Abstract
Background
Management of existing aortic insufficiency (AI) and mechanical aortic valves in patients undergoing left ventricular assist device (LVAD) implantation remains controversial. Surgical options to address these issues include closure, repair or replacement of the valve.
Methods
Continuous flow LVAD/BiVAD patients entered into the INTERMACS database between June 2006 to December 2012 were included (n=5,344). Outcomes were compared between patients who underwent aortic valve (AV) closure (n=125), repair (n=95) and replacement (n=85).
Results
Among patients that underwent an aortic valve procedure, actuarial survival was significantly reduced for AV closures (63.2%) compared to AV repairs (76.8%) and replacements (71.8%, p=0.0003). Differences were greater between groups when only INTERMACS level 1-2 patients were analyzed (p=0.003). After multivariate adjustment, aortic valve closure remained a significant risk factor for mortality (HR=1.87, 95% CI=1.39-2.53, p<0.0001). At six to twelve months postoperatively, moderate to severe AI developed in 19%, 5%, 9% and 10% of patients with available echocardiography who underwent repair, closure, replacement and no intervention, respectively (p<0.0001). Competing outcomes demonstrate that at 1-year fewer patients with aortic valve closures were transplanted compared to patients with repairs/replacements (14% vs. 19%). No differences were observed between groups with respect to cause of death, re-hospitalization, right heart failure or stroke.
Conclusions
AV closure was associated with increased mortality when compared to repair or replacement in patients with AI that underwent LVAD insertion. The reasons for this association require further investigation. This is the largest study to date to examine concomitant AV procedures in patients undergoing LVAD insertion.
Keywords: left ventricular assist device, aortic valve, aortic valve closure, aortic valve repair, aortic valve replacement
Introduction
Untreated aortic insufficiency in patients undergoing left ventricular assist device (LVAD) implantation compromises unloading by creating a short circulatory loop whereby blood pumped into the aorta is returned directly to the device through the incompetent valve. Regurgitation through the aortic valve (AV) diminishes systemic perfusion, elevates left heart filling pressures and increases LVAD flow (1, 2). This derangement is compounded by the progression of even minor aortic insufficiency with time on device support (2-8). Furthermore, existing mechanical aortic prostheses convey an increased risk for stroke from thromboembolism (2). For these reasons, moderate or greater aortic regurgitation and existing mechanical aortic valves have historically been considered contraindications to LVAD support (2).
Current strategies to address these problems include AV closure, AV repair and AV replacement, but there is little data on short-or long-term outcomes to guide management (9, 10). This study utilizes the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) Database to compare outcomes for different approaches to concomitant aortic valve procedures during implantation of continuous-flow LVADs (cf-LVAD).
Methods
INTERMACS Database and Study Population
The Interagency Registry of Mechanically Assisted Devices (INTERMACS) is a prospective national registry of approved, durable mechanical cardiac support devices that was established in 2005. INTERMACS represents a collaboration between the NHLBI, the FDA, the Centers for Medicare and Medicaid Services, industry, surgeons and implanting centers (11). Protocols were approved by the institutional review boards at the Data Coordinating Center at the University of Alabama at Birmingham and at each participating hospital, and registry data is monitored by an NIH-appointed independent Observational Study Monitoring Board.
Between June 2006 and December 2012, 6,721 adult patients (aged ≥18) years who received a cf-LVAD were recorded in the INTERMACS database. While INTERMACS does not currently allow publication of brand names for devices, it should be noted that the HeartWare HVAD was only approved in November 2012, so the vast majority of devices in our study were HeartMate II LVADs. After excluding unverified data as described below, 5,204 cf-LVAD patients and 140 BiVAD patients remained in the study for a total cohort of 5,344 patients from 112 different institutions. Of these, 125 patients had an AV closure, 95 had an AV repair, 85 had an AV replacement and 5,039 did not have any AV procedure performed. These four groups constitute the study populations.
INTERMACS Database Audit
Data regarding the type of aortic valve repair and data on aortic valve closure were collected retrospectively. INTERMACS data for patients with a documented concomitant aortic valve repair were audited to determine if patients underwent a repair or closure. Operative notes were reviewed by registry data entry personnel to determine whether the procedure closed the aortic valve completely (closure) or served to eliminate aortic insufficiency while allowing blood to be ejected through the repaired valve. Central closure, or the Park's stitch, was therefore considered a repair (12). Excerpts from operative reports were reviewed by J.O.R. and S.C.S. for all patients listed as having a repair and for any patients for which there was uncertainty as to whether a patient had a closure or a repair. Additionally, data for any patients who were listed in the database as having preoperative moderate to severe aortic insufficiency but no aortic valve procedure were audited for accuracy. We received responses to our audit from 72% of participating centers. Responses were not received from 19 hospitals, and all patients from non-responding hospitals were excluded from the analysis.
Data Collection and Follow-up
Demographic, hemodynamic, comorbidity, and heart failure severity data were collected prior to implant. Data regarding implantation, transplantation or device explant, hospital readmissions and major adverse events (MAEs) were collected prospectively. Routine follow-up data, including echocardiography, was collected at 1 week, 1 month, 3 months, 6 month, 12 months and every 6 months thereafter. Mean follow-up for the study population was 12.33 months. Details regarding any MAEs were reviewed by the INTERMACS Adverse Event Committee and the INTERMACS data monitors, and causes were adjudicated.
Statistics
Summary statistics are presented as percentages for categorical variables and as the mean ± SD for continuous variables. Baseline characteristics were compared between groups using the chi-square test for categorical variables. For continuous variables, the t-test was used for two group comparisons, and a one-way analysis of variance test was used for comparisons of greater than two groups.
The primary outcomes considered were overall and 1-year mortality, rehospitalization, right heart failure, stroke and renal failure. Definitions of these adverse events have been previously published (13). Survival and time to event outcomes were analyzed between groups using Kaplan-Meier plots and curves were compared using the log-rank test. Data were censored at transplantation or device explant due to recovery. Competing outcomes methodology was used to estimate the probability of different possible outcomes over time.
In order to identify risk factors for death and to assess the unadjusted and adjusted effect of aortic valve intervention and aortic valve regurgitation, a multivariable parametric proportional hazard analysis was performed. This method is analogous to Cox proportional hazard regression except that the underlying hazard function is explicitly modeled and tested. A constant hazard function was deemed to be sufficient for the analysis. Stepwise forward selection was utilized to identify the significant (p<0.05) risk factors. Variables examined in this model are detailed in Appendix 1.
Results
Baseline, Clinical and Operative Characteristics
Compared to patients that underwent an AV Procedure (AVP), those without an AVP were younger (p<0.0001), had lower BMIs (p<0.0001), lower BNPs (p=0.002), lower creatinine (p=0.02), less carotid artery disease (p=0.03), fewer strokes (p=0.02), more coronary artery disease (p=0.03), and a less frequent history of prior valve surgery (p<0.0001) (Table 1). Among those without a concomitant AVP, 33.8% of patients had another concomitant surgery. Those without an AVP were also more likely to be listed as BTT (p=0.005), less likely to be listed as destination therapy (p<0.0001), more frequently current smokers (p=0.01) and less commonly married (p=0.0009), male (p=0.0005), and Caucasian (p=0.05) (Table 1). Fewer differences were noted between the different AVP groups (Table 1). Time on cardiopulmonary bypass for each group is listed (Table 1).
Table 1.
Baseline and clinical characteristics
| Pre-implant characteristics | No AV Procedure N=5039 | AV Closure N=125 | AV Repair N=95 | AV Replacement N=85 | p (AVP vs. No AVP) | p (comparing AVP groups) |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age (yrs) | 56.21 ± 13.01 | 62.4 ± 10.23 | 63.8 ± 10.29 | 63.6 ± 11.06 | <0.0001 | 0.58 |
| Male | 79.2% | 86.4% | 88.4% | 88.2% | 0.0005 | 0.88 |
| Married | 66.0% | 73.4% | 74.2% | 79.5% | 0.0009 | 0.58 |
| White | 71.1% | 74.4% | 81.0% | 74.1% | 0.05 | 0.44 |
| Clinical Comorbidities | ||||||
| BMI (kg/m2) | 28.7 ± 6.70 | 27.2 ± 6.53 | 25.2 ± 5.10 | 27.9 ± 5.77 | <0.0001 | 0.006 |
| Aortic Regurgitation (Moderate/Severe) | 2.0% | 35.7% | 38.8% | 47.8% | <0.0001 | 0.27 |
| Mitral Regurgitation (Moderate/Severe) | 58.7% | 55.0% | 62.6% | 47.8% | 0.31 | 0.19 |
| Tricuspid Regurgitation (Moderate/Severe) | 47.1% | 50.9% | 44.2% | 37.3% | 0.56 | 0.21 |
| Carotid artery disease | 8.6% | 10.8% | 15.5% | 12.7% | 0.03 | 0.67 |
| CVA | 6.1% | 12.9% | 8.4% | 5.9% | 0.02 | 0.21 |
| Diabetes | 32.7% | 35.2% | 31.6% | 27.1% | 0.73 | 0.46 |
| Diagnosis CAD | 8.1% | 2.4% | 7.4% | 4.8% | 0.03 | 0.22 |
| History of CABG | 23.3% | 24.0% | 29.5% | 25.9% | 0.25 | 0.66 |
| History of Valve Surgery | 6.6% | 21.6% | 15.8% | 28.2% | <0.0001 | 0.13 |
| ICD | 82.1% | 85.6% | 81.1% | 79.5% | 0.86 | 0.48 |
| NYHA = 4 | 79.0% | 73.6% | 87.0% | 79.2% | 0.81 | 0.06 |
| Pre-COPD | 10.4% | 11.3% | 11.6% | 12.5% | 0.49 | 0.97 |
| Peripheral vascular disease | 6.2% | 9.1% | 11.0% | 6.2% | 0.10 | 0.61 |
| Dialysis | 1.7% | 2.4% | 0.0% | 0.0% | 0.34 | 0.11 |
| RVEF (severely impaired) | 20.3% | 20.6% | 31.6% | 19.1% | 0.26 | 0.25 |
| Current Smoker | 9.3% | 5.7% | 6.5% | 2.5% | 0.01 | 0.45 |
| TIA | 3.1% | 2.4% | 2.1% | 2.4% | 0.45 | 0.99 |
| Preoperative Condition | ||||||
| BTT: Listed | 27.3% | 19.2% | 28.4% | 23.5% | 0.12 | 0.28 |
| BTT: Likely to be listed | 25.2% | 21.6% | 14.7% | 16.5% | 0.005 | 0.38 |
| BTT: Moderately likely to be listed | 10.4% | 9.6% | 6.3% | 8.2% | 0.22 | 0.68 |
| BTT: Unlikely to be listed | 3.3% | 4.0% | 1.1% | 4.7% | 0.99 | 0.32 |
| Destination Therapy | 32.3% | 44.8% | 47.4% | 45.9% | <0.0001 | 0.93 |
| Failure to wean | 1.0% | 0.8% | 0.0% | 1.2% | 0.54 | 0.60 |
| INTERMACS Patient Profile Level 1: Critical Cardiogenic Shock | 14.7% | 15.2% | 10.5% | 9.4% | 0.22 | 0.38 |
| INTERMACS Patient Profile Level 2: Progressive Decline | 40.5% | 36.8% | 48.4% | 34.1% | 0.77 | 0.10 |
| INTERMACS Patient Profile Level 3 | 25.6% | 24.8% | 27.4% | 28.2% | 0.71 | 0.84 |
| INTERMACS Patient Profile Level 4 | 13.3% | 15.2% | 10.5% | 17.7% | 0.58 | 0.38 |
| INTERMACS Patient Profile Level 5 | 3.4% | 3.2% | 3.2% | 5.9% | 0.60 | 0.55 |
| INTERMACS Patient Profile Level 6 | 1.9% | 4.8% | 0.0% | 4.7% | 0.08 | 0.10 |
| INTERMACS Patient Profile Level 7 | 0.7% | 0.0% | 0.0% | 0.0% | 0.16 | 0.99 |
| Inotropes | 81.3% | 77.6% | 85.1% | 72.9% | 0.25 | 0.13 |
| IABP | 29.3% | 33.6% | 29.5% | 20.0% | 0.77 | 0.10 |
| Ventilator | 7.1% | 3.2% | 3.2% | 10.6% | 0.22 | 0.03 |
| ECMO | 2.2% | 2.4% | 0.0% | 3.5% | 0.80 | 0.21 |
| Preoperative Laboratory and Hemodynamic Testing | ||||||
| BNP (pg/mL) | 1213 ± 1102 | 1258 ± 1047 | 1782 ± 1485 | 1506 ± 1425 | 0.002 | 0.20 |
| Cardiac Index (L/min/m2) | 2.29 ± 0.99 | 1.96 ± 0.51 | 2.07 ± 0.88 | 2.32 ± 0.77 | 0.15 | 0.26 |
| Creatinine (mg/dL) | 1.4 ± 0.8 | 1.5 ±0.62 | 1.6 ± 1.24 | 1.46 ± 0.68 | 0.02 | 0.58 |
| INR (international units) | 1.35 ± 0.56 | 1.62 ± 1.73 | 1.38 ± 0.39 | 1.29 ± 0.34 | 0.004 | 0.11 |
| LVEDD | 6.86 ± 1.15 | 6.88 ± 1.15 | 6.9 ± 1.04 | 7.02 ± 0.94 | 0.46 | 0.75 |
| Pulmonary diastolic pressure (mmHg) | 25.5 ± 8.64 | 23.9 ± 9.68 | 26.5 ± 6.90 | 25.6 ± 9.21 | 0.62 | 0.17 |
| Pulmonary systolic pressure (mmHg) | 50.5 ± 14.64 | 47.6 ± 16.11 | 54.7 ± 13.07 | 55.4 ± 15.85 | 0.15 | 0.003 |
| Pulmonary wedge pressure (mmHg) | 24.1 ± 8.36 | 23.6 ± 10.46 | 25.4 ± 7.40 | 26.1 ± 9.51 | 0.22 | 0.33 |
| Pulmonary vascular resistance (woods units) | 2.8 ± 2.45 | 2.3 ± 2.08 | 3.3 ± 2.54 | 2.6 ± 2.01 | 0.57 | 0.06 |
| RA pressure (mmHg) | 13.1 ± 7.83 | 13.2 ± 8.36 | 12.6 ± 7.24 | 12.0 ± 7.55 | 0.52 | 0.67 |
| SGOT/AST (u/L) | 67.3 ± 253.48 | 42.9 ± 41.54 | 75.9 ± 367.6 | 47.9 ± 71.08 | 0.42 | 0.51 |
| SGPT/ALT (u/L) | 77.6 ± 240.36 | 56.8 ± 73.55 | 83.1 ± 437.7 | 51.3 ± 81.12 | 0.35 | 0.67 |
| Total Bilirubin | 1.39 ± 1.56 | 1.35 ± 1.09 | 1.40 ± 1.12 | 1.34 ± 1.58 | 0.80 | 0.94 |
| Operative and Postoperative Variables | ||||||
| Concommitant surgery | 33.8% | 100.0% | 100.0% | 100.0% | <0.0001 | 0.99 |
| CPB Time (minutes) | 91.37 ± 49.6 | 132.7 ± 53.9 | 143.3 ± 73.1 | 161.6 ± 52.8 | < .0001 | .006 |
| ICU/Step Down Unit LOS (days) | 22.7 ± 16.9 | 23.5 ± 17.2 | 27.0 ± 32.1 | 24.2 ± 49.6 | 0.002 | 0.34 |
| Hospital LOS (days) | 24.2 ± 23.6 | 25.2 ± 19.5 | 35.6 ± 31.8 | 29.1 ± 21.21 | 0.006 | 0.05 |
BMI = body mass index, CVA = cerebrovascular accident, CAD =coronary artery disease, CABG = coronary artery bypass grafting, ICD = implantable cardiac defibrillator, NYHA = New York Heart Association, COPD = chronic obstructive pulmonary disease, RVEF = right ventricular ejection fraction, TIA = transient ischemic attack, BTT = bridge to transplant, IABP = intraaortic balloon pump, ECMO = extracorporeal membrane oxygenation, BNP = brain natriuretic peptide, INR = international normalized ratio, LVEDD = left ventricular end diastolic dimension, RA = right atrial, AST = aspartate aminotransferase, ALT = alanine aminotransferase, CPB = cardiopulmonary bypass, ICU = intensive care unit, LOS = length of stay
At least 74.7% (71/95) of AV repair patients had a procedure that included central aortic closure, and 69.5% (66/95) of AV repair patients underwent only central aortic closure. The type of repair was uncertain in 15.8% (15/95) of patients.
RVADs included in this study were all temporary and included 9.3% (13/140) pulsatile devices and 90.7% (127/140) centrifugal devices. Two patients each with temporary RVADs were included in the AV closure (3.2%), repair (2.1%) and replacement (2.4%) groups, and the remaining RVAD patients were part of the group that did not have an AV procedure performed (2.7%).
Patient Survival by Group and Analysis of Competing Outcomes
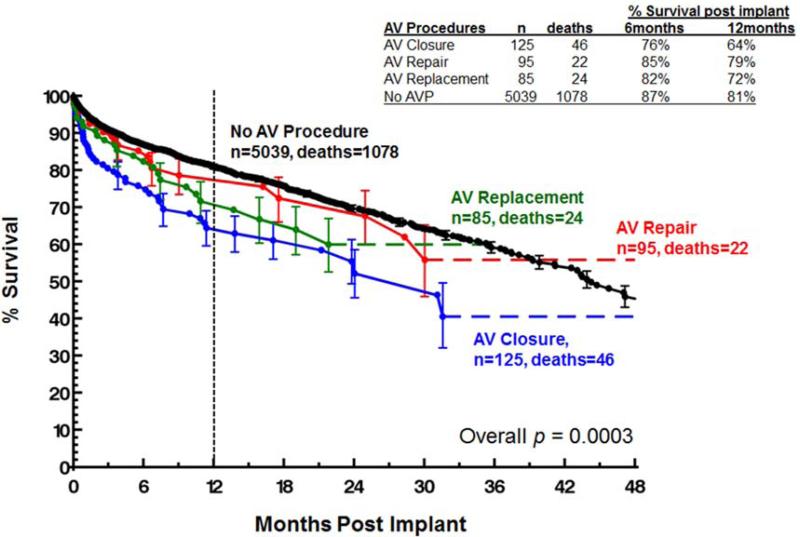
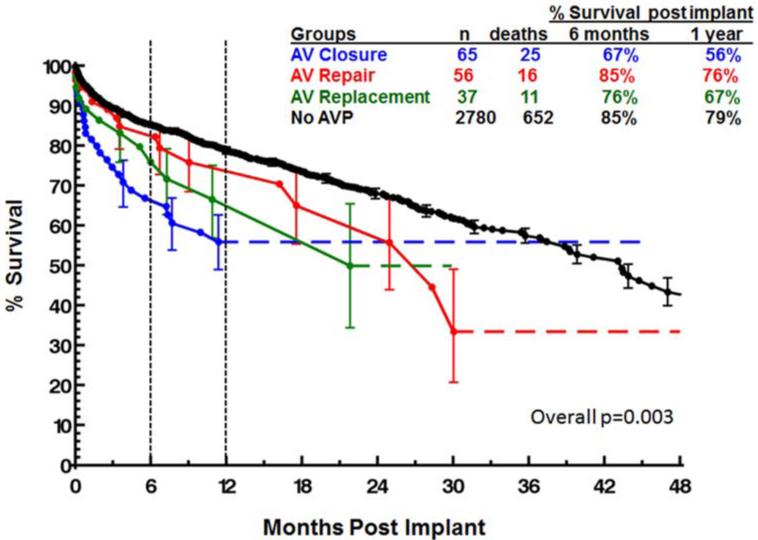
Overall patient survival is compared between groups using a Kaplan-Meier analysis in Figure 1. Survival at 1-year was 81% for patients who did not undergo an AVP, 79% for patients that underwent an AV repair, 72% for patients with an AV replacement, and 64% for those with an AV closure (p=0.0003). Importantly, while there was some late hazard of mortality, the curves separate at the greatest rate within the first three postoperative months. These relative differences held when data was compared between groups for both INTERMACS level 1-2 patients and INTERMACS level 3-7 patients, although the differences were more pronounced in the level 1-2 patients (Figures 2). One year post-implant, survivals for INTERMACS level 1-2 patients with no AVP, AV repair, AV replacement and AV closure were 79%, 76%, 67% and 56% respectively (p=0.003, Figure 2). For these same groups, one year post-implant survivals for INTERMACS level 3-7 patients were 84%, 82%, 75% and 73% (p=0.04).
Figure 1.
Survival by type of aortic valve procedure performed.
Figure 2.
Survival for INTERMACS level 1-2 patients by type of aortic valve procedure.
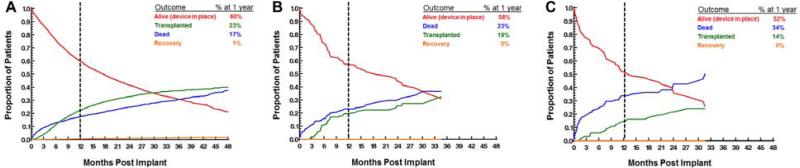
When competing outcomes are analyzed between groups, it is evident that patients with AV closures are substantially less likely to be transplanted by 1-year (14%) when compared to patients with an AV repair/replacement (19%) or no AVP (23%) (Figure 3 A-C). Early (<3 months) and late (≥3 months) causes of mortality are described in Tables 2 and 3. Of note, early mortality in patients with concomitant aortic valve closures is most commonly from respiratory failure (25% [6/24]) and major bleeding (20.8% [5/24]).
Figure 3.
Competing outcomes for patients with (A) no aortic valve procedure, (B) aortic valve repair or replacement and (C) aortic valve closure.
Table 2.
Causes of death <3 months post-implant
| Primary Cause of Death | Closure % (n) | Repair % (n) | Replacement % (n) |
|---|---|---|---|
| Respiratory Failure | 25.0 (6/24) | 0.0 (0/9) | 0.0 (0/10) |
| Major Bleeding | 20.8 (5/24) | 11.1 (1/9) | 10.0 (1/10) |
| Device Malfunction | 0.0 (0/24) | 0.0 (0/9) | 0.0 (0/10) |
| Sudden Unexplained Death | 0.0 (0/24) | 11.1 (1/9) | 0.0 (0/10) |
| Neurological Dysfunction | 8.3 (2/24) | 0.0 (0/9) | 10.0 (1/10) |
| Withdrawal of Support | 4.2 (1/24) | 0.0 (0/9) | 0.0 (0/10) |
| Multisystem Organ Failure | 0.0 (0/24) | 22.2 (2/9) | 0.0 (0/10) |
| Hepatic Dysfunction | 4.2 (1/24) | 0.0 (0/9) | 0.0 (0/10) |
| Renal Dysfunction | 0.0 (0/24) | 11.1 (1/9) | 0.0 (0/10) |
| Major Infection | 0.0 (0/24) | 0.0 (0/9) | 30.0 (3/10) |
| Cardiac Arrhythmia | 0.0 (0/24) | 0.0 (0/9) | 0.0 (0/10) |
| Right Heart Failure | 0.0 (0/24) | 11.1 (1/9) | 0.0 (0/10) |
| Myocardial Infarct | 4.2 (1/24) | 0.0 (0/9) | 0.0 (0/10) |
| Other | 33.3 (8/24) | 33.3 (3/9) | 0.0 (5/10) |
Table 3.
Causes of death ≥3 months post-implant
| Primary Cause of Death | Closure % (n) | Repair % (n) | Replacement % (n) |
|---|---|---|---|
| Respiratory Failure | 4.6 (1/22) | 0.0 (0/13) | 0.0 (0/14) |
| Major Bleeding | 13.6 (3/22) | 0.0 (0/13) | 7.1 (1/14) |
| Device Malfunction | 4.6 (1/22) | 0.0 (0/13) | 7.1 (1/14) |
| Sudden Unexplained Death | 13.6 (3/22) | 30.8 (4/13) | 0.0 (0/14) |
| Neurological Dysfunction | 9.1 (2/22) | 7.7 (1/13) | 7.1 (1/14) |
| Withdrawal of Support | 13.6 (3/22) | 0.0 (0/13) | 0.0 (0/14) |
| Multisystem Organ Failure | 4.6 (1/22) | 7.7 (1/13) | 0.0 (0/14) |
| Hepatic Dysfunction | 0.0 (0/22) | 0.0 (0/13) | 0.0 (0/14) |
| Renal Dysfunction | 0.0 (0/22) | 7.7 (1/13) | 0.0 (0/14) |
| Major Infection | 0.0 (0/22) | 7.7 (1/13) | 14.3 (2/14) |
| Cardiac Arrhythmia | 4.6 (1/22) | 15.4 (2/13) | 0.0 (0/14) |
| Right Heart Failure | 0.0 (0/22) | 0.0 (0/13) | 7.1 (1/14) |
| Myocardial Infarct | 0.0 (0/22) | 0.0 (0/13) | 0.0 (0/14) |
| End Stage Cardiomyopathy | 0.0 (0/22) | 0.0 (0/13) | 14.3 (2/14) |
| Suicide | 0.0 (0/22) | 0.0 (0/13) | 7.1 (1/14) |
| Trauma | 4.6 (1/22) | 0.0 (0/13) | 0.0 (0/14) |
| Other | 27.3 (6/22) | 23.1 (3/13) | 35.7 (5/14) |
Postoperative Complications
Time to rehospitalization, right heart failure, stroke and renal dysfunction were compared between groups using Kaplan-Meier analyses, and no significant differences were noted. While patients that underwent an AV procedure had higher incidences of renal dysfunction at one year compared to those without an AVP (p=0.02), freedom from renal dysfunction was similar between AVP groups (p=0.79). Mean postoperative ICU and hospital lengths of stay are detailed in Table 1.
Multivariate Model
Significant risk factors for postoperative death after multivariate adjustment are listed in Table 4. Of note, AV closure is significantly associated with death in both the unadjusted (HR=2.67 [95% CI: 1.77-4.01], p<0.0001) and adjusted (HR=1.87, [95% CI: 1.39-2.53] p<0.0001) models. After multivariate correction, this risk is on par with the mortality risk imparted from dialysis (HR=1.85, [95% CI: 1.32-2.58] p=0.0003) and only exceeded by that associated with implantation of a Bi-VAD (HR=2.34, [95% CI: 1.80-3.05] p<0.0001).
Table 4.
Multivariate Model for Death After Implant
| Pre-implant Risk Factors for Death after Implant | Hazard Ration (95% CI) | p-value |
|---|---|---|
| Unadjusted | ||
| Aortic regurgitation | 1.10 (0.98, 1.24) | 0.10 |
| AV repair | 0.92 (0.59, 1.43) | 0.70 |
| AV replacement | 1.41 (0.87, 2.30) | 0.17 |
| AV closure | 2.67 (1.77, 4.01) | <0.0001 |
| Adjusted | ||
| Aortic regurgitation | 0.98 (0.87, 1.10) | 0.75 |
| AV repair | 0.83 (0.54, 1.27) | 0.39 |
| AV replacement | 1.36 (0.84, 2.19) | 0.21 |
| AV closure | 1.87 (1.39, 2.53) | <0.0001 |
| Age (years) at time of implant | 1.26 (1.19, 1.35) | <0.0001 |
| Body Mass Index (BMI) | 1.06 (1.01, 1.11) | 0.01 |
| BUN | 1.08 (1.05, 1.11) | <0.0001 |
| Total Bilirubin | 1.33 (1.00, 1.78) | 0.05 |
| Albumin (lower) | 0.32 (0.11, 0.89) | 0.03 |
| Ascites | 1.50 (1.22, 1.84) | 0.0001 |
| INTERMACS Level 1 | 1.23 (1.01, 1.51) | 0.04 |
| INTERMACS Level 2 | 1.30 (1.13, 1.49) | 0.0001 |
| History of CABG | 1.38 (1.21, 1.59) | <0.0001 |
| Dialysis | 1.85 (1.32, 2.58) | 0.0003 |
| Ventilator | 1.28 (1.02, 1.62) | 0.04 |
| Bi-VAD | 2.34 (1.80, 3.05) | <0.0001 |
AV = aortic valve, BUN = blood urea nitrogen, CABG = coronary artery bypass graft, BiVAD = biventricular assist device
Postoperative Development of Aortic Insufficiency
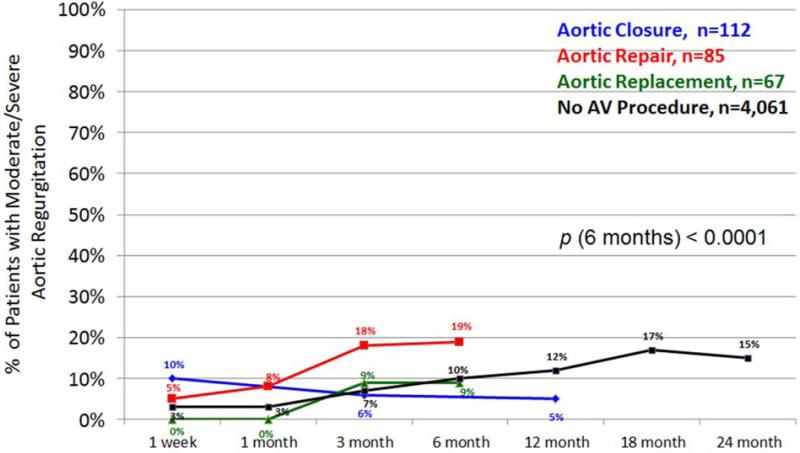
The development of postoperative aortic insufficiency is depicted over time between groups (Figure 4). Despite undergoing an AVP, there is still an incidence of early recurrence of moderate to severe AV insufficiency. By 6 to 12 months, moderate to severe aortic insufficiency has developed in 18% of patients with AV repairs, 10% of patients with no AV procedure, 9% of patients with AV replacements and 5% with AV closures (p<0.0001).
Figure 4.
Postoperative recurrence of moderate/severe aortic insufficiency by type of aortic valve procedure performed. Note: if the total n for a group at any given time point is <20 then it is not plotted in the figure.
Discussion
The best approach for dealing with aortic insufficiency at the time of cf-LVAD implantation has been debated in the literature with no clear consensus on whether to perform AV repair, replacement or closure. The primary finding of this study is that AV closure significantly increases mortality, whereas patients who receive an AV repair have short- and long-term survival that is comparable to cf-LVAD patients that did not undergo an AVP. The limitation to AV repair is a higher incidence of postoperative aortic insufficiency.
Previous studies have disagreed on whether mortality after concomitant AVP increases (9, 14, 15), with some studies demonstrating either equivalent or improved survival (1, 9, 10, 16). However, the majority of reports are from single center studies with fewer than 20 varied AVPs included in their analyses. The largest of these studies utilized data from the HeartMate II bridge to transplant (n=470) and destination therapy (n=636) clinical trials, which completed enrollment in January 2010 and included 80 total AVPs (18 repairs, 32 closures and 30 replacements) (14). In that study, 180-day mortality was significantly higher for patients undergoing isolated AVPs (29.1% vs. 15.9%, p=0.023). Long term survival at 1- and 2- years was also reduced with an AVP (75% vs. 57% and 64% vs. 43%, respectively, p=0.001), and these differences held even after adjusting for baseline risk using the HeartMate II risk score (p=0.002). Mortality in our population as a whole was lower, possibly reflecting the improved results that have been achievable since enrollment in those trials was completed. We similarly see reduced survival with AVPs, but in our study, mortality is primarily driven by AV closure. The HeartMate II trial data shows 30-day mortality that was lowest for AV closure at 6.3% (2/32), followed by replacement at 13% (4/30) and repair at 18% (3/18). The reasons for these variations from our data are unknown; however, the sample sizes and event rates are low enough that the differences seen in the HeartMate II trials are not statistically significant. Importantly, a database audit was performed to ensure data accuracy and all conflicts were adjudicated by the same two authors (J.O.R and S.C.S.) to determine what type of AVP was performed, resulting in internal consistency and accuracy of the present analysis.
While the mortality differences noted in our study are significant between groups for all INTERMACS levels, the differences are most apparent with INTERMACS level 1-2 patients. One prior report has also suggested that outcomes with AVPs may differ according to INTERMACS level. A study by Dranishnikov, et al. examined concomitant AVR for INTERMACS level 1-2 patients (n=7) and INTERMACS level 3-7 patients (n=12) and compared results to patients from the same INTERMACS levels that did not undergo an AVR. INTERMACS level 1-2 AVR patients had significantly increased in-hospital mortality (57% [4/7] vs. 20% [32/162], p=0.038) and a trend towards increased 30-day mortality (29% [2/7] vs. 15% [25/162], p=0.31), but no significant differences were observed for INTERMACS level 3-7 patients. This suggests that proper selection of type of AVP is even more important in sicker patients.
Importantly, the increased mortality observed with AV closures is accompanied by a smaller percentage of patients who are eventually transplanted. Some of the differences observed may be due to surgeon bias in treating aortic insufficiency more aggressively with surgery in destination therapy patients. This bias is suggested by the fact that a higher percentage of those with an AVP were implanted for destination therapy when compared to those without an AVP (45.9% vs. 32.3%, p<0.0001). However, any potential bias in treatment does not explain the observed decrease in transplantation rate (19% to 14%) between aortic valve repair/replacement and aortic valve closure, as those groups were comprised of comparable percentages of destination therapy patients.
The etiology of the observed increased mortality in patients with aortic valve closures is not clear. Early mortality, where we see the largest difference, appears to be driven by respiratory failure and major bleeding in this study. Despite this, AV closures have ICU/step down unit stays and hospital lengths of stay that are comparable to patients without an AV procedure. Our data demonstrating that ICU/step down unit stays are longest with an AV replacement is consistent with data demonstrating significantly prolonged ICU stays (mean: 36 days vs. 13 days, p=0.025) for INTERMACS level 1-2 patients with an AVR (9). Certainly, the fact that AVPs generally require aortic crossclamp and prolonged cardioplegic arrest may contribute to mortality, but if that were the driving factor than we would anticipate that the longer arrest times required for an AVR would confer proportionally greater risk. Postoperative renal dysfunction, one possible correlate of longer pump times, has a greater incidence among patients with an AVP compared to baseline, but no differences are observed between groups. Similarly, there are no differences in time to right heart failure or rehospitalization. Additional data are required to determine the root cause of these mortality differences. Nonetheless, AV closure was an independent predictor of postoperative mortality that was only rivaled by dialysis and placement of a BiVAD.
Use of AV closure as a treatment strategy also has important implications and associated patient risks. AV closure eliminates native ejection through the AV as a backup during equipment malfunction, changeout or with pump dysfunction due to thrombus. Emergency LVAD stoppage is reported in 4% of all fatalities with an LVAD in place (17). Further, aortic closure does not allow for the possibility of myocardial recovery or weaning from the device. Despite these potential problems with valve closure, we did not observe a higher incidence of death from device failure or sudden unexplained death in the AV closure group, and no patients with an AVP were explanted for myocardial recovery.
While AV replacement theoretically retains the ability of the valve to open, the altered hemodynamics of LVAD support impair aortic valve opening and decrease leaflet opening time during systole. Multiple case reports in the literature observed fibrosis leading to complete commissural fusion during the first year of support. Therefore despite differences in operative technique, the ability of the aortic valve to open in LVAD patients with echocardiographically observed “closed aortic valves” is unclear at best. Still, prosthetic aortic valves, particularly mechanical valves, may pose additional thromboembolic risk. Even while anticoagulated, an aortic prosthesis that is inactive and typically remains in the closed position results in blood stasis and can lead to thrombus formation. Embolization may then occur during intentional weaning, intermittent periods of exercise or device malfunction (2). For these reasons, existing mechanical valves are usually either replaced with a biologic valve or closed, and mechanical valves are not typically implanted as a concomitant operation. While less of a concern, reports of bioprosthetic valve thrombosis also exist in the literature (2, 18). Nevertheless, we did not observe a difference in stroke between any of our four groups.
Despite improved survival with AV repair, the durability of this procedure is less than for AV replacement or closure, with an observed incidence of 19% recurrence of moderate to severe aortic insufficiency at 6 months. This is a higher failure rate than has been observed in some single center studies that have reported 0-6.9% recurrence rates of greater than mild AI by echocardiographic assessment at a mean follow-up of greater than 1 year after using a Park's stitch (19, 20). However, our observations are consistent with the 20% (2/10) recurrence reported in another study at a mean follow-up of 118 days (10). The development of de novo AI is well-described postoperatively (5, 21) with an incidence of 4% (1-6%) per month of support in a recent meta-analysis (21). These data did not assess which specific repairs failed during follow-up, only the recurrence rate, and the impact of recurrent AI in these patients cannot be assessed from this analysis, as closures have the lowest rate of recurrent AI and the highest mortality.
Our data support performing repair or replacement when indicated and avoiding AV closure, particularly in INTERMACS level 1-2 patients. However, these data cannot address which patients should receive an AV procedure. Existing data suggests that greater than mild AI requires intervention, whereas consideration should be given to repairing mild AI if death or transplant are not expected within 12 months (19). Moreover, intraoperative assessment of AI before and following implantation of the LVAD should be performed due to the fact that initiation of LVAD support decreases left ventricular end diastolic pressure and increases aortic pressure, resulting in an increased transvalvular gradient and possible worsening of previously trivial or mild AI that was not clinically evident in the setting of severe heart failure limiting regurgitant flow.
Limitations
Our study has several limitations. First, intraoperative echocardiography data is not available. While preoperative echocardiographic data was available, data was not obtained at a uniform time point, and it may not have been representative of the clinical situation at the time of the operation or of the intraoperative studies that were likely used to aid in the decision of whether or not to perform a concomitant AVP. For that reason, outcomes were not analyzed based upon the degree of preoperative AI. Second, hospitals that were non-responders to our audit were excluded from the study. While all data from those institutions, not just unverified data, were excluded to limit bias, hospitals were not excluded in a random fashion and our sample size was ultimately reduced. Importantly, however, the differences reported were still observed when all data (including the unverified data) was included and analyzed. Third, we do not have information on why surgeons selected one AVP over another; and management algorithms, as well as techniques for closure and repair, are likely to have varied between institutions. While we have data available on type of AV repairs performed, we do not have data on the specifics of the AV closures. For the AV replacement group, we also do not have information on who had a previous mechanical AV replacement, as that information was not recorded in the INTERMACS database. Fourth, while we show a strong correlation between AV closures and increased mortality, our data cannot establish a direct cause and effect relationship. Finally, this study cannot be used to comment on who should receive an AVP.
Conclusions
Concomitant AV repairs may be performed during implantation of cf-LVADs with results that are comparable to those for patients that did not undergo an AVP. Aortic valve closures are associated with significant reductions in both short and long term mortality, particularly in INTERMACS level 1-2 patients. The durability of an AV repair, however, is worse than other approaches, and further studies are needed to address how to treat recurrent AI. Importantly, this is the largest study examining concomitant AVPs in cfLVAD patients, and it is the only one powered to compare AV closure, repair and replacement.
Supplementary Material
Acknowledgements and Funding
We have no acknowledgements. This project has been funded in whole or in part with federal funds from the National Heart, Lung and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268201100025C
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This manuscript was presented at the opening plenary session of the 2014 Annual Meeting of the ISHLT. It was the recipient of the Branislav Radovancevic Memorial Best MCS Abstract Award.
Disclosures
S.C.S. is a consultant for Thoratec and HeartWare. The remaining authors have no conflicts of interest.
References
- 1.Adamson RM, Dembitsky WP, Baradarian S, et al. Aortic valve closure associated with HeartMate left ventricular device support: technical considerations and long-term results. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011;30:576–82. doi: 10.1016/j.healun.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Rao V, Slater JP, Edwards NM, Naka Y, Oz MC. Surgical management of valvular disease in patients requiring left ventricular assist device support. The Annals of thoracic surgery. 2001;71:1448–53. doi: 10.1016/s0003-4975(01)02479-1. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal A, Raghuvir R, Eryazici P, et al. The development of aortic insufficiency in continuous- flow left ventricular assist device-supported patients. The Annals of thoracic surgery. 2013;95:493–8. doi: 10.1016/j.athoracsur.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Bryant AS, Holman WL, Nanda NC, et al. Native aortic valve insufficiency in patients with left ventricular assist devices. The Annals of thoracic surgery. 2006;81:e6–8. doi: 10.1016/j.athoracsur.2005.08.072. [DOI] [PubMed] [Google Scholar]
- 5.Cowger J, Pagani FD, Haft JW, Romano MA, Aaronson KD, Kolias TJ. The development of aortic insufficiency in left ventricular assist device-supported patients. Circulation Heart failure. 2010;3:668–74. doi: 10.1161/CIRCHEARTFAILURE.109.917765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman CM, Silver MA, Sobieski MA, Slaughter MS. Management of aortic insufficiency with continuous flow left ventricular assist devices: bioprosthetic valve replacement. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2006;25:1410–2. doi: 10.1016/j.healun.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Hatano M, Kinugawa K, Shiga T, et al. Less frequent opening of the aortic valve and a continuous flow pump are risk factors for postoperative onset of aortic insufficiency in patients with a left ventricular assist device. Circulation journal : official journal of the Japanese Circulation Society. 2011;75:1147–55. doi: 10.1253/circj.cj-10-1106. [DOI] [PubMed] [Google Scholar]
- 8.Samuels LE, Thomas MP, Holmes EC, et al. Insufficiency of the native aortic valve and left ventricular assist system inflow valve after support with an implantable left ventricular assist system: signs, symptoms, and concerns. The Journal of thoracic and cardiovascular surgery. 2001;122:380–1. doi: 10.1067/mtc.2001.114770. [DOI] [PubMed] [Google Scholar]
- 9.Dranishnikov N, Stepanenko A, Potapov EV, et al. Simultaneous aortic valve replacement in left ventricular assist device recipients: single-center experience. The International journal of artificial organs. 2012:0. doi: 10.5301/ijao.5000102. [DOI] [PubMed] [Google Scholar]
- 10.Goda A, Takayama H, Pak SW, et al. Aortic valve procedures at the time of ventricular assist device placement. The Annals of thoracic surgery. 2011;91:750–4. doi: 10.1016/j.athoracsur.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32:141–56. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Park SJ, Liao KK, Segurola R, Madhu KP, Miller LW. Management of aortic insufficiency in patients with left ventricular assist devices: a simple coaptation stitch method (Park's stitch). The Journal of thoracic and cardiovascular surgery. 2004;127:264–6. doi: 10.1016/s0022-5223(03)01301-1. [DOI] [PubMed] [Google Scholar]
- 13.Kirklin JK, Naftel DC, Pagani FD, et al. Long-term mechanical circulatory support (destination therapy): on track to compete with heart transplantation? The Journal of thoracic and cardiovascular surgery. 2012;144:584–603. doi: 10.1016/j.jtcvs.2012.05.044. discussion 597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.John R, Naka Y, Park SJ, et al. Impact of concurrent surgical valve procedures in patients receiving continuous-flow devices. The Journal of thoracic and cardiovascular surgery. 2014;147:581–9. doi: 10.1016/j.jtcvs.2013.10.024. discussion 9. [DOI] [PubMed] [Google Scholar]
- 15.Pal JD, Klodell CT, John R, et al. Low operative mortality with implantation of a continuous-flow left ventricular assist device and impact of concurrent cardiac procedures. Circulation. 2009;120:S215–9. doi: 10.1161/CIRCULATIONAHA.108.844274. [DOI] [PubMed] [Google Scholar]
- 16.Morgan JA, Tsiouris A, Nemeh HW, et al. Impact of concomitant cardiac procedures performed during implantation of long-term left ventricular assist devices. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32:1255–61. doi: 10.1016/j.healun.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. The New England journal of medicine. 2009;361:2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 18.Wagner WR, Johnson PC, Kormos RL, Griffith BP. Evaluation of bioprosthetic valve-associated thrombus in ventricular assist device patients. Circulation. 1993;88:2023–9. doi: 10.1161/01.cir.88.5.2023. [DOI] [PubMed] [Google Scholar]
- 19.Jorde UP, Uriel N, Nahumi N, et al. Prevalence, Significance, and Management of Aortic Insufficiency in Continuous Flow Left Ventricular Assist Device Recipients. Circulation Heart failure. 2014 doi: 10.1161/CIRCHEARTFAILURE.113.000878. [DOI] [PubMed] [Google Scholar]
- 20.McKellar SH, Deo S, Daly RC, et al. Durability of central aortic valve closure in patients with continuous flow left ventricular assist devices. The Journal of thoracic and cardiovascular surgery. 2014;147:344–8. doi: 10.1016/j.jtcvs.2012.09.098. [DOI] [PubMed] [Google Scholar]
- 21.Deo SV, Sharma V, Cho YH, Shah IK, Park SJ. De novo aortic insufficiency during long-term support on a left ventricular assist device: a systematic review and meta-analysis. ASAIO J. 2014;60:183–8. doi: 10.1097/MAT.0000000000000042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.