmodium yoelii-specific T cell clone allows complete clearance of infection in reconstituted mice. Parasite Immunol. 1997;19:111–126. doi: 10.1046/j.1365-3024.1997.d01-187.x. [DOI] [PubMed] [Google Scholar]
- Amante FH, Good MF. Experimental asexual blood stage malaria immunity. Curr Protoc Immunol. 2001;Chapter 19(Unit 19):14. doi: 10.1002/0471142735.im1904s29. [DOI] [PubMed] [Google Scholar]
- Bansal-Pakala P, Jember AG, Croft M. Signaling through OX40 (CD134) breaks peripheral T-cell tolerance. Nature medicine. 2001;7:907–912. doi: 10.1038/90942. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature immunology. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettler T, Choi YS, Salek-Ardakani S, Cheng Y, Moeckel F, Croft M, Crotty S, von Herrath M. Exogenous OX40 stimulation during lymphocytic choriomeningitis virus infection impairs follicular Th cell differentiation and diverts CD4 T cells into the effector lineage by upregulating Blimp-1. J Immunol. 2013;191:5026–5035. doi: 10.4049/jimmunol.1300013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettler T, Moeckel F, Cheng Y, Heeg M, Salek-Ardakani S, Crotty S, Croft M, von Herrath MG. OX40 facilitates control of a persistent virus infection. PLoS pathogens. 2012;8:e1002913. doi: 10.1371/journal.ppat.1002913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nature immunology. 2012;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nature reviews Immunology. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annual review of immunology. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curti BD, Kovacsovics-Bankowski M, Morris N, Walker E, Chisholm L, Floyd K, Walker J, Gonzalez I, Meeuwsen T, Fox BA, et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer research. 2013;73:7189–7198. doi: 10.1158/0008-5472.CAN-12-4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza JB, Williamson KH, Otani T, Playfair JH. Early gamma interferon responses in lethal and nonlethal murine blood-stage malaria. Infection and immunity. 1997;65:1593–1598. doi: 10.1128/iai.65.5.1593-1598.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuretic IM, Levanon D, Negreanu V, Groner Y, Rao A, Ansel KM. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nature immunology. 2007;8:145–153. doi: 10.1038/ni1424. [DOI] [PubMed] [Google Scholar]
- Elliott SR, Kuns RD, Good MF. Heterologous immunity in the absence of variant-specific antibodies after exposure to subpatent infection with blood-stage malaria. Infection and immunity. 2005;73:2478–2485. doi: 10.1128/IAI.73.4.2478-2485.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes AA, Carvalho LJ, Zanini GM, Ventura AM, Souza JM, Cotias PM, Silva-Filho IL, Daniel-Ribeiro CT. Similar cytokine responses and degrees of anemia in patients with Plasmodium falciparum and Plasmodium vivax infections in the Brazilian Amazon region. Clinical and vaccine immunology : CVI. 2008;15:650–658. doi: 10.1128/CVI.00475-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspal FM, Kim MY, McConnell FM, Raykundalia C, Bekiaris V, Lane PJ. Mice deficient in OX40 and CD30 signals lack memory antibody responses because of deficient CD4 T cell memory. J Immunol. 2005;174:3891–3896. doi: 10.4049/jimmunol.174.7.3891. [DOI] [PubMed] [Google Scholar]
- Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165:3043–3050. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- Horne-Debets JM, Faleiro R, Karunarathne DS, Liu XQ, Lineburg KE, Poh CM, Grotenbreg GM, Hill GR, MacDonald KP, Good MF, et al. PD-1 dependent exhaustion of CD8+ T cells drives chronic malaria. Cell reports. 2013;5:1204–1213. doi: 10.1016/j.celrep.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Illingworth J, Butler NS, Roetynck S, Mwacharo J, Pierce SK, Bejon P, Crompton PD, Marsh K, Ndungu FM. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J Immunol. 2013;190:1038–1047. doi: 10.4049/jimmunol.1202438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Miller LH. Cellular mechanisms in immunity to blood stage infection. Immunol Lett. 1990;25:109–114. doi: 10.1016/0165-2478(90)90100-5. [DOI] [PubMed] [Google Scholar]
- Langhorne J. The role of CD4+ T-cells in the immune response to Plasmodium chabaudi. Parasitol Today. 1989;5:362–364. doi: 10.1016/0169-4758(89)90113-0. [DOI] [PubMed] [Google Scholar]
- Langhorne J, Simon-Haarhaus B, Meding SJ. The role of CD4+ T cells in the protective immune response to Plasmodium chabaudi in vivo. Immunol Lett. 1990;25:101–107. doi: 10.1016/0165-2478(90)90099-c. [DOI] [PubMed] [Google Scholar]
- Lee SK, Silva DG, Martin JL, Pratama A, Hu X, Chang PP, Walters G, Vinuesa CG. Interferon-gamma excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity. 2012;37:880–892. doi: 10.1016/j.immuni.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Malleret B, Claser C, Ong AS, Suwanarusk R, Sriprawat K, Howland SW, Russell B, Nosten F, Renia L. A rapid and robust tri-color flow cytometry assay for monitoring malaria parasite development. Scientific reports. 2011;1:118. doi: 10.1038/srep00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott CL, Mackley EC, Ferreira C, Veldhoen M, Yagita H, Withers DR. OX40 controls effector CD4 T-cell expansion, not follicular T helper cell generation in acute Listeria infection. European journal of immunology. 2014 doi: 10.1002/eji.201344211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley MS, Sahu BR, Lotspeich-Cole L, Majam V, Thao Pham P, Sengupta Banerjee A, Kozakai Y, Morris SL, Kumar S. T-bet modulates the antibody response and immune protection during murine malaria. European journal of immunology. 2014;44:2680–2691. doi: 10.1002/eji.201344437. [DOI] [PubMed] [Google Scholar]
- Oestreich KJ, Mohn SE, Weinmann AS. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nature immunology. 2012;13:405–411. doi: 10.1038/ni.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich KJ, Weinmann AS. T-bet employs diverse regulatory mechanisms to repress transcription. Trends in immunology. 2012;33:78–83. doi: 10.1016/j.it.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pombo DJ, Lawrence G, Hirunpetcharat C, Rzepczyk C, Bryden M, Cloonan N, Anderson K, Mahakunkijcharoen Y, Martin LB, Wilson D, et al. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet. 2002;360:610–617. doi: 10.1016/S0140-6736(02)09784-2. [DOI] [PubMed] [Google Scholar]
- Portugal S, Pierce SK, Crompton PD. Young lives lost as B cells falter: what we are learning about antibody responses in malaria. J Immunol. 2013;190:3039–3046. doi: 10.4049/jimmunol.1203067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EM, Wahl S, Perkins DJ, Schofield L. Regulating immunity to malaria. Parasite Immunol. 2006;28:35–49. doi: 10.1111/j.1365-3024.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- Salek-Ardakani S, Moutaftsi M, Crotty S, Sette A, Croft M. OX40 drives protective vaccinia virus-specific CD8 T cells. J Immunol. 2008;181:7969–7976. doi: 10.4049/jimmunol.181.11.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz EG, Mariani L, Radbruch A, Hofer T. Sequential polarization and imprinting of type 1 T helper lymphocytes by interferon-gamma and interleukin-12. Immunity. 2009;30:673–683. doi: 10.1016/j.immuni.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Song J, Salek-Ardakani S, So T, Croft M. The kinases aurora B and mTOR regulate the G1-S cell cycle progression of T lymphocytes. Nature immunology. 2007;8:64–73. doi: 10.1038/ni1413. [DOI] [PubMed] [Google Scholar]
- Song J, So T, Cheng M, Tang X, Croft M. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity. 2005;22:621–631. doi: 10.1016/j.immuni.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Song J, So T, Croft M. Activation of NF-kappaB1 by OX40 contributes to antigen-driven T cell expansion and survival. J Immunol. 2008;180:7240–7248. doi: 10.4049/jimmunol.180.11.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Tran TM, Li S, Doumbo S, Doumtabe D, Huang CY, Dia S, Bathily A, Sangala J, Kone Y, Traore A, et al. An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57:40–47. doi: 10.1093/cid/cit174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- W.H.O. World Malaria Report 2013 2013 [Google Scholar]
- Walker LS, Gulbranson-Judge A, Flynn S, Brocker T, Lane PJ. Co-stimulation and selection for T-cell help for germinal centres: the role of CD28 and OX40. Immunology today. 2000;21:333–337. doi: 10.1016/s0167-5699(00)01636-4. [DOI] [PubMed] [Google Scholar]
- Walker LS, Gulbranson-Judge A, Flynn S, Brocker T, Raykundalia C, Goodall M, Forster R, Lipp M, Lane P. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. The Journal of experimental medicine. 1999;190:1115–1122. doi: 10.1084/jem.190.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.