Abstract
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability worldwide. To date, there are no pharmacologic agents proven to improve outcomes from TBI because all the Phase III clinical trials in TBI have failed. Thus, there is a compelling need to develop treatments for TBI.
Areas covered
The aim of this review is to provide an overview of select cell-based and pharmacological therapies under early development for the treatment of TBI, which seek to enhance cognitive and neurological functional recovery through neuroprotective and neurorestorative strategies.
Expert’s opinion
TBI elicits both complex degenerative and regenerative tissue responses in the brain. TBI can lead to cognitive, behavioral, and motor deficits. Although numerous promising neuroprotective treatment options have emerged from preclinical studies that mainly target the lesion, translation of preclinical effective neuroprotective drugs to clinical trials has proven challenging. Accumulating evidence indicates that the mammalian brain has a significant, albeit limited, capacity for both structural and functional plasticity as well as regeneration essential for spontaneous functional recovery after injury. A new therapeutic approach is to stimulate neurovascular remodeling by enhancing angiogenesis, neurogenesis, oligodendrogenesis, and axonal sprouting, which in concert, may improve neurological functional recovery after TBI.
Keywords: angiogenesis, cell therapy, exosomes, microRNAs, neurogenesis, neuroprotection, neurorestoration, traumatic brain injury
1. Introduction
Traumatic brain injury (TBI) is defined as an alteration in brain function and/or other evidence of brain pathology, caused by a sudden external force [1]. A detailed definition of TBI is provided in a position paper [1]. TBI is a major cause of death and long-term disability worldwide [2], affecting not only athletes and military personnel, but also the general population, ranging from young to old. Globally, at least 10 million TBIs serious enough to result in death or hospitalization occur each year [3, 4]. TBI is a significant health concern and an enormous socioeconomic burden.
TBI is not a single pathophysiological event occurring at the time of injury but a complex continuous disease process [5]. TBI results in structural damage and functional deficits due to both primary and secondary injury mechanisms [6]. Primary injury results from mechanical disruption of brain tissue that occurs at the time of injury and includes contusion, damage to blood vessels (hemorrhage), and axonal shearing, in which the axons of neurons are stretched and torn [7, 8]. The location, nature, and severity of the primary injury, along with preinjury comorbidities including but not restricted to age, gender, pre-existing diseases, use of medication and alcohol, collectively determine brain damage and functional outcome in TBI [9].
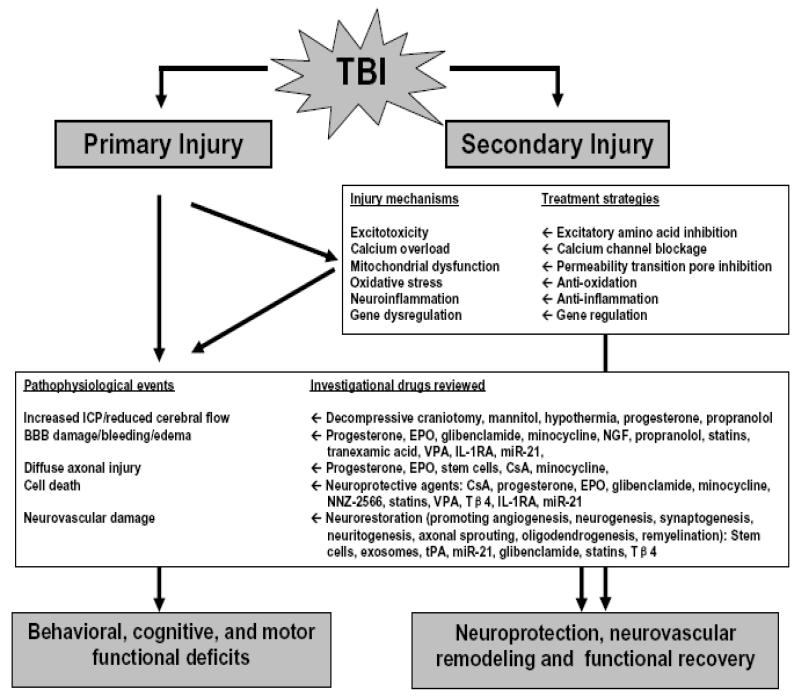
Secondary injury evolves over minutes to months, even years after the primary injury and is the result of biochemical and pathophysiological events which ultimately lead to brain cell death, tissue damage and atrophy [10]. TBI is a complex process of metabolic, cellular, and molecular events including glutamate excitotoxicity, perturbation of cellular calcium homeostasis, increased free radical generation and lipid peroxidation, mitochondrial dysfunction, inflammation, apoptosis, and diffuse axonal injury [11], characterized by a bead-like pattern of beta-amyloid precursor protein in damaged axons and widespread upregulation of this protein in neurons [12]. Collectively, the cascade of secondary injury culminates in neuronal, endothelial, and glial cell death and white matter degeneration (Figure 1-Simplified overview of pathophysiology and recovery of TBI).
Figure 1. Simplified overview of pathophysiology and recovery of TBI.
Sudden external force to the brain causes not only primary injury (that is, mechanical tissue deformation and injury leads to necrotic cell death, shearing and tearing of blood vessels, neuron, glia and axon as well as initiates secondary injury cascade) but also leads to nonspecific depolarization and release of excitatory neurotransmitters including glutamate and aspartate (Excitotoxicity), which bind to glutamate receptors and induce massive influx of calcium (Calcium overload). Calcium overload activates calcium-dependent phospholipases, proteases and endonucleases that damage cell membrane, cytoskeleton and nucleic acids, respectively. Mitochondria (power house of cell) sequester intracellular calcium which may leads to mitochondrial permeability pore opening, energy deficits, free radical formation, and initiation of apoptosis (Mitochondrial dysfunction). After TBI, formation of oxygen and nitrogen reactive species significantly increases, which oxidize lipids, proteins and nuclei acids (Oxidative stress). TBI up-regulates transcription factors, inflammatory mediators, and neuroprotective genes but down-regulates neurotransmitter receptors and neurotransmitter release mechanisms (Gene dysregulation). Increased expression of detrimental cytokines and chemokines induces brain edema, blood-brain barrier damage, and apoptosis (Neuroinflammation). The result of these complex cascades after TBI eventually leads to blood-brain barrier damage, hemorrhage, edema, increased ICP, altered cerebral flow, ischemia/hypoxia, metabolic deficits, apoptosis, diffuse axonal injury, demyelination, progressive atrophy of both grey and white matter, which collectively lead to cell death, brain neurodegeneration, and functional deficits. However, substantial experimental and clinical data have accumulated over the past decade indicating that the adult brain is capable of substantial (limited though) structural and functional reorganization after injury which may contribute to spontaneous functional recovery. Recent new interventions targeting multiple secondary injury mechanisms and promoting neuroplasticity mechanisms improve functional recovery in animal models of TBI.
Abbreviations: TBI: traumatic brain injury; ICP: intracranial pressure; BBB: blood-brain barrier; EPO: erythropoietin; NGF: nerve growth factor; VPA: valproic acid; IL-1RA: interleutin-1receptor antagonist; miR-21: microRNA-21; CsA: cyclosporine A; NNZ-2566: synthetic analogue of the endogenous N-terminus tripeptide glycine-proline-glutamate; Tβ4: thymosin beta 4; tPA: tissue plasminogen activator
TBI leads to behavioral, cognitive and motor deficits. There are two strategic approaches to treat TBI [13]: 1) a neuroprotective treatment that targets the injured brain with a focus on reducing/preventing secondary injury and neural cell death, and reducing the lesion size; and 2) a neurorestorative one, designed to improve neurological recovery by treating the entire central nervous system (CNS) to promote neurovascular remodeling including angiogenesis, neurogenesis, oligodendrogenesis and dendrite/axon outgrowth. For decades, the primary approach and goal of therapy for TBI have been the treatment of the injured tissue, with intervention designed to reduce the lesion size. Enormous effort has gone into the development of neuroprotective agents, including free radical scavengers and glutamate antagonists, among a myriad of others [14]. During the past 3 decades, more than 30 clinical trials for potential treatment of TBI have been initiated, and almost all Phase II/III TBI clinical trials have failed [11, 15]; consequently, no effective treatment options currently exist that improve neurological outcome after TBI.
Until a decade ago, it was believed that once the brain was damaged, there was little, if any, capability for regeneration of axons and formation of new synapses to take place [16]. The brain has a limited ability to exhibit structural and functional plasticity [17]. However, though the capacity for plasticity is limited, the changes may prove to be significant related to functional recovery [13, 16, 17]. Emerging preclinical data indicate that restorative therapies targeting multiple parenchymal cells including cerebral endothelial cells, neural stem cells and oligodendrocyte progenitor cells enhance TBI-induced angiogenesis, neurogenesis, oligodendrogenesis and axonal sprouting, respectively [18, 19]. These interacting neuroplastic events collectively improve neurological function after TBI [19]. Thus, there is a compelling need to develop novel therapeutics specifically designed to stimulate neuroplasticity which subsequently promote cognitive and motor neurological recovery and improve quality of life after TBI.
2. Current status of treatments for TBI
The current approaches include acute interventions to curb the primary insult and minimize secondary injury/complications as well as early rehabilitation to improve residual function, quality of life and independence [20, 21]. Pre-hospital care is designed to restore and maintain airway, breathing and circulation [22]. Numerous preclinical studies have tested therapeutic efficacy of drugs in animal models of TBI by targeting secondary injury mechanisms including calcium channel blockers, corticosteroids, excitatory amino acid inhibitors, N-methyl D-aspartate (NMDA) receptor antagonist, free radical scavengers, magnesium sulfate, and growth factors [23]. Several Phase II clinical trials have shown favorable effects including polyethylene glycol-conjugated superoxide dismutase, moderate hypothermia, nimodopine, triamcinolone [23] and progesterone [24, 25]. Updated meta-analysis supports previous findings that hypothermic therapy constitutes a beneficial treatment of TBI in specific circumstances [26]. Until more evidence from well-conducted trials becomes available, clinicians should continue to exercise caution when considering administering hypothermia for treatment of TBI.
High intracranial pressure (ICP) is still the most frequent cause of death and disability after severe TBI [27]. The efficacy of existing neuroprotective treatments for high ICP after TBI remains uncertain. There is insufficient data supporting pre-hospital administration of mannitol for treatment of high ICP after TBI [28]. Mass lesions and an increase in brain water content (edema) and cerebral blood volume contribute to raised ICP in TBI [29]. However, there is no evidence from randomized controlled trials (RCT) that supports the routine use of decompressive craniotomy (DC) to reduce unfavorable outcomes in adults with severe TBI and refractory high ICP [29]. A recently completed RCT of the DECRA (Decompressive Craniectomy in Patients with Severe TBI) study claims that early DC decreased ICP but was associated with more unfavourable outcomes measured by the score on Extended Glasgow Outcome Scale (GOS-E) at 6 months compared with standard medical therapy [30]. Despite the conclusions of the DECRA study, DC should be considered in certain patients with elevated ICP refractory to first-line therapeutic measures [31]. The ongoing RESCUEicp (Randomized Evaluation of Surgery with Craniectomy for Uncontrollable Elevation of Intra-Cranial Pressure) study hopes to address the issue of clinical efficacy of DC [32, 33].
3. Overview of completed clinical trials
Over the past 40 years, more than 100 RCTs in TBI were conducted [11, 23, 34-41]. In a recent review on 100 RCTs, the majority of acute phase pharmacologic or non-pharmacologic trials (44/55) showed either no effect or adverse effect on TBI outcomes [35]. The acute phase pharmacologic drug trials were grouped based on the treatment mechanisms [35]: calcium channel block agents (n=4), drugs with multiple actions (n=5), glutamate excitotoxicity (n=6), intensive insulin treatment (n=3), steroids (n=5), lipid peroxidation/free radical damage (n=4) and other (n=4). The 23 acute non-pharmacologic trials were grouped according to the treatment mechanisms [35]: decompressive craniotomy (n=3), early nutrition treatment (n=2), hyperbaric oxygen (n=2), hyperventilation treatment (n=1), osmotic therapy (n=5), therapeutic hypothermia (n=9) and pre-hospital rapid sequence intubation (n=1). The majority of post-acute phase trials (36/45), consisting of cognitive rehabilitation, physical rehabilitation and pharmacotherapy, produced various beneficial treatment effects. There were no effective therapeutic treatments for TBI identified from a total of 44 systematic reviews pertaining to therapeutic interventions for acute TBI (21 published in Cochrane Library and 23 in peer-reviewed journals) [42]. In addition, all the acute therapeutic neuroprotective interventions that have been tested thus far in the Phase III TBI trials have failed to clearly show efficacy [40, 41, 43]. Many potential factors contributing to translational failure in neuroprotection for TBI have been identified and corrective recommendations have been provided [11, 13, 37, 44, 45], which will facilitate successful clinical translation in the future. Potential problems include various aspects of the animal models utilized to mimic TBI and the clinical trial design approaches including the heterogeneity of the TBI population, variability of care and patient-specific response that may have impacted the success [11, 37, 45, 46]. Here, we focus on recently completed clinical trials using progesterone and erythropoietin, which showed high therapeutic potential in animal studies but failed in TBI patients.
3.1 Progesterone
Progesterone, a female reproductive hormone, is also synthesized and actively metabolized in the central and peripheral nervous system [47]. After three decades of extensive research on the use of progesterone in TBI, it is clear that progesterone is a neurosteroid that affects multiple mechanisms involved in neuroprotection and repair after various types of brain injury [48, 49]. Both Phase II clinical trials show that progesterone is safe and potentially efficacious in the treatment of TBI [24, 25]. However, two recently completed Phase III clinical trials failed to find progesterone effective in treating severe TBI [50, 51], despite promising preliminary research. The study, named ProTECT III (Progesterone for the TBI: Experimental Clinical Treatment), involved 49 trauma centers across the United States between July 2009 and November 2013 [51]. The study was originally planned to include 1,140 patients, but was terminated after 882 patients due to a non-significant treatment effect. Another study of 1,195 patients, called SyNAPSe (Study of the Neuroprotective Activity of Progesterone in Severe TBIs) showed no clinical benefit of progesterone in patients with severe TBI [50].
These disappointing trials reflect universal translational challenges in neuroprotection for TBI. There are several issues that need to be addressed. The difference seen in females after TBI has conflicting evidence [52]. Some reports indicate that female TBI patients [53, 54] and female TBI animals [55-57] have outcomes similar to or even worse than male counterparts. The differences in treatment protocols and inclusion/exclusion criteria between preclinical and clinical trials of progesterone in TBI, e.g., injury type, injury level, multiple trauma, and initiation intervention time of progesterone, may at least in part underlie the failure of the progesterone clinical trial (Table 1- Comparison of preclinical and clinical trials of progesterone in TBI).
Table 1. Comparison of preclinical and clinical trials of progesterone in TBI.
| Preclinical TBI [180] | Clinical TBI [24, 25, 50, 51] | |
|---|---|---|
| Injury type | Majority in open head injury with cavity formation (CCI or FPI model) [180]; a few in closed head injury (weight drop model) [181, 182] |
Blunt, closed head injury |
| Injury severity | Mainly moderate, homogenous |
Severe only [25, 50] or moderate to severe [24, 51], heterogeneous |
| Multiple trauma | TBI alone | 83% with multiple trauma [50] |
|
Traumatic
subarachnoid hemorrhage |
Not measured | 76%[50] |
| Age | Mainly young adult | 16 to 94 years old, mainly young [24, 25, 50, 51] |
| Sex | Mainly males | Mixed (male sex, 70-80% )[24, 25, 50, 51] |
|
Initial intervention
time |
Most < 1 hr | Average 3.6 - 6.3 hr [24, 25, 50, 51] |
|
Intervention
duration |
12 hr -14 days | 3 days [24], 4 days [51] or 5 days [25, 50] |
| Outcome measures | Edema, lesion size, functional, cytokines |
GOS-E, DRS, mortality at 1, 3, 6 months |
|
Efficacy compared
to placebo |
Effective in majority of animal TBI trials [49, 183, 184]; Worse in female adolescence mice after CCI-TBI [185]; No effect on edema and lesion in rats after CCI-TBI [186] |
Favorable outcome in moderate TBI but not in severe TBI in a Phase II study [24]; Favorable outcome in severe TBI in a Phase II study [25]; No benefits in the Phase III studies [50, 51] |
Note: CCI=Controlled Cortical Impact, FPI=Fluid Percussion Injury; GCS = Glasgow Coma Scale, for classifying TBI severity; GOS = Glasgow Outcome Scale, as a common primary outcome measure in TBI; GOS-E=GOS-Extended (more sensitive than the GOS); DRS=Disability Rating Scale, for tracking the patient’s progress over time.
3.2 Erythropoietin
Erythropoietin (EPO) is a 30-kD glycoprotein that regulates red cell production by binding to an erythroid progenitor cell surface receptor [58]. EPO is used widely for treating anemia of critical illness or anemia induced by chemotherapy [58]. In the human, EPO is produced by peritubular cells in the kidneys of the adult and in hepatocytes in the fetus. EPO is also a multifunctional tissue-protecting agent that exerts antiapoptotic, antiinflammatory, antioxidative, angiogenic, and neurotrophic effects [59]. EPO showed promise as a neuroprotective agent in animal models of TBI [60]. However, in a recent randomized clinical trial of 200 patients (EPO, n = 102; placebo, n = 98) with severe closed head injury enrolled within 6 hr of injury, the administration of EPO failed to improve favorable outcomes by 20% at 6 months [61]. Of note, most preclinical studies employed the moderate controlled cortical impact injury (an open head injury model) TBI to investigate the effect of EPO on lesion reduction, cell death and functional recovery [60]. These limited preclinical results might be inadequate for predicting the treatment response in patients with severe closed head injury. Currently, there are several clinical trials of EPO in TBI to determine the effect of EPO on secondary brain injury and functional recovery (www.clinicaltrials.gov, NCT00987454), and on numbers of circulating endothelial progenitor cells in patients with persistent symptoms during the subacute period after TBI (NCT02148367, NCT02226848).
Carbamylated EPO, an EPO derivative without hematopoietic activity (i.e., without effects on hematocrit and lack of risk for thrombosis), has been shown to improve functional recovery in animal models of TBI [62-64]. The tissue-protective activities of EPO can be mimicked by a small, nonerythropoietic peptide pyroglutamate helix B surface peptide (pHBSP, also known as ARA 290), which inhibits inflammation and improves cognitive function in rats following mild TBI complicated by hemorrhagic shock [65]. pHBSP is safe and effective in the treatment of the neuropathic symptoms of sarcoidosis [66] and type 2 diabetes [67]. pHBSP may hold a therapeutic potential for treatment of TBI because there is lower risk of thrombotic adverse effects.
4. Investigational drugs under early clinical trials
There are many drugs under TBI clinical trials (www.clinicaltrials.gov). We will focus on following the select investigational drugs (Table 2-Select ongoing TBI clinical trials for neuroprotection).
Table 2. Select ongoing clinical trials for neuroprotection in TBI.
| Drugs/cells | Phase | Study population and estimated enrollment |
Primary outcome |
Sponsor |
ClinicalTrials.gov identifier |
|---|---|---|---|---|---|
|
Autologous
bone marrow mononuclear cells |
Phase I, II (adults) |
Severe n = 20 |
Neurological events |
The University of Texas Health Science Center, Houston |
NCT01575470 |
|
Autologous
bone marrow mononuclear cells |
Phase I, II (children) |
Severe n = 50 |
Brain white matter and gray matter preservation |
The University of Texas Health Science Center, Houston |
NCT01851083 |
|
Autologous
bone marrow mononuclear cells |
Phase I (6 months to 65 years) |
Chronic TBI n = 50 |
GOS-E DRS |
Neurogen Brain and Spine Institute, India |
NCT02028104 |
| Cyclosporin A | Phase II | Severe n = 20 |
Pharmacokinetic s |
NeuroVive Pharmaceutical AB |
NCT01825044 |
|
CRASH-III
(TXA) |
Phase III | Moderate to severe n = 10,000 |
Safety | London School of Hygiene and Tropical Medicine |
NCT01402882 |
|
Recombinant
human erythropoietin (EPO) |
Phase II | Moderate to severe n = 30 |
Safety and number of circulating endothelial progenitor cells (EPCs) |
National Institute of Neurological Disorders and Stroke |
NCT02226848 |
| EPO-TBI | Phase II | Moderate to severe n = 30 |
Number of circulating EPCs and biomarkers |
Uniformed Services University of the Health Sciences |
NCT02148367 |
| EPO-TBI | Phase III | Moderate to severe n = 606 |
GOS-E | Australian and New Zealand Intensive Care Research Centre |
NCT00987454 |
|
Glyburide
(RP-1127) |
Phase II | Mild to severe n = 100 |
Safety, brain edema, hemorrhage at 72 hr |
Remedy Pharmaceuticals, Inc. |
NCT01454154 |
|
Intranasal
nerve growth factor |
Phase II | Moderate to severe n = 118 |
Safety, GOS, Neurological functions |
Jinling Hospital, China |
NCT01212679 |
| Minocycline | Phase II | Moderate to severe n = 14 |
Disability rating scale, safety |
Wayne State University |
NCT01058395 |
|
NNZ-2566 (glycine- proline- glutamate analogue) |
Phase II | Moderate to severe n = 260 |
Safety, GOS-E, cognitive, neuropsychologi cal functioning |
Neuren Pharmaceuticals Limited |
NCT00805818 (INTREPID2566) |
| Propranolol | Phase II | Moderate to severe n = 40 |
Safety | Cedars-Sinai Medical Center |
NCT01202110 |
|
Propranolol
(DASH after TBI study) |
Phase II | Severe n = 100 |
Ventilator-free days |
Vanderbilt University | NCT01322048 |
| Valproate acid | Phase I | Severe n = 160 |
Safety | Xijing Hospital, China |
NCT02027987 |
4.1 Cyclosporin A
Cyclosporin A (CsA) is a cyclic nonribosomal 11-amino acid peptide produced by the fungus Tolypocladium inflatum, and is a widely used immunosuppressant [68]. CsA binds to the cytosolic protein cyclophilin in T-cells, and the CsA-cyclophilin complex inhibits calcineurin [69]. Neuroprotection of CsA is independent of immunosuppression [70]. CsA inhibits the opening of the mitochondrial permeability transition pore and apoptosis [71]. The neuroprotection afforded by CsA dose-dependent in several animal models of TBI, and a therapeutic window exists up to 24 hr post-injury [72]. The good safety and tolerability profile of CsA was established in humans when it was administered early after severe TBI [73-75]. A recent Phase II clinical trial demonstrates that the administration of CsA does not improve consciousness and cognitive function, but has no adverse effects [76]. Another small Phase II pharmacokinetics and safety trial of CsA in severe TBI is currently planned (NCT01825044).
4.2 Glibenclamide
Glibenclamide (glyburide) is known best for its use in the treatment of type 2 diabetes mellitus, by blocking pancreatic potassium ATP channels to promote the release of insulin [77]. During the last decade, glibenclamide has received renewed attention due to its pleiotropic protective effects in acute CNS injury including TBI [78]. Glibenclamide reduces edema formation and secondary hemorrhage, inhibits necrotic cell death, exerts potent anti-inflammatory effects and promotes neurogenesis [79]. Treatment with low-dose glibenclamide reduces post-traumatic brain edema and contusion volume following an open head injury in rats [80]. Glibenclamide has long-term protective effects on the hippocampus in rats after mild-to-moderate TBI [78]. Preliminary data from a recent Phase IIa clinical stroke trial (NCT01268683) suggest that glibenclamide significantly reduces cerebral edema and lowers the rate of hemorrhagic conversion following ischemic stroke [81], suggesting the potential use of glibenclamide to improve outcomes in humans after brain injury [82]. A Phase II clinical trial is underway to assess whether patients with severe, moderate, or complicated mild TBI administered glibenclamide will show a decrease in edema and/or hemorrhage, compared to patients administered placebo(NCT01454154).
4.3 Minocycline
The tetracycline derivative minocycline is therapeutically effective in various models of CNS injury and diseases, via mechanisms involving suppression of inflammation and apoptosis [83]. The effect of minocycline in TBI was investigated in a mouse model of closed head injury, indicating that a single dose of minocycline decreases lesion volume and improves short-term (1-day) neurological outcome, which is associated with reduced microglial activation and interleukin-1beta expression [84]. A triple (5 min, 3 hr and 9 hr post-injury) minocycline administration reduced cerebral edema up to 24 hr, and improved long-term (12-weeks) neurological recovery [85]. A Phase I/II clinical trial is ongoing to assess the safety and efficacy of minocycline administration after TBI as a therapeutic agent for severe human TBI (NCT01058395).
4.4 NNZ-2566
NNZ-2566 is a synthetic analogue of the endogenous N-terminus tripeptide, Glycine-Proline-Glutamate (GPE, Neuren Pharmaceuticals), which is proteolytically cleaved from insulin-like growth factor-1 (IGF-1) [86]. GPE has been shown to cross the blood-brain barrier (BBB) and protect against cell death both in vitro and in vivo but is rapidly metabolized [87]. NNZ-2566 has an extended (> 70 minute) half-life for optimizing its therapeutic potential [88]. NNZ-2566 protects against penetrating ballistic-like brain injury (PBBI)-induced inflammation and apoptosis and promotes functional recovery [88]. Two clinical trials are ongoing to investigate the dosing of NNZ-2566 for safety and tolerability with efficacy in non-penetrating TBI (NCT00805818 and NCT01366820). Interestingly, these clinical trials will include non-penetrating TBI while NNZ-2566 efficacy has not been investigated in the animal models of non-penetrating TBI.
4.5 Nerve growth factor
Nerve growth factor (NGF) was the first discovered member of the neurotrophin family [89]. NGF exerts its biological action by challenging the specific receptor tropomyosin kinase receptor A, which is a typical tyrosine kinase receptor [90]. The clinical application of NGF is restricted due to its poor permeability through the BBB [91]. Intranasal delivery is a noninvasive and convenient method which successfully targets NGF to the CNS, bypassing the BBB and minimizing systemic exposure [91]. Intranasal NGF effectively attenuates the hyperphosphorylation of tau after TBI in rats, which may be mediated by an integrated signaling pathway related to nuclear factor-κB (NF-κB) [92], attenuates aquaporin-4-induced edema [93], and ameliorates beta-amyloid deposition [94]. A randomized, double-blind, placebo-controlled trial is underway to investigate the therapeutic effects of intranasal NGF in moderate and severe blunt TBI, with treatment starting between 24 to 72 hr post TBI, and continuing for 2 weeks (NCT01212679).
4.6 Propranolol
A hyper-adrenergic state has long been demonstrated in those patients with severe TBI and is associated with poor outcomes [95]. One strategy to decrease sympathetic hyperactivity is pharmacologic intervention with beta (β)-blockade [96]. In pre-clinical mouse models, propranolol reduces brain edema, increases cerebral perfusion, decreases cerebral hypoxia, and improves neurologic outcomes [97]. The DASH (Decreasing adrenergic or sympathetic hyperactivity after TBI, NCT01322048) study is the first randomized, double-blinded, placebo-controlled trial powered to determine safety and outcomes associated with adrenergic blockade in patients with severe TBI [98]. If the study results in positive trends, this could provide pilot evidence for a larger multicenter randomized clinical trial.
4.7 Statins
Statins, inhibitors of cholesterol biosynthesis used to lower cholesterol levels, induce angiogenesis, neurogenesis and synaptogenesis, and enhance functional recovery following TBI in rats [99, 100]. Simvastatin activates Akt, forkhead transcription factor 1, and NF-κB signaling pathways, which suppress the activation of caspase-3 and apoptotic cell death, and thereby lead to neuronal function recovery after TBI [101]. Simvastatin increases expression of several growth factors and induces neurogenesis in the dentate gyrus of the hippocampus, thereby leading to restoration of cognitive function after TBI in rats [102]. The protective mechanisms of statins may be partly attributed to a reduction in the inflammatory response following TBI [103]. In addition, simvastatin treatment provided long-lasting (3 month) functional improvement following TBI in rats [104]. Given the wide use, favorable safety profile and positive clinical data for statins, the rare occurrence of serious adverse events and the extensive available preclinical data demonstrating neuroprotection and neurorestoration [105], clinical trials are warranted to determine the neuroprotective and neurorestorative properties of statins following TBI. The effect of rosuvastatin on TBI-induced cytokine change is ongoing in a Phase I/II trial (NCT00990028).
4.8 Stem cell therapy
Cell therapies using neural stem/progenitor cells are promising for the treatment of brain injury [106]. However, the clinical use of fetal tissues or embryonic stem cells is limited by ethical considerations and other scientific problems. Multipotent mesenchymal stromal cells (MSCs) are mesoderm-derived cells, primarily resident in adult bone marrow, adipose tissue, skin, umbilical cord blood and peripheral blood as well as other organs [107], and can give rise to neuronal cells as well as many tissue-specific cell phenotypes [108, 109]. Thus, MSCs could represent an alternative source of stem cells for cell replacement therapies.
MSCs administered 24 hr after injury significantly improved functional outcome in animal models of TBI [110, 111]. The benefit of MSCs is unlikely attributable to the very few MSCs that differentiate into brain cells [112]. MSCs secrete various growth factors including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and basic fibroblast growth factor (bFGF) [113-115]. MSCs also induce intrinsic parenchymal cells to produce these growth factors [114]. MSCs influence several neural restorative functions such as synaptogenesis [115], angiogenesis [115, 116], neurogenesis [117], and axonal reorganization [118]. Thus, MSCs act in a pleiotropic way to stimulate brain remodeling. Although MSCs alone do not reduce the lesion volume after TBI, a recent study shows that collagen scaffolds populated with MSCs improve spatial learning and sensorimotor function, reduce the lesion volume, and foster the migration of MSCs into the lesion boundary zone after TBI in rats compared to MSCs without scaffolds [119]. Transplanting human MSCs with the scaffolds down-regulates neurocan and Nogo-A transcription (major forms of growth-inhibitory molecule that suppresses axonal regeneration and neurite regrowth after neural injury) and protein expression [120, 121], which may partially contribute to the enhanced axonal regeneration after TBI.
The safety and feasibility of autologous MSC treatment of TBI patients have been evaluated in a single center [122]. TBI patients received autologous cell transplantation of MSCs isolated by bone marrow aspiration and expanded in culture. A primary administration of MSCs was applied directly to the injured area during the cranial operation followed by a second intravenous dose of MSCs. No immediate or delayed toxicity related to the cell administration was observed within the 6-month follow-up period. Neurologic function was significantly improved at 6 months after MSC therapy [122]. The safety and feasibility of MSC therapy was confirmed by transplanting autologous MSCs into the subarachnoid space via lumbar puncture in another single center [123]. MSCs could be derived from autologous bone marrow, and expanded to yield a clinically relevant therapeutic dose. However, this expansion process could take days or weeks to achieve the dose needed, limiting this approach for acute or even subacute applications. Recent Phase I clinical trials demonstrated that intravenous infusion of autologous bone marrow-derived mononuclear cell therapy (without a need of expansion) within 48 hr after severe TBI in children is feasible and safe [124, 125]. Several Phase I/II clinical trials are ongoing to further study the safety of autologous bone marrow mononuclear cells and their effect on functional outcome in TBI patients of children and adults (NCT01851083, NCT01575470 and NCT02028104).
4.9 Tranexamic acid
Posttraumatic coagulopathy occurs in a third of brain injured patients and is associated with an increased risk of death [126]. Recently, the antifibrinolytic agent tranexamic acid (TXA) demonstrated reduced mortality compared with placebo in severely bleeding trauma patients in the CRASH-2 (Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage) trial, which enrolled 20,211 patients in 40 countries [127]. In addition to the robust data demonstrating clinical benefit in trauma patients with severe bleeding, TXA also has an excellent safety profile and has been shown to be cost-effective [128]. Because of the mechanistic potential for TXA to decrease secondary brain injury, it has been considered as a possible therapy to improve clinically important outcomes in TBI patients. Results from the 2 RCTs demonstrated that TXA significantly reduced intracerebral haemorrhage progression but did not show significant improvement of clinical outcomes in TBI patients [129-132]. Further evidence is required to determine the routine use of TXA in patients with TBI. An ongoing, international, multicenter, Phase III trial (NCT01402882, CRASH-3) evaluating the use of TXA on death and disability in TBI patients, with a planned enrollment of 10,000 patients, will certainly shed light on this particular question [133].
4.10 Valproic acid
Valproic acid (VPA), a histone deacetylase inhibitor, is commonly prescribed to epilepsy and bipolar disorder sufferers for its anticonvulsant and mood-stabilizing effects [134]. In a rat model of TBI, postinjury systemic administration of VPA at high dose (400 mg/kg) reduced cortical contusion volume, decreased BBB permeability, and, of most importance, improved motor function and spatial memory [135]. VPA also dose-dependently increased histone acetylation and reduced glycogen synthase kinase 3β activity in the hippocampus. Valproate at low dose (30 mg/kg) also reduces lesion volume and improves motor function in adult rats after TBI induced by controlled cortical impact, which may be related to its increased histone acetylation, p-ERK, and p-CREB expression in the brain [136].
In a two-year randomized double-blind trial for prevention of posttraumatic seizures, VPA treatment initiated within 24 hr after injury substantially reduced the rate of early seizure, but this benefit was not significant, compared with short-term (one week) treatment with an anti-epileptic drug phenytoin; neither drug prevented late seizures [137] Of note, there was a trend towards increased mortality in the VPA groups although it was not statistically significant compared to phenytoin. In addition, no significant adverse or beneficial effects were associated with VPA in another clinical study, as assessed by a battery of neuropsychological measurements administered 1, 6, and 12 months after TBI [138]. These two clinical trials were conducted over a decade ago, and accumulating evidence for VPA’s robust benefits in preclinical TBI models [135, 136] supports a need to re-examine the clinical effects of VPA in patients with TBI. A clinical trial is underway to evaluate whether VPA at 400 mg/kg could protect brain and recovery of brain function after severe TBI (primary outcome) and to explore whether VPA could prevent late epilepsy after severe non-penetrating TBI (secondary outcome, NCT02027987).
5. Investigational drugs under preclinical studies
Pharmacological treatments have been widely investigated in animal TBI trials to evaluate their efficacy for treatment of TBI [139]. Here, we focus on following select preclinical TBI trials.
5.1 Thymosin beta 4
Thymosin beta 4 (Tβ4), a polypeptide of 43-amino acids, was first isolated from bovine thymus tissue and subsequently found to exist in all mammals studied [140, 141]. The major intracellular function of Tβ4 is G-actin-sequestration, which is necessary for cell motility and organogenesis [142]. Recent studies demonstrate that Tβ4 is a multifunctional peptide, which inhibits inflammation and apoptosis, and promotes tissue repair in skin, cornea, and heart [143]. Tβ4 is an essential paracrine factor of endothelial progenitor cells (EPCs), and Tβ4 promotes angiogenesis after ischemic injury [144]. Tβ4 given intravenously as a single dose or in multiple daily doses for 14 days over a dose range of 42-1260 mg was well tolerated with no evidence of dose limiting toxicity in healthy humans [145].
Tβ4 plays a critical role in many cellular processes including mobility, axonal path-finding, neurite formation, proliferation and neuronal survival [143, 146]. Tβ4 is a potential treatment for TBI. Tβ4 (6 mg/kg) was administered ip starting at day 1 and then every 3 days for an additional 4 doses to the TBI rats [147]. Delayed Tβ4 treatment did not reduce lesion volume but significantly reduced hippocampal cell loss, enhanced angiogenesis and neurogenesis in the injured cortex and hippocampus, increased oligodendrogenesis in the CA3 region, and significantly improved sensorimotor functional recovery and spatial learning compared to the saline treatment [147]. These data demonstrate that administration of Tβ4 significantly improves histological and functional outcomes in rats with TBI, indicating that Tβ4 has considerable therapeutic potential in TBI patients. Further investigation of Tβ4 is warranted for the treatment of TBI.
5.2 Exosomes
Exosomes are endosomal origin small-membrane vesicles with a size of 40-100 nm in diameter [148]. They are generated by many cell types and contain functional mRNAs and miRNAs, as well as proteins [149, 150]. Increasing evidence indicates that exosomes play an important role in cell-to-cell communication [150]. Exosomes are well suited for small functional molecule delivery [151], and may pass the blood-brain barrier [152]. The refinement of MSC therapy from a cell-based to cell-free exosome-based therapy offers several advantages, as it eases the arduous task of preserving cell viability and function, storage and delivery to patient because their bi-lipid membranes can protect their biologically active cargo allowing for easier storage of exosomes, which allows a longer shelf-life and half-life in patients [153]. Exosomes are ideal therapeutic agents because their complex cargo of proteins and genetic materials has diverse biochemical potential to participate in multiple biochemical and cellular processes, an important attribute in the treatment of complex disease such as TBI. Therefore, developing an exosome-based therapy for TBI opens up a wide variety of means to deliver targeted regulatory genes to enhance multifaceted aspects of CNS plasticity and to amplify neurological recovery potentially for a variety of neural injuries and neurodegenerative diseases.
Intravenous administration of exosomes derived from MSCs significantly improves functional outcome, increases angiogenesis, and neurogenesis in an animal model of TBI [154]. Compared with saline-treated controls, exosome-treated TBI rats showed significant improvement in spatial learning at 34-35 days measured by the Morris water maze test, and sensorimotor functional recovery, i.e., reduced neurological deficits and footfault frequency, observed at 14-35 days post injury. Exosome treatment significantly increased the number of newborn endothelial cells in the lesion boundary zone and dentate gyrus, and significantly increased the number of newborn immature and mature neurons in the dentate gyrus as well as reduced neuroinflammation. MSC-generated exosomes effectively improve functional recovery, at least in part, by promoting endogenous angiogenesis and neurogenesis and reducing inflammation in rats after TBI. Thus, MSC-generated exosomes may provide a novel cell-free therapy for TBI and possibly other neurological diseases.
5.3 Interleukin-1 receptor antagonist
Interleukin-1 receptor antagonist (IL-1RA) is an endogenous inhibitor of IL-1 signaling and recombinant IL-1RA is widely used for treatment of autoimmune and inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease [155]. Activation of the IL-1 receptor following TBI contributes to the pathology and that antagonism can reduce both anatomical and functional consequences of neuroinflammation [156]. Perinatal administration of IL-1RA may confer neuroprotective effects during births at high risk for brain injury [157]. IL-1RA can rapidly reach salvageable brain tissue via a subcutaneous administration route that is clinically relevant [158]. Intravenous delivery of IL-1RA leads to increased concentrations in the cerebrospinal fluid of patients with subarachnoid hemorrhage, which might provide therapeutic benefit [159]. A single center, Phase II, open label, randomized-control study in severe TBI provides promising data for recombinant human IL-1RA as a neuroprotective therapeutic candidate by showing safety, brain penetration and a modification of the neuroinflammatory response to TBI in humans for the first time [160].
5.4 Recombinant human tissue plasminogen activator
Recombinant human tissue plasminogen activator (tPA) is the only U.S. Food and Drug Administration (FDA) approved drug for the treatment of acute ischemic stroke [161]. In addition to its well-known function as a thromobolytic enzyme, tPA is also involved in synaptic plasticity, dendritic remodeling and axonal outgrowth in the developing and injured CNS [162]. Among all the neurotrophins, brain-derived neurotrophic factor (BDNF) is the best characterized for its role in regulating synaptic plasticity [163]. tPA, by activating the extracellular protease plasmin, converts the proBDNF to the mature BDNF, and that such conversion is critical for brain neuroplasticity and function [163]. A recent preclinical study investigated efficacy of subacute intranasal tPA in TBI rats treated intranasally with saline or tPA (600 µg/rat) initiated 7 days after injury [164]. Compared with saline treatment, subacute intranasal tPA treatment significantly 1) improved cognitive and sensorimotor functional recovery in rats after TBI, 2) enhanced neurogenesis in the dentate gyrus and axonal sprouting of the corticospinal tract originating from the contralesional cortex into the denervated side of the cervical gray matter, and 3) increased the level of mature brain-derived neurotrophic factor [164]. These data suggest that subacute intranasal tPA treatment improves functional recovery and promotes brain neurogenesis and spinal cord axonal sprouting after TBI, which may be mediated, at least in part, by tPA/plasmin-dependent maturation of BDNF. However, tPA increased brain lesion and hemorrhage in rats when given 15 min after TBI [165]. A catalytically inactive tPA variant, tPA S481A, that acts by competing with wild-type tPA for binding, cleavage, and activation of NMDA receptors provides a novel approach for limiting neuronal toxicity if given iv after TBI [166]. There is potential for use of inactive tPA mutants to treat acute TBI by reducing NMDA-receptor-mediated impairment of cerebral hemodynamics, and enhances excitotoxic neuronal death [166].
5.5 microRNAs
microRNAs (miRNAs) are small (approximately 22 nucleotides) noncoding RNAs that regulate gene expression at the post-transcriptional level, either by translational repression or mRNA degradation [167]. Emerging data indicate that expression levels of miRNAs are altered in the brain of TBI animals [168] and in plasma of TBI patients[169], while therapeutic treatments, including hypothermia [170] and voluntary exercise [171], modified expression of miRNAs. These findings suggest that miRNAs influence the pathophysiological process in brain injury and after treatment. miRNAs (especially derived from plasma and cerebrospinal fluid) can be employed as diagnostic biomarkers [169]. More importantly, miRNAs are potential therapeutic targets in TBI. For example, miR-21, a strong prosurvival miRNA, was up-regulated in adult rat brains after TBI but down-regulated in aged rat brains after TBI [172]. A recent study manipulated the expression level of miR-21 in brain using intracerebroventricular infusion of miR-21 agomir or antagomir to evaluate the potential effect of miR-21 on neurological function in the fluid percussion injury rat model of TBI [173]. Upregulation of miR-21 level in brain conferred a better neurological outcome after TBI by alleviating brain edema and decreasing lesion volume. miR-21 inhibited apoptosis and promoted angiogenesis through regulating the expression of apoptosis- and angiogenesis-related molecules including VEGF, angiopoietin-1 (Ang-1) and Tie-2 (receptor of Ang-1) in brain. In addition, the expression of phosphatase and tensin homolog deleted on chromosome10, a miR-21 target gene, was inhibited and Akt signaling was activated in the brain [173]. These data indicate that miRNAs including miR-21 could be a potential therapeutic target for interventions after TBI.
6. Conclusions
TBI elicits both complex degenerative and regenerative tissue responses in the brain. TBI leads to cognitive, behavioral, and motor deficits. Neuroprotective approaches are the mainstay for development of treatment for TBI. Neuroprotective strategies face challenge to identify and target specific mechanisms involved in the complex secondary injury cascade. In addition to neuroprotective approaches described in this review, the cell-based and pharmacological therapies that enhance endogenous neurorestorative processes by increasing angiogenesis, axonal remodeling, neurogenesis and synaptogenesis represent a promising potential for improving neurological functional recovery following TBI. Although it is still important to further investigate neuroprotective treatments for TBI, an interesting novel research direction is the development of neurorestorative strategies that enhance axonal remodeling, angiogenesis, neurogenesis and synaptogenesis to improve functional recovery of the injured brain.
7. Expert opinion
Despite robust experimental data on efficacy of neuroprotective drugs tested in animal models of TBI, all the Phase III clinical trials of neuroprotection in TBI patients have failed. These failures likely reflect methodological differences between the clinical and animal studies, as well as inadequate pre-clinical evaluation and/or clinical trial designs (Table 3-Translational challenges in TBI and suggestions) [11, 44, 45].
Table 3. Translational challenges in TBI and suggestions.
| Challenges in TBI trial | Comments and potential solutions |
|---|---|
| Complex secondary injury mechanisms |
Important to investigate secondary injury mechanisms at molecular, and cellular levels, and to develop and test multifunctional agents or combination treatments |
| Choice of species, sex and age |
Test therapeutic agents in higher-species animals of TBI, in young and aged subjects of both sexes |
| Comorbidities | Incorporate hypoxia, ischemia, hematoma, alcohol and drug use, systemic trauma and other diseases (e.g., Diabetes) into animal models of TBI |
| Lack of pharmacokinetics and pharmacodynamic |
Obtain pharmacokinetics, pharmacodynamics and brain concentration of the tested treatment, obtain data on dose response, therapeutic window, route, duration, interaction with other drugs |
| Diverse injury type | Test agents in different types of TBI: focal, diffuse, open vs closed, and blast head injury mild, moderate, and severe, repetitive mTBI |
| Neuron-centered neuroprotection |
Block the molecular cascade of injury following TBI. A major limitation of neuroprotection strategies is the short time window in which to deliver the therapy. Test of neuroprotection on neurons alone is inadequate; also require testing on non-neuronal cells. |
| Neurorestoration | Unlike neuroprotection which solely reduces cell death or lesion volume, neurorestorative approaches aim to remodel brain tissue by promoting endogenous neurogenesis, axonal sprouting, synaptogenesis, oligodendrogenesis and angiogenesis, which in concert enhance neuroplasticity and improves functional recovery. Neurorestorative therapy could potentially have a high clinical impact by extending the therapeutic window and targeting an expanded population of patients with TBI. |
| Outcome measures and long-term study |
The outcomes (e.g., mortality, GOS or GOSe) are often measured at 3 or 6 months post injury in TBI patients. Development of new outcome measures and use of well-characterized outcome measures to assess long- term effects of the treatment in animal models of TBI and clinical trials are warranted. |
| Study design and statistical analyses |
Whereas clinical trials generally employ an intent-to-treat analysis, this is virtually never conducted pre-clinically. Whereas clinical studies usually include a range of injury severities with various comorbidities (heterogeneity of the population), preclinical studies employ well- defined, highly controlled animal models of predetermined severity. There is a need to enlarge sample sizes and to improve power as well as to conceal treatment allocation or blinded outcome assessment. |
| Lack of biomarkers | There is a need to identify specific and sensitive biomarkers/imaging makers which are important for the diagnosis, prognosis, and evaluation of treatment efficacy for TBI. |
| Publication bias | Report/publish negative results of preclinical studies on efficacy of therapeutic treatments. |
Significant advances in the understanding of the mechanisms underlying TBI sequelae (behavioral, cognitive, or psychiatric) have been made, and the use of cell-based and pharmacological interventions to improve symptoms, function, and outcome is still under development. Although the results of TBI clinical trials are disappointing, new directions of research on mechanisms underlying TBI-induced brain injury and repair as well as search for innovative approaches targeting neuroprotective and neurorestorative effects will facilitate development of treatment for TBI, with the ultimate goal to reduce brain injury, promote brain repair and remodeling, and eventually improve functional recovery and quality of life.
There are many obstacles to the development of a neuroprotective therapy in TBI. One concern is the choice of species, strain, age or sex of the animal and animal models of TBI and measures of functional outcome and recovery. Preclinical challenges include a lack of complete dose response and therapeutic windows, and an absence of pharmacokinetic, pharmacodynamic, or brain penetration data, which are major factors that need to be addressed. Therefore, prior to the translation of an agent or cell therapy into TBI clinical trials, extensive pharmacokinetic data for agents to treat injured brains should also be obtained, ensuring an adequate concentration in the brain tissue and limiting neurotoxicity. Sufficient preclinical data should be obtained from multiple experiments, preferably in several TBI models, on dose-response, therapeutic windows, optimal administration routes, single dose versus multiple doses, bolus dose versus continuous infusion. In most animal TBI models, genetically identical animals are used with experimental conditions (e.g., age, body weight, sex, injury level, and injury site) well controlled. Animal models of TBI usually examine effects of local contusion or diffuse axonal injury in models that do not include significant ischemia, hypoxia, mass effects, or associated systemic injuries, as often seen in human TBI trials. In contrast to animal studies, human TBI trials have often included a wide range of injury levels. Inclusion of mild injury may lead to a ceiling effect without a very large sample size whereas severe injury may not be amenable to therapeutic interventions. Most preclinical TBI neuroprotective studies use lesion volume and short-term functional recovery as primary outcomes and only 10% of rodent TBI studies evaluate functional outcomes longer than 2 months post-TBI [174]. In contrast, clinical studies have preferentially used long-term behavioral outcomes, such as mortality or disability at 3 or 6 months. Whereas clinical studies generally employ an intent-to-treat analysis, this is never or rarely performed in animal TBI studies. These profound differences in TBI trials between animals and humans may create serious translational obstacles for development of treatments.
Secondary injury involves complex multiple biochemical processes initiated within minutes to days or longer after the insult of TBI, which have been demonstrated in a multitude of animal studies across models and species. Some preclinical studies have examined multiple drug treatments of TBI, reporting that combination treatments have beneficial synergistic effects [175]. Although the combination approach is promising, it faces several challenges in TBI clinical trials given the high cost of neuroprotection trials, selection of effective drugs, and potential drug interactions [176]. For determination of the safety and efficacy, the interaction of agents used in combination therapy should be fully addressed in preclinical studies before translation into clinical trials. Instead, it is feasible to evaluate single compounds that have multifunctional effects on different secondary injury mechanisms. Several promising experimental drug treatments with multipotential actions, which have shown effectiveness across experimental models, have recently failed in randomized TBI clinical trials, e.g., erythropoietin and progesterone. Such studies indicate that even strong experimental data in lower species may not predict therapeutic effectiveness in human TBI. It is recommended to evaluate efficacy in the large animal models of TBI before initiating clinical trials.
As discussed in this review, the multifunctional agents that facilitate endogenous neuroprotective pathways and neurovascular remodeling including angiogenesis, neurogenesis, oligodendrogenesis, and synaptogenesis hold the most promise. To develop exosomes and microRNA as well as other neurorestorative agents as novel approaches for treatment of TBI, is particularly interesting. Exosomes derived from MSCs that carry and transfer their cargo (e.g., miRNAs, mRNAs, proteins, and lipids) to parenchymal cells mediate brain plasticity and improve functional recovery from TBI. Technical issues including purity of exosomes and methods for mass exosome must be addressed. For the modified exosome application, the exosome product needs to be extensively characterized, in order to assess its biological function and to avoid adverse effects. It is essential to improve the drug delivery system to deliver drugs into the brain. Intranasal noninvasive delivery is one of promising pathways.
The enormous burden of TBI clearly supports the need to search for novel neuroprotective and/or neurorestorative agents or approaches. In addition to inadequate preclinical studies noted above, the disappointing clinical Phase III trials may be due to heterogeneity of the population of TBI patients and variability in treatment approaches. Although many pathophysiologic cascades inducing secondary injury have been identified, it remains uncertain which of and where these cascades are actually active in individual TBI patients after injury. Moreover, some pathways may initially be detrimental, but can be protective at later stages. Therefore, effective translation of agents into clinical trials will probably require a more mechanistic approach, i.e., only patients with a particular secondary injury mechanism identified are included in trials evaluating a compound that aims to target this specific mechanism. In this regard, it is very important to identify clinically relevant, sensitive and specific biomarkers for reliably diagnosing TBI and guiding therapeutic development and treatment of TBI. Current preclinical and clinical trials in TBI have primarily targeted neuroprotection, with a focus on preventing neuronal cell death and brain damage during the acute phase of injury. Although reactive astrocytes form an inhibitory glial scar following TBI, astrocytes are involved in neuroprotection [177], axonal remodeling and functional recovery in animal models of neural injury [178, 179]. Development of therapeutic approaches to enhancing protective functions of astrocytes and/or reducing their detrimental effects may improve neurological recovery after TBI. Neurorestorative strategies designed to enhance neurovascular remodeling and long-term functional recovery are promising and warranted especially for treatment of subacute or chronic TBI.
Article highlights.
Traumatic brain injury (TBI) elicits both complex degenerative and regenerative tissue responses in the brain and is one of leading causes for mortality and morbidity worldwide.
There are no pharmacologic agents demonstrated to improve outcomes from TBI because all the Phase III clinical trials in TBI have failed.
Progesterone and erythropoietin showed high therapeutic potential in animal studies but failed in recently completed clinical trials.
Investigational drugs under early clinical trials reviewed include cyclosporin A, glibenclamide, minocycline, NNZ-2566, nerve growth factor, propranolol, statins, stem cell therapy, tranexamic acid and valproic acid.
Other promising investigational biologics and drugs under preclinical development reviewed are thymosin beta 4, exosomes, interleukin-1 receptor antagonist, recombinant human tissue plasminogen activator, microRNAs.
Translational challenges in TBI and potential therapeutic strategies with a focus on neurorestorative approaches are discussed
This box summarizes key points contained in the article
Acknowledgements
We apologize to those researchers whose work has not been cited due to space limitations. Preparation of manuscript was funded in part by NIH grants R01-AG037506 and R01-NS088656 (MC).
Footnotes
The authors have no potential conflicts of interest related to the preparation of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Menon DK, Schwab K, Wright DW, et al. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. 2010;91(11):1637–40. doi: 10.1016/j.apmr.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Chauhan NB. Chronic neurodegenerative consequences of traumatic brain injury. Restor Neurol Neurosci. 2014 doi: 10.3233/RNN-130354. [DOI] [PubMed] [Google Scholar]
- 3.Hyder AA, Wunderlich CA, Puvanachandra P, et al. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 2007;22(5):341–53. [PubMed] [Google Scholar]
- 4.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–8. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma. 2010;27(8):1529–40. doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- 6.Davis AE. Mechanisms of traumatic brain injury: biomechanical, structural and cellular considerations. Crit Care Nurs Q. 2000;23(3):1–13. doi: 10.1097/00002727-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 7•.Cernak I. Animal models of head trauma. NeuroRx. 2005;2(3):410–22. doi: 10.1602/neurorx.2.3.410. Comprehensive review of the animal models of TBI and key factors in development of animal models and neuroprotective approaches
- 8.Gaetz M. The neurophysiology of brain injury. Clin Neurophysiol. 2004;115(1):4–18. doi: 10.1016/s1388-2457(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 9.Moppett IK. Traumatic brain injury: assessment, resuscitation and early management. Br J Anaesth. 2007;99(1):18–31. doi: 10.1093/bja/aem128. [DOI] [PubMed] [Google Scholar]
- 10.Marklund N, Bakshi A, Castelbuono DJ, et al. Evaluation of pharmacological treatment strategies in traumatic brain injury. Curr Pharm Des. 2006;12(13):1645–80. doi: 10.2174/138161206776843340. [DOI] [PubMed] [Google Scholar]
- 11••.Loane DJ, Faden AI. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 2010;31(12):596–604. doi: 10.1016/j.tips.2010.09.005. Critical review on translational challenges in neuroprotection for TBI
- 12.Povlishock JT, Christman CW. The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. J Neurotrauma. 1995;12(4):555–64. doi: 10.1089/neu.1995.12.555. [DOI] [PubMed] [Google Scholar]
- 13••.Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14(2):128–42. doi: 10.1038/nrn3407. Recent comprehensive review on animal models of TBI and translational challenges
- 14.Cernak I, Noble-Haeusslein LJ. Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J Cereb Blood Flow Metab. 2010;30(2):255–66. doi: 10.1038/jcbfm.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janowitz T, Menon DK. Exploring new routes for neuroprotective drug development in traumatic brain injury. Sci Transl Med. 2010;2(27):27rv1. doi: 10.1126/scitranslmed.3000330. [DOI] [PubMed] [Google Scholar]
- 16.Hall ED, Traystman RJ. Role of animal studies in the design of clinical trials. Front Neurol Neurosci. 2009;25:10–33. doi: 10.1159/000209470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chopp M, Li Y, Zhang J. Plasticity and remodeling of brain. J Neurol Sci. 2008;265(1-2):97–101. doi: 10.1016/j.jns.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Xiong Y, Mahmood A, Chopp M. Neurorestorative treatments for traumatic brain injury. Discov Med. 2010;10(54):434–42. [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs. 2010;11(3):298–308. [PMC free article] [PubMed] [Google Scholar]
- 20.Brown JM, Deriso DM, Tansey KE. From contemporary rehabilitation to restorative neurology. Clin Neurol Neurosurg. 2012;114(5):471–4. doi: 10.1016/j.clineuro.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Algattas H, Huang JH. Traumatic Brain Injury pathophysiology and treatments: early, intermediate, and late phases post-injury. Int J Mol Sci. 2014;15(1):309–41. doi: 10.3390/ijms15010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewall J. The ABCs of TBI. Evidence-based guidelines for adult traumatic brain injury care. JEMS. 2010;35(4):54–61. doi: 10.1016/S0197-2510(10)70095-4. quiz 3. [DOI] [PubMed] [Google Scholar]
- 23••.Narayan RK, Michel ME, Ansell B, et al. Clinical trials in head injury. J Neurotrauma. 2002;19(5):503–57. doi: 10.1089/089771502753754037. Informative and insightful review on clinical trials in TBI
- 24.Wright DW, Kellermann AL, Hertzberg VS, et al. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49(4):391–402. e1–2. doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]
- 25.Xiao G, Wei J, Yan W, et al. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit Care. 2008;12(2):R61. doi: 10.1186/cc6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson K, Carson S, Carney N. Hypothermia treatment for traumatic brain injury: a systematic review and meta-analysis. J Neurotrauma. 2008;25(1):62–71. doi: 10.1089/neu.2007.0424. [DOI] [PubMed] [Google Scholar]
- 27.Marmarou A. Increased intracranial pressure in head injury and influence of blood volume. J Neurotrauma. 1992;9(Suppl 1):S327–32. [PubMed] [Google Scholar]
- 28.Wakai A, McCabe A, Roberts I, et al. Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev. 2013;8:CD001049. doi: 10.1002/14651858.CD001049.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahuquillo J, Arikan F. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database Syst Rev. 2006;1:CD003983. doi: 10.1002/14651858.CD003983.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Cooper DJ, Rosenfeld JV, Murray L, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364(16):1493–502. doi: 10.1056/NEJMoa1102077. [DOI] [PubMed] [Google Scholar]
- 31.Sahuquillo J, Martinez-Ricarte F, Poca MA. Decompressive craniectomy in traumatic brain injury after the DECRA trial. Where do we stand? Curr Opin Crit Care. 2013;19(2):101–6. doi: 10.1097/MCC.0b013e32835eba1a. [DOI] [PubMed] [Google Scholar]
- 32.Honeybul S, Ho KM, Lind CR. What can be learned from the DECRA study. World Neurosurg. 2013;79(1):159–61. doi: 10.1016/j.wneu.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Hutchinson PJ, Corteen E, Czosnyka M, et al. Decompressive craniectomy in traumatic brain injury: the randomized multicenter RESCUEicp study ( www.RESCUEicp.com) Acta Neurochir Suppl. 2006;96:17–20. doi: 10.1007/3-211-30714-1_4. www.RESCUEicp.com [DOI] [PubMed] [Google Scholar]
- 34.Maas AI. Neuroprotective agents in traumatic brain injury. Expert Opin Investig Drugs. 2001;10(4):753–67. doi: 10.1517/13543784.10.4.753. [DOI] [PubMed] [Google Scholar]
- 35••.Lu J, Gary KW, Neimeier JP, et al. Randomized controlled trials in adult traumatic brain injury. Brain Inj. 2012;26(13-14):1523–48. doi: 10.3109/02699052.2012.722257. Comprehensive review of all randomized controlled trials in adults with TBI over the past 30 years
- 36.Maas AI, Roozenbeek B, Manley GT. Clinical trials in traumatic brain injury: past experience and current developments. Neurotherapeutics. 2010;7(1):115–26. doi: 10.1016/j.nurt.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Kabadi SV, Faden AI. Neuroprotective strategies for traumatic brain injury: improving clinical translation. Int J Mol Sci. 2014;15(1):1216–36. doi: 10.3390/ijms15011216. Recent comprehensive review on neuroprotective strategies for TBI and critical analysis of the limitations and translational opportunities for developing successful neuroprotective therapies
- 38••.McConeghy KW, Hatton J, Hughes L, et al. A review of neuroprotection pharmacology and therapies in patients with acute traumatic brain injury. CNS Drugs. 2012;26(7):613–36. doi: 10.2165/11634020-000000000-00000. Execellent review on studies reporting TBI outcomes related to neuroprotection, both clinical and experimental published from 1966 through January 2012
- 39.Jain KK. Neuroprotection in traumatic brain injury. Drug Discov Today. 2008;13(23-24):1082–9. doi: 10.1016/j.drudis.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Wheaton P, Mathias JL, Vink R. Impact of pharmacological treatments on cognitive and behavioral outcome in the postacute stages of adult traumatic brain injury: a meta-analysis. J Clin Psychopharmacol. 2011;31(6):745–57. doi: 10.1097/JCP.0b013e318235f4ac. [DOI] [PubMed] [Google Scholar]
- 41.Wheaton P, Mathias JL, Vink R. Impact of early pharmacological treatment on cognitive and behavioral outcome after traumatic brain injury in adults: a meta-analysis. J Clin Psychopharmacol. 2009;29(5):468–77. doi: 10.1097/JCP.0b013e3181b66f04. [DOI] [PubMed] [Google Scholar]
- 42.Lei J, Gao G, Jiang J. Acute traumatic brain injury: is current management evidence based? An empirical analysis of systematic reviews. J Neurotrauma. 2013;30(7):529–37. doi: 10.1089/neu.2012.2548. [DOI] [PubMed] [Google Scholar]
- 43.Doppenberg EM, Choi SC, Bullock R. Clinical trials in traumatic brain injury: lessons for the future. J Neurosurg Anesthesiol. 2004;16(1):87–94. doi: 10.1097/00008506-200401000-00019. [DOI] [PubMed] [Google Scholar]
- 44.Marklund N, Hillered L. Animal modelling of traumatic brain injury in preclinical drug development: where do we go from here? Br J Pharmacol. 2011;164(4):1207–29. doi: 10.1111/j.1476-5381.2010.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roozenbeek B, Lingsma HF, Maas AI. New considerations in the design of clinical trials for traumatic brain injury. Clin Investig (Lond) 2012;2(2):153–62. doi: 10.4155/cli.11.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stein DG. On improving human clinical trials to the level of animal ischemic stroke studies. Metab Brain Dis. 2014 doi: 10.1007/s11011-014-9613-5. [DOI] [PubMed] [Google Scholar]
- 47.Melcangi RC, Giatti S, Calabrese D, et al. Levels and actions of progesterone and its metabolites in the nervous system during physiological and pathological conditions. Prog Neurobiol. 2014;113:56–69. doi: 10.1016/j.pneurobio.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Stein DG. A clinical/translational perspective: can a developmental hormone play a role in the treatment of traumatic brain injury? Horm Behav. 2013;63(2):291–300. doi: 10.1016/j.yhbeh.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Wei J, Xiao GM. The neuroprotective effects of progesterone on traumatic brain injury: current status and future prospects. Acta Pharmacol Sin. 2013;34(12):1485–90. doi: 10.1038/aps.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skolnick BE, Maas AI, Narayan RK, et al. A clinical trial of progesterone for severe traumatic brain injury. N Engl J Med. 2014;371(26):2467–76. doi: 10.1056/NEJMoa1411090. [DOI] [PubMed] [Google Scholar]
- 51.Wright DW, Yeatts SD, Silbergleit R, et al. Very early administration of progesterone for acute traumatic brain injury. N Engl J Med. 2014;371(26):2457–66. doi: 10.1056/NEJMoa1404304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berry C, Ley EJ, Tillou A, et al. The effect of gender on patients with moderate to severe head injuries. J Trauma. 2009;67(5):950–3. doi: 10.1097/TA.0b013e3181ba3354. [DOI] [PubMed] [Google Scholar]
- 53.Farace E, Alves WM. Do women fare worse: a metaanalysis of gender differences in traumatic brain injury outcome. J Neurosurg. 2000;93(4):539–45. doi: 10.3171/jns.2000.93.4.0539. [DOI] [PubMed] [Google Scholar]
- 54.Leitgeb J, Mauritz W, Brazinova A, et al. Effects of gender on outcomes after traumatic brain injury. J Trauma. 2011;71(6):1620–6. doi: 10.1097/TA.0b013e318226ea0e. [DOI] [PubMed] [Google Scholar]
- 55.Hall ED, Gibson TR, Pavel KM. Lack of a gender difference in post-traumatic neurodegeneration in the mouse controlled cortical impact injury model. J Neurotrauma. 2005;22(6):669–79. doi: 10.1089/neu.2005.22.669. [DOI] [PubMed] [Google Scholar]
- 56.Wagner AK, Willard LA, Kline AE, et al. Evaluation of estrous cycle stage and gender on behavioral outcome after experimental traumatic brain injury. Brain Res. 2004;998(1):113–21. doi: 10.1016/j.brainres.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 57.Maghool F, Khaksari M, Siahposht Khachki A. Differences in brain edema and intracranial pressure following traumatic brain injury across the estrous cycle: involvement of female sex steroid hormones. Brain Res. 2013;1497:61–72. doi: 10.1016/j.brainres.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 58.Patel NS, Collino M, Yaqoob MM, et al. Erythropoietin in the intensive care unit: beyond treatment of anemia. Ann Intensive Care. 2011;1:40. doi: 10.1186/2110-5820-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ehrenreich H, Aust C, Krampe H, et al. Erythropoietin: novel approaches to neuroprotection in human brain disease. Metab Brain Dis. 2004;19(3-4):195–206. doi: 10.1023/b:mebr.0000043969.96895.3c. [DOI] [PubMed] [Google Scholar]
- 60.Peng W, Xing Z, Yang J, et al. The efficacy of erythropoietin in treating experimental traumatic brain injury: a systematic review of controlled trials in animal models. J Neurosurg. 2014;121(3):653–64. doi: 10.3171/2014.6.JNS132577. [DOI] [PubMed] [Google Scholar]
- 61.Robertson CS, Hannay HJ, Yamal JM, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. JAMA. 2014;312(1):36–47. doi: 10.1001/jama.2014.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahmood A, Lu D, Qu C, et al. Treatment of traumatic brain injury in rats with erythropoietin and carbamylated erythropoietin. J Neurosurg. 2007;107(2):392–7. doi: 10.3171/JNS-07/08/0392. [DOI] [PubMed] [Google Scholar]
- 63.Gaddam SK, Cruz J, Robertson C. Erythropoietin and cytoprotective cytokines in experimental traumatic brain injury. Methods Mol Biol. 2013;982:141–62. doi: 10.1007/978-1-62703-308-4_9. [DOI] [PubMed] [Google Scholar]
- 64.Xiong Y, Mahmood A, Zhang Y, et al. Effects of posttraumatic carbamylated erythropoietin therapy on reducing lesion volume and hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome in rats following traumatic brain injury. J Neurosurg. 2011;114(2):549–59. doi: 10.3171/2010.10.JNS10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robertson CS, Cherian L, Shah M, et al. Neuroprotection with an erythropoietin mimetic peptide (pHBSP) in a model of mild traumatic brain injury complicated by hemorrhagic shock. J Neurotrauma. 2012;29(6):1156–66. doi: 10.1089/neu.2011.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Velzen M, Heij L, Niesters M, et al. ARA 290 for treatment of small fiber neuropathy in sarcoidosis. Expert Opin Investig Drugs. 2014;23(4):541–50. doi: 10.1517/13543784.2014.892072. [DOI] [PubMed] [Google Scholar]
- 67.Brines M, Dunne AN, Van Velzen M, et al. ARA 290, a non-erythropoietic peptide engineered from erythropoietin, improves metabolic control and neuropathic symptoms in patients with type 2 diabetes. Mol Med. 2014 doi: 10.2119/molmed.2014.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahn EY, Shrestha A, Hoang NH, et al. Structural characterization of cyclosporin A, C and microbial bio-transformed cyclosporin A analog AM6 using HPLC-ESI-ion trap-mass spectrometry. Talanta. 2014;123:89–94. doi: 10.1016/j.talanta.2014.01.067. [DOI] [PubMed] [Google Scholar]
- 69.Kawakami M. Molecular Dissection of Cyclosporin A’s Neuroprotective Effect Reveals Potential Therapeutics for Ischemic Brain Injury. Brain Sci. 2013;3(3):1325–56. doi: 10.3390/brainsci3031325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scheff SW, Sullivan PG. Cyclosporin A significantly ameliorates cortical damage following experimental traumatic brain injury in rodents. J Neurotrauma. 1999;16(9):783–92. doi: 10.1089/neu.1999.16.783. [DOI] [PubMed] [Google Scholar]
- 71.Gajavelli S, Sinha VK, Mazzeo AT, et al. Evidence to support mitochondrial neuroprotection, in severe traumatic brain injury. J Bioenerg Biomembr. 2014 doi: 10.1007/s10863-014-9589-1. [DOI] [PubMed] [Google Scholar]
- 72.Sullivan PG, Rabchevsky AG, Hicks RR, et al. Dose-response curve and optimal dosing regimen of cyclosporin A after traumatic brain injury in rats. Neuroscience. 2000;101(2):289–95. doi: 10.1016/s0306-4522(00)00380-8. [DOI] [PubMed] [Google Scholar]
- 73.Mazzeo AT, Brophy GM, Gilman CB, et al. Safety and tolerability of cyclosporin a in severe traumatic brain injury patients: results from a prospective randomized trial. J Neurotrauma. 2009;26(12):2195–206. doi: 10.1089/neu.2009.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Empey PE, McNamara PJ, Young B, et al. Cyclosporin A disposition following acute traumatic brain injury. J Neurotrauma. 2006;23(1):109–16. doi: 10.1089/neu.2006.23.109. [DOI] [PubMed] [Google Scholar]
- 75.Hatton J, Rosbolt B, Empey P, et al. Dosing and safety of cyclosporine in patients with severe brain injury. J Neurosurg. 2008;109(4):699–707. doi: 10.3171/JNS/2008/109/10/0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aminmansour B, Fard SA, Habibabadi MR, et al. The efficacy of Cyclosporine-A on Diffuse Axonal Injury after Traumatic Brain Injury. Adv Biomed Res. 2014;3:35. doi: 10.4103/2277-9175.125031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luzi L, Pozza G. Glibenclamide: an old drug with a novel mechanism of action? Acta Diabetol. 1997;34(4):239–44. doi: 10.1007/s005920050081. [DOI] [PubMed] [Google Scholar]
- 78.Patel AD, Gerzanich V, Geng Z, et al. Glibenclamide reduces hippocampal injury and preserves rapid spatial learning in a model of traumatic brain injury. J Neuropathol Exp Neurol. 2010;69(12):1177–90. doi: 10.1097/NEN.0b013e3181fbf6d6. [DOI] [PubMed] [Google Scholar]
- 79.Kurland DB, Tosun C, Pampori A, et al. Glibenclamide for the treatment of acute CNS injury. Pharmaceuticals (Basel) 2013;6(10):1287–303. doi: 10.3390/ph6101287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zweckberger K, Hackenberg K, Jung CS, et al. Glibenclamide reduces secondary brain damage after experimental traumatic brain injury. Neuroscience. 2014;272:199–206. doi: 10.1016/j.neuroscience.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 81.Sheth KN, Kimberly WT, Elm JJ, et al. Pilot study of intravenous glyburide in patients with a large ischemic stroke. Stroke. 2014;45(1):281–3. doi: 10.1161/STROKEAHA.113.003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khanna A, Walcott BP, Kahle KT, et al. Effect of glibenclamide on the prevention of secondary brain injury following ischemic stroke in humans. Neurosurg Focus. 2014;36(1):E11. doi: 10.3171/2013.10.FOCUS13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Orsucci D, Calsolaro V, Mancuso M, et al. Neuroprotective effects of tetracyclines: molecular targets, animal models and human disease. CNS Neurol Disord Drug Targets. 2009;8(3):222–31. doi: 10.2174/187152709788680689. [DOI] [PubMed] [Google Scholar]
- 84.Bye N, Habgood MD, Callaway JK, et al. Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp Neurol. 2007;204(1):220–33. doi: 10.1016/j.expneurol.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 85.Homsi S, Piaggio T, Croci N, et al. Blockade of acute microglial activation by minocycline promotes neuroprotection and reduces locomotor hyperactivity after closed head injury in mice: a twelve-week follow-up study. J Neurotrauma. 2010;27(5):911–21. doi: 10.1089/neu.2009.1223. [DOI] [PubMed] [Google Scholar]
- 86.Sara VR, Carlsson-Skwirut C, Bergman T, et al. Identification of Gly-Pro-Glu (GPE), the aminoterminal tripeptide of insulin-like growth factor 1 which is truncated in brain, as a novel neuroactive peptide. Biochem Biophys Res Commun. 1989;165(2:766–71. doi: 10.1016/s0006-291x(89)80032-4. [DOI] [PubMed] [Google Scholar]
- 87.Batchelor DC, Lin H, Wen JY, et al. Pharmacokinetics of glycine-proline-glutamate, the N-terminal tripeptide of insulin-like growth factor-1, in rats. Anal Biochem. 2003;323(2):156–63. doi: 10.1016/j.ab.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 88.Wei HH, Lu XC, Shear DA, et al. NNZ-2566 treatment inhibits neuroinflammation and pro-inflammatory cytokine expression induced by experimental penetrating ballistic-like brain injury in rats. J Neuroinflammation. 2009;6:19. doi: 10.1186/1742-2094-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aloe L, Rocco ML, Bianchi P, et al. Nerve growth factor: from the early discoveries to the potential clinical use. J Transl Med. 2012;10:239. doi: 10.1186/1479-5876-10-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Skaper SD. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol Disord Drug Targets. 2008;7(1):46–62. doi: 10.2174/187152708783885174. [DOI] [PubMed] [Google Scholar]
- 91.Alcala-Barraza SR, Lee MS, Hanson LR, et al. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. J Drug Target. 2010;18(3):179–90. doi: 10.3109/10611860903318134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lv Q, Lan W, Sun W, et al. Intranasal nerve growth factor attenuates tau phosphorylation in brain after traumatic brain injury in rats. J Neurol Sci. 2014;345(1-2):48–55. doi: 10.1016/j.jns.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 93.Lv Q, Fan X, Xu G, et al. Intranasal delivery of nerve growth factor attenuates aquaporins-4-induced edema following traumatic brain injury in rats. Brain Res. 2013;1493:80–9. doi: 10.1016/j.brainres.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 94.Tian L, Guo R, Yue X, et al. Intranasal administration of nerve growth factor ameliorate beta-amyloid deposition after traumatic brain injury in rats. Brain Res. 2012;1440:47–55. doi: 10.1016/j.brainres.2011.12.059. [DOI] [PubMed] [Google Scholar]
- 95.Alali AS, McCredie VA, Golan E, et al. Beta blockers for acute traumatic brain injury: a systematic review and meta-analysis. Neurocrit Care. 2014;20(3):514–23. doi: 10.1007/s12028-013-9903-5. [DOI] [PubMed] [Google Scholar]
- 96.Cotton BA, Snodgrass KB, Fleming SB, et al. Beta-blocker exposure is associated with improved survival after severe traumatic brain injury. J Trauma. 2007;62(1):26–33. doi: 10.1097/TA.0b013e31802d02d0. discussion -5. [DOI] [PubMed] [Google Scholar]
- 97.Ley EJ, Park R, Dagliyan G, et al. In vivo effect of propranolol dose and timing on cerebral perfusion after traumatic brain injury. J Trauma. 2010;68(2):353–6. doi: 10.1097/TA.0b013e3181c8269a. [DOI] [PubMed] [Google Scholar]
- 98.Patel MB, McKenna JW, Alvarez JM, et al. Decreasing adrenergic or sympathetic hyperactivity after severe traumatic brain injury using propranolol and clonidine (DASH After TBI Study): study protocol for a randomized controlled trial. Trials. 2012;13:177. doi: 10.1186/1745-6215-13-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lu D, Goussev A, Chen J, et al. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. Journal of neurotrauma. 2004;21(1):21–32. doi: 10.1089/089771504772695913. [DOI] [PubMed] [Google Scholar]
- 100.Lu D, Qu C, Goussev A, et al. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. Journal of neurotrauma. 2007;24(7):1132–46. doi: 10.1089/neu.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu H, Lu D, Jiang H, et al. Increase in phosphorylation of Akt and its downstream signaling targets and suppression of apoptosis by simvastatin after traumatic brain injury. Journal of neurosurgery. 2008;109(4):691–8. doi: 10.3171/JNS/2008/109/10/0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu H, Lu D, Jiang H, et al. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. Journal of neurotrauma. 2008;25(2):130–9. doi: 10.1089/neu.2007.0369. [DOI] [PubMed] [Google Scholar]
- 103.Li B, Mahmood A, Lu D, et al. Simvastatin attenuates microglial cells and astrocyte activation and decreases interleukin-1beta level after traumatic brain injury. Neurosurgery. 2009;65(1):179–85. doi: 10.1227/01.NEU.0000346272.76537.DC. discussion 85-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mahmood A, Goussev A, Kazmi H, et al. Long-term benefits after treatment of traumatic brain injury with simvastatin in rats. Neurosurgery. 2009;65(1):187–91. doi: 10.1227/01.NEU.0000343540.24780.D6. discussion 91-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wible EF, Laskowitz DT. Statins in traumatic brain injury. Neurotherapeutics. 2010;7(1):62–73. doi: 10.1016/j.nurt.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jain KK. Cell therapy for CNS trauma. Mol Biotechnol. 2009;42(3):367–76. doi: 10.1007/s12033-009-9166-8. [DOI] [PubMed] [Google Scholar]
- 107.Ho AD, Wagner W, Franke W. Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy. 2008;10(4):320–30. doi: 10.1080/14653240802217011. [DOI] [PubMed] [Google Scholar]
- 108.Kassem M, Abdallah BM. Human bone-marrow-derived mesenchymal stem cells: biological characteristics and potential role in therapy of degenerative diseases. Cell Tissue Res. 2008;331(1):157–63. doi: 10.1007/s00441-007-0509-0. [DOI] [PubMed] [Google Scholar]
- 109.Greco SJ, Rameshwar P. Enhancing effect of IL-1alpha on neurogenesis from adult human mesenchymal stem cells: implication for inflammatory mediators in regenerative medicine. J Immunol. 2007;179(5):3342–50. doi: 10.4049/jimmunol.179.5.3342. [DOI] [PubMed] [Google Scholar]
- 110.Lu D, Mahmood A, Wang L, et al. Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport. 2001;12(3):559–63. doi: 10.1097/00001756-200103050-00025. [DOI] [PubMed] [Google Scholar]
- 111.Mahmood A, Lu D, Qu C, et al. Human marrow stromal cell treatment provides long-lasting benefit after traumatic brain injury in rats. Neurosurgery. 2005;57(5):1026–31. doi: 10.1227/01.neu.0000181369.76323.50. discussion -31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lu D, Li Y, Wang L, et al. Intraarterial administration of marrow stromal cells in a rat model of traumatic brain injury. J Neurotrauma. 2001;18(8):813–9. doi: 10.1089/089771501316919175. [DOI] [PubMed] [Google Scholar]
- 113.Chen X, Katakowski M, Li Y, et al. Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: growth factor production. J Neurosci Res. 2002;69(5):687–91. doi: 10.1002/jnr.10334. [DOI] [PubMed] [Google Scholar]
- 114.Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma. 2004;21(1):33–9. doi: 10.1089/089771504772695922. [DOI] [PubMed] [Google Scholar]
- 115.Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1(2):92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 116.Qu C, Mahmood A, Lu D, et al. Treatment of traumatic brain injury in mice with marrow stromal cells. Brain Res. 2008;1208:234–9. doi: 10.1016/j.brainres.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen J, Chopp M. Neurorestorative treatment of stroke: cell and pharmacological approaches. NeuroRx. 2006;3(4):466–73. doi: 10.1016/j.nurx.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jiang Q, Qu C, Chopp M, et al. MRI evaluation of axonal reorganization after bone marrow stromal cell treatment of traumatic brain injury. NMR Biomed. 2011;24(9):1119–28. doi: 10.1002/nbm.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lu D, Mahmood A, Qu C, et al. Collagen scaffolds populated with human marrow stromal cells reduce lesion volume and improve functional outcome after traumatic brain injury. Neurosurgery. 2007;61(3):596–602. doi: 10.1227/01.NEU.0000290908.38438.B2. discussion -3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mahmood A, Wu H, Qu C, et al. Suppression of neurocan and enhancement of axonal density in rats after treatment of traumatic brain injury with scaffolds impregnated with bone marrow stromal cells. J Neurosurg. 2014;120(5):1147–55. doi: 10.3171/2013.12.JNS131362. [DOI] [PubMed] [Google Scholar]
- 121.Mahmood A, Wu H, Qu C, et al. Down-regulation of Nogo-A by collagen scaffolds impregnated with bone marrow stromal cell treatment after traumatic brain injury promotes axonal regeneration in rats. Brain Res. 2014;1542:41–8. doi: 10.1016/j.brainres.2013.10.045. [DOI] [PubMed] [Google Scholar]
- 122.Zhang ZX, Guan LX, Zhang K, et al. A combined procedure to deliver autologous mesenchymal stromal cells to patients with traumatic brain injury. Cytotherapy. 2008;10(2):134–9. doi: 10.1080/14653240701883061. [DOI] [PubMed] [Google Scholar]
- 123.Tian C, Wang X, Wang L, et al. Autologous bone marrow mesenchymal stem cell therapy in the subacute stage of traumatic brain injury by lumbar puncture. Exp Clin Transplant. 2013;11(2):176–81. doi: 10.6002/ect.2012.0053. [DOI] [PubMed] [Google Scholar]
- 124.Liao GP, Harting MT, Hetz RA, et al. Autologous Bone Marrow Mononuclear Cells Reduce Therapeutic Intensity for Severe Traumatic Brain Injury in Children. Pediatr Crit Care Med. 2015 doi: 10.1097/PCC.0000000000000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cox CS, Jr., Baumgartner JE, Harting MT, et al. Autologous bone marrow mononuclear cell therapy for severe traumatic brain injury in children. Neurosurgery. 2011;68(3):588–600. doi: 10.1227/NEU.0b013e318207734c. [DOI] [PubMed] [Google Scholar]
- 126.Harhangi BS, Kompanje EJ, Leebeek FW, et al. Coagulation disorders after traumatic brain injury. Acta Neurochir (Wien) 2008;150(2):165–75. doi: 10.1007/s00701-007-1475-8. discussion 75. [DOI] [PubMed] [Google Scholar]
- 127.Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 128.Guerriero C, Cairns J, Perel P, et al. Cost-effectiveness analysis of administering tranexamic acid to bleeding trauma patients using evidence from the CRASH-2 trial. PLoS One. 2011;6(5):e18987. doi: 10.1371/journal.pone.0018987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zehtabchi S, Abdel Baki SG, Falzon L, et al. Tranexamic acid for traumatic brain injury: a systematic review and meta-analysis. Am J Emerg Med. 2014;32(12):1503–9. doi: 10.1016/j.ajem.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Roberts I, Shakur H, Coats T, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17(10):1–79. doi: 10.3310/hta17100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yutthakasemsunt S, Kittiwatanagul W, Piyavechvirat P, et al. Tranexamic acid for patients with traumatic brain injury: a randomized, double-blinded, placebo-controlled trial. BMC Emerg Med. 2013;13:20. doi: 10.1186/1471-227X-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Effect of tranexamic acid in traumatic brain injury: a nested randomised, placebo controlled trial (CRASH-2 Intracranial Bleeding Study) BMJ. 2011;343:d3795. doi: 10.1136/bmj.d3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dewan Y, Komolafe EO, Mejia-Mantilla JH, et al. CRASH-3 - tranexamic acid for the treatment of significant traumatic brain injury: study protocol for an international randomized, double-blind, placebo-controlled trial. Trials. 2012;13:87. doi: 10.1186/1745-6215-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen S, Wu H, Klebe D, et al. Valproic acid: a new candidate of therapeutic application for the acute central nervous system injuries. Neurochem Res. 2014;39(9):1621–33. doi: 10.1007/s11064-014-1241-2. [DOI] [PubMed] [Google Scholar]
- 135.Dash PK, Orsi SA, Zhang M, et al. Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLoS One. 2010;5(6):e11383. doi: 10.1371/journal.pone.0011383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tai YT, Lee WY, Lee FP, et al. Low dose of valproate improves motor function after traumatic brain injury. Biomed Res Int. 2014;2014:980657. doi: 10.1155/2014/980657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Temkin NR, Dikmen SS, Anderson GD, et al. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. J Neurosurg. 1999;91(4):593–600. doi: 10.3171/jns.1999.91.4.0593. [DOI] [PubMed] [Google Scholar]
- 138.Dikmen SS, Machamer JE, Winn HR, et al. Neuropsychological effects of valproate in traumatic brain injury: a randomized trial. Neurology. 2000;54(4):895–902. doi: 10.1212/wnl.54.4.895. [DOI] [PubMed] [Google Scholar]
- 139••.Wheaton P, Mathias JL, Vink R. Impact of pharmacological treatments on outcome in adult rodents after traumatic brain injury: a meta-analysis. J Psychopharmacol. 2011;25(12):1581–99. doi: 10.1177/0269881110388331. Meta-analytic review on pharmacological treatments administered to adult male rodents after experimental TBI from 1980 to 2009
- 140.Goldstein AL, Hannappel E, Kleinman HK. Thymosin beta4: actin-sequestering protein moonlights to repair injured tissues. Trends Mol Med. 2005;11(9):421–9. doi: 10.1016/j.molmed.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 141.Low TL, Goldstein AL. Thymosins: structure, function and therapeutic applications. Thymus. 1984;6(1-2):27–42. [PubMed] [Google Scholar]
- 142.Crockford D. Development of thymosin beta4 for treatment of patients with ischemic heart disease. Ann N Y Acad Sci. 2007;1112:385–95. doi: 10.1196/annals.1415.051. [DOI] [PubMed] [Google Scholar]
- 143.Morris DC, Chopp M, Zhang L, et al. Thymosin beta4: a candidate for treatment of stroke? Ann N Y Acad Sci. 2010;1194:112–7. doi: 10.1111/j.1749-6632.2010.05469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Smart N, Risebro CA, Melville AA, et al. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445(7124):177–82. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 145.Ruff D, Crockford D, Girardi G, et al. A randomized, placebo-controlled, single and multiple dose study of intravenous thymosin beta4 in healthy volunteers. Ann N Y Acad Sci. 2010;1194:223–9. doi: 10.1111/j.1749-6632.2010.05474.x. [DOI] [PubMed] [Google Scholar]
- 146.Sun W, Kim H. Neurotrophic roles of the beta-thymosins in the development and regeneration of the nervous system. Ann N Y Acad Sci. 2007;1112:210–8. doi: 10.1196/annals.1415.013. [DOI] [PubMed] [Google Scholar]
- 147.Xiong Y, Mahmood A, Meng Y, et al. Treatment of traumatic brain injury with thymosin beta(4) in rats. Journal of neurosurgery. 2010 doi: 10.3171/2010.4.JNS10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stoorvogel W, Kleijmeer MJ, Geuze HJ, et al. The biogenesis and functions of exosomes. Traffic. 2002;3(5):321–30. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- 149.Record M, Carayon K, Poirot M, et al. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta. 2014;1841(1):108–20. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 150.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. Journal of proteomics. 2010;73(10):1907–20. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 151.Wahlgren J, De LKT, Brisslert M, et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40(17):e130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van der Pol E, Boing AN, Harrison P, et al. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64(3):676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 153.Lai RC, Yeo RW, Tan KH, et al. Exosomes for drug delivery - a novel application for the mesenchymal stem cell. Biotechnol Adv. 2013;31(5):543–51. doi: 10.1016/j.biotechadv.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 154.Zhang Y, Chopp M, Meng Y, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015:1–12. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–52. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jones NC, Prior MJ, Burden-Teh E, et al. Antagonism of the interleukin-1 receptor following traumatic brain injury in the mouse reduces the number of nitric oxide synthase-2-positive cells and improves anatomical and functional outcomes. Eur J Neurosci. 2005;22(1):72–8. doi: 10.1111/j.1460-9568.2005.04221.x. [DOI] [PubMed] [Google Scholar]
- 157.Girard S, Sebire H, Brochu ME, et al. Postnatal administration of IL-1Ra exerts neuroprotective effects following perinatal inflammation and/or hypoxic-ischemic injuries. Brain Behav Immun. 2012;26(8):1331–9. doi: 10.1016/j.bbi.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Greenhalgh AD, Galea J, Denes A, et al. Rapid brain penetration of interleukin-1 receptor antagonist in rat cerebral ischaemia: pharmacokinetics, distribution, protection. Br J Pharmacol. 2010;160(1):153–9. doi: 10.1111/j.1476-5381.2010.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Clark SR, McMahon CJ, Gueorguieva I, et al. Interleukin-1 receptor antagonist penetrates human brain at experimentally therapeutic concentrations. J Cereb Blood Flow Metab. 2008;28(2):387–94. doi: 10.1038/sj.jcbfm.9600537. [DOI] [PubMed] [Google Scholar]
- 160.Helmy A, Guilfoyle MR, Carpenter KL, et al. Recombinant human interleukin-1 receptor antagonist in severe traumatic brain injury: a phase II randomized control trial. J Cereb Blood Flow Metab. 2014;34(5):845–51. doi: 10.1038/jcbfm.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Adibhatla RM, Hatcher JF. Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets. 2008;7(3):243–53. doi: 10.2174/187152708784936608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yoshida S, Shiosaka S. Plasticity-related serine proteases in the brain (review) Int J Mol Med. 1999;3(4):405–9. doi: 10.3892/ijmm.3.4.405. [DOI] [PubMed] [Google Scholar]
- 163.Pang PT, Teng HK, Zaitsev E, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306(5695):487–91. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- 164.Meng Y, Chopp M, Zhang Y, et al. Subacute intranasal administration of tissue plasminogen activator promotes neuroplasticity and improves functional recovery following traumatic brain injury in rats. PLoS One. 2014;9(9):e106238. doi: 10.1371/journal.pone.0106238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stein SC, Ganguly K, Belfield CM, et al. Erythrocyte-bound tissue plasminogen activator is neuroprotective in experimental traumatic brain injury. J Neurotrauma. 2009;26(9):1585–92. doi: 10.1089/neu.2008.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Armstead WM, Riley J, Yarovoi S, et al. tPA-S481A prevents neurotoxicity of endogenous tPA in traumatic brain injury. J Neurotrauma. 2012;29(9):1794–802. doi: 10.1089/neu.2012.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–74. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 168.Lei P, Li Y, Chen X, et al. Microarray based analysis of microRNA expression in rat cerebral cortex after traumatic brain injury. Brain Res. 2009;1284:191–201. doi: 10.1016/j.brainres.2009.05.074. [DOI] [PubMed] [Google Scholar]
- 169.Redell JB, Moore AN, Ward NH, 3rd, et al. Human traumatic brain injury alters plasma microRNA levels. J Neurotrauma. 2010;27(12):2147–56. doi: 10.1089/neu.2010.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Truettner JS, Alonso OF, Bramlett HM, et al. Therapeutic hypothermia alters microRNA responses to traumatic brain injury in rats. J Cereb Blood Flow Metab. 2011;31(9):1897–907. doi: 10.1038/jcbfm.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bao TH, Miao W, Han JH, et al. Spontaneous Running Wheel Improves Cognitive Functions of Mouse Associated with miRNA Expressional Alteration in Hippocampus Following Traumatic Brain Injury. J Mol Neurosci. 2014;54(4):622–9. doi: 10.1007/s12031-014-0344-1. [DOI] [PubMed] [Google Scholar]
- 172.Sandhir R, Gregory E, Berman NE. Differential response of miRNA-21 and its targets after traumatic brain injury in aging mice. Neurochem Int. 2014;78:117–21. doi: 10.1016/j.neuint.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ge XT, Lei P, Wang HC, et al. miR-21 improves the neurological outcome after traumatic brain injury in rats. Sci Rep. 2014;4:6718. doi: 10.1038/srep06718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gold EM, Su D, Lopez-Velazquez L, et al. Functional assessment of long-term deficits in rodent models of traumatic brain injury. Regen Med. 2013;8(4):483–516. doi: 10.2217/rme.13.41. [DOI] [PubMed] [Google Scholar]
- 175.Peterson TC, Hoane MR, McConomy K, et al. A Combination Therapy of Nicotinamide and Progesterone Improves Functional Recovery Following Traumatic Brain Injury. J Neurotrauma. 2014 doi: 10.1089/neu.2014.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Margulies S, Hicks R. Combination therapies for traumatic brain injury: prospective considerations. J Neurotrauma. 2009;26(6):925–39. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Benner EJ, Luciano D, Jo R, et al. Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature. 2013;497(7449):369–73. doi: 10.1038/nature12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liu Z, Xin H, Chopp M. Reactive astrocytes promote axonal remodeling and neurological recovery after stroke. Neural Regen Res. 2014;9(21):1874–5. doi: 10.4103/1673-5374.145343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu Z, Li Y, Cui Y, et al. Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia. 2014;62(12):2022–33. doi: 10.1002/glia.22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gibson CL, Gray LJ, Bath PM, et al. Progesterone for the treatment of experimental brain injury; a systematic review. Brain. 2008;131(Pt 2):318–28. doi: 10.1093/brain/awm183. [DOI] [PubMed] [Google Scholar]
- 181.Sarkaki AR, Khaksari Haddad M, Soltani Z, et al. Time- and dose-dependent neuroprotective effects of sex steroid hormones on inflammatory cytokines after a traumatic brain injury. J Neurotrauma. 2013;30(1):47–54. doi: 10.1089/neu.2010.1686. [DOI] [PubMed] [Google Scholar]
- 182.Shahrokhi N, Khaksari M, Soltani Z, et al. Effect of sex steroid hormones on brain edema, intracranial pressure, and neurologic outcomes after traumatic brain injury. Can J Physiol Pharmacol. 2010;88(4):414–21. doi: 10.1139/y09-126. [DOI] [PubMed] [Google Scholar]
- 183.Stein DG. Is progesterone a worthy candidate as a novel therapy for traumatic brain injury? Dialogues Clin Neurosci. 2011;13(3):352–9. doi: 10.31887/DCNS.2011.13.2/dstein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sayeed I, Stein DG. Progesterone as a neuroprotective factor in traumatic and ischemic brain injury. Prog Brain Res. 2009;175:219–37. doi: 10.1016/S0079-6123(09)17515-5. [DOI] [PubMed] [Google Scholar]
- 185.Mannix R, Berglass J, Berkner J, et al. Sex differences in the effect of progesterone after controlled cortical impact in adolescent mice: a preliminary study. J Neurosurg. 2014;121(6):1337–41. doi: 10.3171/2014.8.JNS14715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gilmer LK, Roberts KN, Scheff SW. Efficacy of progesterone following a moderate unilateral cortical contusion injury. J Neurotrauma. 2008;25(6):593–602. doi: 10.1089/neu.2007.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]