Abstract
Elevated Depressive symptoms (DS) are associated with incident mild cognitive impairment and probable dementia in postmenopausal women. We examined the association of elevated DS with domain-specific cognitive changes, and the moderating role of cardiovascular risk factor (CVRF) severity and cardiovascular disease (CVD). 2221 elderly women who participated in the Women's Health Initiative Study of Cognitive Aging were separated into those with (N = 204) and without (N = 2017) elevated DS. DS and multi-domain cognitive outcomes were measured annually for an average follow-up of 5.04 years. Women with elevated DS showed baseline multi-domain cognitive deficits, but longitudinal declines in global cognition only. Persistent DS was related to greater global cognition, and verbal knowledge and fluency, and memory declines. Significant DS-CVD interactions were observed cross-sectionally (but not longitudinally) for figural memory and fine motor speed. Future studies should investigate the role of nonvascular mechanisms linking DS and cognitive decline.
Keywords: Depression, Cognitive decline, Vascular risk factors, Cardiovascular disease, Women, Dementia, Depressive symptoms, Elderly, Cognitive decline
INTRODUCTION
Clinically significant depression occurs in 15% of the elderly and a significant majority of these individuals has milder depressive symptoms (DS) that fall short of the criteria for syndromal late-life depression (LLD). The number of elderly women suffering from elevated DS is expected to increase exponentially in the coming decades.1 Furthermore, the public heath consequences of incident cognitive decline and Alzheimer's Disease (AD) are significantly greater for women than men largely due to women's longer life expectancy.2 While LLD has been associated with an increased risk of incident cognitive decline and Alzheimer's disease (AD),3 the effects of milder DS on future cognitive health is less clear. In the Women's Health Initiative Memory Study (WHIMS), elevated DS was associated with an increased risk of mild cognitive impairment (MCI) and probable dementia in those 65 years of age and older.4 Late-life DS are also associated with longitudinal declines in psychomotor speed and executive functions in mixed gender studies,5-7 although the effects on episodic memory and nonmemory cognitive measures over time in elderly women remains elusive. Moreover, the differential effects of fluctuating and persistently elevated DS on domain-specific cognitive changes are poorly understood.
Of the prospective studies that have examined the longitudinal associations of late-life DS with domain-specific cognitive functions, most did not include a control group, and/or measured cognitive functions at limited timepoints.5, 6 Moreover, only a handful of investigations have examined the effects of persistent DS on the longitudinal cognitive performance, and the findings vary. While persistent DS was associated with only declines in information processing speed in one study, symptom chronicity in patients with coronary heart disease was related to multi-domain cognitive decline in another study.5, 7 Additional investigations of the complex relationships between DS and longitudinal cognitive changes, and the mechanisms that explain their associations in postmenopausal women are critically needed.
Vascular etiologies are hypothesized to play a critical role in linking depression and cognitive decline.8-10 Late-life DS are often co-morbid with cardiovascular risk factors (CVRFs) and cardiovascular and cerebrovascular disease. The co-existence of CVRFs and cardiovascular disease (CVD) with late-life DS is related to worse treatment outcomes and greater cognitive decline.5, 11 Also, there is a bi-directional relationship between LLD and CVRF and vascular disease, and these observations support the ‘vascular depression’ hypothesis.12, 13 While emerging evidence indicates that worsening subclinical cerebrovascular disease in depressed elderly may be associated with future development of dementia, primarily of non-Alzheimer's type,10 others have not found an influence of vascular disease on late-life DS-cognitive impairment relationships.14, 15 CVRFs and CVD are also independently associated with an increased risk of cognitive decline and incident dementia.16 The moderating role of CVRF severity and CVD on the relationship of DS with longitudinal changes in memory and non-memory measures in elderly women remains poorly understood.
The data from a large, well-characterized cohort of postmenopausal women who participated in the Women's Health Initiative Study of Cognitive Aging (WHISCA)17 were utilized here (1) to determine the relationship of DS and domain-specific cognitive changes, and (2) to investigate the moderating effects of CVRF and CVD on various cognitive measures in those with DS, over a seven-year follow-up period.
METHODS
Participants
WHISCA, an ancillary study to the Women's Health Initiative (WHI) randomized, placebo-controlled clinical trial of hormone therapy (HT) regimens based on conjugated equine estrogens, enrolled 2304 postmenopausal women between ages 65 and 79 from 14 of 40 WHI clinical sites. WHISCA assessments began an average of 3 years after the WHI randomization to treatment. The study design, participant eligibility criteria, recruitment procedures, and principal findings of WHISCA have been previously published.17-19 The National Institute of Health (NIH) and the institutional review boards at the participating clinical centers approved WHISCA study protocols. All participants provided informed consent. The WHISCA participants who had completed at least the initial cognitive assessment and contributed complete data on baseline characteristics were included in this analysis (Table 1). In addition, women who were free of MCI and probable dementia at WHISCA enrollment were included in this study. After excluding 83 women who did not meet the above criteria, the final cross-sectional sample was comprised of a total of 2221 participants. Among the 2221 participants, 2123 women finished at least one follow-up assessment for the longitudinal analysis focused on change in cognitive performance from baseline.
Table 1.
Baseline characteristics for WHISCA cohort by depressive symptom status
| Characteristic | No Depressive Symptoms N=2017 | Elevated Depressive Symptoms N=204 | p-value |
|---|---|---|---|
| Age, years, n (%) | 0.96 | ||
| 65-69 | 955 (47) | 95 (46) | |
| 70-74 | 740 (37) | 77 (38) | |
| 75-80 | 322 (16) | 32 (16) | |
| Education, n (%) | < 0.01 | ||
| < high school grad/GED | 91 (5) | 16 (8) | |
| High school grad/GED | 422 (21) | 55 (26) | |
| Some college | 834 (41) | 86 (42) | |
| College grad | 670 (33) | 47 (23) | |
| Race/ethnicity, n (%) | < 0.0001 | ||
| African American | 111 (6) | 26 (13) | |
| Caucasian | 1835 (91) | 167 (82) | |
| Other | 71 (4) | 11 (5) | |
| Marital status, n (%) | 0.35 | ||
| Never married | 64 (3) | 7 (3) | |
| Separated/divorced | 231 (11) | 32 (16) | |
| Widowed | 603 (30) | 59 (29) | |
| Married | 1119 (55) | 106 (52) | |
| Alcohol use, n (%) | < 0.0001 | ||
| Nondrinker | 247 (12) | 19 (9) | |
| Past drinker | 613 (31) | 93 (46) | |
| Current drinker | 1150 (57) | 91 (45) | |
| Smoking, n (%) | 0.27 | ||
| Never | 1107 (55) | 114 (56) | |
| Past | 793 (39) | 73 (36) | |
| Current | 117 (6) | 17 (8) | |
| Leisure physical activity, MET, n (%) | <0.001 | ||
| Lowest tertile | 649 (32) | 94 (46) | |
| Mid tertile | 674 (33) | 61 (30) | |
| Highest tertile | 694 (34) | 49 (24) | |
| Body mass index, kg/m2, n (%) | < 0.01 | ||
| < 25 | 579 (29) | 44 (22) | |
| 25-29 | 749 (37) | 69 (34) | |
| 30+ | 689 (34) | 91 (45) | |
| Hypertension, n (%) | 1094 (54) | 104 (51) | 0.37 |
| Diabetes mellitus, n (%) | 171 (8) | 31 (15) | < 0.01 |
| Dyslipidemia, n (%) | 353 (18) | 34 (17) | 0.76 |
| Cardiovascular disease, n (%) | 304 (15) | 35 (17) | 0.43 |
| Stroke or TIA, n (%) | 89 (4) | 9 (4) | 1.00 |
| Prior hysterectomy, n (%) | 761 (38) | 92 (45) | 0.04 |
| Current use of antidepressant medications, n (%) | 129 (6) | 26 (13) | < 0.001 |
| Prior hormone therapy, n (%) | 918 (46) | 103 (50) | 0.17 |
| WHI treatment assignment, n (%) | 0.64 | ||
| Active | 984 (49) | 96 (47) | |
| Placebo | 1033 (51) | 108 (53) |
Abbreviations: WHI: Women's Health Initiative; WHISCA: Women's Health Initiative Study of Cognitive Aging; MET: Metabolic Equivalent of Task; TIA: Transient Ischemic Attack.
Depressive symptom assessments
DS were assessed annually over up to 7 years using the 15-item Geriatric Depression Scale (GDS).20, 21 A score of 5 or above at WHISCA initial assessment was considered as elevated DS. A cutoff point of 5 have been previously demonstrated as having excellent validity for detecting major depression in the elderly.20, 22, 23 Additionally, women were classified based on the presence or absence of elevated DS at WHISCA year 1 follow-up: Participants were considered to have persistently elevated DS if they scored positive (i.e., > 5 on GDS) at both WHISCA first (i.e., enrollment) and second (i.e., Year 1) visits; fluctuating DS if they scored positive at only one visit, and no DS if they scored negative (i.e., <5 on GDS) at both visits. This analysis cohort consisted of 2046 women who returned one year later for follow-up assessment.
Cardiovascular risk factor score and cardiovascular disease
We calculated the CVRF score closest to WHISCA baseline using the model described previously.24 Briefly, age, systolic blood pressure (BP), use of antihypertensive treatment, current smoking history, history of diabetes mellitus, and body mass index (BMI) were included in this non-laboratory based CVRF model which previously has been shown to predict CV events as accurately as risk factor indices based on laboratory-based values.25 Women with a history of CVD at WHISCA baseline assessment and those having missing variables required for the CVRF score calculation were excluded from this analysis. Therefore, the final cohort for this analysis was comprised of 1820 women. The CVRF score ranged from 0.03 to 0.80.
History of CVD was defined as either a self-report of myocardial infarction, coronary bypass surgery, angioplasty, congestive heart failure, angina, carotid endarterectomy/angioplasty, cardiac catheterization, aortic aneurysm, atrial fibrillation, or cardiac arrest at WHI baseline or occurrence of the incident events between WHI baseline and WHISCA enrollment. These incident events, including myocardial infarction, angina, congestive heart failure, coronary revascularization, carotid artery disease, coronary heart disease, and peripheral artery disease, were ascertained through central adjudication.
Domain-specific cognitive assessments
Detailed cognitive assessments occurred annually at each WHISCA visit.17 The battery of cognitive measures included the Primary Mental Abilities Vocabulary test (PMAVT) to assess verbal knowledge; letter and semantic fluency tests to assess verbal fluency; the Benton Visual Retention Test (BVRT) to assess short-term figural memory; the California Verbal Learning Test to assess verbal memory; the Digit Span Forward and Backward Test to assess attention and working memory; the Card Rotations Test to measure spatial ability; the Finger Tapping Test to assess fine motor speed; and the Modified Mini-mental State (3MS) examination to assess global cognitive function.
Covariates
Demographic information, medical history and lifestyle variables (including smoking and alcohol use) were obtained by self-report and clinical measurements using standardized study forms as detailed elsewhere.17, 19 Current use of antidepressant medications was obtained through a medication inventory conducted at clinic visits. HT assignment was based on whether women were randomized to intervention arms or the placebo groups in the WHI-HT trials. Prior HT use was based on self-report at WHI baseline. History of stroke and transient ischemic attack (TIA) were collected via self-report at WHI baseline and by central adjudication until WHISCA enrollment.
Statistical Analysis
Women classified with and without elevated DS at WHISCA enrollment were compared with respect to demographic, lifestyle, cognitive, and clinical characteristics with chi-square tests and t tests.
Eight cognitive domains (global function, verbal knowledge, verbal fluency, figural memory, verbal memory, attention/working memory, spatial ability, and fine motor speed) were assessed. Individual scores on each outcome measure were first standardized by dividing their difference from the cohort-wide mean at WHISCA enrollment by their standard deviation (SD). For cognitive domains based on more than one outcome measure, the standardized scores of these outcomes were averaged and renormalized. Scores from the BVRT were subtracted from zero so that higher scores signified better performance, consistent with scores for other domains. The analysis focused on assessing three features of the data: 1) whether there was a difference in cognitive function scores associated with DS at WHISCA enrollment (cross-sectional), 2) whether such a difference persisted over follow-up (longitudinal pattern or average scores over time), and 3) whether the magnitude of the difference changed over time (longitudinal change from baseline). Additional modeling was carried out to examine the relationship of persistent and fluctuating levels of DS with domain-specific cognitive changes over time, with and without omitting 81 women who developed MCI or probable dementia during follow-up. This analysis was based on women who had a cognitive assessment at one-year follow-up in addition to enrollment, permitting classification into three groups: women without elevated DS at either time-point; with fluctuating DS; and with persistently elevated DS.
Analysis of covariance (ANCOVA) was used for cross-sectional comparisons. A mixed-model repeated measures ANCOVA incorporating within person correlation was employed to assess whether mean cognitive domain scores and changes from WHISCA initial assessment in scores varied between women with and without DS. We assessed the significance of interaction terms between DS and prior CVD and between DS and CVRF score to explore whether the effects of DS on cognitive domain scores were moderated by either of these two factors. Models included adjustment for potential confounders (age, race/ethnicity, education, marital status, hysterectomy, antidepressant use, prior HT use, smoking, use of cholesterol-lowering medication, alcohol use, body mass index (BMI), hypertension, diabetes, prior CVD including TIA or stroke, physical activity, and treatment arm). The baseline score for each cognitive domain was included as a covariate for the change from baseline in the longitudinal analysis. Because these analyses were exploratory and the cognitive variables are inter-correlated, Type I error was not controlled for multiple outcomes. P<0.01 was considered significant. Statistical analyses were performed with SAS statistical software, version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Of the 2221 participants, 204 (9.2%) women met criteria for elevated DS at WHISCA baseline. Women with elevated DS were more likely to be African-American, have lower education, endorse a history of hysterectomy, diabetes mellitus, past alcohol use, be obese, report being on antidepressants, and less likely to be physically active (Table 1). Among the 155 women reporting antidepressant use at baseline, 71 (46%) were taking selective serotonin reuptake inhibitors (DS: 8.3%; no DS: n=2.7%); 61 (39%) were taking tricyclic antidepressants (DS: n=2.5%; no DS: n=2.8%); and 23 (15%) were taking other classes or were on combination therapy (DS: n=1.96%; no DS: n=0.9%). Women with elevated DS at baseline had significantly lower raw scores on all cognitive measures except fine motor speed (Table 2). Follow-up averaged 5.04 years (range: 0 to 7.50; Mean (SD) length of follow-up for DS: 5.10 (2.07); no DS: 4.46 (2.24); p < 0.0001).
Table 2.
Cognitive test scores at WHISCA enrollment by depressive symptom status
| Mean (standard deviation) | No Depressive Symptoms N=2017 | Elevated Depressive Symptoms N=204 | p-value |
|---|---|---|---|
| Global function | |||
| 3MS | 97.03 (2.73) | 95.97 (3.52) | < 0.0001 |
| Verbal knowledge | |||
| PMAVT | 37.10 (9.51) | 33.35 (10.00) | < 0.0001 |
| Verbal fluency | |||
| Category | 29.15 (6.19) | 27.99 (6.17) | 0.01 |
| Letter | 40.01 (12.45) | 38.08 (12.78) | 0.04 |
| Figural memory | |||
| BVRT | −6.89 (3.58) | −8.26 (4.52) | < 0.0001 |
| Verbal memory | |||
| CVLT-a | 28.94 (6.23) | 27.10 (6.41) | < 0.0001 |
| CVLT-b | 6.61 (2.12) | 6.05 (2.08) | < 0.001 |
| CVLT free short delay | 8.54 (3.03) | 7.56 (3.29) | < 0.0001 |
| CVLT free long delay | 9.28 (2.99) | 8.50 (3.17) | < 0.001 |
| Attention/working memory | |||
| Digit forward | 7.50 (2.06) | 7.05 (2.00) | < 0.01 |
| Digit backward | 6.73 (1.99) | 6.34 (2.18) | < 0.01 |
| Spatial ability | |||
| Card rotation | 56.25 (27.33) | 45.74 (26.87) | < 0.0001 |
| Fine motor speed | |||
| Tapping dominant | 38.65 (7.60) | 37.83 (8.11) | 0.15 |
| Tapping non-dominant | 36.58 (6.40) | 35.83 (6.81) | 0.12 |
Abbreviations: WHISCA: Women's Health Initiative Study of Cognitive Aging; 3MS: Modified Mini-Mental State Examination; PMAVT: Primary Mental Abilities Vocabulary test (PMAVT); BVRT: Benton Visual Retention Test; CVLT: California Verbal Learning Test.
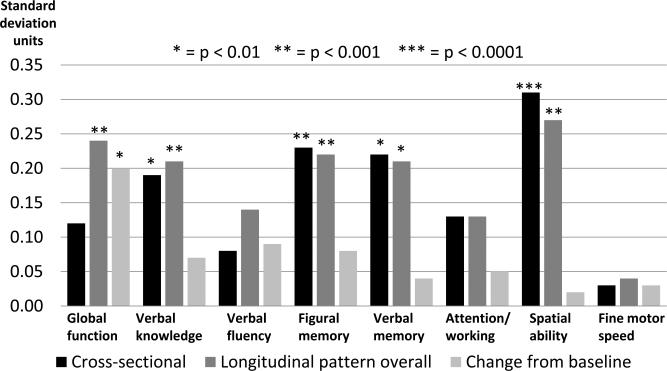
Figure 1 summarizes the mean covariate-adjusted decline in cognitive domain test scores associated with elevated DS (relative to no symptoms) based on the cross-sectional and longitudinal analyses, with the differences averaged across time for the overall longitudinal pattern as well as for the changes from baseline. Elevated DS were associated with lower mean baseline scores in domains including verbal knowledge and verbal memory (p<0.01), figural memory (p<0.001) and visuospatial abilities (p<0.0001). The overall longitudinal pattern also showed lower scores for women with elevated DS: most significant differences were noted for global cognition, verbal knowledge, figural memory and visuospatial abilities (p<0.001), followed by verbal memory (p<0.01). In terms of change from baseline, global cognitive function (p<0.01) declined over time in women with elevated DS.
Figure 1.
Mean decrements associated with elevated depressive symptoms for cognitive domains a
Abbreviation: DS: depressive symptoms.
a With adjustment for age, race/ethnicity, education, marital status, hysterectomy, current use of antidepressant medications, prior use of hormone therapy, smoking, use of cholesterol-lowering medication, alcohol use, body mass index, hypertension, prior cardiovascular/ cerebrovascular disease including stroke or transient ischemic attack, diabetes, physical activity, and Women's Health Initiative hormone therapy trial treatment arm. Cross-sectional refers to baseline differences; longitudinal pattern overall refers to mean differences across time; change from baseline refers to differences in changes from baseline averaged across time.
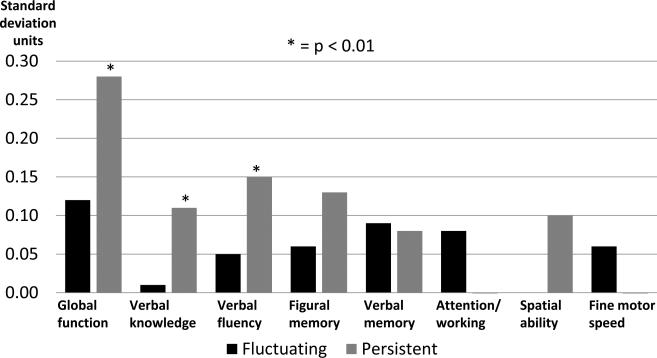
We performed additional analyses to examine the relationship of persistent and fluctuating levels of elevated DS with domain-specific cognitive changes over time. Women were classified into those without elevated DS at either time-point (N=1752); with fluctuating DS (N=202); and with persistently elevated DS (N=92). Mean GDS scores for these groups at baseline were 0.86, 3.90, and 7.32, respectively. At baseline, The fluctuating DS group was more likely to have lower education, be physically inactive, endorse a history of past drinking and be on antidepressants, whereas the persistent DS group was more likely to have lower education, poorer 3MS scores and diabetes, endorse current smoking and past drinking histories, be non-white, physically inactive and on antidepressants, relative to controls (p<0.01).
After full covariate adjustment, women with persistently elevated DS showed greater longitudinal declines in global cognition, verbal knowledge, and verbal fluency (p<0.01), and a trend in figural memory (p<0.05), whereas fluctuating DS course showed no changes (Figure 2). After excluding women who developed MCI/probable dementia (N=81) during follow-up, the persistent DS group findings slightly diminished but remained significant whereas the fluctuating DS group results enhanced slightly. The above results remained essentially unchanged after we repeated the longitudinal analyses controlling for the mean length of follow-up time since baseline.
Figure 2.
Mean longitudinal domain-specific cognitive changes from baseline associated with fluctuating and persistently elevated depressive symptoms versus no symptoms a
a With adjustment for age, race/ethnicity, education, marital status, hysterectomy, current use of antidepressant medications, prior use of hormone therapy, smoking, use of cholesterol-lowering medication, alcohol use, body mass index, hypertension, prior cardiovascular/cerebrovascular disease including stroke or transient ischemic attack, diabetes, physical activity, mean length of follow-up time from baseline, and Women's Health Initiative hormone therapy trial treatment arm.
Moderating effects of CVRF and CVD on DS-cognitive change relationships
We found significant interactions at WHISCA baseline between DS and CVD on figural memory and fine motor speed (p < 0.01), where women with CVD and DS performed worse than each of the other three groups (Table 3). However, no interactive effects of DS with CVD and CVRF scores on the longitudinal cognitive changes were observed. Since the CVRF model used here did not include cholesterol status, we conducted additional analyses and found no significant depressive symptoms-high cholesterol interactions on multiple domain cognitive function (p >0.05).
Table 3.
Interaction effect of depressive symptoms and cardiovascular disease on cognitive domains at baselinea
| Depressive Symptoms/CVD |
No Depressive Symptoms CVD Negative |
No Depressive Symptoms CVD positive |
Elevated Depressive Symptoms CVD Negative |
Elevated Depressive Symptoms CVD Positive |
p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cognitive domain | Adjusted mean |
Standard error |
Adjusted mean |
Standard error |
Adjusted mean |
Standard error |
Adjusted mean |
Standard error |
|
| Global function | 0.07 | 0.02 | 0.06 | 0.05 | −0.05 | 0.06 | −0.09 | 0.14 | 0.85 |
| Verbal knowledge | 0.06 | 0.02 | −0.02 | 0.05 | −0.08 | 0.07 | −0.41 | 0.15 | 0.18 |
| Verbal fluency | 0.01 | 0.02 | 0.11 | 0.06 | −0.01 | 0.07 | −0.24 | 0.16 | 0.07 |
| Figural memory | 0.05 | 0.02 | 0.04 | 0.05 | −0.09 | 0.07 | −0.57 | 0.15 | <0.01b |
| Verbal memory | 0.06 | 0.02 | 0.00 | 0.06 | −0.15 | 0.07 | −0.26 | 0.16 | 0.73 |
| Attention/working memory | 0.03 | 0.02 | 0.04 | 0.06 | −0.03 | 0.08 | −0.47 | 0.17 | 0.02 |
| Spatial ability | 0.04 | 0.02 | 0.04 | 0.06 | −0.19 | 0.07 | −0.61 | 0.16 | 0.02 |
| Fine motor speed | 0.01 | 0.02 | 0.06 | 0.06 | 0.07 | 0.08 | −0.41 | 0.17 | <0.01b |
Abbreviations: CVD: Cardiovascular disease; HT: Hormone Therapy; TIA: Transient Ischemic Attack.
With adjustment for age, race/ethnicity, education, marital status, hysterectomy, current use of antidepressant medications, prior use of Hormone Therapy, smoking, use of cholesterol-lowering medication, alcohol use, body mass index, hypertension, cardiovascular/cerebrovascular disease including stroke or transient ischemic attack, diabetes, physical activity, and Women's Health Initiative hormone therapy trial treatment arm.
Women with elevated depressive symptoms and cardiovascular disease performed worse than each of the other 3 groups for figural memory and fine motor speed (p < 0.01).
DISCUSSION
While elevated DS were cross-sectionally associated with multi-domain cognitive deficits, significant associations with longitudinal declines were only observed for global cognition in a cohort of community-dwelling postmenopausal women free of pathological cognitive impairment at baseline. However, women with persistently elevated DS showed greater declines in global cognition and non-memory measures, and a trend in episodic memory measures whereas those with fluctuating DS course had no changes during follow-up. CVD and CVRF severity histories did not moderate the longitudinal DS-cognitive change relationships.
Elderly women with elevated DS performed poorly on several memory and non-memory-cognitive domains at baseline and longitudinal patterns over time. Our observations in this well-characterized sample of elderly women with elevated DS largely mirror findings reported in those with syndromal LLD.26 Nondemented acutely depressed older adults who perform poorly in memory and executive function measures at baseline may have coexisting MCI and be at higher risk for developing dementia at follow-up, when compared to those with acute LLD who remain cognitively normal over time. 27-29 Our findings suggest that DS, regardless of the severity, frequently co-exist with cognitive deficits that do not meet the criteria for MCI and probable dementia, although the consequence of this co-morbidity is unclear.
The present study findings that elevated DS are related to declines in global cognition measure is consistent with some,7, 30 but not all studies.31 The inconsistencies in findings may be due to the fact that the DS-cognitive decline associations reported previously are largely driven by the course of depressive symptomatology. Our observations of persistent DS being associated with nonmemory declines over time are in accordance with previous mid- and late life studies.7, 32 The vulnerability to the effects of elevated DS on executive function measures appears to be greatest with symptom chronicity and advanced age.7 Elevated DS is likely to persist over time in some older adults, and the risk of developing major depression is significantly higher at follow-up.33 Persistence of LLD is associated with multiple domain cognitive deficits at baseline, suggesting that the presence of baseline cognitive impairment may be prognostic indicators of LLD outcomes.11, 34-36 We extend these prior observations to a cohort of postmenopausal women with milder depressive symptoms, suggesting that both subclinical and syndromal DS, if persistent, can increase the risk of future cognitive decline.
Our observations that fluctuating levels of DS do not detrimentally affect multiple cognitive measures are consistent with the literature.30 On the contrary, remission of DS and syndromal depression has been associated with MCI and probable dementia among elderly women and in mixed gender studies.27, 37 The fluctuating DS course is likely reflective of symptom remission due to the changes in psychosocial stressors, physical ailments and/or remission of depression. We are unable to disentangle the association of these factors with the changes in DS status in this study.
The observations that the CVRF and CVD histories did not detrimentally alter cognitive measures over time in women with DS underscore the complexities in the pathophysiological mechanisms linking DS with subsequent cognitive decline. While our results are also consistent with some studies,14, 15 detrimental effects on cognition when depression co-exists with certain CVRFs have been previously reported. For instance, the relationship of depression with incident cognitive impairment was affected by diabetes in a previous study.38 Executive dysfunction is associated with orbitofrontal volume loss in depressed diabetics.39 Higher CVRF scores have also been shown to predict lesser recovery of working memory deficits and executive dysfunction to treatment in LLD.11 Persistent DS in patients with coronary heart disease may increase the risk of future cognitive decline.5 The DS chronicity after acute coronary syndrome may be associated with decreased white matter integrity in the anterior cingulate, though these findings were no longer significant after controlling for CVRFs.40 While we did not find an interactive relationship of DS with CVRF scores and CVD on cognitive decline, a few points are worthy of mention. First, the smaller sample size in the persistent DS group precluded us from examining the effects of CVRFs and CVD when comorbid with chronic DS on cognition. Second, we did not examine the interactive relationship of DS and individual vascular risk factors separately, rather calculated the CVRF score for each individual by using an all-inclusive model. Therefore, it is still possible that late-life DS, especially when chronic and occurring in those with specific vascular risk factors (for e.g. in diabetics) and coronary heart disease may result in accelerated declines in different cognitive domains. This should be the focus of future investigations.
The lack of influence of CVRF score severity and CVD on the DS-associated memory decline also suggests that this relationship may be mediated by medial temporal lobe neurodegeneration and non-vascular etiologies. LLD is associated with hippocampal volume loss from excessive stress-related glucocorticoid release.41 Also, amyloid and neurofibrillary tangles, genetic heritability, proinflammatory cytokines and oxidative stress have been implicated in the mechanistic underpinnings of both LLD and AD.9, 42- 45 Since DS in the elderly population is a heterogeneous syndrome, future studies that examine the association of DS with incident cognitive impairment should carefully delineate older depressed adults into subtypes based on co-morbidity and neuropsychological deficits.
The strengths of this study include the well-characterized cohort of high functioning, healthy elderly women, the large sample size, the availability of annual domain-specific cognitive measurements and the ability to adjust for multiple potential confounders. However, several limitations need to be addressed. First, since we relied on self reported GDS scores to define DS, it is possible that some women may have met major depressive disorder criteria on a structured clinical interview. Regardless, the mean GDS scores even in women who had persistent DS were in the milder range and therefore the vast majority of women with positive scores had subclinical DS. Second, we are unable to comment on the effects of the age of onset, current episode duration, and number of prior episodes on our study findings. Recent evidence suggests that later onset depression may be a prodromal manifestation of AD, whereas recurrent DS with onset in mid-life may increase the risk of vascular dementia.46 Third, persistent and fluctuating levels of DS were defined based only on two GDS measures similar to prior studies.5 We primarily took this approach due to missing GDS measurements at follow-up and to prevent sample size attrition. Fourth, antidepressant use was associated with attention/working memory, figural memory and fine motor speed (p < 0.05) at baseline. Although we controlled for baseline antidepressant use, we did not include follow-up medication data in our analyses. Future studies should define persistence and fluctuations of DS based on measurements at multiple timepoints, and also factor in the effects of follow-up antidepressant use history on the DS-cognitive health relationships. Fifth, the sample size in the persistently elevated DS group is relatively small, and may have influenced our findings. Sixth, the CVRFs and CVD history was ascertained by self-report, which may have resulted in inaccurate estimation and misclassification of these conditions, and may have reduced the strength when examining for interactions. Sixth, our sample is not population-based and included women who participated in a HT trial, which limit the generalizability of our findings. Finally, since this study was largely exploratory, adjustments were not carried out for multiple comparisons.
In conclusion, persistent DS course resulted in greater declines affecting multiple cognitive domains in our elderly women cohort. CVRF and CVD histories do not appear to influence the association of DS with cognitive decline. A differential course of managing DS in an attempt to reduce the risk of cognitive decline may not be warranted for women with CVRFs and CVD. Future prospective studies should confirm our findings and determine if treatment of DS will reverse cognitive deficits in those women with different degrees of CVRF and CVD severity.
Acknowledgment
The Women's Health Initiative (WHI) program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. The WHI Study of Cognitive Aging (WHISCA) was supported by the Department of Health and Human Services and the National Institute on Aging (NO1-AG-1-2106). SMR is supported by the Intramural Research Program, National Institute on Aging, and National Institutes of Health. JSG is supported by the Alzheimer's Association New Investigator NIRG-11-204070. The active study drug and placebo were supplied by Wyeth-Ayerst Research Laboratories, Philadelphia, Pennsylvania. The Women's Health Initiative Memory Study was funded in part by Wyeth Pharmaceuticals as an ancillary study to the WHI. Wyeth Pharmaceuticals did not participate in the design and conduct of the studies, in the collection, analysis, and interpretation of the data, or in preparation, review or approval of this manuscript.
NIA Program Office. National Institute of Aging, Baltimore, MD: Alan Zonderman, Susan M. Resnick
WHISCA Central Coordinating Center. Wake Forest University Health Sciences, Winston-Salem, NC: Sally Shumaker, Principal Investigator; Stephen Rapp, Mark Espeland, Laura Coker, Deborah Farmer, Anita Hege, Patricia Hogan, Darrin Harris, Cynthia McQuellon, Anne Safrit, Lee Ann Gleiser, Candace Goode, Mary Barr, Carolyn Bell, Linda Allred, Sonya Ashburn
WHISCA Clinical Sites. Women's Health Initiative, Durham, NC, Carol Murphy; Rush Presbyterian-St. Luke's Medical Center, Chicago, IL, Linda Powell; Ohio State University Medical Center, Columbus, OH, Rebecca Jackson; University of California at Davis, Sacramento, CA, John Robbins; University of Iowa College of Medicine, Des Moines, IA, Robert Wallace; University of Florida, Gainesville/Jacksonville, FL, Marian Limacher; University of California at Los Angeles, Los Angeles, CA, Howard Judd (deceased); Medical College of Wisconsin, Milwaukee, WI, Jane Kotchen; The Berman Center for Outcomes and Clinical Research, Minneapolis, MN, Karen Margolis; University of Nevada School of Medicine, Reno, NV, Robert Brunner; Albert Einstein College of Medicine, Bronx, NY, Sylvia Smoller; The Leland Stanford Junior University, San Jose, CA, Marcia Stefanick; The State University of New York, Stony Brook, NY, Dorothy Lane; University of Massachusetts/Fallon Clinic, Worcester, MA, Judith Ockene * The following investigators were the original investigators for these sites: Mary Haan, Davis; Richard Grimm, Minneapolis; Sandra Daugherty (deceased), Nevada
WHI Program Office. National Heart, Lung, and Blood Institute, Bethesda, MD: Barbara Alving, Jacques Rossouw, Linda Pottern
WHI Central Coordinating Center. Fred Hutchinson Cancer Research Center, Seattle, WA: Deborah Bowen, Gretchen Van Lom, Carolyn Burns.
Footnotes
Disclosures: No disclosures to report.
REFERENCES
- 1.Chapman DP, Perry GS. Depression as a major component of public health for older adults. Prev Chronic Dis. 2008 Jan;5(1):A22. [PMC free article] [PubMed] [Google Scholar]
- 2.Thies W, Bleiler L. 2013 Alzheimer's disease facts and figures. Alzheimers Dement. 2013 Mar;9(2):208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006 May;63(5):530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goveas JS, Espeland MA, Woods NF, Wassertheil-Smoller S, Kotchen JM. Depressive symptoms and incidence of mild cognitive impairment and probable dementia in elderly women: the Women's Health Initiative Memory Study. J Am Geriatr Soc. 2011 Jan;59(1):57–66. doi: 10.1111/j.1532-5415.2010.03233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freiheit EA, Hogan DB, Eliasziw M, et al. A dynamic view of depressive symptoms and neurocognitive change among patients with coronary artery disease. Arch Gen Psychiatry. 2012 Mar;69(3):244–255. doi: 10.1001/archgenpsychiatry.2011.1361. [DOI] [PubMed] [Google Scholar]
- 6.Royall DR, Palmer R, Chiodo LK, Polk MJ. Depressive symptoms predict longitudinal change in executive control but not memory. Int J Geriatr Psychiatry. 2012 Jan;27(1):89–96. doi: 10.1002/gps.2697. [DOI] [PubMed] [Google Scholar]
- 7.Dotson VM, Resnick SM, Zonderman AB. Differential association of concurrent, baseline, and average depressive symptoms with cognitive decline in older adults. Am J Geriatr Psychiatry. 2008 Apr;16(4):318–330. doi: 10.1097/JGP.0b013e3181662a9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butters MA, Young JB, Lopez O, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10(3):345–357. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steffens DC, Otey E, Alexopoulos GS, et al. Perspectives on depression, mild cognitive impairment, and cognitive decline. Arch Gen Psychiatry. 2006 Feb;63(2):130–138. doi: 10.1001/archpsyc.63.2.130. [DOI] [PubMed] [Google Scholar]
- 10.Steffens DC, Potter GG, McQuoid DR, et al. Longitudinal magnetic resonance imaging vascular changes, apolipoprotein E genotype, and development of dementia in the neurocognitive outcomes of depression in the elderly study. Am J Geriatr Psychiatry. 2007 Oct;15(10):839–849. doi: 10.1097/JGP.0b013e318048a1a0. [DOI] [PubMed] [Google Scholar]
- 11.Sheline YI, Pieper CF, Barch DM, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010 Mar;67(3):277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997 Oct;54(10):915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997 Apr;154(4):497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- 14.Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry. 2006 Mar;63(3):273–279. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- 15.Ng TP, Niti M, Zaw MH, Kua EH. Depressive symptoms and incident cognitive impairment in cognitively well-functioning older men and women. J Am Geriatr Soc. 2009 Jun;57(6):1058–1063. doi: 10.1111/j.1532-5415.2009.02262.x. [DOI] [PubMed] [Google Scholar]
- 16.Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia: how to move forward? Neurology. 2009 Jan 27;72(4):368–374. doi: 10.1212/01.wnl.0000341271.90478.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resnick SM, Coker LH, Maki PM, Rapp SR, Espeland MA, Shumaker SA. The Women's Health Initiative Study of Cognitive Aging (WHISCA): a randomized clinical trial of the effects of hormone therapy on age-associated cognitive decline. Clin Trials. 2004;1(5):440–450. doi: 10.1191/1740774504cn040oa. [DOI] [PubMed] [Google Scholar]
- 18.Resnick SM, Espeland MA, An Y, et al. Effects of Conjugated Equine Estrogens on Cognition and Affect in Postmenopausal Women with Prior Hysterectomy. Journal of Clinical Endocrinology & Metabolism. 2009 Nov;94(11):4152–4161. doi: 10.1210/jc.2009-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Resnick SM, Maki PM, Rapp SR, et al. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. Journal of Clinical Endocrinology & Metabolism. 2006 May;91(5):1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell AJ, Bird V, Rizzo M, Meader N. Diagnostic validity and added value of the Geriatric Depression Scale for depression in primary care: a meta-analysis of GDS30 and GDS15. J Affect Disord. 2010 Sep;125(1-3):10–17. doi: 10.1016/j.jad.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5(1/2):165–173. [Google Scholar]
- 22.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999 Oct;14(10):858–865. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Lyness JM, Noel TK, Cox C, King DA, Conwell Y, Caine ED. Screening for depression in elderly primary care patients. A comparison of the Center for Epidemiologic Studies-Depression Scale and the Geriatric Depression Scale. Arch Intern Med. 1997 Feb 24;157(4):449–454. [PubMed] [Google Scholar]
- 24.D'Agostino RB, Sr., Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008 Feb 12;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 25.Gaziano TA, Young CR, Fitzmaurice G, Atwood S, Gaziano JM. Laboratory-based versus non-laboratory-based method for assessment of cardiovascular disease risk: the NHANES I Follow-up Study cohort. Lancet. 2008 Mar 15;371(9616):923–931. doi: 10.1016/S0140-6736(08)60418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004 Jun;61(6):587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- 27.Bhalla RK, Butters MA, Becker JT, et al. Patterns of mild cognitive impairment after treatment of depression in the elderly. Am J Geriatr Psychiatry. 2009 Apr;17(4):308–316. doi: 10.1097/JGP.0b013e318190b8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JS, Potter GG, Wagner HR, Welsh-Bohmer KA, Steffens DC. Persistent mild cognitive impairment in geriatric depression. Int Psychogeriatr. 2007 Feb;19(1):125–135. doi: 10.1017/S1041610206003607. [DOI] [PubMed] [Google Scholar]
- 29.Potter GG, Wagner HR, Burke JR, Plassman BL, Welsh-Bohmer KA, Steffens DC. Neuropsychological Predictors of Dementia in Late-Life Major Depressive Disorder. Am J Geriatr Psychiatry. 2012 Mar 11; doi: 10.1016/j.jagp.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paterniti S, Verdier-Taillefer MH, Dufouil C, Alperovitch A. Depressive symptoms and cognitive decline in elderly people. Longitudinal study. Br J Psychiatry. 2002 Nov;181:406–410. doi: 10.1192/bjp.181.5.406. [DOI] [PubMed] [Google Scholar]
- 31.Comijs HC, Jonker C, Beekman AT, Deeg DJ. The association between depressive symptoms and cognitive decline in community-dwelling elderly persons. Int J Geriatr Psychiatry. 2001 Apr;16(4):361–367. doi: 10.1002/gps.343. [DOI] [PubMed] [Google Scholar]
- 32.Singh-Manoux A, Akbaraly TN, Marmot M, et al. Persistent depressive symptoms and cognitive function in late midlife: the Whitehall II study. J Clin Psychiatry. 2010 Oct;71(10):1379–1385. doi: 10.4088/JCP.09m05349gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyness JM, Chapman BP, McGriff J, Drayer R, Duberstein PR. One-year outcomes of minor and subsyndromal depression in older primary care patients. Int Psychogeriatr. 2009 Feb;21(1):60–68. doi: 10.1017/S1041610208007746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butters MA, Becker JT, Nebes RD, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000 Dec;157(12):1949–1954. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- 35.Mojtabai R, Olfson M. Cognitive deficits and the course of major depression in a cohort of middle-aged and older community-dwelling adults. J Am Geriatr Soc. 2004 Jul;52(7):1060–1069. doi: 10.1111/j.1532-5415.2004.52302.x. [DOI] [PubMed] [Google Scholar]
- 36.Sneed JR, Culang ME, Keilp JG, Rutherford BR, Devanand DP, Roose SP. Antidepressant medication and executive dysfunction: a deleterious interaction in late-life depression. Am J Geriatr Psychiatry. 2010 Feb;18(2):128–135. doi: 10.1097/JGP.0b013e3181c796d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goveas JS, Hogan PE, Kotchen JM, et al. Depressive symptoms, antidepressant use, and future cognitive health in postmenopausal women: the Women's Health Initiative Memory Study. Int Psychogeriatr. 2012 Aug;24(8):1252–1264. doi: 10.1017/S1041610211002778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenberg PB, Mielke MM, Xue QL, Carlson MC. Depressive symptoms predict incident cognitive impairment in cognitive healthy older women. Am J Geriatr Psychiatry. 2010 Mar;18(3):204–211. doi: 10.1097/JGP.0b013e3181c53487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watari K, Elderkin-Thompson V, Ajilore O, et al. Neuroanatomical correlates of executive functioning in depressed adults with type 2 diabetes. J Clin Exp Neuropsychol. 2008 May;30(4):389–397. doi: 10.1080/13803390701440486. [DOI] [PubMed] [Google Scholar]
- 40.Rapp MA, Rieckmann N, Lessman DA, et al. Persistent depressive symptoms after acute coronary syndrome are associated with compromised white matter integrity in the anterior cingulate: a pilot study. Psychother Psychosom. 2010;79(3):149–155. doi: 10.1159/000286959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000 Oct;57(10):925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 42.Butters MA, Klunk WE, Mathis CA, et al. Imaging Alzheimer pathology in late-life depression with PET and Pittsburgh Compound-B. Alzheimer Dis Assoc Disord. 2008 Jul-Sep;22(3):261–268. doi: 10.1097/WAD.0b013e31816c92bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geda YE, Knopman DS, Mrazek DA, et al. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol. 2006 Mar;63(3):435–440. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- 44.Kumar A, Ajilore O, Kepe V, Barrio JR, Small G. Mood, cognition and in vivo protein imaging: the emerging nexus in clinical neuroscience. Int J Geriatr Psychiatry. 2008 Jun;23(6):555–563. doi: 10.1002/gps.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar A, Kepe V, Barrio JR, et al. Protein binding in patients with late-life depression. Arch Gen Psychiatry. 2011 Nov;68(11):1143–1150. doi: 10.1001/archgenpsychiatry.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, Whitmer RA. Midlife vs Late-Life Depressive Symptoms and Risk of Dementia: Differential Effects for Alzheimer Disease and Vascular Dementia. Arch Gen Psychiatry. 2012 May;69(5):493–498. doi: 10.1001/archgenpsychiatry.2011.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]