Abstract
Studies that utilize the rodent mammary gland (MG) as an endpoint for assessing the developmental toxicity of chemical exposures typically employ either basic dimensional measurements or developmental scoring of morphological characteristics as a means to quantify MG development. There are numerous means by which to report these developmental changes, leading to inconsistent translation across laboratories. The Sholl analysis is a method historically used for quantifying neuronal dendritic patterns. The present study describes the use of the Sholl analysis to quantify MG branching characteristics. Using this method, we were able to detect significant differences in branching density in MG of peripubertal female Sprague Dawley rats that had been exposed to vehicle or a potent estrogen. These data suggest the Sholl analysis can be an effective tool for quantitatively measuring an important characteristic of MG development and for examining associations between MG growth and density and adverse effects in the breast.
Keywords: Mammary gland, endocrine disruptors, Sholl analysis, reproductive, developmental, toxicology, breast cancer, rodent
1. Introduction
An increasing number of studies have shown a correlation between breast density and breast cancer risk [1-3], but the reasons for such associations are poorly understood. Gaps exist in understanding the role of breast density in the etiology of tumor subtypes and the development of experimental models to examine the role of breast density in breast cancer risk have been recommended [4]. The ability to quantitatively describe branching density would be a highly valuable tool in assessing the effects of chemical exposures on mammary gland development and could also provide important information on the association between breast density and breast cancer risk. Additionally, it is well known that chemical exposures can affect female breast development in both humans [5-7] and animals [8-10] and recently, studies have demonstrated altered mammary gland development in male rats as well [11-14]. Studies that examine the effects of exposure to endocrine disrupting chemicals (EDCs) on mammary gland development in rodents utilize a broad range of methods for interpreting these effects. With the increasing use of the mammary gland as an endpoint in these studies, a common method of assessment is needed to compare data across studies and form the proper interpretations.
Mammogenesis occurs in the latter portion of embryonic development during which epithelial buds sprout and branch to form a rudimentary ductal tree. The gland grows allometrically from birth until puberty when proliferation of terminal end buds results in extensive branching and ductal elongation throughout the fat pad. In the adult, the ductal framework within the mammary epithelium extends to ductules, alveolar buds and alveoli, which will produce milk during pregnancy, and branching continues until the mammary epithelium occupies the entire fat pad. Though there are numerous ways to describe mammary gland development, it is typically quantified through basic dimensional measurements and the counting of structures or through observational scoring of morphological characteristics (Fig. 1). However, glands can vary considerably in size and shape and both dimensional measurements and morphological scoring can not only vary based upon the evaluators’ experience and interpretation, but there also exists a potential for bias if the study is not properly blinded. Standardized or widely-accepted methods for assessing mammary gland development in the rodent model are needed in order to accurately compare developmental differences across studies and across contract/research laboratories.
Figure 1.
Examples of criteria considered when evaluating mammary gland ductal outgrowth. Image shown is a mammary gland whole mount from a PND25 Charles River Sprague Dawley rat. LN = lymph node, MEA = mammary epithelial area, MG4 = 4th inguinal mammary gland, MG5 = 5th inguinal mammary gland, TEB = terminal end bud.
Mammary gland branching is an example of a commonly used indicator of gland development that is difficult to quantify objectively. Herein we describe a method for quantifying branching density in 2D images of the peripubertal rat mammary gland based upon the method of Sholl [15]. This life stage was chosen as it is most often available in test guideline studies around the time of weaning, when animals are being assigned to end point groups. The Sholl analysis method is widely used in neurobiology for measuring neuronal dendritic arborization [16-20] and as both neurons and mammary glands exhibit a similar tree-like structure (Fig. 2), we propose this method can also be employed to measure mammary epithelial branching. The procedure allows for quantitative evaluation of mammary gland development in adolescent to young adult male and female rats by digitally measuring epithelial branching in thresholded and skeletonized images of mammary gland whole mounts. The Sholl analysis exists as a plugin for publicly available NIH ImageJ imaging software. The plugin, which was first utilized in Ferreira et al. [21], creates a series of concentric rings around a predefined center (typically the soma of a neuron or the base of attachment of a mammary gland) and counts the number of branch intersections (N) that occur on each of the rings. Mammary epithelial branching density can be determined from the number of intersections and the area of the mammary epithelium, and the Sholl regression coefficient (k) can be used to describe the complexity, or epithelial “fullness”, of the mammary branches. Our primary objective was to provide an efficient, accurate, and relatively high throughput method for assessing important developmental characteristics in mammary gland whole mounts that can be used in non-specialized or contract laboratories using typically available microscopy, computer, and digital imaging equipment. We propose that the Sholl analysis, which is freely available online, is a suitable and sensitive method for quantitatively assessing branching differences in rodent mammary glands.
Figure 2.

Physical similarities between a neuron and rodent mammary gland. A) Pyramidal cortical neuron of adult male rat. Scale bar is 50 μm. B) A mammary gland from a female PND25 CRSD rat. C) A mammary gland from a female PND21 CD-1 mouse. Although branching is more complex in the mammary gland, particularly in the rat, the basic tree-like structure is maintained. Scale bars in B and C are 1 mm.
2. Materials and methods
2.1 Mammary gland whole mounts
Mammary gland whole mounts used in the development of this method were graciously provided by the National Center for Toxicological Research (NCTR, Jefferson AK) and were derived from animals utilized in a larger study conducted by Delclos et al. [22]. All animal use and procedures for that study were approved by the NCTR Laboratory Animal Care and Use Committee and conducted in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility. Animal rooms were maintained at 23 ± 3°C with a relative humidity of 50 ± 20% and a 12 h light/dark cycle, with lights on at 6 a.m., and food and water were available ad libitum. Timed pregnant NCTR Sprague Dawley (NCTRSD) female rats were dosed by oral gavage with vehicle (0.3% carboxymethylcellulose) or ethinyl estradiol (EE; 5 μg/kg body weight (BW)/day) as an estrogenic positive control. Dosing began on gestation day 6 and continued until necropsy on postnatal day (PND) 21. At necropsy on PND21, the 4th and 5th inguinal mammary glands were collected from each side of one female offspring/dam. The glands from the right side of the animal were processed for histology. The contralateral glands were fixed in 10% neutral buffered formalin, stained with carmine alum, and whole mounts prepared according to the method of Muñoz-de-Toro et al. [23], then stored individually in acrylic bags containing methyl salicylate. Methyl salicylate allows for excellent resolution and clarity of whole mount images and whole mounts can be stored for a relatively long period of time without concern for shrinkage, discoloration, or oxidation (pers. obs.). A subset of mammary whole mounts from each treatment group (n=8 for vehicle and n=7 for EE) was provided for analysis.
2.2 Image preparation
Whole mounts were viewed under a Leica Z16 APO macroscope and 2D images were captured with a Leica DFC295 digital camera using Leica Application Suite version 3.1 imaging software (Leica Microsystems, Wetzlar, Germany). It is important that all images be captured at the same magnification. The whole mount image is prepared for Sholl analysis (Fig. 3; described in detail in Suppl. Data Image Preparation, Sec. 3) using ImageJ imaging software (NIH, USA; http://rsbweb.nih.gov/ij/; v1.49c). Briefly, the image size is minimized by removing the portion of the image surrounding the glandular epithelium (Suppl. Data Image Preparation Sec. 3A-B), color channels are separated (Suppl. Data Image Preparation Sec. 3C) and noise is removed using various methods provided by ImageJ software (Suppl. Data Image Preparation Sec. 3D-E), the image is then thresholded and skeletonized (Suppl. Data Image Preparation Sec. 3F-H), and the skeletonized image is dilated one time to fill in structural gaps produced by the skeletonization (Suppl. Data Image Preparation Sec. 3H). An overlay of the skeletonized image on the original image can be created to ensure the skeleton accurately depicts the glandular epithelium (Fig. 4; Suppl. Data Image Preparation Sec. 3I). This skeletonized image is then used for measuring mammary epithelial parameters and for the Sholl analysis.
Figure 3.

Stepwise ImageJ processing of mammary gland whole mount image. A) Original whole mount image. B) Binary thresholded image. C) Skeletonized image. D) Overlay of skeletonized image onto original whole mount image demonstrating accuracy of ImageJ processing. E) Analyzed image painted as a heat map according to the Sholl profile. An enlargement of Figure 3D is provided in Figure 4 to show detail.
Figure 4.
Enlargement of Figure 3D showing the detail of a skeletonized image overlayed onto the original whole mount image.
2.3 Mammary epithelial parameters
Mammary gland physical parameters were measured using ImageJ software. These parameters include longitudinal growth and mammary epithelial area (MEA) (Fig. 1). Longitudinal growth is the distance in mm from the base of attachment of the epithelial tree to the outermost point of ductal growth. The base of attachment refers to an arbitrary point just proximal to the first bifurcation of the primary duct. In glands where there is not a clear start point for the primary duct (i.e. Fig. 5A), a centralized point can be chosen. Although a precise starting point is not crucial for the Sholl analysis and small variations in the location of the starting point do not significantly affect the outcome of the analysis (data not shown), care should be taken to choose similar starting points for consistency in measuring longitudinal growth. The length of the line created to measure longitudinal growth is also the ending radius for the Sholl analysis (Suppl. Data Sholl Analysis Sec. 1E). The MEA is defined by the perimeter of the glandular epithelium and is reported in mm2.
Figure 5.
Mammary glands from PND 21 NCTRSD rats. A) MG4 from vehicle rat, B) MG4 from rat treated with 5 μg EE/kg BW. Images are representative of group means for mammary gland physical parameters and Sholl analysis. Scale bars are 1 mm.
2.4 Sholl analysis
Branching density and complexity of the MEA were assessed using a modified Sholl analysis (http://fiji.sc/Sholl_Analysis#) and the procedure for running the analysis is described in detail in Suppl. Data Sholl Analysis. In modifying the Sholl analysis to the mammary gland, we determined the relevant endpoints derived explicitly from the analysis itself to be the number of radial intersections (N) and the Sholl regression coefficient (k). The number of intersections can then be used to ascertain branching density as the fundamental endpoint.
Branching density is determined by normalizing N to the MEA using the formula
| (1) |
where Sum N is the sum of the radial intersections for the respective gland. In cases where the glandular epithelium had extended beyond the lymph node, the MEA includes the area occupied by the lymph node, but the lymph node prevents the analysis from counting intersections in this area. Therefore, the area occupied by the lymph node (LNA) must be subtracted from the MEA when calculating the branching density. In cases where the epithelium had not reached the lymph node, the LNA was set to zero.
Branching complexity is the extent to which branching continues in the distal regions of the glandular epithelium and this complexity can be described by determining the rate of decay (Sholl regression coefficient; k). Branching decay is a distal decrease in epithelial branching and the rate of decay (k) is measured as the slope of the line of the decrease in intersections plotted against the distance over which this decrease occurs. Therefore, the formula for determining the Sholl regression coefficient is the slope of the line of the plot of log(N/S) vs. radial distance (r) such that
| (2) |
where N is the number of intersections for each respective ring of radius r and area S (πr2), and m is the intercept. The slope -k indicates the rate of decay of the intersections. Thus, lower values of k (a shallower slope) denote a slower rate of decay and more consistent/complex branching throughout the entire epithelium. Because k is a measure of branching decay, it follows that the ductal ends must be present in the whole mount image in order to obtain a value for this coefficient. As long as the ductal ends are present, k can be calculated.
Radius step size is a user-defined parameter that directly affects the total number of intersections. Decreasing the radius step size increases the number of concentric rings produced, which in turn increases the total number of intersections. In practice, the smallest step size is one pixel (0.0125 mm for the skeletons in this study). It is recommended to begin at the lowest step size as this gives the most detailed descriptions of branching as a function of distance from the base of the gland. A larger step size could be used if the amount of Sholl output generated or computational issues become a concern of the user.
2.5 Statistical analysis
All data are reported as mean ± standard error (SEM) and differences in the mean were assessed by t-test using SAS Enterprise 4.3 (SAS Institute, Inc., Cary, NC). Variances were assessed for equality by the F-test and a value of p<0.05 was used to indicate statistically significant differences.
3. Results
3.1 Histopathology
Histopathological evaluation revealed no significant occurrence of adverse effects in mammary glands of either vehicle-exposed (n=16) or EE-exposed (n=20) female offspring (as reported in [22]).
3.2 Mammary epithelial parameters
Mammary epithelial parameters are presented in Table 1 and a mammary gland that is representative of the means of each group is illustrated in Figure 5. Both the mean longitudinal growth and the mean MEA were significantly greater in mammary glands of rats treated with 5 μg EE/kg BW compared to vehicle rats. There was no significant difference in BW between groups (data not shown; see [22]). Individual animal data for MEA are provided in Suppl. Data Individual Animal Data Figure 1.
Table 1.
Mammary epithelial parameters
| Treatment | Long. Growth (mm) | CV (%) | MEA (mm2) | CV (%) |
|---|---|---|---|---|
| Vehicle (8) | 8.6 ± 0.7 | 21.4 | 55.3 ± 4.9 | 25.1 |
| 5 μg/kg EE (7) | 14.4 ± 0.7 **** | 13.1 | 204.2 ± 15.0 **** | 19.5 |
Values are means ± SEM; (n); Long. Growth = longitudinal growth determined as the distance from base of attachment of the epithelial tree to most distal edge of the epithelium; MEA = mammary epithelial area determined as the area enclosed by a line traced around the perimeter of the glandular epithelium; CV = coefficient of variation.
p<0.0001 by t-test.
3.3 Sholl analysis
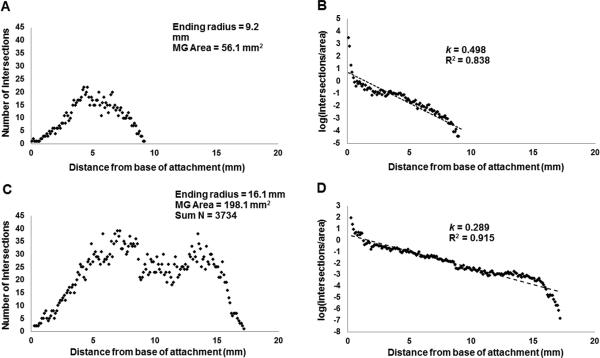
Means calculated using Sholl data are presented in Table 2. Mammary glands of EE-exposed rats exhibited significantly more intersections and a significantly greater branching density compared to mammary glands of untreated rats. Sholl profiles for mammary glands representative of vehicle- and EE-treatment groups are presented in Figure 6. Differences in branching density were confirmed using an alternative method of comparison (described in Suppl. Data Branching Density). The coefficient of variation (CV) for total intersections ranged between 27.7% in vehicle controls and 20.1% in the EE tissue. When adjusted for MEA, the CV was 13.8% and 3.7%, respectively. There were no significant differences in CV%. Mammary glands of EE-exposed rats exhibited more consistent branching throughout the epithelium as indicated by a significantly lower Sholl regression coefficient compared to that of vehicle rats. Individual animal data for N, N/mm2, and k are provided in Suppl. Data Individual Animal Data Fig. 2-4.
Table 2.
Sholl analysis parameters
| Treatment | Sum N | CV (%) | N/mm2 | CV (%) | k | CV (%) |
|---|---|---|---|---|---|---|
| Vehicle (8) | 996 ± 97 | 27.7 | 18.1 ± 0.9 | 13.8 | 0.549 ± 0.046 | 23.7 |
| 5 μg/kg EE (7) | 3936 ± 298 *** | 20.1 | 20.3 ± 0.3 * | 3.7 | 0.323 ± 0.030 ** | 24.5 |
Values are means ± SEM; (n); Sum N = total intersections in MEA; N/mm2 = intersections/MEA less the area occupied by the lymph node; k = Sholl regression coefficient; CV = coefficient ofvariation.
p<0.05
p<0.01
p<0.001 by t-test.
Figure 6.
Sholl profiles for NCTRSD rats. Profiles were derived from images in Figure 5 and are representative of group means. A) Linear plot of N vs. distance and B) semi-log plot of log(N/mm2) vs. distance for vehicle-treated NCTRSD rat. C) Linear plot of N vs. distance and D) semi-log plot of log(N/mm2) vs. distance for EE-treated NCTRSD rat. Parameters indicated are data for the individual animal.
4. Discussion
The ability to objectively quantify mammary gland branching density would be a useful tool for assessing the effect of exposure to EDCs on mammary gland development and also for examining the association between breast density and breast cancer. Here we have described the use of the Sholl analysis as an effective method to quantitatively measure branching characteristics in the rodent mammary gland. This computer-assisted method allows for relatively rapid analysis of skeletonized images of mammary gland whole mounts without the need for specialized microscopy equipment or commercial imaging software. We utilized the Sholl method to count the total number of intersections, which was then used to calculate the epithelial branching density, and to determine the branching complexity (Sholl regression coefficient; k) of mammary glands of peripubertal female rats. The method was sensitive enough to detect significant differences in these branching characteristics in mammary glands of females rats exposed to ethinyl estradiol compared to mammary glands of their untreated counterparts.
There are certain quality assurance aspects that must be addressed when preparing mammary gland whole mounts for Sholl analysis. Although branching density can be determined for the existing regions of the glandular epithelium if the entire epithelium is not present, the entire epithelium, including the ductal ends, must be excised in order to determine the Sholl regression coefficient. Because branching density is directly related to the MEA, it is essential that the mammary glands be stretched to their original size on charged slides in a consistent manner and that they remain adhered to the slide. Mounting and coverslipping can also effect the analysis due to color changes in the mounting media and oxidation of the gland over time, which can result in artifacts that add noise and make thresholding more difficult or even impossible. Mammary whole mounts that are not uniformly attached to the slide, exhibit artifacts that prevent clear visualization of the entire glandular epithelium, or that are torn, damaged or where the integrity of the gland has been compromised in any way, should not be used for the analysis. A thorough review of this protocol, which addresses these and other issues relevant to mammary gland whole mount preparation, is provided in Davis and Fenton [24]. Though we describe these quality control aspects here with respect to the Sholl analysis, they are applicable to all studies utilizing mammary whole mounts and should be practiced universally.
It is common practice to capture images of mammary gland whole mounts at the highest magnification possible so that measurements can be made accurately and the greatest detail is attained for observational scoring. However, when conducting the Sholl analysis it is important that all whole mount images be captured at the same magnification. Preliminary Sholl analysis of skeletonized whole mount images captured at different magnifications failed to reveal significant differences in epithelial branching density in glands of vehicle- and EE-exposed females. However, differences in branching density were clearly evident visually and observational scoring of these images conducted by three individual scorers indicated significant differences did exist (data not shown). Furthermore, line transect sampling of the skeletonized images also indicated significant differences in branching density (described in Suppl. Data Branching Density; it should be noted that while line transect sampling is useful for providing generalized information about branching density, such as confirming or refuting general density measurements, it is not valid for accurately reporting changes in branching density for the purposes intended here). It was determined that data derived from Sholl analysis of images captured at different magnifications cannot be compared to one another. We hypothesize that images captured at higher magnification contain greater detail than those captured at lower magnification and that this detail remains with the image as it is thresholded and skeletonized so that when the Sholl analysis is conducted, a higher number of intersections may be recorded. The ensuing paradox is that smaller glands captured at higher magnification can exhibit a sufficient number of intersections that when compared to larger glands captured at lower magnification, significant differences in branching density are not detected, even though visual differences may be clearly evident. Capturing all whole mount images at the same magnification ensures that the same detail exists in all images through skeletonization and that equivalent comparisons of the Sholl analysis are made.
The most time intensive portion of the process is the editing of whole mount images to minimize noise prior to thresholding and skeletonization. ImageJ provides options for noise removal (described in Suppl. Data Image Preparation Sec. 3E) that can be easily used following familiarization with the process. Once a skeletonized image is obtained, the Sholl plugin is automated so that only point and click measurements and parameter input are required.
Mammary ductal outgrowth typically has not yet extended to the lymph node in pre/peripubertal rats (as in Fig. 5A) and Sholl analysis of glands from rats of this age can be conducted straightforwardly. However, in older rats, or in those that exhibit precocious mammary gland development (as in Fig. 5B), ductal outgrowth will encompass the lymph node. This creates a “void space” in the epithelium where branching occurs but intersections cannot be measured. In order to account for this, the lymph node should be excised from the whole mount image prior to thresholding. After the skeletonized image has been produced, ImageJ can be used to measure the area previously occupied by the lymph node, which should then be subtracted from the total epithelial area prior to calculating branching density.
Although mammary glands four and five had not grown together (overlapped) in any of the whole mounts analyzed in the present study, this can be the case when examining glands that exhibit precocious development or are at a more advanced stage of development. Because contact inhibition prevents extensive migration of either ductal tree into the other, the analysis can still be performed on glands where this has occurred. The image can be prepared simply by tracing around the glandular epithelium (Suppl. Data Image Preparation Sec. 3) as distally as possible on the mammary gland of interest taking care not to include any of the adjacent mammary gland. The branching density is then reported as a measure of the MEA that was analyzed. It is important to realize, however, that the Sholl regression coefficient cannot be calculated in these cases as the ductal ends would not be present.
In rats, mammary epithelial density increases with age to a point where it is difficult to threshold the image with a resolution sufficient to obtain a skeletonized image that is an accurate representation of the gland. Therefore, this method is ideally suited for glands from rats age PND20-30 and, at this time, is not recommended for glands from rats older than PND40. McGinley and Thompson [25] demonstrated quantitative assessment of mammary gland density in rats up to PND63. In that study, the authors used a motorized microscope to collect Z-stack images of mammary whole mounts. It is possible that the signal intensity in a 2D image created from flattened Z-stack images is great enough to allow for thresholding with enough resolution to generate an accurate skeleton. Though this analysis tool was developed expressly for use with non-specialized microscopy equipment, the use of such equipment is certainly valid and the ability to utilize the Sholl analysis to quantify branching density and complexity in older glands would allow for an even broader application of the current method. Because mammary glands in mice do not grow as dense as those in rats, we propose that the procedure can be used on mammary glands from mice of any age. Though branching in mouse mammary glands can be counted manually, this automated method is both faster and much less labor intensive. However, its application in mice has not yet been examined.
This is not the first study to describe digital assessment of mammary gland development. In 1997, Thompson et al. [26] reported mammary gland branching effects in whole mounts of 50 day-old rats by measuring the optical density of the mammary epithelium. However, their method utilized commercial software and they restricted the region of assessment to the portion of the gland cephalic to the anterior-most lymph node. Arganda-Carreras et al. [27] described a method to create a 3D image of the ductal tree by digitally reconstructing serial histological sections of mammary gland tissue. This method is highly informative and useful when analyzing both structural and cellular changes in mammary gland development and also utilizes a number of plug-ins freely available in ImageJ. However, collecting serial histological sections can be expensive and highly time consuming and the process of reconstruction is also lengthy and requires numerous computational corrections to ensure an accurate model has been recreated. The process is clearly not designed for rapid throughput analysis, but for more specialized or focused investigations of mammary gland biology. McGinley and Thompson [25] used digital image analysis to quantitatively assess mammary gland density in rats aged 21-63 days. In that study, mammary epithelial density, as well as mammary epithelial and fat pad area and volume, was measured using commercially available imaging software. As previously mentioned, their use of flattened Z-stack images may have allowed for sufficient thresholding of images with dense epithelium so that a high resolution digital mask could be generated. Though advantageous, specialized microscopy equipment is still required for capturing Z-stack images. Perhaps the most informative quantitative image analysis was conducted by Law et al. [28]. In that study, the authors describe two frameworks of analysis; one for analyzing the entire mammary epithelium as a whole and another for analyzing a skeleton-based image derived from a higher magnification image of the mammary ductal tree. Though the analyses provide information on epithelial area, bifurcation and side-branching points, endpoints, and ductal length, they are mathematically complex and the authors attest to various inaccuracies. The authors state this method would be available in a software package called MammoQuant and a website for access is provided in their publication, but the website was not available at the time of preparation of this manuscript. Each of the aforementioned studies provide useful methods for digital analysis of murine mammary gland whole mounts, but each requires the use of either specialized microscopy equipment and/or commercial software to conduct the analysis. The significant advantages of the current method compared to these studies are that specialized microscopy equipment and commercial imaging software are not required for the Sholl analysis. Furthermore, the Sholl analysis allows for measuring mammary branching complexity as an additional endpoint.
The growing concern regarding the adverse effects of exposure to EDCs and their role in breast cancer has resulted in the increased use of the mammary gland as an endpoint for assessing the developmental toxicity of these chemicals. It has been proposed that mammary gland assessment be enhanced in guideline toxicology studies [29] and the mammary gland has recently been included as a recommended endpoint in both Organisation for Economic Co-operation and Development [30] and National Toxicology Program [31] standardized reproductive studies. While the number of studies examining mammary gland development continues to rise, a standardized method for quantifying development does not currently exist. Differences across laboratories exist both in mammary whole mount preparation [32-36] and in developmental assessment [37-40]. The Sholl analysis provides a standardized protocol for objectively characterizing mammary gland branching characteristics that is simple, quick, and cost effective and can be utilized across a broad range of laboratories. The application of this method to a peripubertal time point is particularly useful as this life stage of development coincides with recommended endpoints in test guideline studies for mammary whole mount preparation. Future efforts for improving or extending this method might include establishing its utility in whole mounts of mice, assessing the utility of flattened 3D images from rats, or assessing the outcomes by measuring the true epithelial area instead of just the space occupied by the ductal tree.
Supplementary Material
Highlights.
A modification of the Sholl analysis has been developed for quantitative assessment of rat mammary gland development.
The method allows for measuring mammary epithelial branching density and rate of branching decay (Sholl regression coefficient) in 2D images of peripubertal rat mammary whole mounts.
The method is relatively rapid and does not require specialized equipment or software.
The software is a free download at http://fiji.sc/Sholl_Analysis#.
Acknowledgments
The authors would like to extend our gratitude to Dr. Barry Delclos and the NCTR (Jefferson, AK) for providing the mammary gland whole mounts. We would also like to thank Drs. John Gensel (Univ. of Kentucky, Lexington, KY) and Jennifer Marks (Olympus America, Raleigh, NC) for their assistance with MetaMorph imaging software, Dr. Grace Kissling for statistical assistance, Drs. Jean Harry and Julie Foley for their technical assistance in reviewing this manuscript, and a special thank you to Dr. Tiago Ferreira (McGill University, Montreal, Quebec, Canada) for his continual assistance with the Sholl application.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boyd NF, Martin LJ, Bronskill M, Yaffe MJ, Duric N, Minkin S. Breast tissue composition and susceptibility to breast cancer. Journal of the National Cancer Institute. 2010;102:1224–37. doi: 10.1093/jnci/djq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llobet R, Pollan M, Anton J, Miranda-Garcia J, Casals M, Martinez I, et al. Semi-automated and fully automated mammographic density measurement and breast cancer risk prediction. Computer methods and programs in biomedicine. 2014 doi: 10.1016/j.cmpb.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen M, Vachon CM, Scott CG, Chernoff K, Karemore G, Karssemeijer N, et al. Mammographic texture resemblance generalizes as an independent risk factor for breast cancer. Breast cancer research : BCR. 2014;16:R37. doi: 10.1186/bcr3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Committee IBCaERC Prioritizing Prevention. 2013 [Google Scholar]
- 5.Byrne C, Divekar SD, Storchan GB, Parodi DA, Martin MB. Metals and breast cancer. Journal of mammary gland biology and neoplasia. 2013;18:63–73. doi: 10.1007/s10911-013-9273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calaf GM, Echiburu-Chau C. Synergistic effect of malathion and estrogen on mammary gland carcinogenesis. Oncology reports. 2012;28:640–6. doi: 10.3892/or.2012.1817. [DOI] [PubMed] [Google Scholar]
- 7.Palmer JR, Boggs DA, Hatch EE, Troisi R, Titus-Ernstoff L, Strohsnitter WC, et al. Prenatal DES exposure in relation to breast size. Cancer causes & control : CCC. 2013;24:1757–61. doi: 10.1007/s10552-013-0248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acevedo N, Davis B, Schaeberle CM, Sonnenschein C, Soto AM. Perinatally administered bisphenol a as a potential mammary gland carcinogen in rats. Environmental health perspectives. 2013;121:1040–6. doi: 10.1289/ehp.1306734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reilly MP, Saca JC, Hamilton A, Solano RF, Rivera JR, Whitehouse-Innis W, et al. Prepubertal exposure to arsenic(III) suppresses circulating insulin-like growth factor-1 (IGF-1) delaying sexual maturation in female rats. Reproductive toxicology. 2014;44:41–9. doi: 10.1016/j.reprotox.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinhouse C, Anderson OS, Bergin IL, Vandenbergh DJ, Gyekis JP, Dingman MA, et al. Dose-Dependent Incidence of Hepatic Tumors in Adult Mice following Perinatal Exposure to Bisphenol A. Environmental health perspectives. 2014;122:485–91. doi: 10.1289/ehp.1307449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delclos KB, Bucci TJ, Lomax LG, Latendresse JR, Warbritton A, Weis CC, et al. Effects of dietary genistein exposure during development on male and female CD (Sprague-Dawley) rats. Reproductive toxicology. 2001;15:647–63. doi: 10.1016/s0890-6238(01)00177-0. [DOI] [PubMed] [Google Scholar]
- 12.Kass L, Durando M, Altamirano GA, Manfroni-Ghibaudo GE, Luque EH, Munoz-de-Toro M. Prenatal Bisphenol A exposure delays the development of the male rat mammary gland. Reproductive toxicology. 2014 doi: 10.1016/j.reprotox.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Latendresse JR, Bucci TJ, Olson G, Mellick P, Weis CC, Thorn B, et al. Genistein and ethinyl estradiol dietary exposure in multigenerational and chronic studies induce similar proliferative lesions in mammary gland of male Sprague-Dawley rats. Reproductive toxicology. 2009;28:342–53. doi: 10.1016/j.reprotox.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Miousse IR, Gomez-Acevedo H, Sharma N, Vantrease J, Hennings L, Shankar K, et al. Mammary gland morphology and gene expression signature of weanling male and female rats following exposure to exogenous estradiol. Experimental biology and medicine. 2013;238:1033–46. doi: 10.1177/1535370213497322. [DOI] [PubMed] [Google Scholar]
- 15.Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. Journal of anatomy. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- 16.Arguello A, Cheyette BN. Dapper Antagonist of Catenin-1 (Dact1) contributes to dendrite arborization in forebrain cortical interneurons. Communicative & integrative biology. 2013;6:e26656. doi: 10.4161/cib.26656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Binley KE, Ng WS, Tribble JR, Song B, Morgan JE. Sholl analysis: a quantitative comparison of semi-automated methods. Journal of neuroscience methods. 2014;225:65–70. doi: 10.1016/j.jneumeth.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Day JS, O'Neill E, Cawley C, Aretz NK, Kilroy D, Gibney SM, et al. Noradrenaline acting on astrocytic beta(2)-adrenoceptors induces neurite outgrowth in primary cortical neurons. Neuropharmacology. 2014;77:234–48. doi: 10.1016/j.neuropharm.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Segura LM, Perez-Marquez J. A new mathematical function to evaluate neuronal morphology using the Sholl analysis. Journal of neuroscience methods. 2014;226:103–9. doi: 10.1016/j.jneumeth.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Wable GS, Barbarich-Marsteller NC, Chowdhury TG, Sabaliauskas NA, Farb CR, Aoki C. Excitatory synapses on dendritic shafts of the caudal basal amygdala exhibit elevated levels of GABAA receptor alpha4 subunits following the induction of activity-based anorexia. Synapse. 2014;68:1–15. doi: 10.1002/syn.21690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreira TA, Iacono LL, Gross CT. Serotonin receptor 1A modulates actin dynamics and restricts dendritic growth in hippocampal neurons. The European journal of neuroscience. 2010;32:18–26. doi: 10.1111/j.1460-9568.2010.07283.x. [DOI] [PubMed] [Google Scholar]
- 22.Delclos KB, Camacho L, Lewis SM, Vanlandingham MM, Latendresse JR, Olson GR, et al. Toxicity evaluation of bisphenol A administered by gavage to Sprague Dawley rats from gestation day 6 through postnatal day 90. Toxicological sciences : an official journal of the Society of Toxicology. 2014;139:174–97. doi: 10.1093/toxsci/kfu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munoz-de-Toro M, Markey CM, Wadia PR, Luque EH, Rubin BS, Sonnenschein C, et al. Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology. 2005;146:4138–47. doi: 10.1210/en.2005-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis BJaF, S.E. The Mammary Gland. In: Haschek WM, Rousseaux CG, Wallig MA, editors. Haschek and Rousseaux's Handbook of Toxicologic Pathology. 3rd ed. Elsevier, Inc., Academic Press; USA: 2013. pp. 2665–94. [Google Scholar]
- 25.McGinley JN, Thompson HJ. Quantitative assessment of mammary gland density in rodents using digital image analysis. Biological procedures online. 2011;13:4. doi: 10.1186/1480-9222-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson HJ, McGinley JN, Rothhammer K, Singh M. Rapid induction of mammary intraductal proliferations, ductal carcinoma in situ and carcinomas by the injection of sexually immature female rats with 1-methyl-1-nitrosourea. Carcinogenesis. 1995;16:2407–11. doi: 10.1093/carcin/16.10.2407. [DOI] [PubMed] [Google Scholar]
- 27.Arganda-Carreras I, Fernandez-Gonzalez R, Munoz-Barrutia A, Ortiz-De-Solorzano C. 3D reconstruction of histological sections: Application to mammary gland tissue. Microscopy research and technique. 2010;73:1019–29. doi: 10.1002/jemt.20829. [DOI] [PubMed] [Google Scholar]
- 28.Law YN, Racine V, Ang PL, Mohamed H, Soo PC, Veltmaat JM, et al. Development of MammoQuant: An Automated Quantitative Tool for Standardized Image Analysis of Murine Mammary Gland Morphogenesis. J Med Imag Health In. 2012;2:352–65. [Google Scholar]
- 29.Makris SL. Current assessment of the effects of environmental chemicals on the mammary gland in guideline rodent studies by the U.S. Environmental Protection Agency (U.S. EPA), Organisation for Economic Co-operation and Development (OECD), and National Toxicology Program (NTP). Environmental health perspectives. 2011;119:1047–52. doi: 10.1289/ehp.1002676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.OECD OECD Guidelines for the Testing of Chemicals, Section 4: Health Effects, Test No. 443: Extended One-Generation Reproductive Toxicity Study. 2012 [Google Scholar]
- 31.Program NT Specifications for the Conduct of Studies to Evaluate the Reproductive and Developmental Toxicity of Chemical, Biological, and Physical Agents in Laboratory Animals for the National Toxicology Progam (NTP) 2011 [Google Scholar]
- 32.Fenton SE, Hamm JT, Birnbaum LS, Youngblood GL. Persistent abnormalities in the rat mammary gland following gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p dioxin (TCDD). Toxicological sciences : an official journal of the Society of Toxicology. 2002;67:63–74. doi: 10.1093/toxsci/67.1.63. [DOI] [PubMed] [Google Scholar]
- 33.Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure. Reproductive toxicology. 2007;23:383–90. doi: 10.1016/j.reprotox.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson MD, Mueller SC. Three dimensional multiphoton imaging of fresh and whole mount developing mouse mammary glands. BMC cancer. 2013;13:373. doi: 10.1186/1471-2407-13-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moral R, Santucci-Pereira J, Wang R, Russo IH, Lamartiniere CA, Russo J. In utero exposure to butyl benzyl phthalate induces modifications in the morphology and the gene expression profile of the mammary gland: an experimental study in rats. Environmental health : a global access science source. 2011;10:5. doi: 10.1186/1476-069X-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan J, Buache E, Alpy F, Daguenet E, Tomasetto CL, Ren GS, et al. Stromal matrix metalloproteinase-11 is involved in the mammary gland postnatal development. Oncogene. 2013 doi: 10.1038/onc.2013.434. [DOI] [PubMed] [Google Scholar]
- 37.Enoch RR, Stanko JP, Greiner SN, Youngblood GL, Rayner JL, Fenton SE. Mammary gland development as a sensitive end point after acute prenatal exposure to an atrazine metabolite mixture in female Long-Evans rats. Environmental health perspectives. 2007;115:541–7. doi: 10.1289/ehp.9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hovey RC, Coder PS, Wolf JC, Sielken RL, Jr., Tisdel MO, Breckenridge CB. Quantitative assessment of mammary gland development in female Long Evans rats following in utero exposure to atrazine. Toxicological sciences : an official journal of the Society of Toxicology. 2011;119:380–90. doi: 10.1093/toxsci/kfq337. [DOI] [PubMed] [Google Scholar]
- 39.Mandrup KR, Hass U, Christiansen S, Boberg J. Perinatal ethinyl oestradiol alters mammary gland development in male and female Wistar rats. International journal of andrology. 2012;35:385–96. doi: 10.1111/j.1365-2605.2012.01258.x. [DOI] [PubMed] [Google Scholar]
- 40.Welsch CW, O'Connor DH. Influence of the type of dietary fat on developmental growth of the mammary gland in immature and mature female BALB/c mice. Cancer research. 1989;49:5999–6007. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.