Abstract
Background
Within cells, there is a narrow concentration threshold that governs whether reactive oxygen species (ROS) induce toxicity or act as second messengers.
Scope of review
We discuss current understanding of how ROS arise, facilitate cell signaling, cause toxicities and disease related to abnormal cell differentiation and those (primarily) sulfur based pathways that provide nucleophilicity to offset these effects.
Primary conclusions
Cellular redox homeostasis mediates a plethora of cellular pathways that determine life and death events. For example, ROS intersect with GSH based enzyme pathways to influence cell differentiation, a process integral to normal hematopoiesis, but also affecting a number of diverse cell differentiation related human diseases. Recent attempts to manage such pathologies have focused on intervening in some of these pathways, with the consequence that differentiation therapy targeting redox homeostasis has provided a platform for drug discovery and development.
General Significance
The balance between electrophilic oxidative stress and protective biomolecular nucleophiles predisposes the evolution of modern life forms. Imbalances of the two can produce aberrant redox homeostasis with resultant pathologies. Understanding the pathways involved provides opportunities to consider interventional strategies.
Keywords: Diseases of Cellular Differentiation, Glutathione, Glutathione S-transferase, Reactive oxygen species, Redox, Redox active drugs
Oxygen
Earth’s atmosphere presently contains 78% nitrogen and 21% oxygen. Life has evolved within this biosphere such that higher eukaryotes derive much of their energy requirements through oxidative metabolism, to date the most efficient means of generating ATP and sustaining life. During the Precambrian epoch, oxygen was present at trace levels, but at given points in an evolving geology, increased and decreased, reaching a maximum of 35% during the Carboniferous period. Obviously, life has adapted (and presumably continues) to such significant changes in oxygen availability. Indeed, giant insects of the Carboniferous could only exist because of proportionally higher oxygen ratios allowing for greater diffusion rates in a spiracle dominated breathing physiology. Given that oxygen is now an obligate requirement in mammals, paradoxically, it also carries considerable toxicities. Chemical, and for our purposes biological, conversion of oxygen or nitrogen can lead to the production of reactive oxygen species (ROS) or reactive nitrogen species (RNS), families of chemically active molecules that contain free radicals and key contributors to cellular redox state [1]. In general, the fate of a mammalian cell is almost entirely contingent upon intracellular and extracellular levels of ROS/RNS. Co-opted during the evolutionary process, relatively low levels of ROS/RNS may function as signals to promote such activities as cell proliferation and differentiation, whereas high levels more likely lead to apoptosis and cell death. As such, redox pathways are essential in maintaining cellular homeostasis and as a consequence, within these pathways, a great deal of functional redundancy has evolved. An adequate balance between formation and elimination of ROS/RNS is maintained in cells via pro- and anti-oxidant enzymatic pathways. A variety of endogenous factors regulate generation of ROS/RNS and in turn, these contribute to cell physiology by influencing such events as proliferation, differentiation, apoptosis, autophagy and senescence. In virtually every case, subtle threshold effects determine the biological consequences of redox homeostatic pathways. The difference between too much and too little ROS will be subtle and yet determine the fate of many pathways critical to cell survival and proliferation. Such events will be of considerable influence on natural selection and it is apparent that many of the complex pathways that underlie redox homeostasis are evolutionarily well conserved. Therein lies the teleological beauty of sulfur biochemistry, for the variable valence and nucleophilic nature of the element provides much needed biological flexibility. In this review, we explore this dual nature of ROS/RNS and how sulfur and selenium can provide maintenance of a balanced oxidative: reductive environment conducive to an oxygen dependent lifestyle. Partly as a consequence of human adaptations to oxygen, we introduce concepts as to how ROS might influence human pathologies, particularly those linked with differentiation pathways.
Sources of ROS/free radicals
Although molecular oxygen has two unpaired electrons in different orbitals, it is not per se a free radical, which by definition contain a single unpaired electron. The term ROS refers to a number of chemically reactive molecules derived from O2, while RNS are derivative of nitrogen and oxygen, particularly nitric oxide (NO). In general the half-lives of RNS are longer than ROS [2, 3]. Three of the most common and biologically important ROS are O2·− (superoxide anions), H2O2 (hydrogen peroxide) and OH• (hydroxyl radicals) [4]. Of these, the hydroxyl radical is invariably toxic, superoxide is a byproduct of mitochondrial oxidation reactions and hydrogen peroxide has evolved into an important intermediary signaling molecule. A summary of how ROS/RNS might be formed, their associated pathways and cellular effects are shown in Table 1.
Table 1.
Examples of free radicals and their biological relevance.
| Radical | Reaction | Biology/function | Refs. |
|---|---|---|---|
| Superoxide O2·− |
O2 + e− → O2·− | Mainly produced by the reaction of O2 with an escaped electron from mitochondria; also produced by xanthine oxidase, lipoxygenase, cyclooxygenase and NADPH dependent oxygenase. | [3, 6, 7] |
| Hydrogen peroxide H2O2 |
2O2·− + 2H+ → 2H2O2 + O2 | An intermediate detoxification of O2·− by SOD; comparatively low intrinsic toxicity and biological half-life make H2O2 well suited to act as an intracellular signaling molecule; involved in remodeling the structure of cells and activation of transcription factors. | [8–12] |
| Hypochlorous acid HOCl |
H+ + Cl− + H2O2 → HOCl+H2O | Formed by myeloperoxidase reaction of H+, Cl−, and H2O2; can terminate bacterial DNA replication by destroying DNA anchoring at the membrane. | [13] |
| Hydroxyl radical HO· |
HOCl+ O2·− → HO·+O2 + Cl− HOCl+ Fe2+ → HO·+Fe3+ + Cl− HOCl+ Cu+ → HO·+Cu2+ + Cl− H2O2+ Fe2+ → HO·+ Fe3+ + OH− H2O2+ Cu+ → HO·+ Cu2+ + OH− H2O2+ O2·− → HO·+O2 + Cl− |
Produced spontaneously by HOCl with O2·− or metal ions; also produced from H2O2 through Fenton reactions. Because of its high reactivity, short half-life and irreversible modification of macromolecules, HO· has high biological toxicity. | [3] [14] |
| Nitric oxide NO |
L-arginine+O2+NADPH → L-citrulline+NO+NADP++e− | Synthesized enzymatically by NOS; can function as a free radical scavenger as it has a long half-life compared with O2·− and HO·. At normal physiological concentrations, NO is an intracellular messenger for guanylate cyclase and protein kinases. NO conjugates with GSH. | [2, 15–17] |
| Peroxynitrite ONOO− |
NO + O2·− → ONOO− | In cells with high NO cells (e.g. stimulated leukocytes), reaction can be faster than the dismutation of O2·− by SOD, then ONOO− can undergo hemolysis to form HO·. | [2] |
| Nitrogen dioxide NO2 |
ONOO− + H+ → HO·+NO2 2NO+ O2 → 2NO2 |
Increased formation of ONOO− can lead to autohomolysis into HO· and NO2; NO2 can also be produced by direct oxidation of NO by O2. | [2, 18] |
Both exogenous (e.g. pollutants, tobacco, smoke, drugs, xenobiotics or radiation [5]) and endogenous sources contribute to intracellular ROS/RNS levels. As illustrated in Figure 1, primary sites of intracellular ROS include the mitochondrial electron transport chain (ETC), endoplasmic reticulum (ER) and NADPH oxidase (NOX) complex. Significantly, a number of human pathologies are associated with dysfunction within mitochondria or ER. Because approximately 1% to 2% of electrons flow through the ETC (generally within complexes I and III) in mitochondria [19], this organelle is a primary site of superoxide production [20]. ROS is produced in complex I during reverse electron transport, where electrons enter complex I through coenzyme Q binding [21]. Mitochondrial complex III catalyzes the electron transfer from ubiquinol to ferricytochrome c, which is coupled to proton translocation for ATP synthesis [22]. Mitochondrial membrane potentials and enhanced proton-motive forces increase ROS formation [7, 21]. In addition, oxygen concentrations, whether hyperoxic or hypoxic may increase ROS levels [23].
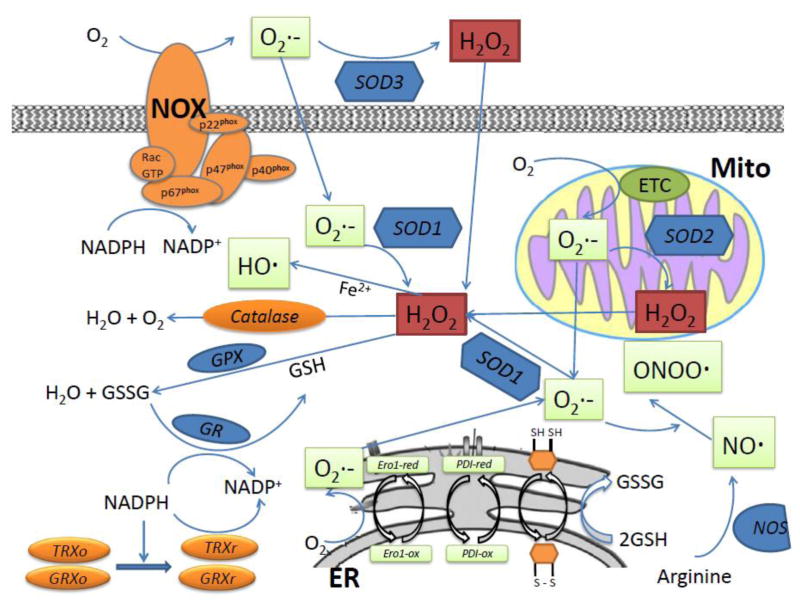
Figure 1.
Cellular ROS, consisting of radical and non-radical oxygen species, are mainly generated in three sites: mitochondrial ETC; ER and NOX complex. Cells have evolved a system to maintain a homeostatic balance between pro- and anti-oxidant processes. Cellular ROS are eliminated by constitutively expressing an arsenal of detoxifying enzymes, including those directly destroying ROS such as SOD, catalase, and GPx. NADPH and GSH are primary contributors to the maintenance of a reduced cellular redox state. In a reduced state, both TRx and GRx reduce disulfides and become oxidized. Oxidized GRx is converted back into a reduced state using GSH, which is regenerated by NADPH-GR, whereas oxidized TRx is converted back into a reduced state using NADPH-TRxR. Nitric oxide synthases are key enzymes for the production and regulation of nitric oxide.
Secondarily, ROS can be produced from ER during oxidative stress. The ER is a well-orchestrated protein-folding machine containing various chaperones and sensors that detect the presence of mis-folded or unfolded proteins. ROS may be generated as byproducts of the protein folding machinery in the ER [24]. Protein disulfide isomerase (PDI) and ER oxidoreductin 1 (Ero1) are two enzymes responsible for regulating oxidative protein folding in the ER. Disulfide bond formation is driven by a protein relay involving Ero1, a conserved FAD-dependent enzyme, which can be oxidized by molecular oxygen and in turn can act as a specific oxidant of PDI, which can then directly oxidize disulfide bonds [25]. Assuming one molecule of ROS is produced per disulfide formed, Ero1-mediated oxidation could account for up to 25% of cellular ROS produced during protein synthesis, a principle drain on cellular energy resources [26]. PDI has enzymatic functions that facilitate correct folding of proteins in the ER, e.g. isomerase activity that catalyzes the rearrangement of incorrectly formed disulfide bonds and oxidase activity that introduces disulfides into proteins [27]. PDI receives electrons and is converted to the reduced form, which then transfers electrons to Ero1 recycling itself. Based on flavin-dependent redox chemistry, ROS are generated when molecular O2 accepts electrons from Ero1 (Figure 1). Consequent to these ER-mediated processes, ROS also arise when glutathione (GSH) reduces unstable and improper disulfide bonds and is thereby depleted [24]. GSH (L-γ-glutamyl-L -cysteinyl glycine) is the most abundant cellular antioxidant preventing thiol groups from oxidation either directly by reducing reactive species or indirectly through catalytic systems such as glutathione peroxidases (GPx) [28]. ER stress can lead to the unfolded protein response (UPR) eliciting calcium leakage from the ER into cytosol, subsequently triggering the production of ROS in mitochondria [29]. Protein folding and refolding are ATP-dependent processes and ATP depletion induced ER stress can stimulate mitochondrial oxidative phosphorylation to increase the generation of ATP, ultimately further forming ROS [24].
The other main source of cellular ROS is NOX, the only enzymes whose primary function is to generate ROS. NADPH oxidases are multi-subunit enzyme complexes highly conserved across phyla with signi cant homology among higher-order mammals. The family presently constitutes 7 members: NOX1-5, Dual oxidase 1 (DUOX1) and DUOX2 [30]. Their function, tissue distribution and sub-cellular localization have been substantially reviewed [31, 32]. The catalytic NOX protein contains FAD and NADPH binding sites, two heme molecules and six transmembrane spanning alpha helices with cytosolic N- and C-termini. The DUOX isoforms also contain the same domains; however, a seventh transmembrane domain and peroxidase homology are present. In addition to the catalytic NOX protein, NOX1-4 complex require a number of other regulatory proteins that are important for enzyme localization, stability and activation, including p22phox, p47phox, p67phox, p40phox and Rac GTPase. Once activated, cytosolic NADPH transfers its electrons to FAD and then sequentially to the two hemes and ultimately to molecular O2, forming the superoxide anion O2·− [33]. The specific cellular and sub-cellular distributions of NADPH oxidases underlie their importance in regulating various functions that require ROS mediated processing, for example cell motility [34].
A wide range of enzymes, including xanthine oxidase, lipoxygenase, cyclooxygenase, NADPH dependent oxygenase, and Nitric oxide synthases (NOS) can also produce ROS/RNS (Table 1, Figure 1), with quantitative contributions contingent upon tissue/cell location.
Cellular antioxidant systems
Excessive or uncontrolled production of ROS can cause damage to nucleic acids, proteins and lipids and this is closely associated with human disease pathogenesis. Here, the salient point is that ROS need not be harmful to normal cellular functions as long as redox homeostasis is iteratively regulated; indeed, ROS/RNS are important signaling messengers for proliferation, differentiation, apoptosis and other critical events during development. Growth in multicellular organisms depends on maintaining the proper balance between cell division and differentiation. Under physiologic conditions, cellular ROS accumulation is controlled by a battery of redundant endogenous antioxidant defense systems, both enzymatic and non-enzymatic, which either prevent or scavenge ROS. Non-enzymatic antioxidants include Vitamins C and E, carotenoids, avonoids; antioxidant enzymes include superoxide dismutase (SOD), catalase, GPx, glutathione S-transferase (GST) and peroxiredoxin (Prx). Low molecular weight cofactor or peptides, such as GSH, thioredoxins (Trx) and NADPH also play an important role in the antioxidant defense [35]. Finally, selenium has a critical role in maintaining antioxidant defenses. In terms of its biological functions, selenium is a fascinating element. In mammals there is a relatively narrow window of selenium homeostasis. Too much dietary selenium causes serious toxicities and death. Too little interferes with the synthesis of the approximately 25 proteins that use selenocysteine. Selenium has a role in maintaining normal immune responses and has preventative roles in cancer prevention. Other indications are that imbalance impacts male fertility, cardiovascular disease mortality and regulation of inflammatory mediators in asthma [36].
As the 21st amino acid, there is a major outlay of energy in maintaining the complex selenocysteine-insertion machinery. As such there must be a strong biological rational to maintain selenocysteine in enzymes. It has been argued that this chemicobiological function is the ability of selenoenzymes to resist inactivation by irreversible oxidation, a property not evident in sulfur. Selenocysteine can confer resistance to oxidation through the superior ability of the oxidized form of selenocysteine (Sec-SeO2-, seleninic acid) to be recycled back to its parent form (Sec-SeH, selenocysteine) in comparison to the same cycling of cysteine-sulfinic acid to cysteine (Cys-SO2- to Cys-SH) [37].
Superoxide dismutase and catalases
As a first line of defense against superoxide free radicals, SOD catalyzes the dismutation of O2·− to H2O2. There are three metal containing isoforms of SOD found in different cellular compartments [38]: SOD1 (copper-zinc SOD) is in the cytoplasm, nucleus and plasma membrane; SOD2 (manganese SOD) is mainly in mitochondria [5]; and SOD3 (copper-zinc SOD) is unique in scavenging superoxide in the extracellular compartment, dismutating superoxides generated during the inflammatory cascade [39]. Bone marrow-derived human mesenchymal stem cells (MSC) specifically secrete SOD3 directly promoting cell survival by reducing toxic ROS [40]. MSC differentiation through the adipogenic, chondrogenic, and osteogenic lineages influences the expression of SOD3 [39]. SOD operates in conjunction with catalase to maintain hydrogen peroxide levels resulting from SOD catalysis. Catalase is abundant in the mammalian tissues, particularly in red blood cells, and overexpression of human catalase targeted to the mitochondria has been shown to be both cardioprotective and neuroprotective and to extend lifespan in transgenic mice [41–43]. In mammals, SOD and catalase always co-exist and their concerted catalytic activities may tighten the control on ROS signaling providing tumor cells with growth advantages [44]. This might include optimal protection against ROS-mediated apoptosis and catalytic dismutation of superoxide anions to hydrogen peroxide providing a central autocrine proliferation stimulus [45]. In addition to catalase, there are other two thiol-based systems to catalyze H2O2 reduction, GPx (with GSH) and Prx (with Trx). For either system, NADPH acts as the hydrogen anion donor helps to recycle oxidized GSH and Trx through glutathione reductase (GR) and thioredoxin reductase (TrxR) respectively [46].
Glutathione peroxidases and GSH
Historically, GPx (the first 4 discovered GPx1-4) were defined by their capacity to catalyze the reduction of H2O2 through selenocysteine (SeCys) at the catalytic site [47]. Similar to the NOX families, their antioxidant functions are contingent upon their subcellular localization. GPx1 is present in the cytosol and mitochondria in most mammalian cells and is more effective than catalase at removing intracellular peroxides under many physiological conditions [48]. Besides the SeCys-containing GPx, non-selenium GPx are widely represented in mammals as well as most vertebrates and some lower phyla [49]. GPx5, 7 and 8 are cysteine-containing GPx, while GPx6 has a selenocysteine active site in humans but a cysteine in rodents and other species. For the reasons discussed above, cysteine-containing GPxs are much less efficient at detoxifying peroxides than seleno-dependent GPx [47]. The weak affinity of non-selenium GPx for GSH has led many authors to reconsider their reducing cofactor. For example, in P. falciparum Trx is the principle reducing cofactor. Since more than 700 cysteine containing GPx homologues have been described, the term glutathione peroxidase actually only describes a small proportion of GPx, leading to a re-classification of non-selenium GPx as thioredoxin peroxidases [50].
Peroxiredoxins and thioredoxins
Prx are ubiquitous peroxidases that use a conserved Cys residue to reduce peroxide substrates. Peroxides oxidize the peroxidatic Cys–SH to Cys–SOH, which then reacts with another cysteine residue to form a disulfide that is subsequently reduced by an appropriate electron donor [51]. The first Prx, described in 1968, was called “torin” [19], subsequently changed to thiol-specific antioxidant and then to thioredoxin peroxidase [52]. Mammalian 1-Cys peroxidase uses GSH rather than Trx as a reductive electron source [53]. More recently, additional substrates such as lipid hydroperoxides and peroxynitrites have been identified for this family of proteins [54]. Peroxiredoxin was selected as a name to more appropriately represent the family of peroxidases in which cysteine is the primary site of oxidation during the reduction of peroxides [51].
Trx is a multifunctional selenoprotein containing two redox-active cysteines and a conserved CXXC active site motif (Cys-Gly-Pro-Cys) [55], and through its redox-active cysteine residues the dithiol Trx contributes to maintain a reduced environment. The dithiol moieties of Trx are reduced by receiving electrons from NADPH through TrxR. Trx serve to reduce oxidized proteins through intermediate disulfide bond formation, transferring electrons from its reactive cysteines through thiol-disulfide exchange reactions. Three mammalian Trx have been described: Trx1 (cytosolic), Trx2 (mitochondrial), and Trx3, a Trx variant localizes in spermatozoa [55, 56].
Glutaredoxins (Grx) also contain the conserved CXXC active site motif and function to reduce protein disulfides. Grx are oxidized by protein disulfide substrates, and reduced non-enzymatically by glutathione. Both Grx and Trx have distinct and overlapping functions [56]. Compared to Trx, Grx not only catalyze the reversible reduction of disulfides by the dithiol mechanism (requiring both active site thiol groups in Grx or Trx), but can also uniquely catalyze this reaction through the monothiol mechanism, originally proposed in 1978 [57], requiring only the N-terminal cysteinyl residue of the active site. Three Grx (Grx1-3) reduce protein disulfides by GSH/GPx system using the cofactor NADPH [58].
Glutathione S-transferases and S-glutathionylation
Glutathione S-transferases catalyze the formation of thioether conjugates of a number of small molecule agents with GSH. In mammals there are six different cytosolic GST isoforms: alpha, mu, pi, theta, omega and zeta. In general, < 10% of the primary sequence is conserved, but all GST isozymes have similar topologies and two domains responsible for protein folding. The glutathione S-transferase pi (GSTP) isozyme is expressed at high levels in many cancers and increased expression has been linked to acquired resistance to cancer drugs [59]. GSTP has four allelic variants, GSTP*A-D and there is evidence that individual expression patterns influence response to oxidative stress [60], implying that there may be pharmacogenetic differences in human populations.
More recently investigations have suggested that GSTP has a diversity of functions in cancer cells, some of which are unrelated to its capacity to detoxify chemicals or drugs. For example, GSTP1 plays a role in controlling stress response, apoptosis and proliferation through direct binding to c-Jun NH2-terminal kinase (JNK), a stress activated protein kinase implicated in pro-apoptotic signaling. In non-stressed cells, low JNK activity is maintained due to sequestration of the kinase in a GSTP1-JNK complex [61]. Under ROS, GSTP1 dissociates from the complex and elicits subsequent induction of apoptosis [62]. In this sense, over-expression of GSTP1 may well-serve the purpose by strengthening sequestration of JNK, and facilitates tumor progression when the ROS or therapeutic drug is not a substrate for GSH conjugation, explaining from another angle how increased levels of GSTP1 in tumor cells might contribute to drug resistance.
ROS/RNS cause reduced cysteine residues (-SH) that have a low pKa to become oxidized into protein sulfenic acids (P-SOH), nitrosylated (P-SNO) and eventually S-glutathionylated (P-SSG). S-glutathionylation is a regulated post-translational modification where GSH is conjugated to cysteine in redox-sensitive proteins. The modification leads to structural and functional changes in proteins, primarily because the host protein is increased in molecular mass and picks up a net negative charge from the addition of the tripeptide. Regulation through S-glutathionylation has been ascribed to a large number of proteins that fall into a number of functional clusters and a variety of recent reviews have addressed the importance of these [63, 64]. While the proximal donor for the S-glutathionylation reaction is not always defined, it is tacitly assumed that high levels of GSH would be consistent with increased levels of S-glutathionylation. The forward reaction can occur spontaneously or through GST catalysis, but the kinetics and magnitude of protein S-glutathionylation are greatly enhanced by the presence of GSTP. Cells expressing mutants of GSTP that lack enzyme activity have diminished levels of S-glutathionylation in response to ROS [65]. In terms of a specific target, GSTP has also been shown to play a necessary role in the S-glutathionylation of 1-cys Prx. Oxidation of the catalytic cysteine of 1-cys Prx has been associated with its loss of peroxidase activity; however heterodimerization of 1-cys Prx with GSTP mediates the S-glutathionylation of the previously oxidized cysteine thus restoring its peroxidase activity [66]. Such a reaction is critical to maintain a defense against ROS and also influences susceptibility to various pathologies. Of relevance, S-glutathionylation of viral peptides is important in presentation of the antigens to the immune system and to altering T cell recognition [67]. Moreover, S-glutathionylation of the Slingshot-1L-binding protein regulates its degradation and contributes to the migration of monocytes [68]. These two recent observations add to the growing body of evidence that this post-translational modification is a critical intermediate in immune system and bone marrow physiology.
Human Pathologies influenced by ROS and differentiation pathways
There is a growing body of literature supporting crucial roles for ROS in the pathogenesis of many diseases, including those related to cell differentiation (e.g. cancer due to loss of differentiation, bone loss-associated disorders due to osteoclast differentiation or type 2 diabetes due to beta-cell dedifferentiation) (Table 2). Accumulation of ROS together with depletion of reducing molecules shifts the cellular redox environment to a more oxidized state. ROS-mediated redox regulation may be direct or indirect, the latter occurring through redox modifications of proteins mainly at cysteine residues, e.g. from reduced-SH to disulfide, sulfenic acid or S-glutathionylation form. Table 2 lists some of the diseases linked with abnormal cell differentiation. Regulation of cell differentiation by ROS through redox-sensitive signaling pathways and transcription factors is covered. Details of some of the diseases are discussed in the accompanying text.
Table 2.
Role of ROS in diseases of cellular differentiation
| Disease | Pathology related to differentiation | Potential differentiation therapy | Regulation of cell differentiation by ROS | Remarks |
|---|---|---|---|---|
| Acute myeloid leukemia | Loss of granulocyte or monocyte differentiation | Inducing leukemic cell differentiation |
|
Despite many encouraging results, the only successful clinical application of differentiation therapy is all-trans retinoic acid for acute promyelocytic leukemia. Differentiation therapy targeting redox homeostasis is likely to provide opportunities for improved pharmacological intervention. |
| Neuroblastoma | Neuroblastoma differentiation block | Inducing neuroblastoma differentiation |
|
In neuroblastoma, hypoxia and ROS have been linked with either progression or spontaneous regression. A better understanding of the opposing mechanisms would likely be informative. |
| Atherosclerosis | Foam cell differentiation | Inhibiting monocyte-to- macrophage/foam cell differentiation |
|
Studies aimed at identifying the intracellular targets of ROS involved in redox signaling in macrophages and at elucidating the redox signaling mechanisms that control differentiation, may lead to the development of novel preventive and therapeutic strategies for atherosclerosis. |
| Bone loss-associated disorders (e.g. osteoporosis, rheumatoid arthritis, Paget’s disease, periodontal disease, osteosarcoma and cancer bone metastasis) | Osteoclast differentiation | Inhibiting osteoclast differentiation |
|
Pharmaceutical inhibition of osteoclast differentiation is one therapeutic strategy to mitigate the extent of bone loss-associated disorders. Several chemical-, natural product-, and biological- based inhibitors are now in clinical trials [84]. |
Degenerative fibrotic disease:
|
Myofibroblast differentiation | Inhibiting or reversing fibroblast-to-myofibroblast differentiation |
|
Despite the considerable morbidity and mortality, there are currently no effective treatments and no approved antifibrotic therapies. Redox signaling plays a fundamental and integral role in the molecular pathogenesis of fibrosis and represents a potential therapeutic target for the treatment of different fibrotic disorders. |
Neurological disorders:
|
Loss of neurons and glial cells | Transplantation of stem cell- differentiated neuroblasts or inducing endogenous neurogenesis [87–90] |
|
A detailed understanding of the redox regulation of neurogenesis is critical for guiding efforts to restore function and ameliorate the devastating effects of acute cerebral injury and chronic neurodegenerative disease. |
| Obesity | Adipocyte differentiation | Inhibiting adipocyte differentiation |
|
More studies are needed to understand the precise roles of ROS and redox signaling mechanisms in the pathogenesis of obesity and related metabolic disorders. |
| Type 2 diabetes | Beta-cell dedifferentiation | Restoring beta-cell differentiation |
|
The exact mechanisms of beta-cell dedifferentiation remain to be worked out, which may help to develop a new class of type 2 diabetes therapies based on the prevention or reversal of beta-cell dedifferentiation. |
Only selected diseases are presented to best serve the focus of this chapter. Details of some of the diseases are discussed in the text.
Obesity
Obesity is a complex disorder involving an excessive accumulation of adipose tissue which can occur through an increase in adipocyte size (hypertrophy) and/or number (hyperplasia). It results in dysregulation of glucose and lipid metabolism, as a consequence of increased production of proinflammatory adipocytokines, destroys energy balance and increases risk factors for type 2 diabetes, hypertension and hyperlipidemia, leading causes of morbidity and mortality [105, 106]. Understanding adipogenesis (adipocyte differentiation) is an important forerunner to gain insight into the metabolic pathologies associated with obesity. In addition to adipocytes, adipose tissue is composed of MSC, T regulatory cells, endothelial precursor cells, macrophages and preadipocytes in various stages of development [107]. MSC have the capacity to commit to preadipocytes and these can further differentiate into mature adipocyte, conferring a constant functional plasticity, which contributes to the tissue’s ability to expand throughout the entire lifespan [108, 109]. Adipocyte differentiation is a tightly controlled process that includes the coordination of a complex network of transcription factors (e.g. proliferator-activated receptor gamma (PPARγ), and CCAAT/enhancer binding proteins (C/EBP)), cofactors (e.g. PPAR-binding protein (TRAP220), cyclic AMP response element-binding proteins (CREB) and histone deacetylases (HDAC)), and signaling intermediates (e.g. insulin/insulin-like growth factor-1 (IGF-1), Wnt proteins, and transforming growth factor beta (TGFβ) from numerous pathways) [109, 110].
Several studies have suggested that obesity can be both cause and effect linked with systemic oxidative stress and ROS production, at the same time promoting fat tissue development both in adipocyte culture and in vivo [111]. Intracellular ROS derived from NOX, mitochondria and ER stress promotes adipocyte differentiation [98]. Moreover, it has been shown that adipogenesis is sensitive to the extracellular redox environment, enhanced under oxidizing conditions and inhibited under reducing conditions [112]. NOX4 is a major source of ROS in preadipocytes [113] and is up-regulated systemically in obesity [114]. Down-regulation of NOX4 inhibits ROS production and adipogenic differentiation in both 10T1/2 MSC and 3T3-L1 preadipocytes [115, 116], through transcriptional activation of CREB [116], up-regulation of mitogen-activated protein kinase phosphatase-1 (MKP-1) [98], or oxidative inhibition of protein-tyrosine phosphatase PTP1B [117]. NOX4 over-expression yields the opposite effect [115]. In obese mice, treatment with NOX inhibitors reduces ROS production in adipose tissue, attenuates the dysregulation of adipocytokines and improves symptoms associated with diabetes, hyperlipidemia and hepatic steatosis [100]. These results imply that NOX4 may promote the adipogenic process; however, its role in vivo has yet to be fully defined. For example, Li et al. showed that NOX4 deficiency accelerated the development of obesity and insulin resistance in mice on a high-fat diet [118].
The role of mitochondria or ER stress-generated ROS in adipocyte differentiation is not fully understood. Adipocyte differentiation is characterized by an increase in mitochondrial metabolism [119], but whether this is essential for differentiation or a byproduct of the differentiation process remains to be seen. In the early phases of adipocyte differentiation in human MSC, there was an increase in mitochondrial metabolism and ROS generation and that mitochondrial ROS (mtROS) production was required to initiate adipocyte differentiation [120]. Moreover, mtROS scavengers significantly reduced adipocyte differentiation [121]. Several studies have demonstrated that obesity may induce ER stress and activate the unfolded protein response and this in turn can lead to suppression of insulin signaling and inflammation in adipocytes [122–124]. However, relatively little is known about the precise role of ER stress induced oxidative stress in adipogenesis.
Regardless of the specific sources of intracellular ROS production, the close relationship between ROS and adipogenesis has been confirmed by a number of recent studies. In general, a more oxidized intracellular environment favors differentiation of progenitor or stem cells into mature adipocytes. Lee et al. demonstrated that H2O2 accelerated hormone-induced adipogenic differentiation in 3T3-L1 preadipocytes by accelerating mitotic clonal expansion. This effect was explained through the positive regulation of key transcription factors C/EBPβ and PPARγ, which are able to promote the expression of genes involved in adipocyte differentiation [101]. Meanwhile antioxidants such as genistein, resveratrol, and N-acetylcysteine (NAC) suppressed ROS levels and inhibited C/EBPβ and PPARγ expression, adipocyte differentiation and fat accumulation in 3T3-L1 preadipocytes [101, 102]. Intriguingly, there is evidence that the redox status of specific cysteine residues may affect the activities of C/EBPβ and PPARγ. Oxidation-induced dimerization of doubly phosphorylated C/EBPβ/liver activating protein increases DNA binding activity of C/EBPβ, whereas mutation of the C-terminal Cys(296), a residue adjacent to the leucine zipper and Cys(143), just upstream of the DNA binding domain, eliminated phosphorylation-, oxidation-, and dimerization-dependent DNA binding activities [96]. Nitro-fatty acids, which are endogenous products of nitric oxide and nitrite-mediated redox reactions, may increase PPARγ activity by covalent modification of redox sensitive Cys-285 [97]. The link with oxidative stress is further strengthened by recent data that demonstrate that Nrf2 and Nrf2-targeted antioxidant genes (e.g. NQO1, GPx and GCLM) also play critical roles in the biology of adipose tissue [99]. Collectively, these results suggest that working to understand the redox state in adipose tissue could lead to a variety of possible treatments for obesity and obesity-associated metabolic syndromes.
Neuroblastoma
Neuroblastoma is the most common extracranial solid malignancy in childhood and the most common cancer in infants [125, 126]. It is an embryonal tumor originating from neural crest-derived precursors of the sympathetic nervous system and shows remarkable biological and clinical heterogeneity with immature cells forming more aggressive tumors. Approximately 50% of patients have poorly differentiated metastatic tumors (stroma poor neuroblastoma) at diagnosis correlated with poor prognoses; whereas the remaining cases show localized presentation with more differentiated tumors (ganglioneuroblastoma or ganglioneuroma) associated with more favorable prognosis [75]. Although improved overall 5-year survival of patients with neuroblastoma has been noted over the past decade, survival rates among children with high-risk neuroblastoma remains below 50% despite aggressive chemotherapy [127]. Therefore, new therapeutic strategies are still needed to improve not only the cure rate but also the patient quality of life. Unlike most other cancers, neuroblastoma is characterized by a unique capacity for spontaneous regression, a phenomenon reflecting the activation of apoptotic and/or differentiation programs, and is therefore regarded as a cancer susceptible to cell differentiation blockades [128]. Several signaling pathways have been shown to play a role in inducing neuroblastoma differentiation, including nerve growth factor/tyrosine kinase receptor-A (TrkA), glial cell line-derived neurotrophic factor/RET receptor, protein kinase C/Ras/ERK, Fyn kinase, and tissue transglutaminase (TG2). Conversely, N-Myc can repress neuroblastoma differentiation partly through recruiting HDAC1 and thereby blocking TrkA and TG2 expression [128, 129].
The concept that redox status may play an important role in neurogenesis has gained traction in recent years [91]. During embryonic, perinatal or adult neurogenensis, changes in the levels of oxygen and ROS appear as crucial signaling factors for the generation of new neurons. Neural stem cells (NSC) continually generate new neurons in highly specialized microenvironments (niches) which tightly regulate NSC quiescence, self-renewal and differentiation [130]. Oxygen concentration is one of the more crucial environmental conditions that regulate cell proliferation and differentiation both in vitro and in vivo. In the central nervous system, physiological concentrations of oxygen range from 0.55 to 8%. In NSC niches, the oxygen concentration is estimated to be 2.5 to 3% [131]. Dynamic control of O2 availability may be a component of the in vivo NSC niches. Conditions of lower O2 tensions stimulate proliferation and self-renewal, whereas increasing O2 tension promotes differentiation or apoptosis of NSC and progenitors [132]. There is evidence that hypoxia-inducible factors (HIF) play a role in both NSC proliferation and differentiation [130, 132]. Proliferative, self-renewing NSC maintain a high ROS status which appears to be required to maintain their self-renewal and neurogenesis perhaps through maintaining adequate levels of PI3K signaling [133]. ROS generation could be closely related to alterations in O2 levels and a growing body of evidence highlights ROS as key factors in neuronal differentiation. Indeed, it was shown that endogenously produced ROS activate PI3K/Akt, p38 and ERK signaling during neuronal differentiation [93, 133]. Intracellular ROS derived from NOX promoted neuronal differentiation. Conversely, inhibition of endogenous ROS production by NOX inhibition or mutation negatively regulated NSC differentiation [133]. Within this model, it was proposed that mitochondrial ROS production at complex III is especially important during hypoxia [130].
The role of oxygen and ROS in neuronal differentiation is also crucial for neuroblastoma development [130]. Solid tumors often contain regions with significant oxygen deficiency or hypoxia and tumor hypoxia generally predicts for poor clinical outcomes. Hypoxic tumor cells appear to be highly tumorigenic and poorly differentiated, with expression of multiple stem cell markers [134, 135]. Hypoxic conditions of 1% O2 in neuroblastoma cells can lead to dedifferentiation both in human neuroblastoma cell culture and in vivo. Hypoxia decreased the expression of neuronal marker genes but induced genes expressed in immature neural crest sympathetic progenitors, partly through activation of Notch signaling in a HIF1α/2α dependent way, resulting in malignant progression (higher capacity of invasion and metastasis) of tumors [74, 136]. Conversely, knockdown of HIF2α induced partial sympathetic neuronal differentiation, indicating that HIF2α contributes to maintain an undifferentiated state in neuroblastomas [137]. Additionally, hypoxia decreases the tumor sensitivity to chemotherapy [138]. These observations reveal a strong correlation between hypoxia pathways and cancer stem cell characteristics and emphasize the need to maintain a tight balance between hypoxia and ROS. Hypoxia stimulates ROS generation, at the same time ROS participate in the activation of homeostatic responses to hypoxia [130]. Collectively, these results suggest that targeting hypoxia and ROS production of neuroblastoma cells can have positive therapeutic impact.
Bone Marrow Niches and Myelodysplastic Syndromes/Acute myeloid leukemia
Loss of differentiation is an important component in the pathogenesis of many cancers. One of the most salient examples is acute myeloid leukemia (AML), an aggressive, age dependent hematopoietic malignancy characterized by an accumulation of granulocyte or monocyte precursors in the bone marrow and blood [72, 139]. Myelodysplastic syndromes (MDS) can be considered representative premalignant hematopoietic disorders that can transform to AML [140]. Even after standard chemotherapy and stem cell transplantation, patients with AML inevitably experience relapse, most of which occur in patients with poor prognosis indices. Current therapeutic strategies appear to have reached the limit of their effectiveness; thus, novel strategies are needed to target leukemic cells exclusively [72]. Targeted therapy in AML was initially proposed in the late twentieth century by trying to force malignant cells to differentiate. Many cancer subtypes displayed alterations in the normal program of differentiation and growth; thus, the use of specific agents that might trigger differentiation of leukemic cells along normal hematopoietic lineages seemed a logical approach [141, 142]. All-trans retinoic acid (ATRA, also known as Tretinoin) was the first clinically useful cyto-differentiating agent employed in the treatment of acute promyelocytic leukemia (APL, a unique subtype of AML) [143]. Besides retinoids, several other agents (e.g. adenanthin [144, 145], antiocidin [146] and shikonin [147]) were found to inhibit proliferation and induce terminal differentiation of leukemic cells. Moreover, there is increasing evidence that redox therapies exploiting the role of ROS in signaling pathways and targeting redox homeostasis and regulation have become an attractive field [72]. For example, the mechanisms of several differentiation-inducing agents actually involve modulation of the intracellular redox homeostasis (e.g. targeting Prx I and Prx II thus increasing ROS production by adenanthin [144], and regulating Nrf2/ARE pathway by shikonin [147]), thus facilitating differentiation.
ROS have been implicated as chemical mediators in normal hematopoiesis. NOX and mitochondria are the major ROS-generating enzymes in bone marrow-derived hematopoetic stem cells (HSC) [148–151]. Those pathways behind HSC intracellular ROS regulation are currently the subject of ongoing investigations. The majority of the evidence suggests that the PTEN/PI3K/Akt pathway via the forkhead box-O (FoxO) family of transcription factors plays a crucial role in maintaining long-term regenerative HSC pools. FoxO protects quiescent HSC from oxidative stress either through the up-regulation of ROS detoxifying genes or deregulation of the H2O2-p38MAPK pathway [152, 153]. Studies in the 1950s identified the importance of cysteines and thiols in bone marrow cell proliferation [154]. The bone marrow produces all the differentiated hematopoietic cells for peripheral blood. Long-term, self-renewing HSC have low levels of intracellular ROS. However, high ROS, which can occur during chemotherapy, may result in senescence. Specifically, elevated ROS appears to drive HSC out of quiescence and reduces self-renewal capacity, resulting in rapid bone marrow failure. Within the bone marrow environment, HSC may reside either at the bone-bone marrow interface (osteoblastic niche), where the microenvironment encourages quiescence or close to the blood vessels (vascular niche), where the microenvironment favors proliferation and/or differentiation [155–157] (Figure 2). Each population is defined by the expression of adhesive molecules, cytokines and chemokine signaling. Chemokine interactions can govern cellular niche behavior. For example, CXCL12 regulates HSC migration to the vascular niche while depletion of CXCR4 depletes HSC in the vascular niche [158]. Calcium gradients also influence HSC migration [159]. As a tissue, bone marrow is relatively hypoxic (1% to 2% O2) [160] and oxygen levels are higher closer to the vascular niche, presenting an oxygen gradient. The hypoxic osteoblastic environment encourages quiescence in HSC and movement to the more oxygenated vascular niche promotes HSC differentiation, mobilizing myeloid and lymphoid hematopoietic cells to the peripheral blood [161]. The possibility that a redox gradient may influence HSC migration and differentiation does not seem unreasonable. Older mice show accumulation of HSCs more distantly in the endosteum as well as increased levels of DNA damage in their HSCs [162], perhaps reflecting the accumulation of oxidative stress associated with the aging process. The distinct features of niche microenvironment and HSC sub-populations may present opportunities for the development of targeted therapeutics.
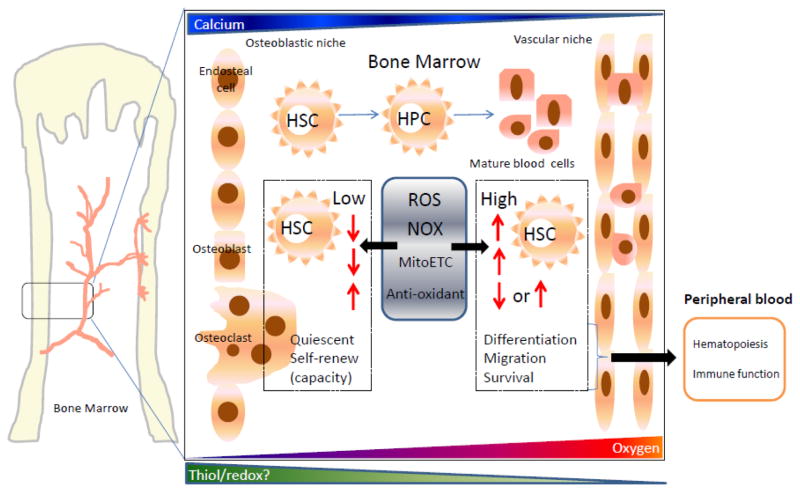
Figure 2.
Bone marrow niche microenvironments. Various gradients help to define the osteoblastic and vascular niches of the marrow compartment. Movement between one niche and another can be facilitated by microenvironment gradients of calcium, oxygen and potentially thiol/redox state. Hematopoietic stem and progenitor cells traverse this space and mature towards the variety of blood cells that present the circulating blood lineages. Two types of HSC exist. Depicted are low and high ROS populations. As ROS formation requires oxygen, ROS levels in HSC or HPC correspond with oxygen availability. For HSC, when cells are quiescent, both ROS levels and NOX enzyme expression are low. During differentiation and migration of HSC, high ROS levels occur and act as a signaling response to promote cell migration and differentiation, ultimately contributing to maintaining hematopoiesis and immune function.
Excessive ROS production or oxidative DNA damage is frequently observed in hematopoietic malignancy [69–71]. Human HSC express multiple isoforms of NADPH oxidases, NOX1, NOX2 and NOX4. These have the capacity to produce constitutive ROS, which can serve as redox messengers that control HSC proliferation and differentiation [150]. Recent studies reported that NOX1, 2 and 4 isoforms are expressed in MDS/AML cell lines and MDS samples. Interestingly, NOX4 interacts with ERK and Akt1 within the nuclear speckle domain and contributes to nuclear ROS production, implying pathophysiological effects in MDS through modulation of nuclear signaling and DNA damage [34]. NOX-derived ROS are strongly elevated (> 10-fold) in more than 60% of AML patients and strongly promote both AML proliferation and survival. High ROS AML cells show depleted antioxidant defenses but evade the oxidative stress response through suppression of p38MAPK signaling [163]. In fact, the combination of a NOX inhibitor, histamine dihydrochloride, with the T-cell-derived cytokine IL-2 has been approved as remission maintenance immunotherapy in AML [164]. Constitutive activation of the serine/threonine protein kinase Akt followed by FoxO inhibition and increased ROS levels are critical factors in the survival of AML blast cells [165, 166]. ROS production induced by growth factors or cytokines (e.g. G-CSF or IL-3) can protect leukemic cells from apoptosis [72]. Taken together, leukemic cells may develop a degree of “ROS addiction” to promote their survival and targeting ROS to suppress the high levels of ROS may be of clinical benefit in treatment of AML. Another approach to target ROS might involve amplification of existing ROS stress or inhibition of intracellular antioxidants (possibly augmented in response to increased oxidative stress). Such treatments could cause catastrophic chemical damage only in malignant cells where oxidative stress preexists [73]. Indeed, several compounds with pro-oxidant capacity appear to be effective against AML. For example, arsenic trioxide is currently approved for treatment of relapsed APL and the mechanism of ROS generation by arsenic trioxide can involve TrxR inhibition [167], augmentation of mitochondrial ROS [168] or NOX activation [169].
Anticancer drug development using the platforms of GSH, GSTP and pathways that maintain thiol homeostasis have produced a number of leads [170]. TLK 199 (Telintra®), as a GSH peptidomimetic inhibitor of GSTP, stimulates myeloproliferation in both rodents [171] and man [172, 173] and has shown positive results (decreased requirements for red blood cell, platelet and growth factor support) in ongoing Phase 2 clinical trials for MDS patients [172, 173]. Even in the absence of Telintra, GSTP-null animals have increased HSC differentiation and proliferation [174, 175] and the molecular mechanism(s) may involve deregulation of JNK and JAK/STAT pathways [174] and thiol homeostasis (e.g. protein S-glutathionylation responses, free protein thiols, GSH and GSSG levels) [175]. Meanwhile, we demonstrated for the first time that Matrix-Assisted Laser Desorption/Ionization-Mass Spectrometry (MALDI-MS) bone imaging can be used for the simultaneous in situ visualization of both GSH and GSSG in sectioned bones with an intact bone marrow compartment (Figure 3) [175]. MALDI-MS imaging is a powerful label-free technique mapping the distribution of a large range of biomolecules in tissue, including proteins, lipids, and drug metabolites, it has been used to generate 2-D or 3-D molecular maps directly from the tissue surface, allowing the display of the relative quantification and distribution of individual molecular moieties in tissues [176–179]. Basically, MALDI can record the spatial distribution of high mass molecules using the chemically specific molecular ions with typical spatial resolutions 25 μm or more. More detailed information has been extensively reviewed by McDonnell et al [180]. Refinements of this approach may be helpful in mapping those molecule gradients that contribute to the maintenance of myeloproliferative pathways. Collectively, these results suggest that redox-based therapies provide a clinical opportunity in the treatment of AML patients.
Figure 3.
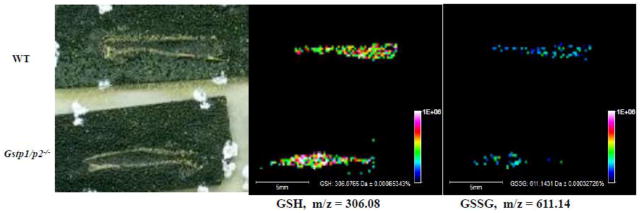
Representative MALDI-MS images of sectioned femurs showing niche distribution in bone marrow of GSH and GSSG in WT and Gstp1/p2−/− mice. From left to right: scanned image of matrix sprayed MALDI slide of mouse femur with bone marrow; corresponding images of: GSH ions at m/z = 306.08; GSSG ions at m/z = 611.14. Color heat map of the data points in the GSH and GSSG images represent averaged individual ion signal intensities of the spots (taken from [175]).
Redox active Drugs used for differentiation/redox therapy
Despite many encouraging results obtained both in vivo and in vitro, the only existing successful clinical application of differentiation therapy is ATRA for acute promyelocytic leukemia. ATRA is the most important active metabolite of vitamin A controlling segmentation in the developing organism and the homeostasis of various tissues in the adult. ATRA as well as natural and synthetic derivatives, collectively known as retinoic acid, plays an important role in mediating the growth and differentiation of both normal and transformed cells [181]. It has been reported that retinoid neural differentiation is via increasing intracellular ROS production and decreasing thiol levels in the cell. ROS scavengers and flavoprotein inhibitors reduced the retinoid induced differentiation and expression of N-Cadherin and the β-III tubulin subunit [182]. A growing body of evidence suggests that ROS generation and signaling are involved in cellular differentiation in many diseases. Differentiation therapy targeting redox homeostasis may provide opportunities for improved pharmacological intervention. Table 3 lists some of the clinical and preclinical redox active agents used for differentiation-based therapies through targeting redox/signaling pathways. While this list is not designed to be complete, it does serve to exemplify some of the issues where redox biology has provided a platform for drug discovery and development.
Table 3.
Redox active drugs used for differentiation diseases therapy.
| Drugs | Differentiation Diseases | Redox Target and/or mechanism | refs |
|---|---|---|---|
| Adenanthin | AML | Directly targets the conserved resolving cysteines of PrxI/II, and inhibits their peroxidase activities. Consequently, cellular H2O2 is increased and causes the activation of ERK1/2, inducing expression of C/EBPβ, which has been widely shown to drive AML cell differentiation. | [144, 145] [183] |
| Amifostine | AML MDS Neuroblastoma |
Thiol-phosphate pro-drug subject to activation by blood and tissue alkaline phosphatase to the thiol WR-1065, which may function mechanistically through repair of DNA damage induced by ROS, induction of cellular hypoxia, inhibition of apoptosis and direct covalent binding of cytotoxic drugs. | [184–188] |
| As2O3 | AML | Thiol-reactive pro-oxidant; redox chemotherapeutic agent. The anti- proliferative and apoptogenic effects are linked with the pro-oxidant effects through covalent cross-linking of vicinal thiols; | [167, 189–192] |
| Lung fibrosis | Block TGFβ-induced H2O2 and NOX4 mRNA expression. | [193] | |
| BHA | Neuroblastoma | Antioxidant, acting directly as free radical scavengers, and directly reduces the level of ROS. | [194–196] |
| Edaravone | Acute brain ischemia Parkinson’s | Acts as a potent antioxidant and strongly scavenges free radicals, protecting against oxidative stress and neuronal apoptosis. | [197, 198] |
| Nox inhibitors: e.g. | Bone loss-associated disorders | Nox inhibitor. Inhibition of NOX expression and activity is critical for prevention of chronic EtOH-induced osteoblast-dependent bone loss; | [199] |
| Diphenylene iodonium | Atherosclerosis | Inhibition of NOX2 and/or NOX1 contributes to the prevention and treatment of atherosclerosis. | [200] |
| Degenerative fibrotic disease Neurological disorders |
NOX involved in fibroblast/myofibroblast transition and death of epithelial cells; NOX inhibitors block TGFβ-induced H2O2 and NOX mRNA; | [201–205] | |
| NAC | Obesity | Antioxidant and cysteine precursor. Enhances GSH synthesis. Suppresses | [101, 102] |
| Neuroblastoma | C/EBPβ and PPARγ expression, which inhibit adipocyte differentiation. | [206] | |
| Bone loss-associated disorders | Suppression of ROS in response to Nox enzymes triggers down-regulation of the essential osteoclastogenic factor RANKL, critical for prevention of EtOH- induced bone loss; | [199] | |
| NOV-002 | MDS | Glutathione disulfide mimetic alters cellular redox, causes phosphorylation of JNK, p38 and ERK with roles in cell proliferation. | [207–209] |
| Telintra | MDS | GSTP inhibitor causes dissociation of GSTP from the JNK/c-Jun complex, leading to JNK/c-Jun activation which contributes to hematopoietic progenitor cell proliferation and maturation. | [210, 211] |
| Vorinostat | AML MDS |
HDAC inhibitor causes cancer cell apoptosis by induction of DNA damage and genomic instability through generation of ROS. | [212–214] |
Adenanthin
Adenanthin, a diterpenoid isolated from the leaves of Isodon adenanthus, has been reported to possess anti-leukemic activity through targeting peroxiredoxin I/II [144, 145]. Adenanthin directly targets the conserved resolving cysteines of Prx1/II, and inhibits their peroxidase activities. Consequently, cellular H2O2 is increased and causes the activation of ERK1 and ERK2, inducing the expression of C/EBPβ, which has been widely shown to drive AML cell differentiation [183]. Adenanthin may serve as a lead compound for the development of PrxI/II-targeted therapeutic agents, which may provide a promising redox=based differentiation therapy for APL cells.
Amifostine
Amifostine (WR-2721; Ethyol) is a cytoprotective adjuvant used in cancer radiotherapy and with electrophilic chemotherapeutic agents. While it has bene cial effects on alleviating neurotoxicity, nephrotoxicity and hematologic toxicities of the various cytotoxic drugs, it was initially approved by the FDA for prevention of xerostomia in head and neck cancer patients undergoing radiotherapy [215]. Amifostine is converted to its active metabolite (WR-1065) after dephosphorylation by alkaline phosphatase, an enzyme presents at higher relative concentrations in normal cells, therefore higher levels of the active WR-1065 metabolite accumulate in non-neoplastic tissue [216, 217]. Amifostine protects primitive hematopoietic progenitors against chemotherapy cytotoxicity [184], suppresses apoptosis in AML/MDS patients [187] and has been used in the management of MDS [188]. In normal bone marrow progenitors, amifostine stimulates the growth of granulocyte, erythroid, macrophage, megakaryocyte colony-forming units and erythroid bursts and inhibits apoptosis in cytokine-deficient conditions [185]. Activation of NF-κB/Rel factors can counteract apoptosis in the hematopoietic compartment, raising the possibility that, in addition to amifostine, other NF-κB/Rel–stimulating strategies could improve hematopoietic progenitor cell survival [186].
Arsenic trioxide (As2O3)
Despite the well-known toxicity of arsenic, arsenic trioxide has long been of biomedical interest. Under the trade name Trisenox (and approved by FDA in 2000), it is an important class of thiol-reactive pro-oxidant redox chemotheraputic agents that can target APL [218]. The antiproliferative and apoptogenic effects are linked with the pro-oxidant effects through covalent cross-linking of vicinal thiols [219]. The irreversible inhibition of mammalian TrxR by arsenic trioxide causes growth inhibition in breast cancer cells, and GSH depletion further enhances the drug-induced cell death [167]. Moreover, micro RNA let-7d and miR-766 might be involved in the regulation of arsenic trioxide activity in APL cells. Expression of caspase-3 and Bax, which are targets of let-7d and miR-766, respectively, were increased in treated APL cells [191]. Although generally considered as a relatively safe therapeutic strategy, numerous clinical reports have indicated that chronic exposure to a therapeutic dose of arsenic trioxide can evoke severe cardiac side effects [189, 190]. Efforts to reduce the cardiac side effects have involved using natural antioxidants, with combination treatments of either genistein or resveratrol and arsenic trioxide in the treatment of APL [192].
Butylated hydroxyanisole
Butylated hydroxyanisole (BHA) is an antioxidant primarily used as a food additive. It is an inducer of the CBR1 gene, which is regulated by Nrf2 [220]. Through their antioxidant properties, both vitamin E and BHA enhance the effect of irradiation on mouse neuroblastoma [194]. Uncoupling protein 1 plays a role in generating heat by uncoupling oxidative phosphorylation in the mitochondrial inner membrane of mammalian brown adipose tissue. Overexpression of sestrins, a family of stress-inducible proteins that regulate metabolic homeostasis, increase fat accumulation through suppressing ROS [195]. Similar results were obtained by using either BHA or NAC [196].
N-acetyl cysteine
NAC is perhaps the simplest redox active agent, which can be deacetylated in tissue/cells to form cysteine, the limiting amino acid in GSH biosynthesis [221], replenishing GSH stores. NAC is both a drug and dietary supplement used primarily as a mucolytic agent and in the management of acetaminophen overdose. As an antioxidant, NAC suppressed C/EBPβ and PPARγ expression, which can inhibit adipocyte differentiation and fat accumulation [101, 102]. Expression of obesity-related proteins, such as monoamine oxidase A, heat shock protein 70, aminoacylase-1 and transketolase, is suppressed by NAC [222]. Positive clinical responses obtained during NAC therapy in neurodegenerative diseases have provided supportive evidence for the role of ROS in pathological processes. NAC promoted neuron differentiation of ES cells in a dose- and time-dependent manner. Notably, NAC suppressed cell death caused by retinoic acid during neuron differentiation. In addition, neurite extension of SCG neurons was greatly stimulated in the presence of NAC, these results indicated that NAC enhanced both neuron differentiation and neuritogenesis [206].
NOV-002
NOV-002, a complex of GSSG and cisplatin at ratio of 1000:1, is a glutathione disulfide mimetic that alters intracellular GSH/GSSG ratios by increasing serum and tissue levels of stable GSSG, creating a mild, transient oxidative intracellular signal that can induce S-glutathionylation [207, 208]. Preclinical and clinical studies indicated that NOV-002 modulates signaling pathways involved in tumor cell proliferation and metastasis and enhances anti-tumor immune responsiveness in tumor models [207, 209, 223, 224]. NOV-002 altered intracellular levels of GSH and GSSG by >35% as well as caused a time- and concentration-dependent increase in the phosphorylation of three kinases that play direct roles in cell proliferation (JNK, p38, and ERK) and in AKT in pre-myeloid HL-60 cells, all involved in the regulation of hematopoiesis/myeloproliferation [207]. In addition, increased peripheral blood counts of leukocytes, monocytes, lymphocytes, T-suppressor cells, IL-2 receptor-expressing T cells and natural killer cells were seen in the patient group receiving NOV-002 in combination with chemotherapy, indicating that NOV-002 has myeloproliferative properties [208]. In animals, NOV-002 attenuated the decrease of red blood cells, platelets, total white blood cells, absolute lymphocyte and neutrophil counts, hematocrit values and hemoglobin content following radiotherapy with whole body γ-rays [209].
Telintra
TLK199 [γ-glutamyl-s-(benzyl)-cysteinyl-R-(−) phenyl glycine diethyl ester], now called Telintra or Ezatiostat is a glutathione peptidomimetic inhibitor of the enzyme GSTP. While early preclinical testing focused on overcoming GSTP-associated drug resistance, preclinical studies in rodents revealed that the drug also increased circulating blood cells of all lineages [171]. Furthermore, GSTP null mice exhibit an increase in myeloid cell differentiation and proliferation, evidenced by elevated leukocyte numbers and bone marrow derived dendritic cells [174, 175]. Telintra causes dissociation of GSTP from the JNK/c-Jun complex, leading to JNK activation by phosphorylation. Activated JNK phosphorylates c-Jun, which ultimately contributes to the stimulation of hematopoietic progenitor cell (HPC) proliferation and maturation [174]. Phase 2 studies of Telintra showed significant therapeutic responses in a proportion of patients with low- to intermediate-risk MDS [211]. The Telintra response profile contains two miRNAs (under-expressed miR-129 and over-expressed miR-155) that regulate expression of genes known to be implicated in MDS disease pathology. Moreover, the JNK/c-Jun pathway is under-expressed in Telintra responding patients and over-expressed in non-responding patients, suggesting that patients whose cells do not under-express the JNK/c-Jun pathway are not likely to benefit from additional activation by Telintra. These signature genes and signaling pathways positively correlate with the known mechanism of action of Telintra and could potentially be developed into a predictive diagnostic test for MDS patients who may be sensitive to Telintra [210].
Vorinostat
Vorinostat (suberoylanilide hydroxamic acid, Zolinza) is a HDAC inhibitor that promotes cell cycle arrest, growth inhibition, apoptosis and differentiation of cells from AML and MDS patients [212]. Vorinostat received FDA approval for use in cutaneous T-cell lymphomas in 2006. This drug increased expression of genes previously found to be down regulated in MDS and/or AML (cFOS, COX2, IER3, p15, RAI3) and suppressed expression of genes over-expressed in these malignancies (AXL, c-MYC, Cyclin D1) [214]. One mechanism by which HDAC inhibitors can induce cancer cell apoptosis is by induction of DNA damage and genomic instability through generation of ROS [213]. Therapeutic index is enhanced because sensitivity to normal cells is lower than tumor cells [225].
Conclusion
Cellular redox homeostasis mediates a plethora of cellular pathways that determine life and death events. For example, ROS intersect with GSH based enzyme pathways to influence cell differentiation, a process integral to normal hematopoiesis, but also affecting a number of diverse cell differentiation related human diseases. Recent attempts to manage such pathologies have focused on intervening in some of these pathways, with the consequence that redox biology has provided a platform for drug discovery and development.
Highlights.
Redox homeostasis mediates cell pathways that determine life and death events
Aberrant redox homeostasis causes pathologies linked with differentiation
ROS intersects with GSH based enzyme pathways to influence cell differentiation
Redox targeted differentiation therapy provides a drug discovery platform
Acknowledgments
This work was supported by grants from the National Institutes of Health (CA08660, CA117259, NCRR P20RR024485 - COBRE in Oxidants, Redox Balance and Stress Signaling) and support from the South Carolina SmartState program and was conducted in a facility constructed with the support from the National Institutes of Health, Grant Number C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources. Supported in part by the Drug Metabolism and Clinical Pharmacology shared Resource, Hollings Cancer Center, Medical University of South Carolina. J. Z. was supported by the Swedish Research Council (No. 524-2011-6998).
Abbreviations
- AML
acute myeloid leukemia
- APL
acute promyelocytic leukemia
- ATRA
all-trans retinoic acid
- BHA
butylated hydroxyanisole
- C/EBP
CCAAT/enhancer binding proteins
- CREB
cyclic AMP response element-binding proteins
- CSF
cerebrospinal fluid
- DUOX
dual oxidase
- ER
endoplasmic reticulum
- Ero1
ER oxidoreductin 1
- ETC
electron transport chain
- FoxO
forkhead box-O
- GPx
glutathione peroxidases
- GR
glutathione reductase
- Grx
Glutaredoxins
- GSH
glutathione
- GST
glutathione S-transferase
- GSTP
glutathione S-transferase pi
- HDAC
histone deacetylases
- HIF
hypoxia-inducible factors
- HPC
hematopoietic progenitor cell
- HSC
hematopoetic stem cells
- IGF-1
insulin-like growth factor-1
- JNK
c-Jun NH2-terminal kinase
- MDS
myelodysplastic syndromes
- MKP-1
mitogen-activated protein kinase phosphatase-1
- MSC
mesenchymal stem cells
- (mtROS)
mitochondrial ROS
- NAC
N-acetylcysteine
- NOS
Nitric oxide synthases
- NOX
NADPH oxidases
- NSC
Neural stem cells
- PDI
protein disulfide isomerase
- PPARγ
proliferator-activated receptor gamma
- Prx
peroxiredoxins
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SeCys
selenocysteine
- SOD
superoxide dismutase
- TBI
traumatic brain injury
- TG2
tissue transglutaminase
- TGFβ
transforming growth factor beta
- TrkA
tyrosine kinase receptor-A
- Trx
thioredoxins
- TrxR
thioredoxin reductase
- UPR
unfolded protein response
Footnotes
Conflict of interest
All authors state that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang Y, Du Y, Le W, Wang K, Kieffer N, Zhang J. Redox control of the survival of healthy and diseased cells. Antioxid Redox Signal. 2011;15:2867–2908. doi: 10.1089/ars.2010.3685. [DOI] [PubMed] [Google Scholar]
- 2.Balazy M, Nigam S. Aging, lipid modifications and phospholipases--new concepts. Ageing Res Rev. 2003;2:191–209. doi: 10.1016/s1568-1637(02)00065-x. [DOI] [PubMed] [Google Scholar]
- 3.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 4.Halliwell B. Free radicals and metal ions in health and disease. Proc Nutr Soc. 1987;46:13–26. doi: 10.1079/pns19870004. [DOI] [PubMed] [Google Scholar]
- 5.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuppusamy P, Zweier JL. Characterization of free radical generation by xanthine oxidase. Evidence for hydroxyl radical generation. J Biol Chem. 1989;264:9880–9884. [PubMed] [Google Scholar]
- 7.Holmstrom KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 8.Lee C, Lee SM, Mukhopadhyay P, Kim SJ, Lee SC, Ahn WS, Yu MH, Storz G, Ryu SE. Redox regulation of OxyR requires specific disulfide bond formation involving a rapid kinetic reaction path. Nat Struct Mol Biol. 2004;11:1179–1185. doi: 10.1038/nsmb856. [DOI] [PubMed] [Google Scholar]
- 9.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 10.Fridovich I. Superoxide anion radical (O2−.), superoxide dismutases, and related matters. J Biol Chem. 1997;272:18515–18517. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- 11.Yost FJ, Jr, Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1973;248:4905–4908. [PubMed] [Google Scholar]
- 12.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 13.Rossi F, Bellavite P, Berton G, Grzeskowiak M, Papini E. Mechanism of production of toxic oxygen radicals by granulocytes and macrophages and their function in the inflammatory process. Pathol Res Pract. 1985;180:136–142. doi: 10.1016/S0344-0338(85)80161-8. [DOI] [PubMed] [Google Scholar]
- 14.Mikkelsen RB, Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003;22:5734–5754. doi: 10.1038/sj.onc.1206663. [DOI] [PubMed] [Google Scholar]
- 15.Bredt DS. Endogenous nitric oxide synthesis: biological functions and pathophysiology. Free Radic Res. 1999;31:577–596. doi: 10.1080/10715769900301161. [DOI] [PubMed] [Google Scholar]
- 16.Beck KF, Eberhardt W, Frank S, Huwiler A, Messmer UK, Muhl H, Pfeilschifter J. Inducible NO synthase: role in cellular signalling. J Exp Biol. 1999;202:645–653. doi: 10.1242/jeb.202.6.645. [DOI] [PubMed] [Google Scholar]
- 17.Hussain S, Slikker W, Jr, Ali SF. Age-related changes in antioxidant enzymes, superoxide dismutase, catalase, glutathione peroxidase and glutathione in different regions of mouse brain. Int J Dev Neurosci. 1995;13:811–817. doi: 10.1016/0736-5748(95)00071-2. [DOI] [PubMed] [Google Scholar]
- 18.Meli R, Nauser T, Latal P, Koppenol WH. Reaction of peroxynitrite with carbon dioxide: intermediates and determination of the yield of CO3*- and NO2*. Journal of biological inorganic chemistry: JBIC: a publication of the Society of Biological Inorganic Chemistry. 2002;7:31–36. doi: 10.1007/s007750100262. [DOI] [PubMed] [Google Scholar]
- 19.Harris JR. Release of a macromolecular protein component from human erythrocyte ghosts. Biochim Biophys Acta. 1968;150:534–537. doi: 10.1016/0005-2736(68)90157-0. [DOI] [PubMed] [Google Scholar]
- 20.Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 2010;45:466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YR, Zweier JL. Cardiac mitochondria and reactive oxygen species generation. Circ Res. 2014;114:524–537. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 25.Tu BP, Ho-Schleyer SC, Travers KJ, Weissman JS. Biochemical basis of oxidative protein folding in the endoplasmic reticulum. Science. 2000;290:1571–1574. doi: 10.1126/science.290.5496.1571. [DOI] [PubMed] [Google Scholar]
- 26.Princiotta MF, Finzi D, Qian SB, Gibbs J, Schuchmann S, Buttgereit F, Bennink JR, Yewdell JW. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity. 2003;18:343–354. doi: 10.1016/s1074-7613(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 27.Townsend DM, Manevich Y, He L, Xiong Y, Bowers RR, Jr, Hutchens S, Tew KD. Nitrosative stress-induced s-glutathionylation of protein disulfide isomerase leads to activation of the unfolded protein response. Cancer Res. 2009;69:7626–7634. doi: 10.1158/0008-5472.CAN-09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mills GC. The purification and properties of glutathione peroxidase of erythrocytes. J Biol Chem. 1959;234:502–506. [PubMed] [Google Scholar]
- 29.Csordas G, Hajnoczky G. SR/ER-mitochondrial local communication: calcium and ROS. Biochim Biophys Acta. 2009;1787:1352–1362. doi: 10.1016/j.bbabio.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coso S, Harrison I, Harrison CB, Vinh A, Sobey CG, Drummond GR, Williams ED, Selemidis S. NADPH oxidases as regulators of tumor angiogenesis: current and emerging concepts. Antioxid Redox Signal. 2012;16:1229–1247. doi: 10.1089/ars.2011.4489. [DOI] [PubMed] [Google Scholar]
- 31.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 33.Pendyala S, Usatyuk PV, Gorshkova IA, Garcia JG, Natarajan V. Regulation of NADPH oxidase in vascular endothelium: the role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid Redox Signal. 2009;11:841–860. doi: 10.1089/ars.2008.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guida M, Maraldi T, Beretti F, Follo MY, Manzoli L, De Pol A. Nuclear Nox4-derived reactive oxygen species in myelodysplastic syndromes. Biomed Res Int. 2014;2014:456937. doi: 10.1155/2014/456937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou D, Shao L, Spitz DR. Reactive oxygen species in normal and tumor stem cells. Adv Cancer Res. 2014;122:1–67. doi: 10.1016/B978-0-12-420117-0.00001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown KM, Arthur JR. Selenium, selenoproteins and human health: a review. Public Health Nutr. 2001;4:593–599. doi: 10.1079/phn2001143. [DOI] [PubMed] [Google Scholar]
- 37.Hondal RJ, Ruggles EL. Differing views of the role of selenium in thioredoxin reductase. Amino Acids. 2011;41:73–89. doi: 10.1007/s00726-010-0494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Limon-Pacheco J, Gonsebatt ME. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat Res. 2009;674:137–147. doi: 10.1016/j.mrgentox.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Nightingale H, Kemp K, Gray E, Hares K, Mallam E, Scolding N, Wilkins A. Changes in expression of the antioxidant enzyme SOD3 occur upon differentiation of human bone marrow-derived mesenchymal stem cells in vitro. Stem Cells Dev. 2012;21:2026–2035. doi: 10.1089/scd.2011.0516. [DOI] [PubMed] [Google Scholar]
- 40.Kemp K, Gray E, Mallam E, Scolding N, Wilkins A. Inflammatory cytokine induced regulation of superoxide dismutase 3 expression by human mesenchymal stem cells. Stem Cell Rev. 2010;6:548–559. doi: 10.1007/s12015-010-9178-6. [DOI] [PubMed] [Google Scholar]
- 41.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 42.Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao AC, Craver BM, Tseng BP, Tran KK, Parihar VK, Acharya MM, Limoli CL. Mitochondrial-targeted human catalase affords neuroprotection from proton irradiation. Radiat Res. 2013;180:1–6. doi: 10.1667/RR3339.1. [DOI] [PubMed] [Google Scholar]
- 44.Bauer G. Targeting extracellular ROS signaling of tumor cells. Anticancer Res. 2014;34:1467–1482. [PubMed] [Google Scholar]
- 45.Bauer G. Tumor cell-protective catalase as a novel target for rational therapeutic approaches based on specific intercellular ROS signaling. Anticancer Res. 2012;32:2599–2624. [PubMed] [Google Scholar]
- 46.Filomeni G, Rotilio G, Ciriolo MR. Cell signalling and the glutathione redox system. Biochem Pharmacol. 2002;64:1057–1064. doi: 10.1016/s0006-2952(02)01176-0. [DOI] [PubMed] [Google Scholar]
- 47.Brigelius-Flohe R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 48.Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;15:1957–1997. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herbette S, Roeckel-Drevet P, Drevet JR. Seleno-independent glutathione peroxidases. FEBS Journal. 2007;274:2163–2180. doi: 10.1111/j.1742-4658.2007.05774.x. [DOI] [PubMed] [Google Scholar]
- 50.Sztajer H, Gamain B, Aumann KD, Slomianny C, Becker K, Brigelius-Flohe R, Flohe L. The putative glutathione peroxidase gene of Plasmodium falciparum codes for a thioredoxin peroxidase. J Biol Chem. 2001;276:7397–7403. doi: 10.1074/jbc.M008631200. [DOI] [PubMed] [Google Scholar]
- 51.Rhee SG, Woo HA. Multiple functions of peroxiredoxins: peroxidases, sensors and regulators of the intracellular messenger H(2)O(2), and protein chaperones. Antioxid Redox Signal. 2011;15:781–794. doi: 10.1089/ars.2010.3393. [DOI] [PubMed] [Google Scholar]
- 52.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 53.Fisher AB, Dodia C, Manevich Y, Chen JW, Feinstein SI. Phospholipid hydroperoxides are substrates for non-selenium glutathione peroxidase. J Biol Chem. 1999;274:21326–21334. doi: 10.1074/jbc.274.30.21326. [DOI] [PubMed] [Google Scholar]
- 54.Peshenko IV, Shichi H. Oxidation of active center cysteine of bovine 1-Cys peroxiredoxin to the cysteine sulfenic acid form by peroxide and peroxynitrite. Free Radic Biol Med. 2001;31:292–303. doi: 10.1016/s0891-5849(01)00579-2. [DOI] [PubMed] [Google Scholar]
- 55.Mahmood DF, Abderrazak A, El Hadri K, Simmet T, Rouis M. The thioredoxin system as a therapeutic target in human health and disease. Antioxid Redox Signal. 2013;19:1266–1303. doi: 10.1089/ars.2012.4757. [DOI] [PubMed] [Google Scholar]
- 56.Lillig CH, Holmgren A. Thioredoxin and related molecules--from biology to health and disease. Antioxid Redox Signal. 2007;9:25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- 57.Holmgren A. Glutathione-dependent enzyme reactions of the phage T4 ribonucleotide reductase system. J Biol Chem. 1978;253:7424–7430. [PubMed] [Google Scholar]
- 58.Holmgren A, Aslund F. Glutaredoxin. Methods Enzymol. 1995;252:283–292. doi: 10.1016/0076-6879(95)52031-7. [DOI] [PubMed] [Google Scholar]
- 59.Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994;54:4313–4320. [PubMed] [Google Scholar]
- 60.Manevich Y, Hutchens S, Tew KD, Townsend DM. Allelic variants of glutathione S-transferase P1-1 differentially mediate the peroxidase function of peroxiredoxin VI and alter membrane lipid peroxidation. Free radical biology & medicine. 2013;54:62–70. doi: 10.1016/j.freeradbiomed.2012.10.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ, Wolf CR, Davis RJ, Ronai Z. Regulation of JNK signaling by GSTp. The EMBO journal. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yin Z, Ivanov VN, Habelhah H, Tew K, Ronai Z. Glutathione S-transferase p elicits protection against H2O2-induced cell death via coordinated regulation of stress kinases. Cancer research. 2000;60:4053–4057. [PubMed] [Google Scholar]
- 63.Zhang J, Grek C, Ye Z-W, Manevich Y, Tew KD, Townsend DM. Chapter Four - Pleiotropic Functions of Glutathione S-Transferase P. In: Danyelle MT, Kenneth DT, editors. Adv Cancer Res. Vol. 122. Academic Press; 2014. pp. 143–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grek CL, Zhang J, Manevich Y, Townsend DM, Tew KD. Causes and consequences of cysteine S-glutathionylation. The Journal of biological chemistry. 2013;288:26497–26504. doi: 10.1074/jbc.R113.461368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Townsend DM, Manevich Y, He L, Hutchens S, Pazoles CJ, Tew KD. Novel role for glutathione S-transferase pi. Regulator of protein S-Glutathionylation following oxidative and nitrosative stress. J Biol Chem. 2009;284:436–445. doi: 10.1074/jbc.M805586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manevich Y, Feinstein SI, Fisher AB. Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with pi GST. Proc Natl Acad Sci U S A. 2004;101:3780–3785. doi: 10.1073/pnas.0400181101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trujillo JA, Croft NP, Dudek NL, Channappanavar R, Theodossis A, Webb AI, Dunstone MA, Illing PT, Butler NS, Fett C, Tscharke DC, Rossjohn J, Perlman S, Purcell AW. The Cellular Redox Environment Alters Antigen Presentation. J Biol Chem. 2014 doi: 10.1074/jbc.M114.573402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim HS, Ullevig SL, Nguyen HN, Vanegas D, Asmis R. Redox regulation of 14-3-3zeta controls monocyte migration. Arterioscler Thromb Vasc Biol. 2014;34:1514–1521. doi: 10.1161/ATVBAHA.114.303746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghoti H, Amer J, Winder A, Rachmilewitz E, Fibach E. Oxidative stress in red blood cells, platelets and polymorphonuclear leukocytes from patients with myelodysplastic syndrome. Eur J Haematol. 2007;79:463–467. doi: 10.1111/j.1600-0609.2007.00972.x. [DOI] [PubMed] [Google Scholar]
- 70.Novotna B, Bagryantseva Y, Siskova M, Neuwirtova R. Oxidative DNA damage in bone marrow cells of patients with low-risk myelodysplastic syndrome. Leuk Res. 2009;33:340–343. doi: 10.1016/j.leukres.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 71.Zhou FL, Zhang WG, Wei YC, Meng S, Bai GG, Wang BY, Yang HY, Tian W, Meng X, Zhang H, Chen SP. Involvement of oxidative stress in the relapse of acute myeloid leukemia. J Biol Chem. 2010;285:15010–15015. doi: 10.1074/jbc.M110.103713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou F, Shen Q, Claret FX. Novel roles of reactive oxygen species in the pathogenesis of acute myeloid leukemia. J Leukoc Biol. 2013;94:423–429. doi: 10.1189/jlb.0113006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hole PS, Darley RL, Tonks A. Do reactive oxygen species play a role in myeloid leukemias? Blood. 2011;117:5816–5826. doi: 10.1182/blood-2011-01-326025. [DOI] [PubMed] [Google Scholar]
- 74.Jogi A, Ora I, Nilsson H, Lindeheim A, Makino Y, Poellinger L, Axelson H, Pahlman S. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci U S A. 2002;99:7021–7026. doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marengo B, Raffaghello L, Pistoia V, Cottalasso D, Pronzato MA, Marinari UM, Domenicotti C. Reactive oxygen species: biological stimuli of neuroblastoma cell response. Cancer Lett. 2005;228:111–116. doi: 10.1016/j.canlet.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 76.Tavakoli S, Asmis R. Reactive oxygen species and thiol redox signaling in the macrophage biology of atherosclerosis. Antioxid Redox Signal. 2012;17:1785–1795. doi: 10.1089/ars.2012.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qiao M, Zhao Q, Lee CF, Tannock LR, Smart EJ, LeBaron RG, Phelix CF, Rangel Y, Asmis R. Thiol oxidative stress induced by metabolic disorders amplifies macrophage chemotactic responses and accelerates atherogenesis and kidney injury in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2009;29:1779–1786. doi: 10.1161/ATVBAHA.109.191759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Srinivasan S, Koenigstein A, Joseph J, Sun L, Kalyanaraman B, Zaidi M, Avadhani NG. Role of mitochondrial reactive oxygen species in osteoclast differentiation. Ann N Y Acad Sci. 2010;1192:245–252. doi: 10.1111/j.1749-6632.2009.05377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, Kim N, Lee SY. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106:852–859. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- 80.Ha H, Kwak HB, Lee SW, Jin HM, Kim HM, Kim HH, Lee ZH. Reactive oxygen species mediate RANK signaling in osteoclasts. Exp Cell Res. 2004;301:119–127. doi: 10.1016/j.yexcr.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 81.Lean JM, Jagger CJ, Kirstein B, Fuller K, Chambers TJ. Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology. 2005;146:728–735. doi: 10.1210/en.2004-1021. [DOI] [PubMed] [Google Scholar]
- 82.Kanzaki H, Shinohara F, Kajiya M, Kodama T. The Keap1/Nrf2 protein axis plays a role in osteoclast differentiation by regulating intracellular reactive oxygen species signaling. J Biol Chem. 2013;288:23009–23020. doi: 10.1074/jbc.M113.478545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Almeida M. Unraveling the role of FoxOs in bone--insights from mouse models. Bone. 2011;49:319–327. doi: 10.1016/j.bone.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim SH, Moon SH. Osteoclast differentiation inhibitors: a patent review (2008 – 2012) Expert Opin Ther Pat. 2013;23:1591–1610. doi: 10.1517/13543776.2013.842556. [DOI] [PubMed] [Google Scholar]
- 85.Sampson N, Berger P, Zenzmaier C. Redox signaling as a therapeutic target to inhibit myofibroblast activation in degenerative fibrotic disease. Biomed Res Int. 2014;2014:131737. doi: 10.1155/2014/131737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sampson N, Berger P, Zenzmaier C. Therapeutic targeting of redox signaling in myofibroblast differentiation and age-related fibrotic disease. Oxid Med Cell Longev. 2012;2012:458276. doi: 10.1155/2012/458276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders--time for clinical translation? J Clin Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lemmens R, Steinberg GK. Stem cell therapy for acute cerebral injury: what do we know and what will the future bring? Curr Opin Neurol. 2013;26:617–625. doi: 10.1097/WCO.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sundberg M, Bogetofte H, Lawson T, Jansson J, Smith G, Astradsson A, Moore M, Osborn T, Cooper O, Spealman R, Hallett P, Isacson O. Improved cell therapy protocols for Parkinson’s disease based on differentiation efficiency and safety of hESC-, hiPSC-, and non-human primate iPSC-derived dopaminergic neurons. Stem Cells. 2013;31:1548–1562. doi: 10.1002/stem.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Magnusson JP, Goritz C, Tatarishvili J, Dias DO, Smith EM, Lindvall O, Kokaia Z, Frisen J. A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science. 2014;346:237–241. doi: 10.1126/science.346.6206.237. [DOI] [PubMed] [Google Scholar]
- 91.Kennedy KA, Sandiford SD, Skerjanc IS, Li SS. Reactive oxygen species and the neuronal fate. Cell Mol Life Sci. 2012;69:215–221. doi: 10.1007/s00018-011-0807-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aoyama K, Nakaki T. Impaired glutathione synthesis in neurodegeneration. Int J Mol Sci. 2013;14:21021–21044. doi: 10.3390/ijms141021021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.da Paulsen BS, da Silveira MS, Galina A, Rehen SK. Pluripotent stem cells as a model to study oxygen metabolism in neurogenesis and neurodevelopmental disorders. Arch Biochem Biophys. 2013;534:3–10. doi: 10.1016/j.abb.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 94.Ostrakhovitch EA, Semenikhin OA. The role of redox environment in neurogenic development. Arch Biochem Biophys. 2013;534:44–54. doi: 10.1016/j.abb.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 95.Manevich Y, Hutchens S, Halushka PV, Tew KD, Townsend DM, Jauch EC, Borg K. Peroxiredoxin VI oxidation in cerebrospinal fluid correlates with traumatic brain injury outcome. Free Radic Biol Med. 2014;72:210–221. doi: 10.1016/j.freeradbiomed.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim JW, Tang QQ, Li X, Lane MD. Effect of phosphorylation and S-S bond-induced dimerization on DNA binding and transcriptional activation by C/EBPbeta. Proc Natl Acad Sci U S A. 2007;104:1800–1804. doi: 10.1073/pnas.0611137104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schopfer FJ, Cole MP, Groeger AL, Chen CS, Khoo NK, Woodcock SR, Golin-Bisello F, Motanya UN, Li Y, Zhang J, Garcia-Barrio MT, Rudolph TK, Rudolph V, Bonacci G, Baker PR, Xu HE, Batthyany CI, Chen YE, Hallis TM, Freeman BA. Covalent peroxisome proliferator-activated receptor gamma adduction by nitro-fatty acids: selective ligand activity and anti-diabetic signaling actions. J Biol Chem. 2010;285:12321–12333. doi: 10.1074/jbc.M109.091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu GS, Chan EC, Higuchi M, Dusting GJ, Jiang F. Redox mechanisms in regulation of adipocyte differentiation: beyond a general stress response. Cells. 2012;1:976–993. doi: 10.3390/cells1040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schneider KS, Chan JY. Emerging role of Nrf2 in adipocytes and adipose biology. Adv Nutr. 2013;4:62–66. doi: 10.3945/an.112.003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee H, Lee YJ, Choi H, Ko EH, Kim JW. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem. 2009;284:10601–10609. doi: 10.1074/jbc.M808742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Calzadilla P, Sapochnik D, Cosentino S, Diz V, Dicelio L, Calvo JC, Guerra LN. N-acetylcysteine reduces markers of differentiation in 3T3-L1 adipocytes. Int J Mol Sci. 2011;12:6936–6951. doi: 10.3390/ijms12106936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dor Y, Glaser B. beta-cell dedifferentiation and type 2 diabetes. N Engl J Med. 2013;368:572–573. doi: 10.1056/NEJMcibr1214034. [DOI] [PubMed] [Google Scholar]
- 104.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nakagami H. The mechanism of white and brown adipocyte differentiation. Diabetes Metab J. 2013;37:85–90. doi: 10.4093/dmj.2013.37.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Son YH, Ka S, Kim AY, Kim JB. Regulation of Adipocyte Differentiation via MicroRNAs. Endocrinol Metab (Seoul) 2014;29:122–135. doi: 10.3803/EnM.2014.29.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:139239. doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.James AW. Review of Signaling Pathways Governing MSC Osteogenic and Adipogenic Differentiation. Scientifica (Cairo) 2013;2013:684736. doi: 10.1155/2013/684736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 110.Fernandez-Real JMM-NaJM. Adipocyte Differentiation. In: Symonds ME, editor. Adipose Tissue Biology. Springer Science+ BUsiness Media, LLC; 2012. pp. 17–38. [Google Scholar]
- 111.De Marchi E, Baldassari F, Bononi A, Wieckowski MR, Pinton P. Oxidative stress in cardiovascular diseases and obesity: role of p66Shc and protein kinase C. Oxid Med Cell Longev. 2013;2013:564961. doi: 10.1155/2013/564961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Imhoff BR, Hansen JM. Extracellular redox environments regulate adipocyte differentiation. Differentiation. 2010;80:31–39. doi: 10.1016/j.diff.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 113.Mouche S, Mkaddem SB, Wang W, Katic M, Tseng YH, Carnesecchi S, Steger K, Foti M, Meier CA, Muzzin P, Kahn CR, Ogier-Denis E, Szanto I. Reduced expression of the NADPH oxidase NOX4 is a hallmark of adipocyte differentiation. Biochim Biophys Acta. 2007;1773:1015–1027. doi: 10.1016/j.bbamcr.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 114.Jiang F, Lim HK, Morris MJ, Prior L, Velkoska E, Wu X, Dusting GJ. Systemic upregulation of NADPH oxidase in diet-induced obesity in rats. Redox Rep. 2011;16:223–229. doi: 10.1179/174329211X13049558293713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schroder K, Wandzioch K, Helmcke I, Brandes RP. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler Thromb Vasc Biol. 2009;29:239–245. doi: 10.1161/ATVBAHA.108.174219. [DOI] [PubMed] [Google Scholar]
- 116.Kanda Y, Hinata T, Kang SW, Watanabe Y. Reactive oxygen species mediate adipocyte differentiation in mesenchymal stem cells. Life Sci. 2011;89:250–258. doi: 10.1016/j.lfs.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 117.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li Y, Mouche S, Sajic T, Veyrat-Durebex C, Supale R, Pierroz D, Ferrari S, Negro F, Hasler U, Feraille E, Moll S, Meda P, Deffert C, Montet X, Krause KH, Szanto I. Deficiency in the NADPH oxidase 4 predisposes towards diet-induced obesity. Int J Obes (Lond) 2012;36:1503–1513. doi: 10.1038/ijo.2011.279. [DOI] [PubMed] [Google Scholar]
- 119.De Pauw A, Tejerina S, Raes M, Keijer J, Arnould T. Mitochondrial (dys)function in adipocyte (de)differentiation and systemic metabolic alterations. Am J Pathol. 2009;175:927–939. doi: 10.2353/ajpath.2009.081155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, Chandel NS. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011;14:537–544. doi: 10.1016/j.cmet.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim JH, Kim SH, Song SY, Kim WS, Song SU, Yi T, Jeon MS, Chung HM, Xia Y, Sung JH. Hypoxia induces adipocyte differentiation of adipose-derived stem cells by triggering reactive oxygen species generation. Cell Biol Int. 2014;38:32–40. doi: 10.1002/cbin.10170. [DOI] [PubMed] [Google Scholar]
- 122.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 123.Chaudhari N, Talwar P, Parimisetty A, Lefebvre d’Hellencourt C, Ravanan P. A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front Cell Neurosci. 2014;8:213. doi: 10.3389/fncel.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jiao P, Ma J, Feng B, Zhang H, Diehl JA, Chin YE, Yan W, Xu H. FFA-induced adipocyte inflammation and insulin resistance: involvement of ER stress and IKKbeta pathways. Obesity (Silver Spring) 2011;19:483–491. doi: 10.1038/oby.2010.200. [DOI] [PubMed] [Google Scholar]
- 125.Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Davidoff AM. Neuroblastoma. Semin Pediatr Surg. 2012;21:2–14. doi: 10.1053/j.sempedsurg.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ashton DTLaLJ. Genetic Factors Influencing the Risk and Clinical Outcome of Neuroblastoma. In: Shimada H, editor. Neuroblastoma - Present and Future. InTech; 2012. pp. 3–28. [Google Scholar]
- 128.Andrew Tee PYL, Marshall Glenn M, Liu Tao. Neuroblastoma: A Malignancy Due to Cell Differentiation Block. In: Shimada H, editor. Neuroblastoma - Present and Future. InTech; 2012. pp. 79–84. [Google Scholar]
- 129.Leondaritis XK George, Johnson Shalini, Li Chengjun, Florakis Andreas, Dimas Konstantinos, Sakellaridis Nikos, Mangoura Dimitra. Interplay Between Protein Kinase C Isoforms Alpha and Epsilon, Neurofibromin, and the Ras/MAPK Pathway in Neuroblastoma Differentiation. In: Shimada H, editor. Neuroblastoma - Present and Future. InTech; 2012. [Google Scholar]
- 130.Vieira HL, Alves PM, Vercelli A. Modulation of neuronal stem cell differentiation by hypoxia and reactive oxygen species. Prog Neurobiol. 2011;93:444–455. doi: 10.1016/j.pneurobio.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 131.Santilli G, Lamorte G, Carlessi L, Ferrari D, Rota Nodari L, Binda E, Delia D, Vescovi AL, De Filippis L. Mild hypoxia enhances proliferation and multipotency of human neural stem cells. PLoS One. 2010;5:e8575. doi: 10.1371/journal.pone.0008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. J Cell Physiol. 2009;220:562–568. doi: 10.1002/jcp.21812. [DOI] [PubMed] [Google Scholar]
- 133.Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, Kornblum HI. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8:59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chang J, Erler J. Hypoxia-mediated metastasis. Adv Exp Med Biol. 2014;772:55–81. doi: 10.1007/978-1-4614-5915-6_3. [DOI] [PubMed] [Google Scholar]
- 135.Yun Z, Lin Q. Hypoxia and regulation of cancer cell stemness. Adv Exp Med Biol. 2014;772:41–53. doi: 10.1007/978-1-4614-5915-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lofstedt T, Jogi A, Sigvardsson M, Gradin K, Poellinger L, Pahlman S, Axelson H. Induction of ID2 expression by hypoxia-inducible factor-1: a role in dedifferentiation of hypoxic neuroblastoma cells. J Biol Chem. 2004;279:39223–39231. doi: 10.1074/jbc.M402904200. [DOI] [PubMed] [Google Scholar]
- 137.Pietras A, Hansford LM, Johnsson AS, Bridges E, Sjolund J, Gisselsson D, Rehn M, Beckman S, Noguera R, Navarro S, Cammenga J, Fredlund E, Kaplan DR, Pahlman S. HIF-2alpha maintains an undifferentiated state in neural crest-like human neuroblastoma tumor-initiating cells. Proc Natl Acad Sci U S A. 2009;106:16805–16810. doi: 10.1073/pnas.0904606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jogi A, Vallon-Christersson J, Holmquist L, Axelson H, Borg A, Pahlman S. Human neuroblastoma cells exposed to hypoxia: induction of genes associated with growth, survival, and aggressive behavior. Exp Cell Res. 2004;295:469–487. doi: 10.1016/j.yexcr.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 139.Tenen DG. Disruption of differentiation in human cancer: AML shows the way. Nat Rev Cancer. 2003;3:89–101. doi: 10.1038/nrc989. [DOI] [PubMed] [Google Scholar]
- 140.Mijovic A, Mufti GJ. The myelodysplastic syndromes: towards a functional classification. Blood Rev. 1998;12:73–83. doi: 10.1016/s0268-960x(98)90018-2. [DOI] [PubMed] [Google Scholar]
- 141.Sachs L. Cell differentiation and bypassing of genetic defects in the suppression of malignancy. Cancer Res. 1987;47:1981–1986. [PubMed] [Google Scholar]
- 142.Marchwicka A, Cebrat M, Sampath P, Sniezewski L, Marcinkowska E. Perspectives of differentiation therapies of acute myeloid leukemia: the search for the molecular basis of patients’ variable responses to 1,25-dihydroxyvitamin d and vitamin d analogs. Front Oncol. 2014;4:125. doi: 10.3389/fonc.2014.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L, Gu LJ, Wang ZY. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–572. [PubMed] [Google Scholar]
- 144.Liu CX, Yin QQ, Zhou HC, Wu YL, Pu JX, Xia L, Liu W, Huang X, Jiang T, Wu MX, He LC, Zhao YX, Wang XL, Xiao WL, Chen HZ, Zhao Q, Zhou AW, Wang LS, Sun HD, Chen GQ. Adenanthin targets peroxiredoxin I and II to induce differentiation of leukemic cells. Nat Chem Biol. 2012;8:486–493. doi: 10.1038/nchembio.935. [DOI] [PubMed] [Google Scholar]
- 145.Liu CX, Zhou HC, Yin QQ, Wu YL, Chen GQ. Targeting peroxiredoxins against leukemia. Exp Cell Res. 2013;319:170–176. doi: 10.1016/j.yexcr.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 146.Tuszynski GP, Rothman VL. Angiocidin induces differentiation of acute myeloid leukemia cells. Exp Mol Pathol. 2013;95:249–254. doi: 10.1016/j.yexmp.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 147.Zhang B, Chen N, Chen H, Wang Z, Zheng Q. The critical role of redox homeostasis in shikonin-induced HL-60 cell differentiation via unique modulation of the Nrf2/ARE pathway. Oxid Med Cell Longev. 2012;2012:781516. doi: 10.1155/2012/781516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu J, Cao L, Chen J, Song S, Lee IH, Quijano C, Liu H, Keyvanfar K, Chen H, Cao LY, Ahn BH, Kumar NG, Rovira, Xu XL, van Lohuizen M, Motoyama N, Deng CX, Finkel T. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature. 2009;459:387–392. doi: 10.1038/nature08040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Liu Y, Zheng P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Piccoli C, D’Aprile A, Ripoli M, Scrima R, Lecce L, Boffoli D, Tabilio A, Capitanio N. Bone-marrow derived hematopoietic stem/progenitor cells express multiple isoforms of NADPH oxidase and produce constitutively reactive oxygen species. Biochem Biophys Res Commun. 2007;353:965–972. doi: 10.1016/j.bbrc.2006.12.148. [DOI] [PubMed] [Google Scholar]
- 151.Piccoli C, Ria R, Scrima R, Cela O, D’Aprile A, Boffoli D, Falzetti F, Tabilio A, Capitanio N. Characterization of mitochondrial and extra-mitochondrial oxygen consuming reactions in human hematopoietic stem cells. Novel evidence of the occurrence of NAD(P)H oxidase activity. J Biol Chem. 2005;280:26467–26476. doi: 10.1074/jbc.M500047200. [DOI] [PubMed] [Google Scholar]
- 152.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 153.Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, Chen C, Hosokawa K, Nakauchi H, Nakayama K, Nakayama KI, Harada M, Motoyama N, Suda T, Hirao A. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 154.Baldini M, Sacchetti C. Effect of cystine and cysteine on human bone marrow cultured in medium deficient in amino acids. Revue d’hematologie. 1953;8:3–19. [PubMed] [Google Scholar]
- 155.Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, Lio P, Macdonald HR, Trumpp A. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 156.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 157.Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97:2293–2299. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- 158.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 159.Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 160.Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood. 1993;82:2031–2037. [PubMed] [Google Scholar]
- 161.Iwasaki H, Suda T. Cancer stem cells and their niche. Cancer science. 2009;100:1166–1172. doi: 10.1111/j.1349-7006.2009.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kohler A, Schmithorst V, Filippi MD, Ryan MA, Daria D, Gunzer M, Geiger H. Altered cellular dynamics and endosteal location of aged early hematopoietic progenitor cells revealed by time-lapse intravital imaging in long bones. Blood. 2009;114:290–298. doi: 10.1182/blood-2008-12-195644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hole PS, Zabkiewicz J, Munje C, Newton Z, Pearn L, White P, Marquez N, Hills RK, Burnett AK, Tonks A, Darley RL. Overproduction of NOX-derived ROS in AML promotes proliferation and is associated with defective oxidative stress signaling. Blood. 2013;122:3322–3330. doi: 10.1182/blood-2013-04-491944. [DOI] [PubMed] [Google Scholar]
- 164.Martner A, Thoren FB, Aurelius J, Soderholm J, Brune M, Hellstrand K. Immunotherapy with histamine dihydrochloride for the prevention of relapse in acute myeloid leukemia. Expert Rev Hematol. 2010;3:381–391. doi: 10.1586/ehm.10.30. [DOI] [PubMed] [Google Scholar]
- 165.Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972–980. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- 166.Tothova Z, Gilliland DG. FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell Stem Cell. 2007;1:140–152. doi: 10.1016/j.stem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 167.Lu J, Chew EH, Holmgren A. Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc Natl Acad Sci U S A. 2007;104:12288–12293. doi: 10.1073/pnas.0701549104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pelicano H, Feng L, Zhou Y, Carew JS, Hileman EO, Plunkett W, Keating MJ, Huang P. Inhibition of mitochondrial respiration: a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J Biol Chem. 2003;278:37832–37839. doi: 10.1074/jbc.M301546200. [DOI] [PubMed] [Google Scholar]
- 169.Wang J, Li L, Cang H, Shi G, Yi J. NADPH oxidase-derived reactive oxygen species are responsible for the high susceptibility to arsenic cytotoxicity in acute promyelocytic leukemia cells. Leuk Res. 2008;32:429–436. doi: 10.1016/j.leukres.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 170.Townsend DM, Findlay VL, Tew KD. Glutathione S-transferases as regulators of kinase pathways and anticancer drug targets. Methods Enzymol. 2005;401:287–307. doi: 10.1016/S0076-6879(05)01019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ruscoe JE, Rosario LA, Wang T, Gate L, Arifoglu P, Wolf CR, Henderson CJ, Ronai Z, Tew KD. Pharmacologic or genetic manipulation of glutathione S-transferase P1–1 (GSTpi) influences cell proliferation pathways. J Pharmacol Exp Ther. 2001;298:339–345. [PubMed] [Google Scholar]
- 172.Lyons RM, Wilks ST, Young S, Brown GL. Oral ezatiostat HCl (Telintra(R), TLK199) and idiopathic chronic neutropenia (ICN): a case report of complete response of a patient with G-CSF resistant ICN following treatment with ezatiostat, a glutathione S-transferase P1-1 (GSTP1-1) inhibitor. J Hematol Oncol. 2011;4:43. doi: 10.1186/1756-8722-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Raza A, Galili N, Callander N, Ochoa L, Piro L, Emanuel P, Williams S, Burris H, 3rd, Faderl S, Estrov Z, Curtin P, Larson RA, Keck JG, Jones M, Meng L, Brown GL. Phase 1-2a multicenter dose-escalation study of ezatiostat hydrochloride liposomes for injection (Telintra, TLK199), a novel glutathione analog prodrug in patients with myelodysplastic syndrome. J Hematol Oncol. 2009;2:20. doi: 10.1186/1756-8722-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gate L, Majumdar RS, Lunk A, Tew KD. Increased myeloproliferation in glutathione S-transferase pi-deficient mice is associated with a deregulation of JNK and Janus kinase/STAT pathways. J Biol Chem. 2004;279:8608–8616. doi: 10.1074/jbc.M308613200. [DOI] [PubMed] [Google Scholar]
- 175.Zhang J, Ye ZW, Gao P, Reyes L, Jones EE, Branham-O’Connor M, Blumer JB, Drake RR, Manevich Y, Townsend DM, Tew KD. Glutathione S-Transferase P Influences Redox and Migration Pathways in Bone Marrow. PLoS One. 2014;9:e107478. doi: 10.1371/journal.pone.0107478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ait-Belkacem R, Berenguer C, Villard C, Ouafik L, Figarella-Branger D, Chinot O, Lafitte D. MALDI imaging and in-source decay for top-down characterization of glioblastoma. Proteomics. 2014;14:1290–1301. doi: 10.1002/pmic.201300329. [DOI] [PubMed] [Google Scholar]
- 177.Powers TW, Jones EE, Betesh LR, Romano PR, Gao P, Copland JA, Mehta AS, Drake RR. Matrix assisted laser desorption ionization imaging mass spectrometry workflow for spatial profiling analysis of N-linked glycan expression in tissues. Anal Chem. 2013;85:9799–9806. doi: 10.1021/ac402108x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Enthaler B, Pruns JK, Wessel S, Rapp C, Fischer M, Wittern KP. Improved sample preparation for MALDI-MSI of endogenous compounds in skin tissue sections and mapping of exogenous active compounds subsequent to ex-vivo skin penetration. Anal Bioanal Chem. 2012;402:1159–1167. doi: 10.1007/s00216-011-5562-6. [DOI] [PubMed] [Google Scholar]
- 179.Seeley EH, Caprioli RM. Molecular imaging of proteins in tissues by mass spectrometry. Proc Natl Acad Sci U S A. 2008;105:18126–18131. doi: 10.1073/pnas.0801374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.McDonnell LA, Heeren RM. Imaging mass spectrometry. Mass Spectrom Rev. 2007;26:606–643. doi: 10.1002/mas.20124. [DOI] [PubMed] [Google Scholar]
- 181.Soprano DR, Teets BW, Soprano KJ. Role of retinoic acid in the differentiation of embryonal carcinoma and embryonic stem cells. Vitam Horm. 2007;75:69–95. doi: 10.1016/S0083-6729(06)75003-8. [DOI] [PubMed] [Google Scholar]
- 182.Shinjyo N, Kita K. Relationship between reactive oxygen species and heme metabolism during the differentiation of Neuro2a cells. Biochem Biophys Res Commun. 2007;358:130–135. doi: 10.1016/j.bbrc.2007.04.071. [DOI] [PubMed] [Google Scholar]
- 183.Duprez E. A new role for C/EBPbeta in acute promyelocytic leukemia. Cell Cycle. 2004;3:389–390. doi: 10.4161/cc.3.4.771. [DOI] [PubMed] [Google Scholar]
- 184.List AF, Heaton R, Glinsmann-Gibson B, Capizzi RL. Amifostine protects primitive hematopoietic progenitors against chemotherapy cytotoxicity. Semin Oncol. 1996;23:58–63. [PubMed] [Google Scholar]
- 185.List AF, Heaton R, Glinsmann-Gibson B, Capizzi RL. Amifostine stimulates formation of multipotent and erythroid bone marrow progenitors. Leukemia. 1998;12:1596–1602. doi: 10.1038/sj.leu.2401151. [DOI] [PubMed] [Google Scholar]
- 186.Romano MF, Lamberti A, Bisogni R, Garbi C, Pagnano AM, Auletta P, Tassone P, Turco MC, Venuta S. Amifostine inhibits hematopoietic progenitor cell apoptosis by activating NF-kappaB/Rel transcription factors. Blood. 1999;94:4060–4066. [PubMed] [Google Scholar]
- 187.Erikci AA, Ozturk A, Karagoz B, Bilgi O, Turken O, Top C, Kandemir EG. Results of combination therapy with amifostine, pentoxifylline, ciprofloxacin and dexamethasone in patients with myelodysplastic syndrome and acute myeloid leukemia. Hematology. 2008;13:289–292. doi: 10.1179/102453308X343428. [DOI] [PubMed] [Google Scholar]
- 188.Schanz J, Jung H, Wormann B, Gassmann W, Petersen T, Hinke A, Haase D. Amifostine has the potential to induce haematologic responses and decelerate disease progression in individual patients with low- and intermediate-1-risk myelodysplastic syndromes. Leuk Res. 2009;33:1183–1188. doi: 10.1016/j.leukres.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 189.Drolet B, Simard C, Roden DM. Unusual effects of a QT-prolonging drug, arsenic trioxide, on cardiac potassium currents. Circulation. 2004;109:26–29. doi: 10.1161/01.CIR.0000109484.00668.CE. [DOI] [PubMed] [Google Scholar]
- 190.Mumford JL, Wu K, Xia Y, Kwok R, Yang Z, Foster J, Sanders WE. Chronic arsenic exposure and cardiac repolarization abnormalities with QT interval prolongation in a population-based study. Environ Health Perspect. 2007;115:690–694. doi: 10.1289/ehp.9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liang H, Li X, Wang L, Yu S, Xu Z, Gu Y, Pan Z, Li T, Hu M, Cui H, Liu X, Zhang Y, Xu C, Guo R, Lu Y, Yang B, Shan H. MicroRNAs contribute to promyelocyte apoptosis in As2O3-treated APL cells. Cell Physiol Biochem. 2013;32:1818–1829. doi: 10.1159/000356615. [DOI] [PubMed] [Google Scholar]
- 192.Fan Y, Chen M, Meng J, Yu L, Tu Y, Wan L, Fang K, Zhu W. Arsenic trioxide and resveratrol show synergistic anti-leukemia activity and neutralized cardiotoxicity. PLoS One. 2014;9:e105890. doi: 10.1371/journal.pone.0105890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Luo F, Zhuang Y, Sides MD, Sanchez CG, Shan B, White ES, Lasky JA. Arsenic trioxide inhibits transforming growth factor-beta1-induced fibroblast to myofibroblast differentiation in vitro and bleomycin induced lung fibrosis in vivo. Respir Res. 2014;15:51. doi: 10.1186/1465-9921-15-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sarria A, Prasad KN. dl-alpha-Tocopheryl succinate enhances the effect of gamma-irradiation on neuroblastoma cells in culture. Proc Soc Exp Biol Med. 1984;175:88–92. doi: 10.3181/00379727-175-41772. [DOI] [PubMed] [Google Scholar]
- 195.Lee JH, Budanov AV, Karin M. Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metab. 2013;18:792–801. doi: 10.1016/j.cmet.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ro SH, Nam M, Jang I, Park HW, Park H, Semple IA, Kim M, Kim JS, Park H, Einat P, Damari G, Golikov M, Feinstein E, Lee JH. Sestrin2 inhibits uncoupling protein 1 expression through suppressing reactive oxygen species. Proc Natl Acad Sci U S A. 2014;111:7849–7854. doi: 10.1073/pnas.1401787111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Higashi Y, Jitsuiki D, Chayama K, Yoshizumi M. Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), a novel free radical scavenger, for treatment of cardiovascular diseases. Recent Pat Cardiovasc Drug Discov. 2006;1:85–93. doi: 10.2174/157489006775244191. [DOI] [PubMed] [Google Scholar]
- 198.Yoshida H, Yanai H, Namiki Y, Fukatsu-Sasaki K, Furutani N, Tada N. Neuroprotective effects of edaravone: a novel free radical scavenger in cerebrovascular injury. CNS Drug Rev. 2006;12:9–20. doi: 10.1111/j.1527-3458.2006.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chen JR, Lazarenko OP, Shankar K, Blackburn ML, Lumpkin CK, Badger TM, Ronis MJ. Inhibition of NADPH oxidases prevents chronic ethanol-induced bone loss in female rats. J Pharmacol Exp Ther. 2011;336:734–742. doi: 10.1124/jpet.110.175091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Crestani B, Besnard V, Boczkowski J. Signalling pathways from NADPH oxidase-4 to idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2011;43:1086–1089. doi: 10.1016/j.biocel.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 202.Samarakoon R, Dobberfuhl AD, Cooley C, Overstreet JM, Patel S, Goldschmeding R, Meldrum KK, Higgins PJ. Induction of renal fibrotic genes by TGF-beta1 requires EGFR activation, p53 and reactive oxygen species. Cell Signal. 2013;25:2198–2209. doi: 10.1016/j.cellsig.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 203.Samarakoon R, Overstreet JM, Higgins PJ. TGF-beta signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal. 2013;25:264–268. doi: 10.1016/j.cellsig.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jarman ER, Khambata VS, Cope C, Jones P, Roger J, Ye LY, Duggan N, Head D, Pearce A, Press NJ, Bellenie B, Sohal B, Jarai G. An inhibitor of NADPH oxidase-4 attenuates established pulmonary fibrosis in a rodent disease model. Am J Respir Cell Mol Biol. 2014;50:158–169. doi: 10.1165/rcmb.2013-0174OC. [DOI] [PubMed] [Google Scholar]
- 205.Paik YH, Kim J, Aoyama T, De Minicis S, Bataller R, Brenner DA. Role of NADPH oxidases in liver fibrosis. Antioxid Redox Signal. 2014;20:2854–2872. doi: 10.1089/ars.2013.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Qian HR, Yang Y. Neuron differentiation and neuritogenesis stimulated by N-acetylcysteine (NAC) Acta Pharmacol Sin. 2009;30:907–912. doi: 10.1038/aps.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Townsend DM, He L, Hutchens S, Garrett TE, Pazoles CJ, Tew KD. NOV-002, a glutathione disulfide mimetic, as a modulator of cellular redox balance. Cancer Res. 2008;68:2870–2877. doi: 10.1158/0008-5472.CAN-07-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Townsend DM, Pazoles CJ, Tew KD. NOV-002, a mimetic of glutathione disulfide. Expert Opin Investig Drugs. 2008;17:1075–1083. doi: 10.1517/13543784.17.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Salama S, Montaser S. Possible modulating impact of glutathione disulfide mimetic on physiological changes in irradiated rats. Hum Exp Toxicol. 2014 doi: 10.1177/0960327114529452. [DOI] [PubMed] [Google Scholar]
- 210.Galili N, Tamayo P, Botvinnik OB, Mesirov JP, Brooks MR, Brown G, Raza A. Prediction of response to therapy with ezatiostat in lower risk myelodysplastic syndrome. J Hematol Oncol. 2012;5:20. doi: 10.1186/1756-8722-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Raza A, Galili N, Smith SE, Godwin J, Boccia RV, Myint H, Mahadevan D, Mulford D, Rarick M, Brown GL, Schaar D, Faderl S, Komrokji RS, List AF, Sekeres M. A phase 2 randomized multicenter study of 2 extended dosing schedules of oral ezatiostat in low to intermediate-1 risk myelodysplastic syndrome. Cancer. 2012;118:2138–2147. doi: 10.1002/cncr.26469. [DOI] [PubMed] [Google Scholar]
- 212.Pan LN, Lu J, Huang B. HDAC inhibitors: a potential new category of anti-tumor agents. Cell Mol Immunol. 2007;4:337–343. [PubMed] [Google Scholar]
- 213.Eot-Houllier G, Fulcrand G, Magnaghi-Jaulin L, Jaulin C. Histone deacetylase inhibitors and genomic instability. Cancer Lett. 2009;274:169–176. doi: 10.1016/j.canlet.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 214.Silva G, Cardoso BA, Belo H, Almeida AM. Vorinostat induces apoptosis and differentiation in myeloid malignancies: genetic and molecular mechanisms. PLoS One. 2013;8:e53766. doi: 10.1371/journal.pone.0053766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Culy CR, Spencer CM. Amifostine: an update on its clinical status as a cytoprotectant in patients with cancer receiving chemotherapy or radiotherapy and its potential therapeutic application in myelodysplastic syndrome. Drugs. 2001;61:641–684. doi: 10.2165/00003495-200161050-00012. [DOI] [PubMed] [Google Scholar]
- 216.Yuhas JM. Active versus passive absorption kinetics as the basis for selective protection of normal tissues by S-2-(3-aminopropylamino)-ethylphosphorothioic acid. Cancer Res. 1980;40:1519–1524. [PubMed] [Google Scholar]
- 217.Shaw LM, Turrisi AT, Glover DJ, Bonner HS, Norfleet AL, Weiler C, Kligerman MM. Human pharmacokinetics of WR-2721. Int J Radiat Oncol Biol Phys. 1986;12:1501–1504. doi: 10.1016/0360-3016(86)90203-8. [DOI] [PubMed] [Google Scholar]
- 218.Wondrak GT. Redox-directed cancer therapeutics: molecular mechanisms and opportunities. Antioxid Redox Signal. 2009;11:3013–3069. doi: 10.1089/ars.2009.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111:2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 220.Miura T, Taketomi A, Nishinaka T, Terada T. Regulation of human carbonyl reductase 1 (CBR1, SDR21C1) gene by transcription factor Nrf2. Chem Biol Interact. 2013;202:126–135. doi: 10.1016/j.cbi.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 221.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Calzadilla P, Gomez-Serrano M, Garcia-Santos E, Schiappacasse A, Abalde Y, Calvo JC, Peral B, Guerra LN. N-Acetylcysteine affects obesity-related protein expression in 3T3-L1 adipocytes. Redox Rep. 2013;18:210–218. doi: 10.1179/1351000213Y.0000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gumireddy K, Li A, Cao L, Yan J, Liu L, Xu X, Pazoles C, Huang Q. NOV-002, A Glutathione Disulfide Mimetic, Suppresses Tumor Cell Invasion and Metastasis. J Carcinog Mutagen. 2013;2013 doi: 10.4172/2157-2518.S7-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Montero AJ, Diaz-Montero CM, Deutsch YE, Hurley J, Koniaris LG, Rumboldt T, Yasir S, Jorda M, Garret-Mayer E, Avisar E, Slingerland J, Silva O, Welsh C, Schuhwerk K, Seo P, Pegram MD, Gluck S. Phase 2 study of neoadjuvant treatment with NOV-002 in combination with doxorubicin and cyclophosphamide followed by docetaxel in patients with HER-2 negative clinical stage II-IIIc breast cancer. Breast Cancer Res Treat. 2012;132:215–223. doi: 10.1007/s10549-011-1889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gaymes TJ, Padua RA, Pla M, Orr S, Omidvar N, Chomienne C, Mufti GJ, Rassool FV. Histone deacetylase inhibitors (HDI) cause DNA damage in leukemia cells: a mechanism for leukemia-specific HDI-dependent apoptosis? Mol Cancer Res. 2006;4:563–573. doi: 10.1158/1541-7786.MCR-06-0111. [DOI] [PubMed] [Google Scholar]