Abstract
Background
In the ALIAS Part 2 Multicenter Trial, 85% of subjects received standard-of-care intravenous tPA, and 21% received some form of endovascular thrombolysis. The overall rate of symptomatic intracranial hemorrhage was within the expected range but was higher in albumin- than in saline-treated subjects.
Aims and Methods
Using the trial’s Public Use Dataset, we analyzed factors contributing to symptomatic (sICH) and asymptomatic intracranial hemorrhage in the “safety sample” of 830 subjects.
Results
Four hundred sixteen subjects received ALB therapy, and 414 received saline. Intravenous tPA was given to 68.2%; IV tPA plus endovascular intervention in 16.4%; and endovascular therapy alone in 4.3%. sICH occurred in 41 subjects – within the first 12 hours in one-third of cases, and within the first day in ~60%. Intravenous tPA had been used in 78% of sICH subjects – no higher than in the overall cohort. In contrast, 48.8% of subjects with sICH had received endovascular therapy – markedly higher than the 20.7% rate in the entire cohort (p=0.0001). 68.3% of subjects with sICH had received ALB, and 31.7% saline (risk ratio 2.14, p=0.025). Other factors associated with sICH were baseline NIHSS and ASPECTS scores and the SEDAN score. 41.4% of subjects with sICH died. The odds ratio (OR) for sICH was 3.89 (95% CI 2.04–7.41) with endovascular therapy and 2.15 (CI 1.08–4.25) with albumin.
Conclusions
Endovascular thrombolysis was the major factor predisposing to sICH, and albumin contributed to this predisposition. The latter may be mediated by albumin’s influence on platelet aggregation or collateral perfusion.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Identifier, NCT00235495
Keywords: intracranial hemorrhage, ischemic stroke, albumin, endovascular therapy, thrombolysis, neuroprotection
The ALIAS (Albumin in Acute Stroke) Part 2 multicenter clinical trial was a phase III, randomized placebo-controlled investigation to determine whether the early administration of high-dose human albumin would improve the neurological and functional outcome of patients with acute ischemic stroke. As presented in the primary report of the trial’s results,1 no overall clinical benefit was demonstrated. Standard-of-care intravenous thrombolytic therapy with tPA (alteplase) was administered to 712 (85%) of the 841 subjects in the intent-to-treat population; and 176 subjects (21%) received some form of endovascular thrombolytic intervention (mechanical and/or drug). As the trial progressed to Part 2,2 endovascular stroke therapy was provided to an increasing proportion of stroke patients as part of routine care. This was permitted by the trial’s protocol and was a concurrent secular evolution in acute stroke treatment.
As albumin has properties potentially useful in preventing reperfusion injury, we suspected that ALB therapy might reduce sICH.3 Although the overall rate of symptomatic intracranial hemorrhage (sICH) within 24 hours of randomization (2.9%) was within the clinically acceptable range,4 it was unexpectedly more frequent in subjects treated with albumin (ALB) than with saline-placebo (SAL) (relative risk 2.42, 95% CI 1.02–5.78). In this report, we present a detailed analysis of both symptomatic and asymptomatic ICH in the ALIAS Part 2 trial and explore contributory factors.
METHODS
The details of the trial’s design, inclusion and exclusion criteria, and outcomes have been reported previously.1 In brief, subjects aged 18 through 83 with baseline National Institutes of Health Stroke Scale (NIHSS) scores of 6 or greater were randomized 1:1 to treatment with either 25% albumin (ALB, 2 g/kg) or a comparable volume of isotonic saline (SAL) started within 5 hours of stroke onset. Exclusions were chiefly cardiovascular in nature. A favorable primary outcome was defined as a score of 0 or 1 on either the NIHSS or the modified Rankin Scale (mRS), or both, at 90 days post-randomization. The present analysis was conducted on the protocol-defined “safety sample” of 830 subjects who had received at least 20% of the weight-based study-drug dose. The data were derived from the trial’s Public Use Dataset.
In univariate analyses, we compared baseline and treatment-related factors hypothesized to be possibly contributory to sICH development: age, baseline NIHSS score, baseline plasma glucose, study drug (ALB or SAL), use of IV tPA, use of endovascular thrombolysis, baseline ASPECTS score, and presence of hyperdense MCA sign on baseline neuroimaging. We used the Mann-Whitney rank sum test and chi-square or Fisher’s exact test as appropriate. We also considered the SEDAN score to estimate the extent of sICH risk in patients receiving IV thrombolysis;5, 6 this score was derived 974 ischemic stroke patients and validated in another >800 subjects but did not include patients with endovascular therapy.6 The SEDAN score ranges from 0 to 6 and incorporates blood glucose level, early ischemic signs and hyperdense cerebral artery sign on admission CT scan, age > 75 years, and NIHSS score of 10 or above. In the analysis of Strbian et al,6 a SEDAN score of 3 would predict a sICH rate of ~11%.
We derived a multivariable model using logistic regression to obtain an adjusted estimate of effect size. Within this model we assessed the possible interaction of ALB and endovascular treatment, after adjusting for the prognostic variables listed above. Significance was assessed at the p<0.05 level. Statistical analyses utilized IBM SPSS Statistics, version 22; and SigmaPlot for Windows, version 11.0.
RESULTS
The baseline and treatment characteristics of the entire safety cohort are presented in Appendix Table 1. A total of 416 subjects received ALB, and 414 received SAL (placebo). Mean age was 64; median baseline NIHSS score was 10–11; and the median interval from stroke onset to initiation of study drug was 199 min. Intravenous tPA was administered to 566 subjects (68.2%); IV tPA plus an endovascular intervention in 136 (16.4%); and endovascular therapy alone in 36 (4.3%). The remaining 92 subjects (11.1%) received no thrombolytic treatment. Intravenous tPA therapy was begun, on average, 52 ± 24 (SD) min prior to randomization and 135 ± 48 min following stroke onset.
Appendix Table 1.
Baseline and Treatment Characteristics of the Subjects in Safety Sample*
| Albumin (N=416) | Saline Control (N=414) | |
|---|---|---|
| Demographics | ||
| Age (mean, SD) | 63.5 (12.9) | 64.9 (12.9) |
| Sex, male (%, n) | 52.9% (220) | 56.0% (232) |
| Caucasian (%, n) | 74.5% (310) | 72.8% (302) |
| Black (%, n) | 16.1% (67) | 20.3% (83) |
| Asian (%, n) | 6.0% (25) | 4.8% (20) |
| Medical History | ||
| Hypertension | 71.9% (299) | 70.5% (292) |
| Atrial fibrillation | 18.8% (78) | 18.6% (77) |
| Past congestive heart failure | 4.1% (17) | 6.3% (26) |
| Past myocardial infarction | 10.6% (44) | 12.1% (50) |
| Past stroke | 20.9% (87) | 18.6% (77) |
| Past transient ischemic attack | 12.3% (51) | 11.8% (49) |
| Diabetes mellitus | 18.5% (77) | 22.5% (93) |
| Hyperlipidemia | 46.6% (194) | 51.0% (211) |
| Peripheral vascular disease | 5.0% (21) | 6.0% (25) |
| Clinical Factors | ||
| NIHSS score (median, iqr) | 10 (9) | 11 (9) |
| ASPECTS > 7 (%, n/N) | 77.4% (319/412) | 78.0% (323/410) |
| OCSP clinical stroke type | ||
| TACS | 24.3% (101) | 23.4% (97) |
| PACS | 55.3% (230) | 56.0% (232) |
| LACS | 12.0% (50) | 11.1% (46) |
| POCS | 8.4% (35) | 9.4% (39) |
| Thrombolysis | 83.2% (246) | 86.0% (356) |
| Intravenous tPA only | 66.6% (277) | 69.8% (289) |
| Intravenous tPA plus any endovascular procedure | 16.6% (69) | 16.2% (67) |
| Any endovascular procedure only | 5.8% (24) | 2.9% (12) |
| No thrombolysis | 11.1% (46) | 11.1% (46) |
| Systolic BP, mm Hg (mean, SD) | 155 (28) | 157 (30) |
| Glucose, mmol/L (mean, SD) | 7.1 (2.5) | 7.7 (3.7) |
| Hemoglobin, g/L (mean, SD) | 139 (17) | 140 (18) |
| Creatinine, μmol/L (mean, SD) | 86.3 (23.1) | 90.3 (26.9) |
| ECG at baseline shows normal sinus rhythm (%, n) | 72.3% (297) | 72.9% (296) |
| Process Measures | ||
| Stroke onset to initiation of study drug infusion (min) [median, iqr] | 200 (82) | 198 (75) |
| Stroke onset to initiation of intravenous tPA (min) [median, iqr] | 126 (71) | 131 (68) |
| Initiation of IV tPA to initiation of study drug infusion (min) [median, iqr] | 60 (31) | 60 (32) |
| Post-treatment – Fluids and Cardiac Status | ||
| Total IV fluids administered within 48 hours of randomization (ml) (mean (SD); [min, max]) | 3284 (1669); [416,15006] | 3249 (1629); [432, 11235] |
| ECG at 24 hours shows normal sinus rhythm (%, n) | 70.3% (275) | 78.3% (313) |
tPA = tissue plasminogen activator; OCSP = Oxfordshire Community Stroke Project stroke classification (TACS = total anterior circulation syndrome; PACS = partial anterior circulation syndrome; LACS = lacunar syndrome; POCS = posterior circulation syndrome); BP = blood pressure; IV = intravenous; ECG = electrocardiogram
Hill MD, Martin RH, Palesch YY, Moy CS, Tamariz D, Ryckborst KJ, Jones EB, Weisman D, Pettigrew C, Ginsberg MD: Safety analysis of the ALIAS Part 2 Multicenter Trial (manuscript in preparation)
Race and ethnic group were self-reported.
The National Institutes of Health Stroke Scale (NIHSS) is a 42-point scale that quantifies neurological deficits in 11 categories, with 0 indicating normal function without deficits, and higher scores indicating greater severities of deficit.
The Alberta Stroke Program Early Computed Tomography Score (ASPECTS) uses computed tomography to assess 10 regions of the brain; a score of 1 indicates a normal region and 0 indicates a region showing signs of ischemia. Total scores range from 10 (no evidence of early ischemia) to 0 (all 10 regions of the affected hemisphere show early ischemic changes).
Symptomatic Intracranial Hemorrhage (sICH)
Treatment-related symptomatic ICH (sICH) was defined per-protocol as intracranial hemorrhage proven by neuroimaging and associated with deterioration in neurological status; the site investigator must have adjudicated the ICH to be the primary cause of the subject’s deterioration. sICH occurred in 41 subjects, whose clinical features are presented in Table 1. The mean age was 68 ± 9 (SD) years; median baseline NIHSS was 15. These values were both somewhat higher than for the safety cohort as a whole.
Table 1.
Characteristics of subjects with symptomatic Intracranial hemorrhage
| Sub- ject |
Day of sICH Onset |
Age | Gen- der |
bNIHSS | Baseline ASPECTS |
Treat- ment |
IV tPA | Endo- vascular: Mechan- ical Device |
Endo- vascular: Intra- arterial tPA |
NETT site |
90-day NIHSS |
90-day mRS |
Favorable Primary Outcome |
Death | Day of Death |
SEDAN score |
Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.1 | 81 | F | 9 | 6 | ALB | X | X | X | x | X | 9 | 3 | b,c | |||
| 2 | 0.1 | 74 | F | 8 | 10 | ALB | X | X | 3 | 1 | X | 2 | d | ||||
| 3 | 0.1 | 65 | M | 18 | ALB | X | X | x | X | 2 | 1 | ||||||
| 4 | 0.2 | 65 | M | 22 | 9 | ALB | X | X | 4 | 3 | c | ||||||
| 5 | 0.2 | 56 | M | 7 | 10 | ALB | X | x | X | 7 | 2 | a,c,d,f | |||||
| 6 | 0.2 | 63 | F | 10 | 8 | ALB | X | X | X | X | 1 | 1 | b | ||||
| 7 | 0.2 | 79 | M | 11 | 8 | SAL | X | X | 8 | 4 | b | ||||||
| 8 | 0.2 | 69 | F | 17 | 10 | SAL | X | X | X | X | 1 | 3 | a,d | ||||
| 9 | 0.3 | 72 | M | 7 | 9 | ALB | X | x | X | 9 | 2 | b,c | |||||
| 10 | 0.3 | 60 | M | 15 | 7 | SAL | X | X | X | 3 | 2 | 4 | d | ||||
| 11 | 0.3 | 66 | M | 9 | 10 | ALB | X | x | 19 | 5 | 3 | ||||||
| 12 | 0.4 | 51 | F | 15 | 10 | ALB | X | X | X | 1 | 2 | c,d | |||||
| 13 | 0.4 | 52 | M | 11 | 9 | ALB | X | 8 | 4 | 2 | |||||||
| 14 | 0.5 | 55 | M | 17 | 8 | ALB | X | x | 20 | 4 | 3 | ||||||
| 15 | 0.5 | 79 | F | 20 | 8 | ALB | X | X | 2 | 5 | |||||||
| 16 | 0.5 | 65 | F | 16 | 4 | ALB | X | x | X | 6 | 2 | ||||||
| 17 | 0.7 | 72 | M | 8 | 9 | SAL | X | x | X | 68 | 2 | ||||||
| 18 | 0.7 | 75 | F | 15 | 6 | SAL | X | X | x | 17 | 4 | 3 | c | ||||
| 19 | 0.8 | 52 | M | 9 | 8 | ALB | X | X | X | x | 7 | 3 | 1 | c | |||
| 20 | 0.9 | 61 | F | 15 | 10 | SAL | X | 1 | 2 | X | 2 | ||||||
| 21 | 0.9 | 64 | F | 10 | 8 | ALB | X | x | 13 | 4 | 3 | ||||||
| 22 | 0.9 | 65 | M | 29 | 8 | ALB | x | 3 | 2 | ||||||||
| 23 | 0.9 | 81 | F | 10 | 7 | ALB | X | 8 | 3 | X | 154 | 3 | |||||
| 24 | 1 | 78 | M | 10 | 9 | SAL | X | x | 5 | 1 | X | 3 | |||||
| 25 | 1 | 65 | F | 27 | 7 | ALB | X | X | x | 9 | 4 | 3 | b | ||||
| 26 | 1.1 | 72 | F | 23 | 7 | SAL | X | X | x | 11 | 3 | 3 | a,c | ||||
| 27 | 1.2 | 78 | F | 21 | 9 | SAL | X | X | 9 | 4 | |||||||
| 28 | 1.2 | 71 | M | 12 | 9 | ALB | X | X | 4 | 2 | 3 | d | |||||
| 29 | 1.6 | 67 | M | 6 | 9 | ALB | X | X | X | 8 | 2 | e | |||||
| 30 | 1.7 | 74 | M | 17 | 8 | ALB | X | X | 3 | 3 | |||||||
| 31 | 1.8 | 75 | M | 15 | 8 | SAL | X | 11 | 3 | 2 | |||||||
| 32 | 2.1 | 76 | F | 21 | 8 | ALB | X | X | X | 7 | 3 | ||||||
| 33 | 3.5 | 79 | F | 25 | 5 | ALB | X | x | X | 16 | 4 | c | |||||
| 34 | 7.9 | 80 | M | 20 | 10 | SAL | X | X | x | 9 | 4 | 3 | b | ||||
| 35 | 17.3 | 48 | M | 7 | 10 | ALB | X | 1 | 2 | X | 3 | ||||||
| 36 | 19 | 73 | F | 11 | 8 | ALB | X | 4 | 4 | 3 | |||||||
| 37 | 35.3 | 74 | M | 6 | 10 | ALB | X | x | 3 | 1 | X | 1 | |||||
| 38 | 46.3 | 77 | F | 22 | 3 | ALB | X | x | 22 | 4 | 4 | ||||||
| 39 | 59.7 | 60 | M | 19 | 6 | ALB | x | 10 | 4 | 4 | |||||||
| 40 | 131.1 | 68 | F | 9 | 8 | SAL | 0 | 2 | X | 2 | |||||||
| 41 | 161.3 | 59 | M | 24 | 10 | SAL | 11 | 3 | 2 |
bNIHSS, baseline NIHSS score; ALB, albumin; SAL, saline-placebo; NETT, Neurological Emergencies Treatment Trials consortium; mRS, modified Rankin Scale score
Notes:
balloon angioplasty
Concentric device
Penumbra device
Intracranial stent
Eptifibitide
Abciximab
Thirty-five subjects with sICH, and 698 without sICH, had received some form of thrombolytic therapy (IV tPA and/or endovascular) (p=0.62, Fisher exact test). Intravenous tPA had been administered to 32 subjects, or 78% - no more than for the entire safety cohort. In contrast, 20 of the 41 subjects (48.8%) with sICH had received some form of endovascular thrombolysis – a percentage markedly higher than the 20.7% in the entire safety cohort (p=0.0001, Fisher exact test). Twenty-eight subjects with sICH (68.3%) had received ALB, and 13 (31.7%) had received SAL (risk ratio, 2.14; p=0.025, Fisher exact test).
As shown in Table 1, ~60% of all sICH events occurred within the first day following randomization; one-third of cases within the first 12 hours; and only 7 of 41 occurred after day 8. Endovascular thrombolysis had been employed in 11 of the 16 cases with onset within the first half-day, and death resulted in 10 of these subjects. Overall, the median time from randomization to sICH onset was 0.9 days (interquartile range, 0.3–1.8 days), and the overall rate of death was 41.4%. Only rarely did subjects with early sICH (defined as sICH onset < day 8) attain a favorable primary outcome (3 of 34 subjects, or 9%) (Table 1).
Table 2 compares descriptive variables in subjects with vs. without early sICH (i.e., onset < day 8). The following factors were associated with early sICH: baseline NIHSS score (p=0.025); baseline ASPECTS (p=0.005); SEDAN score (p=0.011); and use of endovascular thrombolytic therapies (p<0.0001). ALB therapy (p=0.053) and age (p=0.057) were of borderline significance in these univariate analyses. Use of intravenous tPA, intervals from stroke onset to randomization and to start of thrombolytic therapies, gender, baseline glucose, and order of randomization into the trial were not associated with a propensity to early sICH. Failure to achieve a favorable primary outcome, and likelihood of death, were markedly increased in subjects with early sICH (p<0.0001).
Table 2.
Comparison of subjects with and without early sICH (onset < day 8).
|
|
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Early sICH (onset < day 8) | No early sICH | |||||||
| Numerical Descriptors | Median | Interquartile range | Median | Interquartile range | p value * | |||
| Age (years) | 70.0 | 63.8 | 76.5 | 65.5 | 54.4 | 75.0 | 0.057 | |
| Baseline NIHSS score | 15.0 | 9.8 | 20.0 | 11.0 | 8.0 | 17.0 | 0.025 | |
| Baseline ASPECTS | 8.0 | 7.5 | 9.0 | 10.0 | 8.0 | 10.0 | 0.005 | |
| Baseline Plasma Glucose (mmol/L) | 6.4 | 5.6 | 6.9 | 6.5 | 5.7 | 7.8 | 0.378 | |
| SEDAN score | 3.0 | 2.0 | 3.0 | 2.0 | 1.0 | 3.0 | 0.011 | |
| Interval from stroke onset to randomization (min) | 179 | 150 | 248 | 190 | 155 | 235 | 0.988 | |
| Interval from stroke onset to start of IV tPA (min) | 114 | 91 | 176 | 129 | 98 | 167 | 0.522 | |
| Interval from stroke onset to start of endovascular therapy † | 256 | 166 | 308 | 248 | 196 | 304 | 0.903 | |
| Order of randomization into trial | 472 | 151 | 655 | 423 | 215 | 633 | 0.803 | |
| Dichotomous Descriptors | [yes] | [no] | [yes] | [no] | p value ** | |||
| Male gender | 18 | 16 | 434 | 362 | 0.862 | |||
| Use of IV tPA | 28 | 6 | 674 | 122 | 0.634 | |||
| Use of endovascular therapy | 20 | 14 | 152 | 644 | <0.0001 | |||
| Received albumin | 23 | 11 | 393 | 403 | 0.053 | |||
| Favorable primary outcome at 90 days | 3 | 31 | 370 | 426 | <0.0001 | |||
| Death | 17 | 17 | 97 | 659 | <0.0001 | |||
|
|
|
|||||||
Notes:
Mann-Whitney rank sum test
Fisher exact test
For endovascular subjects receiving both intra-arterial tPA and a mechanical device, start-time refers to the last-employed modality
Using binary logistic regression, we derived adjusted estimates of the effect of endovascular therapy on early sICH (onset < day 8) and on any sICH; we accounted for IV tPA treatment, drug (ALB, SAL), age, baseline NIHSS score (bNIHSS), and the possibility of multiplicative interaction of endovascular therapy and drug. IV tPA and bNIHSS did not influence the model. Table 3 shows these analyses. Endovascular therapy was a strong predictor of both early sICH (odds ratio 5.94; CI 2.9–12.1) and any sICH (OR 3.98; 2.0–7.4) (p<0.001). ALB therapy increased the odds of both early sICH and any sICH by ~2-fold in this model but was significant only for the latter. Older age increased the odds of sICH to a significant but minor degree (OR 1.03–1.04) (Table 3). There was no demonstrable interaction of endovascular therapy and study drug.
Table 3.
Binary logistic regression – symptomatic intracranial hemorrhage
| Early sICH (onset < day 8) | |||||||
|---|---|---|---|---|---|---|---|
| Coefficient | S.E. | Wald statistic | P = | Odds Ratio | 95% CI – lower bound | 95% CI -- upper bound | |
| Any endovascular therapy | 1.782 | 04.364 | 24.0 | <0.001 | 5.94 | 2.91 | 12.12 |
| Drug (ALB, Sal) | 0.697 | 0.382 | 3.3 | 0.068 | 2.01 | 0.95 | 4.24 |
| Age | 0.035 | 0.016 | 4.6 | 0.031 | 1.04 | 1.00 | 1.07 |
| Any sICH | |||||||
|---|---|---|---|---|---|---|---|
| Coefficient | S.E. | Wald statistic | P = | Odds Ratio | 95% CI – lower bound | 95% CI -- upper bound | |
| Any endovascular therapy | 1.358 | 0.329 | 17.1 | <0.001 | 3.89 | 2.04 | 7.41 |
| Drug (ALB, Sal) | 0.764 | 0.348 | 4.8 | 0.028 | 2.15 | 1.08 | 4.25 |
| Age | 0.030 | 0.014 | 4.5 | 0.035 | 1.03 | 1.00 | 1.06 |
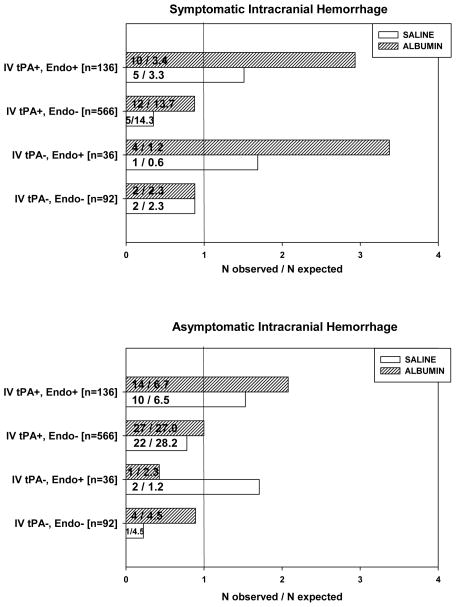
In additional analyses, we categorized thrombolytic interventions according to the four combinations used in the trial (i.e., IV tPA alone; IV tPA plus endovascular therapy [mechanical and/or intra-arterial tPA]; endovascular therapy alone; and neither IV nor endovascular therapy), and we explored the relationship of each of these to study drug (ALB or SAL) (Figure 1). Among the 41 subjects with sICH, chi-square analysis revealed highly significant differences of sICH in subjects receiving endovascular therapy; and, in particular, when endovascular therapy was used in conjunction with ALB (χ2 = 26.9, 7 df, p=0.0004). Separate analyses of sICH subjects receiving ALB vs. SAL (corrected for multiple comparisons) revealed significant sICH differences from expected levels associated with endovascular therapy in ALB subjects (p<0.001), but not in SAL subjects (p=0.13).
Figure 1.
Results of chi-square analysis of symptomatic (upper panel) and asymptomatic (lower panel) intracranial hemorrhage by treatment (ALB, saline) in the 4 categories of thrombolytic interventions employed in the ALIAS Part 2 Trial (IV tPA plus endovascular therapy; IV tPA without endovascular; endovascular without IV tPA; and neither therapy). The fractions shown represent (number of observed cases)/(number of expected cases based upon the proportion of the respective thrombolytic category within the entire sample). Observed numbers of symptomatic ICH (sICH) cases significantly exceed expected numbers in subjects receiving endovascular therapies together with ALB. (See text for statistical details.)
Asymptomatic Intracranial Hemorrhage (asx-ICH)
Clinically asymptomatic ICH (asx-ICH) was detected by neuroimaging in 81 subjects. Seventy-six subjects with asx-ICH, and 657 without asx-ICH, had received some form of thrombolytic therapy (IV tPA and/or endovascular) (p=0.14, Fisher exact test). Mann-Whitney rank sum tests revealed that asx-ICH was significantly associated with the following factors: baseline NIHSS score (p<0.001); baseline ASPECTS (p<0.001); use of endovascular therapy (p=0.003); and SEDAN score (p<0.001). Median bNIHSS was 14 (IQR, 10–19) in asx-ICH subjects and 11 (IQR 8–16.5) in subjects without asx-ICH. Age, study drug (ALB, SAL), and IV tPA use were not associated with asx-ICH development.
Chi-square analysis was used to assess asx-ICH in relation to thrombolytic interventions and study drug in a manner similar to that described above for sICH. This also revealed significant differences chiefly attributable to the use of endovascular therapy (Figure 1) (χ2 = 15.2, 7 df, p=0.034). Separate analyses of asx-ICH subjects receiving ALB vs. SAL revealed that this trend was attributable to endovascular therapy in ALB subjects (p=0.034), but not in SAL subjects (p=0.089) (Figure 1).
DISCUSSION
These results show that endovascular thrombolytic therapies in the ALIAS Part 2 Trial constituted the major risk factor for the development of symptomatic intracranial hemorrhage (sICH); that the majority of these sICH cases developed within 12–24 hours of randomization (Table 1); and that treatment with ALB accentuated the predisposition to sICH development (Table 3; Figure 1).
While there is abundant published information as to the risk of sICH following intravenous thrombolysis, fewer data are available for endovascular therapy (with or without IV tPA). In a comprehensive survey of sICH following IV thrombolysis for ischemic stroke, Seet and Rabinstein4 compiled data from 7 randomized controlled trials, 7 stroke registries and 10 cohort studies. Overall, mean age was 69 years; baseline NIHSS was 12.5 (range 9–15); sICH incidence was 5.6 ± 2.3%; and mortality 14.7 ± 4.8%. In the 7 major randomized controlled trials of IV tPA (N=2124) – in which sICH definitions differed as to interval from treatment, extent of neurological decline, and neuroimaging criteria -- mean sICH rate was 7.5 ± 1.5%, and mortality 15.7 ± 6.9%.4 The criteria for sICH used in the ALIAS Trial most closely resembled those of the 1995 NINDS multicenter trial of IV tPA – i.e., neuroimaging-proven hemorrhage associated in the investigator’s judgment with neurological worsening within 36h following IV thrombolysis.7 In the 7 summarized reports utilizing the NINDS criteria, the mean sICH rate was 7.61 ± 0.94%.4
It is more difficult to form a unitary concept of hemorrhagic complications following endovascular therapy for stroke, particularly in view of the evolution of improved mechanical endovascular devices over the past decade. The early Merci device had a 7.8% sICH rate;8 and the MultiMERCI trial, utilizing an improved retriever within 8 hours after initial IV tPA therapy, reported a 9.8% sICH rate.9 In the multicenter SWIFT trial, in which the Merci retriever was compared to the newer Solitaire device (the latter not reportedly used in ALIAS), the respective sICH rates differed markedly -- 10.9% for Merci vs. 1.7% for Solitaire.10 Correspondingly, the odds ratio for successful recanalization with Solitaire compared to Merci was >5. The Penumbra device was associated with a 10% sICH rate.11 Reports of the combined use of IV tPA and endovascular therapy, in general, note incidences of sICH comparable to IV tPA alone.12 In the landmark Interventional Management of Stroke (IMS) III multicenter trial comparing IV plus endovascular therapy with IV therapy alone, sICH rates within 30 hours were similar for the two treatments (6.2% and 5.9%, respectively), as were outcomes.13 In a meta-analysis of 5 randomized trials comparing endovascular therapy (with or without IV tPA) to standard-of-care IV tPA alone, sICH rates did not differ by treatment modality (risk ratio 0.99), and the analysis failed to show superiority of endovascular therapy over IV tPA.14
In the ALIAS Part 2 Trial, the overall sICH rate within 24 hours in both the intent-to-treat analysis1 and the safety cohort was 2.9% -- thus, as low as or lower than in other reported thrombolytic trials (see above). Nonetheless, this rate differed significantly by treatment: 4.1% in ALB-treated subjects vs. 1.7% with SAL (relative risk, 2.42, 95% CI 1.02–5.78).1 In a recent review, Jickling et al15 comprehensively surveyed the factors associated with hemorrhagic transformation after ischemic stroke in experimental and clinical studies. A factor of possible relevance to ALIAS relates to the use of antiplatelet agents in close temporal relationship to tPA treatment.16, 17 This is pertinent in that several studies indicate that albumin may exert a platelet anti-aggregatory effect. Under conditions of thromboxane synthase inhibition, serum albumin enhances the impairment of platelet aggregation by increasing the formation of the anti-aggregatory prostaglandin, PGD2.18 Local concentrations of albumin exert a profound influence on the potency of endogenously released platelet-activating factor (PAF).19 Albumin also exerts a dose-dependent role in preventing histone-induced platelet aggregation.20 In ex-vivo studies of canine carotid arteries, albumin pretreatment significantly reduced platelet and red cell deposition on damaged arteries.21 Albumin-coating of extracorporeal pump tubing reduced platelet adherence.22
The status of the collateral circulation is also relevant to hemorrhagic transformation after ischemic stroke.15 Poor collaterals are associated with larger infarcts and worse functional outcome,23 and, in the setting of recanalization, they confer an increased risk of hemorrhagic transformation.24, 25 Bang et al26, 27 reported a 2-center study of >200 acute ischemic stroke patients receiving endovascular therapy, in whom there was a 19% rate of symptomatic hemorrhagic transformation. When revascularization was achieved, patients with poorer collateral grade on baseline angiography were more likely to undergo sICH (odds ratio, 2.7, 95% CI 1.2–6.1)26 as well as a lower rate of complete revascularization and greater infarct growth.27
The ALIAS trials were not designed to assess the status of the collateral circulation. Several experimental studies from our group, however, indicate that high-dose albumin administration in focal cerebral ischemia influences cerebral blood flow (CBF) and collateral perfusion. In autoradiographic studies of temporary MCA occlusion, high-dose ALB improved perfusion to regions of critically reduced local CBF.28 In a model of permanent MCA occlusion, high-dose ALB also increased cortical perfusion.29 In a confocal microscopic study of rats with reversible MCA occlusion, ALB therapy increased post-ischemic microvascular perfusion and was associated with the partial clearance of adherent thrombotic material from cortical venules.30 In vivo studies employing two-photon laser-scanning microscopy demonstrated that ALB (but not saline) reversed impaired microvascular flow velocity distal to a laser-induced arteriolar thrombus31 and augmented the effect of sub-lytic doses of reteplase in this model.32
Definitive evidence of ALB’s effect on collateral perfusion was obtained in a study utilizing two inbred mouse strains that differed in their degree of collateral circulation, and in which cortical perfusion was studied by in vivo laser speckle contrast flow imaging following distal MCA occlusion.33 In the BALB/c mouse strain, which has sparse brain collaterals, ALB treatment (but not SAL) enhanced perfusion by ~2-fold in both the ischemic-core and penumbral regions, while in the better-collateralized CD-1 strain, this effect was not present.
Based on the above considerations, we suggest that ALB therapy in the ALIAS Part 2 Trial may have induced an “unwanted” enhancement of collateral perfusion in subjects with poor baseline collateralization such that, when some degree of recanalization was then induced by endovascular therapy, ALB may have enhanced the propensity to sICH and poor outcome. This hypothesis would be amenable to clinical investigation by employing sequential neurovascular imaging in relation to ALB or saline therapy.
Acknowledgments
Acknowledgments and Funding:
The ALIAS Multicenter Trial was supported by cooperative agreements from the National Institute of Neurological Disorders and Stroke, National Institutes of Health (U01NS040406, University of Miami; U01NS054630, Medical University of South Carolina; U01NS056975, University of Michigan). The Data Coordination Unit, Medical University of South Carolina, prepared the ALIAS Part 2 Trial’s Public Use Dataset.
Footnotes
Disclosures: none
References
- 1.Ginsberg MD, Palesch YY, Hill MD, et al. High-dose albumin treatment for acute ischaemic stroke (ALIAS) part 2: A randomised, double-blind, phase 3, placebo-controlled trial. Lancet Neurol. 2013;12:1049–1058. doi: 10.1016/S1474-4422(13)70223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ginsberg MD, Palesch YY, Martin RH, et al. The Albumin in Acute Stroke (ALIAS) Multicenter Clinical Trial: Safety analysis of part 1 and rationale and design of part 2. Stroke. 2011;42:119–127. doi: 10.1161/STROKEAHA.110.596072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill MD, Moy CS, Palesch YY, et al. The Albumin in Acute Stroke Trial (ALIAS); design and methodology. Int J Stroke. 2007;2:214–219. doi: 10.1111/j.1747-4949.2007.00143.x. [DOI] [PubMed] [Google Scholar]
- 4.Seet RC, Rabinstein AA. Symptomatic intracranial hemorrhage following intravenous thrombolysis for acute ischemic stroke: A critical review of case definitions. Cerebrovasc Dis (Basel) 2012;34:106–114. doi: 10.1159/000339675. [DOI] [PubMed] [Google Scholar]
- 5.Lyden PD. Stroke. Haemorrhage risk after thrombolysis--the SEDAN score. Nature Rev Neurol. 2012;8:246–247. doi: 10.1038/nrneurol.2012.66. [DOI] [PubMed] [Google Scholar]
- 6.Strbian D, Engelter S, Michel P, et al. Symptomatic intracranial hemorrhage after stroke thrombolysis: The SEDAN score. Ann Neurol. 2012;71:634–641. doi: 10.1002/ana.23546. [DOI] [PubMed] [Google Scholar]
- 7.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 8.Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: Results of the Merci trial. Stroke. 2005;36:1432–1438. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 9.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: Final results of the Multi Merci trial. Stroke. 2008;39:1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 10.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci retriever in patients with acute ischaemic stroke (SWIFT): A randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 11.Hussain SI, Zaidat OO, Fitzsimmons BF. The Penumbra system for mechanical thrombectomy in endovascular acute ischemic stroke therapy. Neurology. 2012;79:S135–141. doi: 10.1212/WNL.0b013e31826958a8. [DOI] [PubMed] [Google Scholar]
- 12.Mazighi M, Serfaty JM, Labreuche J, et al. Comparison of intravenous alteplase with a combined intravenous-endovascular approach in patients with stroke and confirmed arterial occlusion (RECANALISE study): A prospective cohort study. Lancet Neurol. 2009;8:802–809. doi: 10.1016/S1474-4422(09)70182-6. [DOI] [PubMed] [Google Scholar]
- 13.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh B, Parsaik AK, Prokop LJ, Mittal MK. Endovascular therapy for acute ischemic stroke: A systematic review and meta-analysis. Mayo Clin Proc. 2013;88:1056–1065. doi: 10.1016/j.mayocp.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jickling GC, Liu D, Stamova B, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. 2014;34:185–199. doi: 10.1038/jcbfm.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 17.Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: A secondary analysis of the European-Australasian Acute Stroke Study (ECASS II) Stroke. 2001;32:438–441. doi: 10.1161/01.str.32.2.438. [DOI] [PubMed] [Google Scholar]
- 18.Gresele P, Deckmyn H, Huybrechts E, Vermylen J. Serum albumin enhances the impairment of platelet aggregation with thromboxane synthase inhibition by increasing the formation of prostaglandin D2. Biochem Pharmacol. 1984;33:2083–2088. doi: 10.1016/0006-2952(84)90577-x. [DOI] [PubMed] [Google Scholar]
- 19.Grigoriadis G, Stewart AG. Albumin inhibits platelet-activating factor (PAF)-induced responses in platelets and macrophages: Implications for the biologically active form of PAF. Brit J Pharmacol. 1992;107:73–77. doi: 10.1111/j.1476-5381.1992.tb14465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam FW, Cruz MA, Leung HC, et al. Histone induced platelet aggregation is inhibited by normal albumin. Thromb Res. 2013;132:69–76. doi: 10.1016/j.thromres.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Morley D, Santamore WP, Bove AA. Inhibition of platelet and red blood cell accumulation on damaged arterial surfaces with albumin pretreatment. Thromb Res. 1989;56:265–276. doi: 10.1016/0049-3848(89)90168-0. [DOI] [PubMed] [Google Scholar]
- 22.Borgdorff P, van den Berg RH, Vis MA, van den Bos GC, Tangelder GJ. Pump-induced platelet aggregation in albumin-coated extracorporeal systems. J Thor Cardiovasc Surg. 1999;118:946–952. doi: 10.1016/s0022-5223(99)70066-8. [DOI] [PubMed] [Google Scholar]
- 23.Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132:2231–2238. doi: 10.1093/brain/awp155. [DOI] [PubMed] [Google Scholar]
- 24.Brunner F, Tomandl B, Hanken K, Hildebrandt H, Kastrup A. Impact of collateral circulation on early outcome and risk of hemorrhagic complications after systemic thrombolysis. Int J Stroke. 2012;10:1747–49. doi: 10.1111/j.1747-4949.2012.00922.x. [DOI] [PubMed] [Google Scholar]
- 25.Christoforidis GA, Karakasis C, Mohammad Y, Caragine LP, Yang M, Slivka AP. Predictors of hemorrhage following intra-arterial thrombolysis for acute ischemic stroke: The role of pial collateral formation. AJNR Am J Neuroradiol. 2009;30:165–170. doi: 10.3174/ajnr.A1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bang OY, Saver JL, Kim SJ, et al. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke. 2011;42:2235–2239. doi: 10.1161/STROKEAHA.110.604603. [DOI] [PubMed] [Google Scholar]
- 27.Bang OY, Saver JL, Kim SJ, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42:693–699. doi: 10.1161/STROKEAHA.110.595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huh PW, Belayev L, Zhao W, Busto R, Saul I, Ginsberg MD. The effect of high-dose albumin therapy on local cerebral perfusion after transient focal cerebral ischemia in rats. Brain Res. 1998;804:105–113. doi: 10.1016/s0006-8993(98)00674-x. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Belayev L, Zhao W, Busto R, Belayev A, Ginsberg MD. Neuroprotective effect of treatment with human albumin in permanent focal cerebral ischemia: Histopathology and cortical perfusion studies. Eur J Pharmacol. 2001;428:193–201. doi: 10.1016/s0014-2999(01)01255-9. [DOI] [PubMed] [Google Scholar]
- 30.Belayev L, Pinard E, Nallet H, et al. Albumin therapy of transient focal cerebral ischemia: In vivo analysis of dynamic microvascular responses. Stroke. 2002;33:1077–1084. doi: 10.1161/hs0402.105555. [DOI] [PubMed] [Google Scholar]
- 31.Nimmagadda A, Park HP, Prado R, Ginsberg MD. Albumin therapy improves local vascular dynamics in a rat model of primary microvascular thrombosis: A two-photon laser-scanning microscopy study. Stroke. 2008;39:198–204. doi: 10.1161/STROKEAHA.107.495598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park HP, Nimmagadda A, DeFazio RA, Busto R, Prado R, Ginsberg MD. Albumin therapy augments the effect of thrombolysis on local vascular dynamics in a rat model of arteriolar thrombosis: A two-photon laser-scanning microscopy study. Stroke. 2008;39:1556–1562. doi: 10.1161/STROKEAHA.107.502195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Defazio RA, Zhao W, Deng X, Obenaus A, Ginsberg MD. Albumin therapy enhances collateral perfusion after laser-induced middle cerebral artery branch occlusion: A laser speckle contrast flow study. J Cereb Blood Flow Metab. 2012;32:2012–2022. doi: 10.1038/jcbfm.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]