Summary
Patients with Langerhans cell histiocytosis (LCH) refractory to conventional chemotherapy have a poor outcome. There are currently two promising treatment strategies for high-risk patients: the first involves the combination of 2-chlorodeoxyadenosine and cytarbine; the other approach is allogeneic haematopoietic stem cell transplantation (HSCT). Here we evaluated 87 patients with high-risk LCH who were transplanted between 1990–2013. Prior to the year 2000, most patients underwent HSCT following myeloablative conditioning (MAC): only 5 of 20 patients (25%) survived with a high rate (55%) of transplant-related mortality (TRM). After the year 2000 an increasing number of patients underwent HSCT with reduced intensity conditioning (RIC): 49/67 (73%) patients survived, however, the improved survival was not overtly achieved by the introduction of RIC regimens with similar 3-year probability of survival after MAC (77%) and RIC transplantation (71%). There was no significant difference in TRM by conditioning regimen intensity but relapse rates were higher after RIC compared to MAC regimens (28% vs. 8%, p=0.02), although most patients relapsing after RIC transplantation could be salvaged with further chemotherapy. HSCT may be a curative approach in 3 out of 4 patients with high risk LCH refractory to chemotherapy: the optimal choice of HSCT conditioning remains uncertain.
Keywords: Langerhans cell histiocytosis, allogeneic transplantation, conditioning regimen intensity, treatment failure, survival
INTRODUCTION
Langerhans cell histiocytosis (LCH) is a rare proliferative disease of cells that share phenotypic characteristics with Langerhans cells (LC), the primary antigen presenting cell of the epidermis. The pathogenesis of LCH is not well understood yet and there is controversy concerning the key question as to whether LCH is a reactive inflammatory or a neoplastic disease (Laman et al, 2003). Recent molecular analysis of human LCH samples as well as mouse models suggests that the cell of origin may not be the epidermal LC itself but a myeloid-derived precursor (Berres et al, 2014). Furthermore, genomic screening has revealed the presence of activating BRAF mutations in the majority of patient specimens, and more recently ARAF mutations (Nelson et al, 2014) and somatic MAP2KI mutations (Brown et al, 2014) in BRAF-negative LCH patients. Taken together, these observations now point to LCH as a myeloid neoplasm (Badalian-Very et al, 2012).
The clinical presentation of LCH is highly variable, ranging from benign localized disease to a disseminated aggressive disease that causes significant mortality. Patients less than 2 years of age at onset with “risk organ” involvement including the haematopoietic system, liver, spleen or lung, and whose disease is refractory to conventional chemotherapy have a poor outcome, with survival rates less than 30% at 2 years (The French Langerhans' Cell Histiocytosis Study Group, 1996; Minkov et al, 2002; Maria Postini et al, 2012). The pattern of disease progression is unpredictable, with partial responses to treatment followed by further progression and deterioration. In patients with disseminated disease, liver dysfunction with coagulopathy, together with refractory thrombocytopenia and sometimes a secondary haemophagocytic syndrome, is associated with a high risk of lethal haemorrhage. Profound neutropenia and a poor nutritional status due to malabsorption and enteral protein loss contribute to the increased risk of sepsis (Favara et al, 2002).
There are currently two promising treatment strategies for these high-risk patients. The first involves the combination of 2-chlorodeoxyadenosine and cytarbine (Bernard et al, 2005; Weitzman et al, 2009). 2-chlorodeoxyadenosine is a deoxyadenosine analogue that is phosphorylated by the enzyme deoxyctidine kinase, leading to the formation of nucleotides that are resistant to deoxyadenosine aminase, and their accumulation results in inhibition of DNA synthesis and cell death. Cytarabine is a drug also phosphorlyated by deoxyctidine kinase and has been used successfully together with vincristine and prednisolone in children with disseminated LCH and organ dysfunction (Egeler et al, 1993). The main limitation of the study combining and cytarabine was the small number of patients enrolled, nevertheless the results were significantly better than the reported historical outcome for this group of patients (Imamura et al, 2010).
The other promising approach is allogeneic haematopoietic stem cell transplantation (HSCT) because of its strong immunomodulatory effects (Ringdén et al, 1987; Ringdén et al, 1997). Our review of the literature identified 29 paediatric patients with risk organ involvement who underwent allogeneic HSCT using myeloablative conditioning (MAC) (Steiner et al, 2005). The overall survival was 48% but transplant-related mortality (TRM) was exceedingly high, at 45%. Reduced intensity conditioning (RIC) regimens have been developed to reduce morbidity and TRM in paediatric patients with non-malignant disorders, particularly in patients with significant co-morbidities (Chiesa & Veys, 2014). This approach was published by Steiner et al. (2005) in nine high-risk LCH patients. The conditioning was generally well tolerated. Two patients died at 50 and 69 days after transplantation, and 7 patients survived free of disease to a median follow-up of 390 days after transplantation, including one patient who experienced graft rejection with autologous reconstitution. Similarly, good outcomes with RIC regimens and allogeneic transplantation have also been confirmed in a further 13 LCH patients (Kudo et al, 2010; Cooper et al, 2008; Hatakeyama et al, 2010).
A prospective trial set up to examine the use RIC transplant regimens in LCH (LCH-HCT-2006) had to be closed due to poor recruitment, although many centres applied the proposed RIC protocol. On the one hand, RIC regimens may reduce the previously reported high rates of TRM, but on the other hand if LCH is in fact a myeloid malignancy some studies in adult acute myeloid leukaemia have suggested an increased risk of relapse after RIC (Schmid et al, 2012). The data of 87 HSCTs for LCH reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) and the European Blood and Marrow Transplant (EBMT), two large transplant registries, shows that in about one third RIC regimens had been used. In order to examine the influence of conditioning intensity on outcome of transplant with particular reference to TRM, donor chimerism and disease status we performed a retrospective analysis of these patients.
PATIENTS AND METHODS
Data Source
The CIBMTR and EBMT are voluntary working groups of transplant centres that contribute data on consecutive allogeneic and autologous transplants. Participating centres are required to report all transplants consecutively to avoid any selection bias. Patients are followed longitudinally until death or lost to follow-up. All patients provided written informed consent for data submission and research participation. The Institutional Review Boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study.
Eligibility Criteria
Patients with the diagnosis of LCH and who had received allogeneic transplantation were identified in the databases of the CIBMTR and EBMT. Donors included human leucocyte antigen (HLA)-matched siblings, HLA-mismatched relatives and HLA-matched or mismatched unrelated donors. Transplants occurred in Europe or North America between 1990 and 2013.
Outcomes
The primary outcome of interest was TRM, defined as death in the absence of disease recurrence or progressive disease. Secondary outcomes included haematopoietic recovery (time to neutrophil recovery, ≥0.5 × 109/l for 3 consecutive days, and platelet recovery, ≥20 × 109/l without transfusion for 7 days), acute graft-versus-host disease (GVHD) (Prezpiorka et al, 1995), chronic GVHD (Flowers et al, 1999), donor chimerism, recurrent disease and overall survival. Death from any cause was considered an event for overall survival and surviving patients were censored at last follow up.
Statistical Methods
The incidence of neutrophil and platelet recovery, acute and chronic GVHD, TRM and relapse was calculated using the cumulative incidence estimator. For neutrophil and platelet recovery and acute and chronic GVHD, death without the event was the competing risk. For TRM, progressive or recurrent disease was the competing risk, and for relapse, TRM was the competing risk (Gooley et al, 1999). The probability of overall survival was calculated using the Kaplan-Meier estimator (Klein & Moeschberger, 2003). The 95% confidence interval (CI) was generated using log transformation. All p-values were two-sided and were considered significant when ≤0.05. All analyses were performed in SAS version 9.3 (Cary, NC, USA).
RESULTS
Patient and transplant characteristics
Patients were transplanted between 1990 and 2013. As RIC regimens were predominantly used after 1999, Tables IA and IB shows the characteristics of the study population by transplant period, i.e., prior to 2000 and thereafter.
Table 1.
| A. Characteristics of patients with Langerhans' cell histiocytosis transplanted between 1990 and 1999 | ||
|---|---|---|
| Myeloablative Conditioning |
Reduced intensity Conditioning |
|
| Characteristics | N | N |
| Patients | 18 | 2 |
| Transplant centres | 16 | 1 |
| Age, years | ||
| ≤ 2 | 16 | 1 |
| 3 – 5 | 2 | 1 |
| Sex, male | 11 | 2 |
| Interval between diagnosis and transplant | ||
| < 1 year | 9 | - |
| 1 – 2 years | 9 | 1 |
| > 2 years | 2 | 1 |
| Conditioning regimen | ||
| Total body irradiation + cyclophosphamide* | 7 | 2 |
| Total body irradiation + other agent | 1 | - |
| Busulfan + cyclophosphamide** | 9 | - |
| Busulfan + other agent | 1 | - |
| Graft-versus-host disease prophylaxis | ||
| Ex vivo T-cell depletion | 2 | - |
| Ciclosporin + methotrexate | 7 | 1 |
| Ciclosporin + steroids | 5 | 1 |
| Ciclosporin alone | 3 | - |
| Not reported | 1 | - |
| Donor source | ||
| HLA-matched sibling | 8 | - |
| HLA-mismatched relative | 2 | 2 |
| HLA-matched unrelated adult donor | 3 | - |
| HLA-mismatched adult donor / cord blood | 5 | - |
| Graft source | ||
| Bone marrow | 16 | - |
| Peripheral blood | - | 2 |
| Umbilical cord blood | 2 | - |
| B. Characteristics of patients transplanted between 2000 and 2013. | ||
|---|---|---|
| Myeloablative Conditioning |
Reduced intensity Conditioning |
|
| Characteristics | Number | Number |
| Patients | 41 | 26 |
| Transplant centres | 33 | 19 |
| Age, years | ||
| ≤ 2 | 25 | 20 |
| 3 – 5 | 8 | 5 |
| 6 – 10 | 5 | - |
| 11 – 20 | 2 | 1 |
| > 20 | 1 | - |
| Interval between diagnosis and transplant | ||
| < 1 year | 20 | 12 |
| 1 – 2 years | 11 | 12 |
| 2 – 4 years | 5 | 2 |
| > 4 years | 5 | - |
| Conditioning regimen | ||
| Total body irradiation + cyclophosphamide* | 3 | 1 |
| Total body irradiation + other agent** | 4 | 1 |
| Busulfan + cyclophosphamide*** | 22 | - |
| Busulfan + fludarabine**** | 9 | 1 |
| Busulfan + other agent | 1 | - |
| Melphalan + fludarabine***** | 2 | 22 |
| Cyclophosphamide + fludarabine | - | 1 |
| Graft-versus-host disease prophylaxis | ||
| Ex vivo T-cell depletion | 3 | - |
| Ciclosporin + methotrexate | 15 | 4 |
| Ciclosporin + mycophenolate | 4 | 9 |
| Ciclosporin + steroids | 5 | 4 |
| Ciclosporin alone | 7 | 7 |
| Tacrolimus + methotrexate | 1 | 1 |
| Other agents | 4 | 1 |
| Not reported | 2 | - |
| Donor / graft source | ||
| HLA-matched sibling | 16 | 10 |
| Bone marrow | 13 | 10 |
| Peripheral blood | 2 | - |
| Cord blood | 1 | - |
| Donor / graft source | ||
| HLA-mismatched relative | 1 | 3 |
| Bone marrow | 1 | __ |
| Peripheral blood | __ | 3 |
| HLA-matched unrelated donor | 8 | 4 |
| Bone marrow | 4 | 1 |
| Peripheral blood | 1 | 1 |
| Cord blood | 3 | 2 |
| HLA-mismatched unrelated donor | 9 | 2 |
| Bone marrow | 1 | __ |
| Peripheral blood | __ | 1 |
| Cord blood | 8 | 1 |
| HLA-matching not reported unrelated donor | 7 | 7 |
| Bone marrow | 2 | 1 |
| Peripheral blood | 3 | 3 |
| Cord blood | 2 | 3 |
N=3 received anti-thymocyte globulin
N=1 received anti-thymocyte globulin
Myeloablative conditioning regimens
N=1 received anti-thymocyte globulin
N=2 received anti-thymocyte globulin
N=10 received anti-thymocyte globulin; N=2 received alemtuzumab
N=6 received anti-thymocyte globulin; N=1 received alemtuzumab
N=2 received alemtuzumab
Reduced intensity conditioning regimens
N=1 received anti-thymocyte globulin
N=1 received anti-thymocyte globulin
N=4 received anti-thymocyte globulin; N=14 received alemtuzumab
HLA, human leucocyte antigen
Twenty patients were transplanted prior to 2000. Of these, 18 patients received MAC regimens (Table IA). The median age at transplantation for this cohort was 2 years (range 1 – 3 years). Patients received total body irradiation (TBI)-containing regimens (n=8) or busulfan-containing non-irradiation regimens (n=10). Most patients received bone marrow grafts and ciclosporin-containing GVHD prophylaxis regimens. The median follow-up of patients was 14.5 years. During this period only two patients received RIC; these patients were aged 2 years and 3 years at transplantation and both received peripheral blood progenitor cells from HLA-mismatched relatives. Both patients received low dose TBI, cyclophosphamide with anti-thymocyte globulin and ciclosporin-containing GVHD prophylaxis.
Table IB shows the characteristics of patients transplanted between 2000 and 2013 by transplant conditioning regimen intensity. Forty-one patients received MAC regimens and 26, RIC regimens. The median age at transplantation for both treatment groups was 2 years. Eighty percent (33 of 41) patients in the MAC group and all except one patient (25 of 26) in the RIC group were aged less than or equal to 5 years at transplantation. Most patients (36 of 41) in the MAC group and all patients in the RIC group were transplanted within 4 years of their diagnosis. Busulfan with cyclophosphamide or fludarabine was the predominant regimen for MAC and for RIC it was melphalan with fludarabine. Donors were matched siblings in one third and about 50% of the patients were transplanted from matched unrelated donors. In half of the cases the graft was bone marrow, 20 patients received cord blood grafts. GVHD prophylaxis consisted of ciclosporin in most cases. The median follow-up of patients was 6 years after MAC and 5 years after RIC transplantation.
Outcomes
Transplant period prior to 2000
Of the 18 patients who received MAC regimens, 14 achieved neutrophil recovery but only seven patients achieved platelet recovery. Grade II – IV acute GVHD was reported for 8 patients (n = 2 grade II and n = 6 grade III) and chronic GVHD was reported for 2 patients. Thirteen of 18 patients have died; of these 5 had achieved full donor chimerism, 1, mixed chimerism and 1 graft failure. Chimerism was not available for 6 patients. Three patients died from recurrent disease and 10 from transplant-related complications (infection, n=2, GVHD, n=1, interstitial pneumonitis, n=1, organ failure, n=2, other causes, n=4). Of the 5 patients who are alive, with a median follow-up of 14.5 years, 4 patients displayed full donor chimerism and all were disease-free at last contact. Although both patients who received a RIC regimen achieved neutrophil recovery, neither achieved platelet recovery. One patient reported grade II acute GVHD. Both patients died early after transplantation, one from infection, and the cause of death is unknown in one case.
Transplant period 2000 – 2013
There were no significant differences in haematopoietic recovery rates after MAC compared to RIC transplantation, but recovery rates were slow for both treatment groups with most patients engrafting by two months after transplantation (Table II). The incidence of grade II – IV acute GVHD was marginally higher after MAC compared to RIC regimens. However, there were no differences in either the incidence of grade III – IV acute GVHD or chronic GVHD (Table II).
Table II.
Univariate analysis of transplantation outcomes for the period 2000 – 2013.
| Myeloablative conditioning | Reduced intensity conditioning | ||||
|---|---|---|---|---|---|
| Outcome | Number events/evaluable |
Probability (95% CI) |
Number events/evaluable |
Probability (95% CI) |
p-value |
| Neutrophil recovery | 40/41 | 20/25 | 0.17 | ||
| Day 28 | 78% (64–89) | 64% (45–81) | |||
| Day 60 | 95% (87–99) | 80% (62–93) | |||
| Platelet recovery | 28/34 | 13/17 | 0.35 | ||
| Day 100 | 76% (61–89) | 65% (42–85) | |||
| Grade II–IV acute GVHD | 14/39 | 2/23 | 0.05 | ||
| Day 100 | 31% (17–46) | 9% (1–23) | |||
| Grade III–IV acute GVHD | 8/40 | 2/23 | 0.35 | ||
| Day 100 | 18% (7–31) | 9% (1–23) | |||
| Chronic GVHD | 10/41 | 5/26 | 0.53 | ||
| 3 years | 27% (14–42) | 21% (6–41) | |||
| Transplant-related mortality | 8/41 | 5/26 | 0.78 | ||
| Day 100 | 13% (4–24) | 13% (3–28) | |||
| 3 years | 15% (6–28) | 21% (7–40) | |||
| Recurrent disease | 3/41 | 6/26 | 0.05 | ||
| 3 years | 8% (2–18) | 28% (11–49) | |||
| Disease-free survival | 11+/41 | 11+/26 | 0.15 | ||
| 3 years | 77% (63–89) | 51% (30–71) | |||
| Overall survival | 11++/41 | 7++/26 | 0.89 | ||
| Day 100 | 83% (70–93) | 85% (69–96) | |||
| 3 years | 77% (62–89) | 71% (52–87) | |||
Denotes patients with disease recurrence or death
Denotes death
95% CI, 95% confidence interval; GVHD, graft-versus-host disease
Thirty of 41 patients after MAC transplantation are alive. Of the 11 patients who died, 9 patients had achieved 100% donor chimerism, 1 patient experienced graft failure and chimerism status was not reported for 1 patient. Three patients died from recurrent disease and 8 patients from transplant-related complications (infection, n=2, GVHD, n=1, interstitial pneumonitis, n=3, organ failure, n=1 and secondary malignancy, n=1). All patients who relapsed died, including one patient who underwent a second transplant.
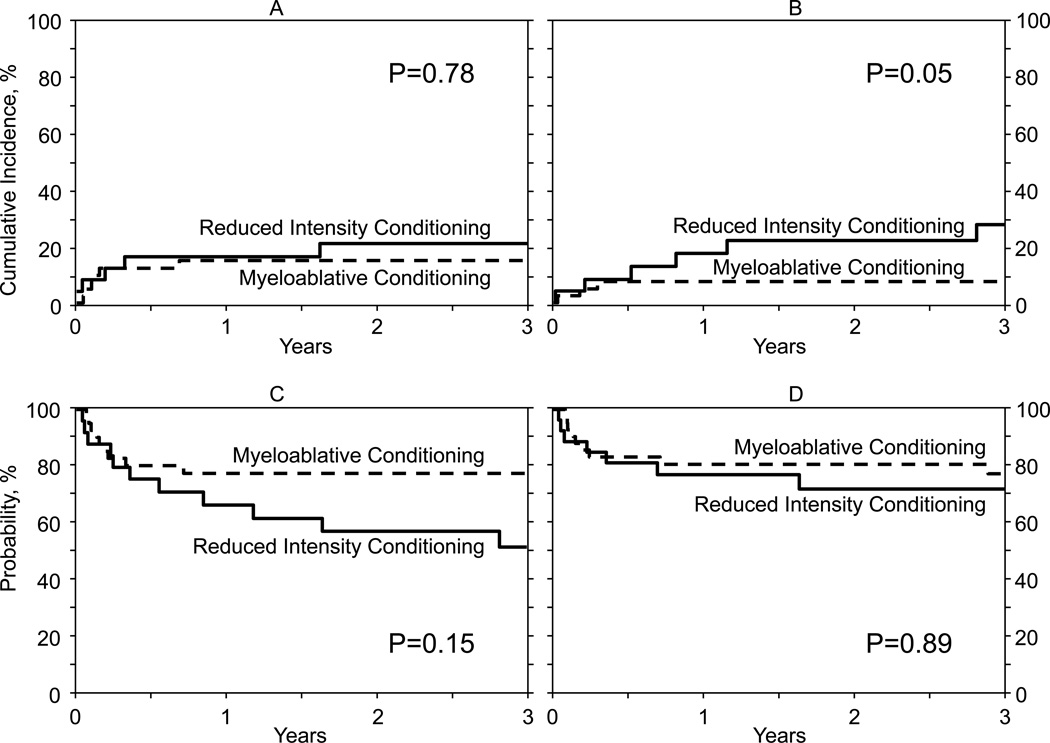
Nineteen of 26 patients are alive after RIC transplantation. This includes 4 of 6 patients who relapsed after HSCT but remain alive after receiving chemotherapy as salvage treatment. Of the 7 patients who died, 1 died from recurrent disease and the remaining 6, from transplant-related causes (GVHD, n=1, infection, n=2, organ failure, n=1, and, n=2 unknown but disease status at last follow-up was reported as being in remission). The probabilities of overall and disease-free survival after MAC and RIC transplantation were similar. There were no significant differences in terms of TRM, however, relapse rates after RIC transplantation were marginally higher compared to after MAC transplantation (Table II, Figure 1A–D).
Figure 1.
A: The cumulative incidence of transplant-related mortality by conditioning regimen intensity
B: The cumulative incidence of relapse by conditioning regimen intensity
C: The probability of disease-free survival by conditioning regimen intensity
D: The probability of overall survival by conditioning regimen intensity
Second allogeneic transplant for transplant period 1990 – 2013
Six patients received a second transplantation for graft failure (n=4) or recurrent disease (n=2). Of the patients re-transplanted for graft failure, one had undergone MAC and 3 patients RIC regimens for their first transplantation. One of three patients who received a RIC regimen for first transplantation died within 3 months after the second transplantation and the remaining two patients are alive, 9 years and 2 years after their first transplantation (interval between first and second transplantation was 1 month and 2 months, respectively). The patient with graft failure following MAC died within 3 months after a second transplant. Of the two patients who underwent a second transplant for recurrent disease, one died 18 months after the second transplant and the other is alive, 8 years after the first transplant and 6 years after the second transplantation.
DISCUSSION
Here we present the largest study examining the use of HSCT in patients with multisystem refractory LCH. Although we did not conduct a comparative analysis of outcomes by transplant period it is worth noting that survival has improved over time, with 75% of patients surviving after the year 2000 compared to 28% in the earlier cohort. We hypothesize that the better survival after 2000 is attributed to better supportive care after transplantation rather than the increasing use of RIC transplant conditioning regimens, as there were no significant differences in rates of TRM after MAC and RIC transplantation. However, it is possible that clinicians elected to treat higher risk patients with RIC procedures due to concern over the risk of TRM, and this might also explain the apparent lack of benefit of a RIC procedure. Relapse rates were marginally higher after RIC transplantation compared to that after MAC transplantation. This could also be explained by the inclusion of higher risk patients in the RIC cohort. Alternatively, if there was no selection bias in the patient groups, this could also reflect the behaviour of myeloid malignancy with an increased relapse rate after RIC transplantation (Schmid et al, 2012). It is of note, however, that 4 of 6 patients who relapsed after RIC HSCT attained a further remission with chemotherapy, implying these patients are relatively easy to salvage. The overall survival of patients in the cohort transplanted after 2000 is similar to that reported in a small single centre study of 15 patients from Japan, which also included RIC and MAC regimens (Kudo et al, 2010). Neither that report nor the current analysis found differences in survival between patients receiving MAC and RIC transplantation.
The observed lower incidence of acute GVHD in the setting of RIC transplantation despite the higher number of patients receiving peripheral blood progenitor cells reflects the increased use of serotherapy with RIC regimens. The use of serotherapy might also explain the trend to higher infectious-related deaths in the RIC regimen group although the numbers are very small. Most patients achieved 100% donor chimerism; amongst those with mixed donor chimerism, there was a significant increase in disease progression or relapse; this is again reminiscent of the behaviour of a malignant disease, although, amongst 6 patients who had autologous reconstitution following graft rejection, only one had recurrence of LCH: a finding previously reported in a single patient by Steiner et al (2005). The majority of patients achieved sufficient donor chimerism to cure a genetic disease, such as haemophagocytic lymphohistiocytosis (HLH), where 10–20% donor chimerism usually secures ongoing disease remission (Terrell & Jordan, 2013). Relapse despite good levels of donor chimerism and continuous remission despite autologous reconstitution distinguish LCH from HLH where disease control would be expected with mixed donor chimerism and disease recurrence in all patients following graft rejection (Ardeshna et al, 2001).
RIC transplantation has been shown to have a survival advantage in HLH (Marsh et al, 2010) mainly due the reduction of TRM. Given the caveats above, the results of the current analysis do not overtly suggest that TRM is reduced in LCH by the use of RIC transplantation and, indeed, the incidence of disease relapsing after transplantation may be increased in the RIC setting. If LCH is confirmed to be a myeloid malignancy and on the basis of the findings of this study, another approach to HSCT in LCH might be via the use of myeloablative but reduced toxicity protocols, such as the addition of thiotepa to fludarabine and melphalan (Lang et al, 2014) or treosulfan/fludarbine/thiotepa (Bernardo et al, 2012).
One of the major limitations of this study was that the precise indication and/or timing of HSCT and details of preceding therapy in each patient were not specified. Kudo et al (2010) reported a 10-year overall survival rate amongst 9 patients with risk organ involvement at diagnosis of 55.6% (7/9 undergoing HSCT within 12 months of diagnosis), whereas 6 patients without risk organ involvement have all survived with no evidence of disease (3/6 patients undergoing HSCT 7 years or later after diagnosis). Bernard et al (2005) reported that 7/10 patients with refractory LCH had achieved sustained complete remission after treatment with 2-chlorodeoxyadenosine and cytarabine alone. In the study by Kudo et al (2010), 2 patients who failed to respond to the combination of 2-chlorodeoxyadenosine and cytarabine underwent HSCT, and one is alive with no disease after RIC transplantation. Consequently both 2-chlorodeoxyadenosine /cytarabine salvage therapy or HSCT are reasonable approaches in refractory LCH with disease in risk organs. At several of the institutions represented by the authors of this manuscript the current policy is to treat refractory LCH with 2-chlorodeoxyadenosine and cytarabine. At the same time a search is initiated for potential HSCT donors. If there is no response after 2 courses of salvage chemotherapy and a donor has been identified, patients proceed to HSCT. If there is a partial response to initial treatment, 4 courses of 2-chlorodeoxyadenosine / cytarabine are given and HSCT considered if response inadequate. Both salvage options, 2-chlorodeoxyadenosine/cytarabine and HSCT following a conditioning regimen with alemtuzumab, fludarabine and melphelan, will be studied prospectively in the LCH-IV International Collaborative Treatment Protocol. Unfortunately, the expected numbers are too small to allow a randomization between the two treatment options, and both options will be available depending on physician’s choice and donor availability.
In summary, HSCT may be a curative therapy in 3 out of 4 children with high risk LCH that is refractory to chemotherapy, however the optimal choice of conditioning intensity remains uncertain. Although the current analysis showed similar survival rates after MAC and RIC transplantation, we are unable to adjust for potential selection bias and several unknown or unmeasured factors that may have influenced survival. The optimal study to explore the effects of regimen intensity on transplantation can be best achieved through carefully controlled clinical trials.
Acknowledgments
FUNDING SOURCE
Public Health Service Grant U24-CA076518 from the National Cancer Institute, the National Heart, Lung and Blood Institute and the National Institute of Allergy and Infectious Diseases and contract HHSH250201200016C with Health Resources and Services Administration.
Footnotes
AUTHORSHIP
Contribution: PAV and ME designed the study. WH and ME analysed and interpreted the data. PAV and ME drafted the manuscript. VN, SB, WH, GB, AB, AD, JHD, GME, RME, AHF, AF, RF, HJ, SK, RK, EL, WHL, SM, GM, PJO, AP, OR, PGS, AS, KV critically reviewed the manuscript. All authors approved the manuscript.
Competing interests: the authors have no competing interests
REFERENCES
- Ardeshna K, Hollifield J, Chessells JM, Veys P, Webb DKH. Outcome for children after failed transplant for primary haemophagocytic lymphohistiocytosis. British Journal of Haematology. 2001;115:949–952. doi: 10.1046/j.1365-2141.2001.03177.x. [DOI] [PubMed] [Google Scholar]
- Badalian-Very G, Vergilio JA, Degar BA, Rodriguez-Galindo C, Rollins BJ. Recent advances in the understanding of Langerhans cell histiocytosis. British Journal of Haematology. 2012;156:163–172. doi: 10.1111/j.1365-2141.2011.08915.x. [DOI] [PubMed] [Google Scholar]
- Bernard F, Thomas C, Bertrand Y, Munzer M, Landman Parker J, Ouache M, Colin VM, Perel Y, Chastagner P, Vermylen C, Donadieu J. Multi-centre pilot study of 2-chlorodeoxyadenosine and cytosine arabinoside combined chemotherapy in refractory Langerhans cell histiocytosis with haematological dysfunction. European Journal of Cancer. 2005;41:2682–2689. doi: 10.1016/j.ejca.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Bernardo ME, Piras E, Vacca A, Giorgiani G, Zecca M, Bertaina A, Pagliara D, Contoli B, Pinto RM, Caocci G, Mastronuzzi A, La Nasa G, Locatelli F. Allogeneic hematopoietic stem cell transplantation in thalassemia major: results of a reduced-toxicity conditioning regimen based on the use of treosulfan. Blood. 2012;120:473–476. doi: 10.1182/blood-2012-04-423822. [DOI] [PubMed] [Google Scholar]
- Berres ML, Lim KP, Peters T, Price J, Takizawa H, Salmon H, Idoyaga J, Ruzo A, Lupo PJ, Hicks MJ, Shih A, Simko SJ, Abhyankar H, Chakraborty R, Leboeuf M, Beltrão M, Lira SA, Heym KM, Bigley V, Collin M, Manz MG, McClain K, Merad M, Allen CE. BRAF-V600E expression in precursor versus differentiated dendritic cells defines clinically distinct LCH risk groups. The Journal of Experimental Medicine. 2014;211:669–683. doi: 10.1084/jem.20130977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NA, Furtado LV, Betz BL, Kiel MJ, Weigelin HC, Lim MS, Elenitoba-Johnson KS. High prevalence of somatic MAP2K1 mutations in BRAF V600E-negative Langerhans cell histiocytosis. Blood. 2014;124:1655–1658. doi: 10.1182/blood-2014-05-577361. [DOI] [PubMed] [Google Scholar]
- Chiesa R, Veys P. Reduced Intensity Conditioning in Paediatric haematopoietic cell Transplantation. In: Thomas AE, Halsey C, editors. Controversies in Paediatric and Adolescent Haematology. Basel, Switzerland: Karger; 2014. pp. 116–134. [Google Scholar]
- Cooper N, Rao K, Goulden N, Webb D, Amrolia P, Veys P. The use of reduced-intensity stem cell transplantation in haemophagocytic lymphohistiocytosis and Langerhans cell histiocytosis. Bone Marrow Transplant. 2008;42(Suppl 2):S47–S50. doi: 10.1038/bmt.2008.283. [DOI] [PubMed] [Google Scholar]
- Egeler RM, de Kraker J, Voute PA. Cytosine-arabinoside, vincristine, and prednisolone in the treatment of children with disseminated Langerhans cell histiocytosis with organ dysfunction: experience at a single institution. Medical and Pediatric Oncology. 1993;21:265–270. doi: 10.1002/mpo.2950210406. [DOI] [PubMed] [Google Scholar]
- Favara BE, Jaffe R, Egeler RM. Macrophage activation and hemophagocytic syndrome in langerhans cell histiocytosis: report of 30 cases. Pediatric and Developmental Pathology. 2002;5:130–140. doi: 10.1007/s10024001-0159-2. [DOI] [PubMed] [Google Scholar]
- Flowers ME, Kansu E, Sullivan KM. Pathophysiology and treatment of graft-versus-host disease. Hematology/Oncology Clinics of North America. 1999;13:1091–1112. doi: 10.1016/s0889-8588(05)70111-8. [DOI] [PubMed] [Google Scholar]
- Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statistics in Medicine. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Hatakeyama N, Hori T, Yamamoto M, Inazawa N, Hirako Y, Tsutsumi H, Suzuki N. Successful treatment of refractory Langerhans cell histiocytosis with pulmonary aspergillosis by reduced-intensity conditioning cord blood transplantation. Pediatric Transplantation. 2010;14:E4–E10. doi: 10.1111/j.1399-3046.2008.01124.x. [DOI] [PubMed] [Google Scholar]
- Imamura T, Sato T, Shiota Y, Kanegane H, Kudo K, Nakagawa S, Nakadate H.,Tauchi H, Kamizono J, Morimoto A. Outcome of pediatric patients with Langerhans cell histiocytosis treated with 2 chlorodeoxyadenosine: a nationwide survey in Japan. International Journal of Hematology. 2010;91:646–651. doi: 10.1007/s12185-010-0558-0. [DOI] [PubMed] [Google Scholar]
- Klein JP, Moeschberger ML. Survival Analysis: Statistical Methods for Censored and Truncated Data. 2nd ed. New York, NY: Springer-Verlag; 2003. [Google Scholar]
- Kudo K, Ohga S, Morimoto A, Ishida Y, Suzuki N, Hasegawa D, Nagatoshi Y, Kato S, Ishii E. Improved outcome of refractory Langerhans cell histiocytosis in children with hematopoietic stem cell transplantation in Japan. Bone Marrow Transplant. 2010;45:901–906. doi: 10.1038/bmt.2009.245. [DOI] [PubMed] [Google Scholar]
- Laman JD, Leenen PJ, Annels NE, Hogendoorn PC, Egeler RM. Langerhans-cell histiocytosis 'insight into DC biology'. Trends in Immunology. 2003;24:190–196. doi: 10.1016/s1471-4906(03)00063-2. [DOI] [PubMed] [Google Scholar]
- Lang P, Teltschik HM, Feuchtinger T, Müller I, Pfeiffer M, Schumm M, Ebinger M, Schwarze CP, Gruhn B, Schrauder A, Albert MH, Greil J, Urban C, Handgretinger R. Transplantation of CD3/CD19 depleted allografts from haploidentical family donors in paediatric leukaemia. British Journal of Haematology. 2014;165:688–698. doi: 10.1111/bjh.12810. [DOI] [PubMed] [Google Scholar]
- Maria Postini A, del Prever AB, Pagano M, Rivetti E, Berger M, Asaftei SD, Barat V, Andreacchio A, Fagioli F. Langerhans cell histiocytosis, 40 years' experience. Journal of Pediatric Hematology/Oncology. 2012;34:353–358. doi: 10.1097/MPH.0b013e318257a6ea. [DOI] [PubMed] [Google Scholar]
- Marsh RA, Vaughn G, Kim MO, Li D, Jodele S, Joshi S, Mehta PA, Davies SM, Jordan MB, Bleesing JJ, Filipovich AH. Reduced-intensity conditioning significantly improves survival of patients with hemophagocytic lymphohistiocytosis undergoing allogeneic hematopoietic cell transplantation. Blood. 2010;116:5824–5831. doi: 10.1182/blood-2010-04-282392. [DOI] [PubMed] [Google Scholar]
- Minkov M, Grois N, Heitger A, Potschger U, Westermeier T, Gadner H. Response to initial treatment of multisystem Langerhans cell histiocytosis: an important prognostic indicator. Medical and Pediatric Oncology. 2002;39:581–585. doi: 10.1002/mpo.10166. [DOI] [PubMed] [Google Scholar]
- Nelson DS, Quispel W, Badalian-Very G, van Halteren AG, van den Bos C, Bovée JV, Tian SY, Van Hummelen P, Ducar M, MacConaill LE, Egeler RM, Rollins BJ. Somatic activating ARAF mutations in Langerhans cell histiocytosis. Blood. 2014;123:3152–3155. doi: 10.1182/blood-2013-06-511139. [DOI] [PubMed] [Google Scholar]
- Prezpiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Concensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- Ringdén O, Ahström L, Lönnqvist B, Båryd I, Svedmyr E, Gahrton G. Allogeneic bone marrow transplantation in a patient with chemotherapy-resistant progressive histiocytosis X. The New England Journal of Medicine. 1987;316:733–735. doi: 10.1056/NEJM198703193161207. [DOI] [PubMed] [Google Scholar]
- Ringdén O, Lönnqvist B, Holst M. 12-year follow-up of allogeneic bone-marrow transplant for Langerhans' cell histiocytosis. The Lancet. 1997;349:476. doi: 10.1016/S0140-6736(05)61189-0. [DOI] [PubMed] [Google Scholar]
- Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R, Stadler M, Kuball J, Cornelissen J, Vorlicek J, Socié G, Falda M, Vindeløv L, Ljungman P, Jackson G, Kröger N, Rank A, Polge E, Rocha V, Mohty M Acute Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119:1599–1606. doi: 10.1182/blood-2011-08-375840. [DOI] [PubMed] [Google Scholar]
- Steiner M, Matthes-Martin S, Attarbaschi A, Minkov M, Grois N, Unger E, Holter W, Vormoor J, Wawer A, Ouachee M, Woessmann W, Gadner H. Improved outcome of treatment-resistant high-risk Langerhans cell histiocytosis after allogeneic stem cell transplantation with reduced-intensity conditioning. Bone Marrow Transplantation. 2005;36:215–225. doi: 10.1038/sj.bmt.1705015. [DOI] [PubMed] [Google Scholar]
- Terrell CE, Jordan MB. Mixed hematopoietic or T-cell chimerism above a minimal threshold restores perforin-dependent immune regulation in perforin-deficient mice. Blood. 2013;122:2618–2621. doi: 10.1182/blood-2013-06-508143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The French Langerhans' Cell Histiocytosis Study Group. A multicentre retrospective survey of Langerhans' cell histiocytosis, 348 cases observed between 1983 and 1993. Archives of Disease in Childhood. 1996;75:17–24. doi: 10.1136/adc.75.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman S, Braier J, Donadieu J, Egeler RM, Grois N, Ladisch S, Pötschger U, Webb D, Whitlock J, Arceci RJ. 2'-Chlorodeoxyadenosine (2-CDA) as salvage therapy for Langerhans cell histiocytosis (LCH). Results of the LCH-S-98 protocol of the Histiocyte Society. Pediatric Blood & Cancer. 2009;53:1271–1276. doi: 10.1002/pbc.22229. [DOI] [PubMed] [Google Scholar]