Abstract
Rapid advances in molecular biology and genome sequencing have enabled the generation of new technology and resources for cryptococcal research. RNAi-mediated specific gene knock down has become routine and more efficient by utilizing modified shRNA plasmids and convergent promoter RNAi constructs. This system was recently applied in a high-throughput screen to identify genes involved in host-pathogen interactions. Gene deletion efficiencies have also been improved by increasing rates of homologous recombination through a number of approaches, including a combination of double-joint PCR with split-marker transformation, the use of dominant selectable markers and the introduction of Cre-Loxp systems into Cryptococcus. Moreover, visualization of cryptococcal proteins has become more facile using fusions with codon-optimized fluorescent tags, such as green or red fluorescent proteins or, mCherry. Using recent genome-wide analytical tools, new transcriptional factors and regulatory proteins have been identified in novel virulence-related signaling pathways by employing microarray analysis, RNA-sequencing and proteomic analysis.
Keywords: Cryptoccocus neoformans, Cryptococcus gattii, cryptococcosis, pathogenic fungus, RNAi, yeast, technology, microscopy, experimental model
Crytococcus neoformans is a life-threatening opportunistic pathogen that causes both pulmonary disease and meningoencephalitis not only in immunocompromised patients, but in healthy individuals, as well. Cases of crytococcosis have increased dramatically over the past 30 years and currently results in approximately half a million deaths per year (Park et al., 2009). In survivors, the disease is a major cause of neurological sequelae in HIV patients, causing substantial morbidity (Banerjee et al., 2001). Because of its significant impact on human health as well as the recent development of robust genetic and disease models, C.neoformans has become an ideal model system for researching mechanisms of fungal pathogenesis. Two varieties of C. neoformans have been recognized. C. neoformans var. neoformans and var. grubii, the latter representing the dominant strain in clinical isolates. A former variety of C. neoformans var. gattii was reclassified as a separate species Cryptococcus gattii (Kwon-Chung et al., 2002). As a second major pathogenic Cryptococcus, it is capable of causing infections in previously healthy individuals although a subgroup has been recently shown to possess immunosuppressing autoantibodies to granulocyte-monocyte stimulating factor, GMCSF (Rosen et al., 2013). The outbreak of C. gattii in Vancouver Island, BC, Canada, has led to increased recognition of cryptococcosis in non-HIV infected individuals.
C. neoformans grows in culture as haploid yeast forms and reproduces by budding. During mating, C. neoformans generates hyphae, fuses with opposite or, rarely, the same mating types (Fraser et al., 2005; Lin et al., 2005) and produces spores. One of the unique features of Cryptococcus is the polysaccharide capsule that enlarges during infection or under infection-related conditions, such as low glucose, low nitrogen, serum and low iron (O'Meara and Alspaugh, 2012). Based on antigenic differences in the polysaccharide capsule, Cryptococcus is traditionally subdivided into 4 serotypes, A, B, C, and D as well as a hybrid serotype, AD.
Formerly, molecular studies of C. neoformans were challenging, due to the lack of genetic tools and the low efficiency of homologous recombination. To overcome these difficulties, researchers have made progress in the areas of genome sequencing, gene silencing and disruption, transformation and imaging. The completion of genome sequencing of two related strains of C. neoformans-- serotype D (JEC21 and B3501A) was first reported by Loftus and his colleagues in 2005 (Loftus et al., 2005) and has been extended to the reference serotype A isolate, H99 (www.broad.mit.edu)(Janbon et al., 2014), as well as C. gattii (http://www.bcgsc.ca/project/cryptococcus). The genome of C. neoformans is approximately 19Mb and consists of 14 chromosomes. The genome of C. gattii strains WM276 and R265 are also assembled into 14 chromosomes with a size of 18.4Mb. When compared with the genome of C. neoforman strain B3501A, the WM276 and R265 genomes show 87% and 85.6% identity respectively (Janbon et al., 2014). In addition, many chromosomal rearrangements were observed between C. gattii and C. neoformans especially in chromosomes 4, 9, 10 in the two strains (D'Souza et al., 2011). These advances in the sequencing field have provided researchers an important resource to apply newly available molecular technologies to silence, delete or mutate specific genes by homologous recombination or random insertion of exogenous DNA (Edman and Kwon-Chung, 1990; Erickson et al., 2001b; Lodge et al., 1994; Toffaletti et al., 1993). In this article, we review new molecular and genetic tools that have been applied to the regulation of gene and protein expression as well as protein localization and interactions, which have become useful experimental methods for Cryptococcus research.
RNA interference
Progress in molecular biology has enabled the silencing of gene expression in a regulated and specific way through introduction of double-stranded small interfering RNA (siRNAs) to target complementary messenger RNA (mRNA) for degradation. The RNAi machinery is evolutionary conserved in most eukaryotes, including the pathogenic fungus C. neoformans (Nakayashiki et al., 2006). In this highly-conserved regulatory process, siRNA duplexes of 20–25 nucleotides are produced from long double-stranded RNAs (dsRNAs) or small hairpin RNAs (shRNAs) by an endoribonuclease Dicer. The duplexes of siRNA activate and bind the RNA-induced silencing complex (RISC), in which the sense strand is cleaved by endonucleases, and the remaining antisense-RISC complex can target homologous mRNA sequences for RNA degradation. Since shRNA can be synthesized intracellularly from a plasmid, one of the valuable molecular tools for C. neoformans is to silence targeted genes via shRNA.
In 2002, Gorlach and his colleagues provided the first evidence that RNA-mediated silencing functions in C. neoformans (Gorlach et al., 2002). They were able to repress the expression of calcineurin A (CNA1) in serotype D and laccase (LAC1) in serotype A using plasmids carrying genes’ cDNA sequence in an antisense orientation. In the same year, a successful shRNA-mediated silencing of CAP59 and ADE2 was reported by Liu et al. (Liu et al., 2002), which was later widely used in the Cryptococcus field. An additional advance consisted of using a cryptococcal intron in a ‘one step’ plasmid construction approach, rather than a random fragment of DNA to bridge the sense and anti-sense strands (Fig.1) (Hu et al., 2008; Panepinto et al., 2009; Panepinto et al., 2005; Reese and Doering, 2003). This allows efficient formation of dsRNA hairpins after cryptococcal intron splicing and more effective eukaryotic silencing but successful recovery of the shuttle vector from E. coli due to lack of splicing in prokaryotes. However the application of these methods has been limited by the efficiency of RNAi constructs transformation and time required for generation of silencing plasmids; a simpler RNAi construct with two cryptococcal promoters in opposing orientation was thus developed. The two convergent promoters initiate the transcription of both sense and antisense strands of the target gene sequences. Each newly synthesized transcript anneals with its complementary strand to form a double-stranded RNA which activates the RNAi system (Bose and Doering, 2011). Cryptococcus researchers have used both shRNAi and convergent promoter RNAi constructs to study the function of genes involved in C. neoformans virulence.
Figure 1. The cryptococcal shuttle vector, KUTAP.
It contains a URA5 selective marker. After linearized by Sce1, it can be transformed for effective RNAi-mediated suppression of target genes.
RNAi mediated gene silencing has several advantages over gene disruption. For example, different promoters can be used to regulate the synthesis of dsRNA in C. neoformans. They can be either constitutively expressed, under promoters such as ACT1 (Cox et al., 1995; Varma and Kwon-Chung, 1999) or inducibly expressed or repressed under galactose-inducible GAL7 promoters, which allows conditional gene silencing for targeted genes (Ory et al., 2004; Ruff et al., 2009; Wickes and Edman, 1995). Moreover, combinations of dsRNA can be used to silence multiple genes at the same time, or to silence the expression of a gene family or paralogous genes using a single antisense sequence (Zhao et al., 2005). Stability of multiple RNAi plasmids also allows simultaneous suppression of a regulator or trafficking gene and localization of its target protein through epitope tagging (Panepinto et al., 2009). Specific transcript variants can also be targeted by a unique sequence which is not present in other transcript isoforms (Celotto et al., 2005). In addition, constitutive RNAi can be used for the study of essential genes since expression is merely reduced rather than eliminated and has a distinct advantage over conditional modalities such as temperature-sensitive mutations in that retained phenotypes after RNAi suppression are likely to be unrelated to the targeted mutation because of continual suppression during all stages of growth. Recently the RNAi system has been used for high-throughput screens in cryptococcal genome analysis and to identify genes involved in host-pathogen interactions (Falschlehner et al., 2010; Prudencio and Lehmann, 2009).
In addition to exogenous RNAi introduced into cells, endogenous RNAi has also been reported in C. neoformans, such as RNAi-mediated transposon regulation and specific gene silencing during sexual reproduction (Janbon et al., 2010; Wang et al., 2010). Recently miRNAs (mIR1 and mIR2) were identified in transgene silencing in C. neoformans (Jiang et al., 2012). The authors found that miR1 or miR2 fused URA5 and CLC1 reporter genes (URA5-miRs and CLC1-miRs) were successfully silenced via RNAi pathway in C. neoformans, then these miRNAs can be useful tools to fuse with a target gene for gene silencing in cryptococcal research.
Gene disruption
Since RNAi typically does not eliminate a targeted gene’s function, a complementary approach for the study of non-essential genes is to completely disrupt a gene’s sequence by replacing it with a gene-disruption cassette (Lodge et al., 1994; Nelson et al., 2001; Salas et al., 1996). However the homologous recombination rate in C. neoformans is much lower than in Saccharomyces cerevisiae and it requires longer flanking sequences (preferentially 500bp or more). The H99 serotype A strains has a rate of up to 50% in homologous integration using biolistic transformation; however, the rate dramatically decreases to a reported 1–4% with serotype D (Davidson et al., 2000). The integration rates for serotype B and C are about 3.4% and 8–12% respectively (Fraser et al., 2003; Narasipura et al., 2003). The soil bacterium Agrobacterium tumefacients has also been used to increase transformation efficiency of C. neoformans (Idnurm et al., 2009; McClelland et al., 2005). Another way to increase the frequency of homologous recombination is to reduce the rate of non-homologous recombination by deleting the Ku proteins which are important for non-homologous end joining processes, although Ku mutations may reduce virulence of the strains (Goins et al., 2006).
In most laboratories, overlap-PCR methods have been used routinely to amplify a gene-disruption cassette with selectable makers, such as nourseothricin acetyltransferase (NAT) (Fig.2) (Davidson et al., 2002). However this type of PCR requires multiple templates and generates long lengths of the final PCR products, which lowers its efficiency. In addition, all insertional constructs have high rates of non-homologous recombination in C. neoformans. To improve the frequency of homologous recombination in recovered transformants, a split-marker transformation method was developed (Fu et al., 2006). It uses a mix of double-joined PCR products including overlapping truncations of the selectable marker for homologous recombination (Fig.2). The homologous recombination rates are reported to be as high as 60% depending on the target gene. However because it uses orotidine monophosphate pyrophosphrylase (OMPPase/URA5) as a selectable marker and ura5 auxotrophic strains for transformation, it may be less applicable to the direct transformation of clinical isolates. Recently, this method was modified by Kim MS et al by replacing the URA5 with a NAT dominant selectable marker in combination with double-Joint PCR (DJ-PCR) (Fig. 2) (Kim et al., 2009). The modified method requires fewer templates and generates shorter PCR products, so it is more convenient and efficient with higher targeted-integration frequencies for gene disruption in C. neoformans.
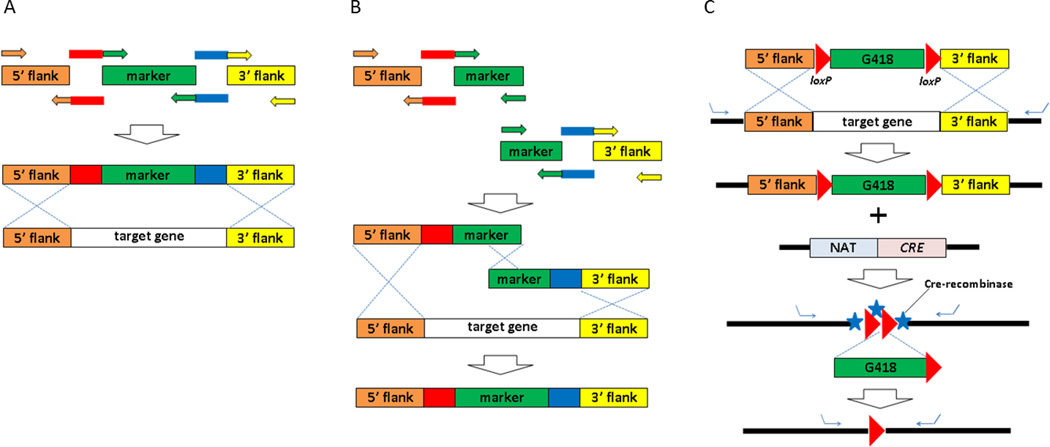
Figure 2. Generation of knockout strains by homologous recombination.
Schematic drawing of overlapping PCR (A), double-joint PCR with a split marker (B), and Cre-lox system (C).
Currently, there are only five dominant selectable makers available from gene deletion cassettes, which limit the number of genes that can be deleted from the genome in the same strain. However the introduction of Cre-Loxp systems in Cryptococcus allows the reuse of the selectable marker (G418), enabling multiple gene deletions in one strain (Baker and Lodge, 2012) (Fig. 2). This Cre-Loxp system is a powerful tool for gene disruption in Cryptococcus. Cre recombinase plasmids can be introduced into strains containing loxp flanked target genes for single, multiple or inducible gene deletions, improving the efficiency of the deletion system.
The genome of C. neoformans encodes an estimated 6,967 genes based on the results of gene prediction programs (Janbon et al., 2014), making the identification of mutant genes created through chemical or ultraviolet radiation difficult. Thus, to improve identification of mutagenized genes responsible for a given phenotype, a signature-tagged mutagenesis (STM) approach was developed for screening avirulent mutants of C. neoformans. The STM strategy was first developed in bacteria, relying on transposon random insertion into the genome of bacteria (Hensel et al., 1995). Each transposon has a unique tag sequence for identification. Researchers have utilized STM to discover virulence-associated genes by injecting a mixture of different random mutants into an animal model for virulence analysis. However there are no active transposon systems present in Cryptococcus. Thus, to isolate virulence mutants of C. neoformans, a plasmid vector, pKB-STM was designed and used for insertional mutagenesis through ectopic integration of foreign DNA into the genome of fungus (Nelson et al., 2001; Saenz and Dehio, 2005). Recently, this approach was modified and used in a high-throughput in vivo screen for pulmonary infectivity (Liu et al., 2008). The new method utilized fusion PCR to create deletion constructs with 48 unique barcodes in a high throughput manner. These constructs was transformed using enhanced biolistic transformation for higher rate of homologous recombination. A total of 1180 gene deletion mutants were created in the screen. Another approach consists of insertional mutagenesis of a pBSK-derived E. coli plasmid containing a cryptococcal URA5 transformation marker. Identification of a mutagenized gene is made by restriction nuclease digestion of fungal genomic DNA, using an enzyme that cuts outside of the insertional vector, followed by re-ligation and recovery as an E. coli shuttle vector. This approach has an advantage that insertions have tended to be single site insertions and has been extensively used in the identification of laccase-associated virulence genes including a vacuolar proton pump, VPH1 and a global regulator, VAD1 (Erickson et al., 2001a; Panepinto et al., 2005).
More recently, targeted genome-wide mutant libraries have being developed with approximately 1500 deletion mutants thus far and the number is increasing every year (Idnurm et al., 2005; Liu et al., 2008). This collection is currently available through the fungal genetic stock center (www.fgsc.org) or ATCC (www.atcc.org) and has been successfully utilized for several screens (He et al., 2012; Tseng et al., 2012). The library of C. neoformans deletion mutants is expected to facilitate the screening of virulent factors important for fungal pathogenicity and the dissection of multiple signaling pathways in Cryptococcus’s. However, other methods of mutagenesis will continue to be valuable as production of high throughput deletion libraries often require parental strains that have higher transformation efficiencies, but may have lost certain virulence features.
Expression plasmids used in C. neoformans
Plasmids have provided an important workhorse for molecular studies in C. neoformans, including protein expression, RNAi gene silencing, protein localization and genomic library constructions (Fox et al., 2003; Vallim et al., 2005; Varma et al., 2006). The first group of plasmids used in C. neoformans carried either a uracil, adenine or lysine synthetic gene as a selected marker, which could complement the corresponding auxotrophic mutant (Chang and Kwon-Chung, 1994; Kwon-Chung et al., 1992; Toffaletti et al., 1993). This method was modified by several groups to construct a new set of plasmids with dominant drug resistant markers such as hygromycin B, NAT, neomycin, geneticin or phleomycin (Cox et al., 1996; Hu and Kronstad, 2006; Hua and et al., 2000; McDade and Cox, 2001).
Constitutively-expressed or regulated plasmids are used to control protein expression under certain circumstances. For example, genes driven by glyceraldehyde-3-phosphate dehydrogenase (GDP) and actin (ACT1) promoters can be constitutively overexpressed (Varma and Kwon-Chung, 1999). Inducible promoters including CTR4 and GAL7 are regulated by copper and galactose respectively (Ory et al., 2004; Wickes and Edman, 1995). Two other galactose-inducible promoters have also been successfully used in C. neoformans var. grubii, which is useful for the analysis of gene functions in the most prevalent serotype A reference strain, H99 (Ruff et al., 2009).
Plasmid transformation in C. neoformans can be achieved by electroporation which tends to retain the plasmid as an extra-chromosomal plasmid (Edman and Kwon-Chung, 1990), but Cryptococcus has low transformation efficiency compared to model yeasts. Alternatively, transformation can be accomplished by biolistic transformation, which delivers DNA more efficiently to Cryptococcus (Davidson et al., 2000; Toffaletti et al., 1993). When co-incubated with C. neoformans, A. tumefacients cells containing a modified Ti plasmid can inject its plasmid into host cells and become integrated into the host genome. Unlike other transformation methods, this method allows host genome integration, but does not mediate homologous recombination. The A. tumefacients mediated transformation efficiency is comparable to either electroporation or biolistic transformation and shows higher efficiency in serotype B and C with stable transformans and a low integration rate (McClelland et al., 2005).
Stability and transformation efficiencies are keys to successful plasmid construction. Previous studies in C. neoformans showed that foreign DNA could be transformed with higher efficiency by adding telomeric repeats to both ends of the DNA. These elements can be inserted into a plasmid as inverted repeats, which when cleaved, generate linearized plasmid telomeric ends that facilitate plasmid transformation and stabilize transformants (Edman, 1992; Edman and Kwon-Chung, 1990). Another feature that stabilizes plasmids of the pPM8 and the pKUTAP class of plasmids is an E.coli-derived STAB1 sequence that allows retention of the plasmid even under non-selective conditions (Mondon et al., 2000). Such plasmids have sufficient stability to enable retention even during mouse passage, enabling virulence studies to be conducted after altered gene dosing by RNAi suppression (Panepinto et al., 2009) or overexpression (Hu et al., 2014).
Microscopy
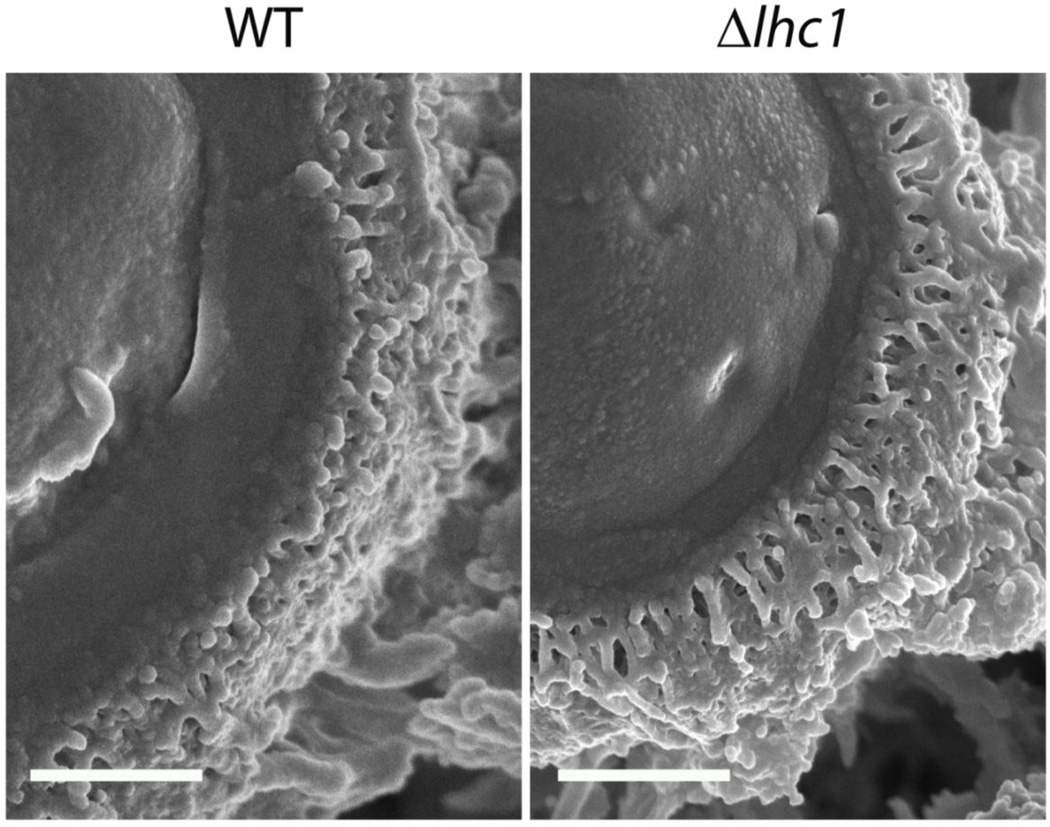
Microcopy has been used extensively in the diagnostic and research fields of Cryptococcus. For example, capsules in cerebral spinal fluid (CSF) can be visualized by India ink stain, which produces a distinctive halo appearance around the cell wall due to capsular exclusion of the microparticular dye. Mucicarmine stain, derived from pigments produced by scale insects of certain Porphyrophora species, is used for Cryptococcus diagnostic visualization in tissues (Lazcano et al., 1991). In frozen or paraffin-embedded tissue sections, cryptococcal cell wall can be detected with calcofluor white, a cotton whitener that fluoresces under ultraviolet light (Giles et al., 2009; Monheit et al., 1984). Lucifer yellow (Srikanta et al., 2011) and eosin Y (Baker et al., 2007) are also used to stain cryptococcal cell walls but none except mucicarmine are specific to Cryptococcus. In addition, ultrathin-section, quick-freeze, deep-etch scanning electron microscopy and differential interference microscopy can be used for characterizing the three-dimensional ultrastructure of C. neoformans (Cleare and Casadevall, 1999; Edwards et al., 1967; Feldmesser et al., 2001) and freeze fracturing methods are especially useful for studying the fine ultrastructure of yeast capsular mutants (Fig.3) (Park et al., 2014).
Figure 3. Three dimensional ultrastructure of C. neoformans capsule.
Cryptococcus neoformans wild-type (H99) and lactonohydrolaes knock out (lhc1Δ) cells were subjected to Cryo-Scanning Electron Microscopy (Cryo-SEM). Bar = 500 nm by the method of Park et al., 2014.
Various antibodies are also used to image Cryptococcus by immunofluorescence staining, such as anti-capsule antibodies (Belay et al., 1997; Casadevall et al., 1994; Feldmesser et al., 2000). The fluorescein or FITC labeled lectins or wheat germ lectin were used to visualize cryptococcal cells (Botts et al., 2009; Fonseca et al., 2013; Rodrigues et al., 2008). Fluorescent-tagged cryptococcal proteins can also be detected under fluorescent microscopy (Ding et al., 2013; Haynes et al., 2011). Several fluorescent-tagged proteins have been codon-optimized and are highly expressed in Cryptococcus, such as green fluorescent protein (GFP, Fig. 4, top panel) (Adler et al., 2011; Liu and Balasubramanian, 2001; Liu et al., 2006; Panepinto et al., 2005; Verma and Idnurm, 2013; Wang et al., 2010), mCherry (Fig.4 bottom panel)(Kozubowski et al., 2013; Park et al., 2014; Waterman et al., 2012) and Dsred (Xue et al., 2006).
Figure 4. Localization of Atg8-GFP and Lhc1-mCherry fusion proteins.
Cryptococcus neoformans cells were grown in the ASN for 2 days at 30°C. Atg8-GFP and Lhc1-mCherry fusion proteins were observed by differential interference contrast microscopy (DIC) or fluorescence microscopy. Bar = 10 µm.
Genome-wide analysis
Whole genome analytical techniques were greatly facilitated by the acquisition and development of multiple cryptococcal genomic sequence annotations and expressed sequence tag (EST) databases. Initial methods for whole genome transcriptional analysis consisted of serial analysis of gene expression (SAGE), developed for rapid and detailed analysis of the overall gene expression profiles between cell populations (Velculescu et al., 1995). Sage was used in Cryptococcus to examine the transcriptome changes in response to temperature and iron concentration manipulations (Hu et al., 2008; Steen et al., 2002). Recently, progress in transcriptional techniques has allowed researchers to analyze gene expression profiling changes to antifungal drugs, iron depletion, CO2 and oxidative stress by DNA microarray analysis in Cryptococcus (Florio et al., 2011; Kim et al., 2010; Upadhya et al., 2013). These microarray analytic techniques have resulted in the identification of new transcription factors and regulatory proteins in signaling pathways (Chun et al., 2011; O'Meara et al., 2010). Transcriptional analysis has also been combined with RNA sequencing (RNA-seq) to explore regulatory networks in cell wall remodeling and capsule regulation (Haynes et al., 2011; O'Meara et al., 2013). RNA-seq has an advantage in being an ‘open method’ in that it is not restricted in sequence output, like microarrays, and can identify novel genes, or compare specific transcript regions or non-coding RNAs. In addition, transcriptional analysis can be used to compare microevolutionary changes within the mammalian host (Hu et al., 2014) and comparative transcriptomics can be used to identify novel signaling pathways in evolutionarily diverse transcriptomes (Adler et al., 2011). By using RNA-seq and transcriptome analysis at the site of human cryptococcal meningitis, Chen and his colleagues identified genes and pathways uniquely regulated by exposure to CSF, which could be important for the survival of C.neoformans in the central nervous system (Chen et al., 2014). Recently, a high-throughput small RNA (sRNA) sequencing technique enabled the discovery of a novel RNAi dependent antifungal drug resistance mechanism (Calo et al., 2014).
In addition to genome-wide transcriptional analysis, proteomics has been also increasingly utilized for cryptococcal research. A recent proteomics study revealed that in C. neoformans, calcineurin interacts with endoplasmic reticulum and Golgi trafficking proteins during heat stress (Kozubowski et al., 2011), suggesting potential mechanisms by which these fungi could be targeted. A global phosphoproteome analysis of C. neoforman var. grubii recovered 1089 phosphopeptides derived from 648 proteins, providing bases for understanding the functions of phosphoproteins in virulence and infection (Selvan et al., 2014). Moreover, Vu and his colleagues identified a secreted metalloprotease (Mpr1) required for central nervous system infection of C. neoformans through a proteomic analysis of the extracellular proteome (Vu et al., 2014). This approach was also used for identifying the substrate of a virulence associated enzyme, components important for the interactions of extracellular vesicles with the cell wall, and metabolic changes in cryptococcal biofilms (Liu and Xue, 2014; Santi et al., 2014; Wolf et al., 2014). An important outcome of proteome analysis has been the identificaiton of potential new drug targets for the treatment of fungal infection. The rapid progress of proteomics in cryptococcal research will certainly shed light on discovering proteins associated with fungal virulence.
Experimental Models of Cryptococcosis for the Fungal Geneticist
The vertebrate models are most commonly used to study cryptococcal pathogenesis since they possess both innate and adaptive immune systems. Among infection models, the mouse infection model is a robust model to study mammalian immunological responses to cryptococcosis, since it is well established and provides numerous genetic backgrounds and mutant strains. Immunological modeling is important to the fungal geneticist as it allows the study of the role of fungal virulence genes in modulating the mammalian immune system beyond simple survival studies. For example, deletion of the global regulator, VAD1 was recently shown to result in infections inducing a more robust innate immune response (Qiu et al., 2013). The cryptococcal infection routes used in mice models includes intranasal, intraperitoneal, intravenous and intratracheal inoculation, the latter introduced either surgically or via pharyngeal inoculation (Clemons et al., 1996; Qiu et al., 2013; Zaragoza et al., 2007). These multiple infection methods allow the study of cryptococcal pathogenesis from different perspectives. This model has been used to identify numerous virulent factors of Crytococcus, including CAP59, LAC1, VPH1, CLC1, VAD1 et al. (Chang and Kwon-Chung, 1994); (Erickson et al., 2001a; Idnurm et al., 2004; Panepinto et al., 2005; Salas et al., 1996). Recently this model was employed to identify FRE3 as a virulence adaptation gene, so called because of its role in the microevolution of cryptococcal virulence during the environmental to mammalian transition (Hu et al., 2014). Furthermore, an immunocompromised rabbit model of CNS infections has been used for the study of meningitis (Perfect et al., 1980). This model is unique in that it allows serial sampling of the infective organism during meningeal infection (Steen et al., 2003). In addition, a guinea pig model has recently been employed in antifungal drug testing (Kirkpatrick et al., 2007). All these in vivo models are very useful for revealing the varied and unique aspects of cryptococcal host-pathogen interactions.
In addition to mouse models, several In vitro cellular models have been developed for evaluating the function of innate immune cells and have been adapted in the high-throughput multistrain analysis of fungal mutants. Monocytes and macrophages play important roles in innate immune responses to cryptococcal infections. Macrophages are the dominant phagocytic cells that interact with Cryptococcus. They can recognize, phagocytose and digest extracellular cryptococcal cells followed by presentation of antigen to T cells (Mansour et al., 2002). Macrophage killing functions in cryptococcal infections have been analyzed using this model. For example, it has been shown that macrophages from HIV/AIDS patients were defective in cryptococcal phagocytosis and failed to induce T cell proliferation when co-cultured with lymphocytes (Monari et al., 1997). This model was also used to evaluate the ability of mutant strains to grow in macrophages (Hu et al., 2008; Tucker and Casadevall, 2002). In addition, genetic markers to measure growth rates of Cryptococcus in macrophages have been identified using live imaging and gene-expression methods in macrophage cell lines (J774 and RAW) as well as primary cells (Alvarez and Casadevall, 2006; Fan et al., 2005; Johnston and May, 2010; Ma et al., 2006).
Similar to the phagocytosis of microbes by human macrophages, soil amoebae feed by phagocytosis of microorganisms. Cryptococcus can cause amoebae to lyse following phagocytosis (Steenbergen et al., 2001). The popular theory for the amoebae model is that the ability of fungi to escape the predator has been adapted to the escape from mammalian phagocytes. This theory was supported by the evidence that factors promoting Cryptococcus survival in Acantamoeba castellanii were also identified as virulence factors that increased Cryptococcus survival in macrophages (Steenbergen et al., 2001). Therefore, the amoebae model is widely used to identify virulence factors in Cryptococcus (Casadevall, 2012; Chrisman et al., 2011; Derengowski Lda et al., 2013).
Besides macrophages, functions of other phagocytes, innate and adaptive immune cells have been also evaluated by in vitro cellular models when co-cultured with the fungi including dendritic cells, neutrophils, eosinophil and lymphocytes (Chaturvedi et al., 1996; Feldmesser et al., 1997; Goulart et al., 2010; Kelly et al., 2005; Mambula et al., 2000; Murphy et al., 1993; Qin et al., 2011). The application of these in vitro cellular models sheds light on the role of the fungus and virulence factors in the innate and adaptive immune responses to Cryptococcus infection in vivo.
Cryptococcal infection models have also been established in invertebrate models using Caenorhabditis elegans, Acantamoeba castellanii and the insects Galleria mellonella and Drosophila melanogaster (Apidianakis et al., 2004; Mylonakis et al., 2002; Swanson and Hammer, 2000). These have advantages in studying fungal mutants which do not grow at mammalian host temperatures.
Concluding remarks
The persistent burden of severe cryptococcosis has prompted researchers to develop more effective tools to study its pathogenicity and characterize its unique features during host-pathogen interactions. In the past decade, the availability of advanced molecular biology tools and the whole genome sequences of C. neoformans and C. gattii have enabled the generation of new technology and resources for cryptococcal research. RNAi-mediated specific gene knock down has become routine and more efficient and has been applied in high-throughput screens for identifying new genes involved in host-pathogen interactions. Gene disruption is a valuable tool in exploring the functions of non-essential genes and its usefulness has been facilitated by increasing rates of homologous recombination and minimizing non-homologous recombination. Modified STM-mediated gene deletion has also been employed in a high-throughput screen to discover virulent associated genes. In addition, new approaches for cellular localization of cryptococcal proteins during infection have been facilitated by the development of codon-optimized fluorescent tags, such as GFP, mCherry and RFP. Recently, multiple genome wide analysis have identified new transcriptional factors and regulatory proteins involved in novel signaling pathways, which sheds light on cryptococcal pathogenesis. Finally the application of multiple experimental infection models allows us to reveal the mechanisms of host-pathogen interactions from different perspectives.
However, many questions remain to be answered about Cryptococcus’ unique biology, its pathogenesis and its interactions with the mammalian host. For example, how do virulence genes cooperate to produce the virulence composite of this formidable pathogen? How do cryptococcal genes help resist innate or adaptive immune defenses and facilitate its neurotropism in animals and in human patients? As homologous recombination rates are still relatively low for Cryptococcus, more efficient tools are required to increase plasmid transformation efficiency, stability and homologous recombination rates and apply these to effective animal modeling and clinical studies to further facilitate the Cryptococcus research field and apply these insights for our patients.
Highlights.
The availability of the whole genome sequences have enabled the generation of new tools for cryptococcal research.
RNAi has become more efficient and been applied in screens for identifying new genes involved in virulence.
Gene disruption efficiencies have been increased. Modified STM has also been employed in screens for virulence genes.
Cellular localization of cryptococcal proteins has been facilitated by optimized tags, such as GFP, mCherry and RFP.
Multiple genome-wide analyses have identified new factors involved in the pathogenesis of Cryptococcus.
Acknowledgements
This work was supported by the intramural research program of the NIH, NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Adler A, Park YD, Larsen P, Nagarajan V, Wollenberg K, Qiu J, Myers TG, Williamson PR. A Novel Specificity Protein 1 (SP1)-like Gene Regulating Protein Kinase C-1 (Pkc1)-dependent Cell Wall Integrity and Virulence Factors in Cryptococcus neoformans. J Biol Chem. 2011;286:20977–20990. doi: 10.1074/jbc.M111.230268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez M, Casadevall A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Current biology : CB. 2006;16:2161–2165. doi: 10.1016/j.cub.2006.09.061. [DOI] [PubMed] [Google Scholar]
- Apidianakis Y, Rahme LG, Heitman J, Ausubel FM, Calderwood SB, Mylonakis E. Challenge of Drosophila melanogaster with Cryptococcus neoformans and role of the innate immune response. Eukaryotic cell. 2004;3:413–419. doi: 10.1128/EC.3.2.413-419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LG, Lodge JK. Multiple gene deletion in Cryptococcus neoformans using the Cre-lox system. Methods in molecular biology. 2012;845:85–98. doi: 10.1007/978-1-61779-539-8_6. [DOI] [PubMed] [Google Scholar]
- Baker LG, Specht CA, Donlin MJ, Lodge JK. Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryotic cell. 2007;6:855–867. doi: 10.1128/EC.00399-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee U, Datta K, Majumdar T, Gupta K. Cryptococcosis in India: the awakening of a giant? Medical mycology. 2001;39:51–67. doi: 10.1080/mmy.39.1.51.67. [DOI] [PubMed] [Google Scholar]
- Belay T, Cherniak R, Kozel TR, Casadevall A. Reactivity patterns and epitope specificities of anti-Cryptococcus neoformans monoclonal antibodies by enzyme-linked immunosorbent assay and dot enzyme assay. Infection and immunity. 1997;65:718–728. doi: 10.1128/iai.65.2.718-728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose I, Doering TL. Efficient implementation of RNA interference in the pathogenic yeast Cryptococcus neoformans. Journal of microbiological methods. 2011;86:156–159. doi: 10.1016/j.mimet.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botts MR, Giles SS, Gates MA, Kozel TR, Hull CM. Isolation and characterization of Cryptococcus neoformans spores reveal a critical role for capsule biosynthesis genes in spore biogenesis. Eukaryotic cell. 2009;8:595–605. doi: 10.1128/EC.00352-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo S, Shertz-Wall C, Lee SC, Bastidas RJ, Nicolas FE, Granek JA, Mieczkowski P, Torres-Martinez S, Ruiz-Vazquez RM, Cardenas ME, et al. Antifungal drug resistance evoked via RNAi-dependent epimutations. Nature. 2014;513:555–558. doi: 10.1038/nature13575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A. Amoeba provide insight into the origin of virulence in pathogenic fungi. Advances in experimental medicine and biology. 2012;710:1–10. doi: 10.1007/978-1-4419-5638-5_1. [DOI] [PubMed] [Google Scholar]
- Casadevall A, DeShaw M, Fan M, Dromer F, Kozel TR, Pirofski LA. Molecular and idiotypic analysis of antibodies to Cryptococcus neoformans glucuronoxylomannan. Infection and immunity. 1994;62:3864–3872. doi: 10.1128/iai.62.9.3864-3872.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celotto AM, Lee JW, Graveley BR. Exon-specific RNA interference: a tool to determine the functional relevance of proteins encoded by alternatively spliced mRNAs. Methods in molecular biology. 2005;309:273–282. doi: 10.1385/1-59259-935-4:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Kwon-Chung KJ. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol Cell Biol. 1994;14:4912–4919. doi: 10.1128/mcb.14.7.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi V, Wong B, Newman S. Oxidative killing of Cryptococcus neoformans by human neutrophils. Evidence that fungal mannitol protects by scavenging reactive oxygen intermediates. J Immunol. 1996;156:3836–3840. [PubMed] [Google Scholar]
- Chen Y, Toffaletti DL, Tenor JL, Litvintseva AP, Fang C, Mitchell TG, McDonald TR, Nielsen K, Boulware DR, Bicanic T, et al. The Cryptococcus neoformans transcriptome at the site of human meningitis. mBio. 2014;5:e01087–e01013. doi: 10.1128/mBio.01087-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrisman CJ, Albuquerque P, Guimaraes AJ, Nieves E, Casadevall A. Phospholipids trigger Cryptococcus neoformans capsular enlargement during interactions with amoebae and macrophages. PLoS pathogens. 2011;7:e1002047. doi: 10.1371/journal.ppat.1002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun CD, Brown JC, Madhani HD. A major role for capsule-independent phagocytosis-inhibitory mechanisms in mammalian infection by Cryptococcus neoformans. Cell host & microbe. 2011;9:243–251. doi: 10.1016/j.chom.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleare W, Casadevall A. Scanning electron microscopy of encapsulated and non-encapsulated Cryptococcus neoformans and the effect of glucose on capsular polysaccharide release. Medical mycology. 1999;37:235–243. [PubMed] [Google Scholar]
- Clemons KV, Azzi R, Stevens DA. Experimental systemic cryptococcosis in SCID mice. J Med Vet Mycol. 1996;34:331–335. doi: 10.1080/02681219680000561. [DOI] [PubMed] [Google Scholar]
- Cox GM, Rude TH, Dykstra CC, Perfect JR. The actin gene from Cryptococcus neoformans: structure and phylogenetic analysis. J Med Vet Mycol. 1995;33:261–266. doi: 10.1080/02681219580000521. [DOI] [PubMed] [Google Scholar]
- Cox GM, Toffaletti DL, Perfect JR. Dominant selection system for use in Cryptococcus neoformans. J Med Vet Mycol. 1996;34:385–391. [PubMed] [Google Scholar]
- D'Souza CA, Kronstad JW, Taylor G, Warren R, Yuen M, Hu G, Jung WH, Sham A, Kidd SE, Tangen K, et al. Genome variation in Cryptococcus gattii, an emerging pathogen of immunocompetent hosts. mBio. 2011;2:e00342–e00310. doi: 10.1128/mBio.00342-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RC, Blankenship JR, Kraus PR, de Jesus Berrios M, Hull CM, D'Souza C, Wang P, Heitman J. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology. 2002;148:2607–2615. doi: 10.1099/00221287-148-8-2607. [DOI] [PubMed] [Google Scholar]
- Davidson RC, Cruz MC, Sia RA, Allen B, Alspaugh JA, Heitman J. Gene disruption by biolistic transformation in serotype D strains of Cryptococcus neoformans. Fungal genetics and biology : FG & B. 2000;29:38–48. doi: 10.1006/fgbi.1999.1180. [DOI] [PubMed] [Google Scholar]
- Derengowski Lda S, Paes HC, Albuquerque P, Tavares AH, Fernandes L, Silva-Pereira I, Casadevall A. The transcriptional response of Cryptococcus neoformans to ingestion by Acanthamoeba castellanii and macrophages provides insights into the evolutionary adaptation to the mammalian host. Eukaryotic cell. 2013;12:761–774. doi: 10.1128/EC.00073-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C, Festa RA, Chen YL, Espart A, Palacios O, Espin J, Capdevila M, Atrian S, Heitman J, Thiele DJ. Cryptococcus neoformans copper detoxification machinery is critical for fungal virulence. Cell host & microbe. 2013;13:265–276. doi: 10.1016/j.chom.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman JC. Isolation of telomerelike sequences from Cryptococcus neoformans and their use in high-efficiency transformation. Mol Cell Biol. 1992;12:2777–2783. doi: 10.1128/mcb.12.6.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman JC, Kwon-Chung KJ. Isolation of the URA5 gene from Cryptococcus neoformans var. neoformans and its use as a selective marker for transformation. Mol Cell Biol. 1990;10:4538–4544. doi: 10.1128/mcb.10.9.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MR, Gordon MA, Lapa EW, Ghiorse WC. Micromorphology of Cryptococcus neoformans. Journal of bacteriology. 1967;94:766–777. doi: 10.1128/jb.94.3.766-777.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson T, Liu L, Gueyikian A, Zhu X, Gibbons J, Williamson P. Multiple virulence factors of Cryptococcus neoformans are dependent on VPH1. Molecular microbiology. 2001a;42:1121–1131. doi: 10.1046/j.1365-2958.2001.02712.x. [DOI] [PubMed] [Google Scholar]
- Erickson T, Liu L, Gueyikian A, Zhu X, Gibbons J, Williamson PR. Multiple virulence factors of Cryptococcus neoformans are dependent on VPH1. Molecular microbiology. 2001b;42:1121–1131. doi: 10.1046/j.1365-2958.2001.02712.x. [DOI] [PubMed] [Google Scholar]
- Falschlehner C, Steinbrink S, Erdmann G, Boutros M. High-throughput RNAi screening to dissect cellular pathways: a how-to guide. Biotechnology journal. 2010;5:368–376. doi: 10.1002/biot.200900277. [DOI] [PubMed] [Google Scholar]
- Fan W, Kraus P, Boily M, Heitman J. Cryptococcus neoformans gene expression during murine macrophage infection. Eukaryotic cell. 2005;4:1420–1433. doi: 10.1128/EC.4.8.1420-1433.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmesser M, Casadevall A, Kress Y, Spira G, Orlofsky A. Eosinophil-Cryptococcus neoformans interactions in vivo and in vitro. Infection and immunity. 1997;65:1899–1907. doi: 10.1128/iai.65.5.1899-1907.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmesser M, Kress Y, Casadevall A. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology. 2001;147:2355–2365. doi: 10.1099/00221287-147-8-2355. [DOI] [PubMed] [Google Scholar]
- Feldmesser M, Rivera J, Kress Y, Kozel TR, Casadevall A. Antibody interactions with the capsule of Cryptococcus neoformans. Infection and immunity. 2000;68:3642–3650. doi: 10.1128/iai.68.6.3642-3650.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio AR, Ferrari S, De Carolis E, Torelli R, Fadda G, Sanguinetti M, Sanglard D, Posteraro B. Genome-wide expression profiling of the response to short-term exposure to fluconazole in Cryptococcus neoformans serotype A. BMC microbiology. 2011;11:97. doi: 10.1186/1471-2180-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca FL, Guimaraes AJ, Kmetzsch L, Dutra FF, Silva FD, Taborda CP, Araujo Gde S, Frases S, Staats CC, Bozza MT, et al. Binding of the wheat germ lectin to Cryptococcus neoformans chitooligomers affects multiple mechanisms required for fungal pathogenesis. Fungal genetics and biology : FG & B. 2013;60:64–73. doi: 10.1016/j.fgb.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox DS, Cox GM, Heitman J. Phospholipid-binding protein Cts1 controls septation and functions coordinately with calcineurin in Cryptococcus neoformans. Eukaryotic cell. 2003;2:1025–1035. doi: 10.1128/EC.2.5.1025-1035.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser JA, Giles SS, Wenink EC, Geunes-Boyer SG, Wright JR, Diezmann S, Allen A, Stajich JE, Dietrich FS, Perfect JR, et al. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature. 2005;437:1360–1364. doi: 10.1038/nature04220. [DOI] [PubMed] [Google Scholar]
- Fraser JA, Subaran RL, Nichols CB, Heitman J. Recapitulation of the sexual cycle of the primary fungal pathogen Cryptococcus neoformans var. gattii: implications for an outbreak on Vancouver Island, Canada. Eukaryotic cell. 2003;2:1036–1045. doi: 10.1128/EC.2.5.1036-1045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Hettler E, Wickes BL. Split marker transformation increases homologous integration frequency in Cryptococcus neoformans. Fungal genetics and biology : FG & B. 2006;43:200–212. doi: 10.1016/j.fgb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Giles SS, Dagenais TR, Botts MR, Keller NP, Hull CM. Elucidating the pathogenesis of spores from the human fungal pathogen Cryptococcus neoformans. Infection and immunity. 2009;77:3491–3500. doi: 10.1128/IAI.00334-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goins CL, Gerik KJ, Lodge JK. Improvements to gene deletion in the fungal pathogen Cryptococcus neoformans: absence of Ku proteins increases homologous recombination, and co-transformation of independent DNA molecules allows rapid complementation of deletion phenotypes. Fungal genetics and biology : FG & B. 2006;43:531–544. doi: 10.1016/j.fgb.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Gorlach JM, McDade HC, Perfect JR, Cox GM. Antisense repression in Cryptococcus neoformans as a laboratory tool and potential antifungal strategy. Microbiology. 2002;148:213–219. doi: 10.1099/00221287-148-1-213. [DOI] [PubMed] [Google Scholar]
- Goulart L, Rosa e Silva LK, Chiapello L, Silveira C, Crestani J, Masih D, Vainstein MH. Cryptococcus neoformans and Cryptococcus gattii genes preferentially expressed during rat macrophage infection. Medical mycology. 2010;48:932–941. doi: 10.3109/13693781003677494. [DOI] [PubMed] [Google Scholar]
- Haynes BC, Skowyra ML, Spencer SJ, Gish SR, Williams M, Held EP, Brent MR, Doering TL. Toward an integrated model of capsule regulation in Cryptococcus neoformans. PLoS pathogens. 2011;7:e1002411. doi: 10.1371/journal.ppat.1002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Lyons DM, Toffaletti DL, Wang F, Qiu Y, Davis MJ, Meister DL, Dayrit JK, Lee A, Osterholzer JJ, et al. Virulence Factors Identified by Cryptococcus neoformans Mutant Screen Differentially Modulate Lung Immune Responses and Brain Dissemination. Am J Pathol. 2012 doi: 10.1016/j.ajpath.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel M, Shea JE, Gleeson C, Jones MD, Dalton E, Holden DW. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- Hu G, Chen SH, Qiu J, Bennett JE, Myers TG, Williamson PR. Microevolution during serial mouse passage demonstrates FRE3 as a virulence adaptation gene in Cryptococcus neoformans. mBio. 2014;5:e00941–e00914. doi: 10.1128/mBio.00941-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Hacham M, Waterman SR, Panepinto J, Shin S, Liu X, Gibbons J, Valyi-Nagy T, Obara K, Jaffe HA, et al. PI3K signaling of autophagy is required for starvation tolerance and virulence of Cryptococcus neoformans. J Clin Invest. 2008;118:1186–1197. doi: 10.1172/JCI32053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Kronstad JW. Gene disruption in Cryptococcus neoformans and Cryptococcus gattii by in vitro transposition. Curr Genet. 2006:1–10. doi: 10.1007/s00294-005-0054-x. [DOI] [PubMed] [Google Scholar]
- Hua J, et al. Development of positive selectable markers for the fungal pathogen Cryptococcus neoformans. Clin Diagn Lab Immunol. 2000;7:125–128. doi: 10.1128/cdli.7.1.125-128.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idnurm A, Bahn YS, Nielsen K, Lin X, Fraser JA, Heitman J. Deciphering the model pathogenic fungus Cryptococcus neoformans. Nat Rev Microbiol. 2005;3:753–764. doi: 10.1038/nrmicro1245. [DOI] [PubMed] [Google Scholar]
- Idnurm A, Reedy JL, Nussbaum JC, Heitman J. Cryptococcus neoformans virulence gene discovery through insertional mutagenesis. Eukaryotic cell. 2004;3:420–429. doi: 10.1128/EC.3.2.420-429.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idnurm A, Walton FJ, Floyd A, Reedy JL, Heitman J. Identification of ENA1 as a virulence gene of the human pathogenic fungus Cryptococcus neoformans through signature-tagged insertional mutagenesis. Eukaryotic cell. 2009;8:315–326. doi: 10.1128/EC.00375-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janbon G, Maeng S, Yang DH, Ko YJ, Jung KW, Moyrand F, Floyd A, Heitman J, Bahn YS. Characterizing the role of RNA silencing components in Cryptococcus neoformans. Fungal genetics and biology : FG & B. 2010;47:1070–1080. doi: 10.1016/j.fgb.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janbon G, Ormerod KL, Paulet D, Byrnes EJ, 3rd, Yadav V, Chatterjee G, Mullapudi N, Hon CC, Billmyre RB, Brunel F, et al. Analysis of the genome and transcriptome of Cryptococcus neoformans var. grubii reveals complex RNA expression and microevolution leading to virulence attenuation. PLoS genetics. 2014;10:e1004261. doi: 10.1371/journal.pgen.1004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N, Yang Y, Janbon G, Pan J, Zhu X. Identification and functional demonstration of miRNAs in the fungus Cryptococcus neoformans. PloS one. 2012;7:e52734. doi: 10.1371/journal.pone.0052734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston SA, May RC. The human fungal pathogen Cryptococcus neoformans escapes macrophages by a phagosome emptying mechanism that is inhibited by Arp2/3 complex-mediated actin polymerisation. PLoS pathogens. 2010;6:e1001041. doi: 10.1371/journal.ppat.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, Chen J, Yauch LE, Levitz SM. Opsonic requirements for dendritic cell-mediated responses to Cryptococcus neoformans. Infection and immunity. 2005;73:592–598. doi: 10.1128/IAI.73.1.592-598.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Kim SY, Yoon JK, Lee YW, Bahn YS. An efficient gene-disruption method in Cryptococcus neoformans by double-joint PCR with NAT-split markers. Biochemical and biophysical research communications. 2009;390:983–988. doi: 10.1016/j.bbrc.2009.10.089. [DOI] [PubMed] [Google Scholar]
- Kim MS, Ko YJ, Maeng S, Floyd A, Heitman J, Bahn YS. Comparative transcriptome analysis of the CO2 sensing pathway via differential expression of carbonic anhydrase in Cryptococcus neoformans. Genetics. 2010;185:1207–1219. doi: 10.1534/genetics.110.118315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick WR, Najvar LK, Bocanegra R, Patterson TF, Graybill JR. New guinea pig model of Cryptococcal meningitis. Antimicrobial agents and chemotherapy. 2007;51:3011–3013. doi: 10.1128/AAC.00085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozubowski L, Thompson JW, Cardenas ME, Moseley MA, Heitman J. Association of calcineurin with the COPI protein Sec28 and the COPII protein Sec13 revealed by quantitative proteomics. PloS one. 2011;6:e25280. doi: 10.1371/journal.pone.0025280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozubowski L, Yadav V, Chatterjee G, Sridhar S, Yamaguchi M, Kawamoto S, Bose I, Heitman J, Sanyal K. Ordered kinetochore assembly in the human-pathogenic basidiomycetous yeast Cryptococcus neoformans. mBio. 2013;4:e00614–e00613. doi: 10.1128/mBio.00614-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung J, Boekhout T, Fell JW, Diaz M. Proposal to conserve the name Cryptococcus gattii against C. hondurianus and C. bacillisporus (Basidomycota, Hymenomycetes, Tremellomycetidae) Toxon. 2002;51:804–806. [Google Scholar]
- Kwon-Chung KJ, Varma A, Edman JC, Bennett JE. Selection of ura5 and ura3 mutants from the two varieties of Cryptococcus neoformans on 5-fluoroorotic acid medium. J Med Vet Mycol. 1992;30:61–69. [PubMed] [Google Scholar]
- Lazcano O, Speights VO, Jr, Bilbao J, Becker J, Diaz J. Combined Fontana-Masson-mucin staining of Cryptococcus neoformans. Archives of pathology & laboratory medicine. 1991;115:1145–1149. [PubMed] [Google Scholar]
- Lin X, Hull CM, Heitman J. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature. 2005;434:1017–1021. doi: 10.1038/nature03448. [DOI] [PubMed] [Google Scholar]
- Liu H, Cottrell T, Pierini L, Goldman W, Doering T. RNA interference in the pathogenic fungus Cryptococcus neoformans. Genetics. 2002;160:463–470. doi: 10.1093/genetics/160.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Balasubramanian MK. 1,3-beta-Glucan synthase: a useful target for antifungal drugs. Curr Drug Targets Infect Disord. 2001;1:159–169. doi: 10.2174/1568005014606107. [DOI] [PubMed] [Google Scholar]
- Liu OW, Chun CD, Chow ED, Chen C, Madhani HD, Noble SM. Systematic genetic analysis of virulence in the human fungal pathogen Cryptococcus neoformans. Cell. 2008;135:174–188. doi: 10.1016/j.cell.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TB, Xue C. Fbp1-mediated ubiquitin-proteasome pathway controls Cryptococcus neoformans virulence by regulating fungal intracellular growth in macrophages. Infection and immunity. 2014;82:557–568. doi: 10.1128/IAI.00994-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hu G, Panepinto J, Williamson P. Role of a VPS41 homolog in starvation response and virulence of Cryptococcus neoformans. Molecular microbiology. 2006;61:1132–1146. doi: 10.1111/j.1365-2958.2006.05299.x. [DOI] [PubMed] [Google Scholar]
- Lodge JK, Jackson-Machelski E, Toffaletti DL, Perfect JR, Gordon JI. Targeted gene replacement demonstrates that myristoyl-CoA: protein N-myristoyltransferase is essential for viability of Cryptococcus neoformans. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:12008–12012. doi: 10.1073/pnas.91.25.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus BJ, Fung E, Roncaglia P, Rowley D, Amedeo P, Bruno D, Vamathevan J, Miranda M, Anderson IJ, Fraser JA, et al. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science. 2005;307:1321–1324. doi: 10.1126/science.1103773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Croudace JE, Lammas DA, May RC. Expulsion of live pathogenic yeast by macrophages. Current biology : CB. 2006;16:2156–2160. doi: 10.1016/j.cub.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Mambula SS, Simons ER, Hastey R, Selsted ME, Levitz SM. Human neutrophil-mediated nonoxidative antifungal activity against Cryptococcus neoformans. Infection and immunity. 2000;68:6257–6264. doi: 10.1128/iai.68.11.6257-6264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour M, Schlesinger L, Levitz S. Optimal T cell responses to Cryptococcus neoformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. Journal of immunology. 2002;168:2872–2879. doi: 10.4049/jimmunol.168.6.2872. [DOI] [PubMed] [Google Scholar]
- McClelland CM, Chang YC, Kwon-Chung KJ. High frequency transformation of Cryptococcus neoformans and Cryptococcus gattii by Agrobacterium tumefaciens. Fungal genetics and biology : FG & B. 2005;42:904–913. doi: 10.1016/j.fgb.2005.07.003. [DOI] [PubMed] [Google Scholar]
- McDade HC, Cox GM. A new dominant selectable marker for use in Cryptococcus neoformans. Medical mycology. 2001;39:151–154. doi: 10.1080/mmy.39.1.151.154. [DOI] [PubMed] [Google Scholar]
- Monari C, Baldelli F, Pietrella D, Retini C, Tascini C, Francisci D, Bistoni F, Vecchiarelli A. Monocyte dysfunction in patients with acquired immunodeficiency syndrome (AIDS) versus Cryptococcus neoformans. J Infect. 1997;35:257–263. doi: 10.1016/s0163-4453(97)93042-5. [DOI] [PubMed] [Google Scholar]
- Mondon P, Chang YC, Varma A, Kwon-Chung KJ. A novel episomal shuttle vector for transformation of Cryptococcus neoformans with the ccdB gene as a positive selection marker in bacteria. FEMS Microbiol Lett. 2000;187:41–45. doi: 10.1111/j.1574-6968.2000.tb09134.x. [DOI] [PubMed] [Google Scholar]
- Monheit JE, Cowan DF, Moore DG. Rapid detection of fungi in tissues using calcofluor white and fluorescence microscopy. Archives of pathology & laboratory medicine. 1984;108:616–618. [PubMed] [Google Scholar]
- Murphy JW, Hidore MR, Wong SC. Direct interactions of human lymphocytes with the yeast-like organism, Cryptococcus neoformans. J Clin Invest. 1993;91:1553–1566. doi: 10.1172/JCI116361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylonakis E, Ausubel FM, Perfect JR, Heitman J, Calderwood SB. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15675–15680. doi: 10.1073/pnas.232568599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayashiki H, Kadotani N, Mayama S. Evolution and diversification of RNA silencing proteins in fungi. Journal of molecular evolution. 2006;63:127–135. doi: 10.1007/s00239-005-0257-2. [DOI] [PubMed] [Google Scholar]
- Narasipura S, Ault J, Behr M, Chaturvedi V, Chaturvedi S. Characterization of Cu,Zn superoxide dismutase (SOD1) gene knock-out mutant of Cryptococcus neoformans var. gattii: role in biology and virulence. Molecular microbiology. 2003;47:1681–1694. doi: 10.1046/j.1365-2958.2003.03393.x. [DOI] [PubMed] [Google Scholar]
- Nelson RT, Hua J, Pryor B, Lodge JK. Identification of virulence mutants of the fungal pathogen Cryptococcus neoformans using signature-tagged mutagenesis. Genetics. 2001;157:935–947. doi: 10.1093/genetics/157.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Meara TR, Alspaugh JA. The Cryptococcus neoformans capsule: a sword and a shield. Clinical microbiology reviews. 2012;25:387–408. doi: 10.1128/CMR.00001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Meara TR, Holmer SM, Selvig K, Dietrich F, Alspaugh JA. Cryptococcus neoformans Rim101 is associated with cell wall remodeling and evasion of the host immune responses. mBio. 2013;4:e00522–e00512. doi: 10.1128/mBio.00522-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Meara TR, Norton D, Price MS, Hay C, Clements MF, Nichols CB, Alspaugh JA. Interaction of Cryptococcus neoformans Rim101 and protein kinase A regulates capsule. PLoS pathogens. 2010;6:e1000776. doi: 10.1371/journal.ppat.1000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory JJ, Griffith CL, Doering TL. An efficiently regulated promoter system for Cryptococcus neoformans utilizing the CTR4 promoter. Yeast. 2004;21:919–926. doi: 10.1002/yea.1139. [DOI] [PubMed] [Google Scholar]
- Panepinto J, Komperda K, Frases S, Park YD, Djordjevic JT, Casadevall A, Williamson PR. Sec6-dependent sorting of fungal extracellular exosomes and laccase of Cryptococcus neoformans. Molecular microbiology. 2009;71:1165–1176. doi: 10.1111/j.1365-2958.2008.06588.x. [DOI] [PubMed] [Google Scholar]
- Panepinto J, Liu L, Ramos J, Zhu X, Valyi-Nagy T, Eksi S, Fu J, Jaffe H, Wickes B, Williamson P. The DEAD-box RNA helicase Vad1 regulates multiple virulence-associated genes in Cryptococcus neoformans. J Clin Invest. 2005;115:632–641. doi: 10.1172/JCI200523048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- Park YD, Shin S, Panepinto J, Ramos J, Qiu J, Frases S, Albuquerque P, Cordero RJ, Zhang N, Himmelreich U, et al. A role for LHC1 in higher order structure and complement binding of the Cryptococcus neoformans capsule. PLoS pathogens. 2014;10:e1004037. doi: 10.1371/journal.ppat.1004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect JR, Lang SD, Durack DT. Chronic cryptococcal meningitis: a new experimental model in rabbits. Am J Pathol. 1980;101:177–194. [PMC free article] [PubMed] [Google Scholar]
- Prudencio M, Lehmann MJ. Illuminating the host - how RNAi screens shed light on host-pathogen interactions. Biotechnology journal. 2009;4:826–837. doi: 10.1002/biot.200900071. [DOI] [PubMed] [Google Scholar]
- Qin QM, Luo J, Lin X, Pei J, Li L, Ficht TA, de Figueiredo P. Functional analysis of host factors that mediate the intracellular lifestyle of Cryptococcus neoformans. PLoS pathogens. 2011;7:e1002078. doi: 10.1371/journal.ppat.1002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Olszewski MA, Williamson PR. Cryptococcus neoformans Growth and Protection from Innate Immunity Are Dependent on Expression of a Virulence-Associated DEAD-Box Protein, Vad1. Infection and immunity. 2013;81:777–788. doi: 10.1128/IAI.00821-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese A, Doering T. Cell wall alpha-1,3-glucan is required to anchor the Cryptococcus neoformans capsule. Molecular microbiology. 2003;50:1401–1409. doi: 10.1046/j.1365-2958.2003.03780.x. [DOI] [PubMed] [Google Scholar]
- Rodrigues ML, Alvarez M, Fonseca FL, Casadevall A. Binding of the wheat germ lectin to Cryptococcus neoformans suggests an association of chitinlike structures with yeast budding and capsular glucuronoxylomannan. Eukaryotic cell. 2008;7:602–609. doi: 10.1128/EC.00307-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen LB, Freeman AF, Yang LM, Jutivorakool K, Olivier KN, Angkasekwinai N, Suputtamongkol Y, Bennett JE, Pyrgos V, Williamson PR, et al. Anti-GM-CSF Autoantibodies in Patients with Cryptococcal Meningitis. Journal of immunology. 2013;190:3959–3966. doi: 10.4049/jimmunol.1202526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff JA, Lodge JK, Baker LG. Three galactose inducible promoters for use in C. neoformans var. grubii. Fungal genetics and biology : FG & B. 2009;46:9–16. doi: 10.1016/j.fgb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz HL, Dehio C. Signature-tagged mutagenesis: technical advances in a negative selection method for virulence gene identification. Current opinion in microbiology. 2005;8:612–619. doi: 10.1016/j.mib.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Salas SD, Bennett JE, Kwon-Chung KJ, Perfect JR, Williamson PR. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. The Journal of experimental medicine. 1996;184:377–386. doi: 10.1084/jem.184.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi L, Beys-da-Silva WO, Berger M, Calzolari D, Guimaraes JA, Moresco JJ, Yates JR., 3rd Proteomic profile of Cryptococcus neoformans biofilm reveals changes in metabolic processes. Journal of proteome research. 2014;13:1545–1559. doi: 10.1021/pr401075f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvan LD, Renuse S, Kaviyil JE, Sharma J, Pinto SM, Yelamanchi SD, Puttamallesh VN, Ravikumar R, Pandey A, Prasad TS, et al. Phosphoproteome of Cryptococcus neoformans. Journal of proteomics. 2014;97:287–295. doi: 10.1016/j.jprot.2013.06.029. [DOI] [PubMed] [Google Scholar]
- Srikanta D, Yang M, Williams M, Doering TL. A sensitive high-throughput assay for evaluating host-pathogen interactions in Cryptococcus neoformans infection. PloS one. 2011;6:e22773. doi: 10.1371/journal.pone.0022773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen B, T L, S Z, WK M, M M, SJ J, JW K. Temperature-Regulated Transcription in the Pathogenic Fungus Cryptococcus neoformans. Genome Res. 2002;12:1386–1400. doi: 10.1101/gr.80202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen BR, Zuyderduyn S, Toffaletti DL, Marra M, Jones SJ, Perfect JR, Kronstad J. Cryptococcus neoformans gene expression during experimental cryptococcal meningitis. Eukaryotic cell. 2003;2:1336–1349. doi: 10.1128/EC.2.6.1336-1349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen JN, Shuman HA, Casadevall A. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:15245–15250. doi: 10.1073/pnas.261418798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson MS, Hammer BK. Legionella pneumophila pathogesesis: a fateful journey from amoebae to macrophages. Annual review of microbiology. 2000;54:567–613. doi: 10.1146/annurev.micro.54.1.567. [DOI] [PubMed] [Google Scholar]
- Toffaletti DL, Rude TH, Johnston SA, Durack DT, Perfect JR. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. Journal of bacteriology. 1993;175:1405–1411. doi: 10.1128/jb.175.5.1405-1411.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng HK, Liu CP, Price MS, Jong AY, Chang JC, Toffaletti DL, Betancourt-Quiroz M, Frazzitta AE, Cho WL, Perfect JR. Identification of genes from the fungal pathogen Cryptococcus neoformans related to transmigration into the central nervous system. PloS one. 2012;7:e45083. doi: 10.1371/journal.pone.0045083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker S, Casadevall A. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc Natl Acad Sci. 2002;99:3165–3170. doi: 10.1073/pnas.052702799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhya R, Campbell LT, Donlin MJ, Aurora R, Lodge JK. Global transcriptome profile of Cryptococcus neoformans during exposure to hydrogen peroxide induced oxidative stress. PloS one. 2013;8:e55110. doi: 10.1371/journal.pone.0055110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallim MA, Nichols CB, Fernandes L, Cramer KL, Alspaugh JA. A Rac homolog functions downstream of Ras1 to control hyphal differentiation and high-temperature growth in the pathogenic fungus Cryptococcus neoformans. Eukaryotic cell. 2005;4:1066–1078. doi: 10.1128/EC.4.6.1066-1078.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma A, Kwon-Chung K. Characterization of the glyceraldehydes-3-phosphate dehydrogenase gene [correction of glyceraldehydes-3-phosphate gene] and the use of its promoter for heterologous expression in Cryptococcus neoformans, a human pathogen. Gene. 1999;232:277. doi: 10.1016/s0378-1119(99)00132-8. [DOI] [PubMed] [Google Scholar]
- Varma A, Wu S, Guo N, Liao W, Lu G, Li A, Hu Y, Bulmer G, Kwon-Chung KJ. Identification of a novel gene, URE2, that functionally complements a urease-negative clinical strain of Cryptococcus neoformans. Microbiology. 2006;152:3723–3731. doi: 10.1099/mic.0.2006/000133-0. [DOI] [PubMed] [Google Scholar]
- Velculescu VE, Zhang L, Vogelstein B, Kinzler KW. Serial analysis of gene expression. Science. 1995;270:484–487. doi: 10.1126/science.270.5235.484. [DOI] [PubMed] [Google Scholar]
- Verma S, Idnurm A. The Uve1 endonuclease is regulated by the white collar complex to protect cryptococcus neoformans from UV damage. PLoS genetics. 2013;9:e1003769. doi: 10.1371/journal.pgen.1003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu K, Tham R, Uhrig JP, Thompson GR, 3rd, Na Pombejra S, Jamklang M, Bautos JM, Gelli A. Invasion of the central nervous system by Cryptococcus neoformans requires a secreted fungal metalloprotease. mBio. 2014;5:e01101–e01114. doi: 10.1128/mBio.01101-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hsueh YP, Li W, Floyd A, Skalsky R, Heitman J. Sex-induced silencing defends the genome of Cryptococcus neoformans via RNAi. Genes & development. 2010;24:2566–2582. doi: 10.1101/gad.1970910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman SR, Park YD, Raja M, Qiu J, Hammoud DA, O'Halloran TV, Williamson PR. Role of CTR4 in the Virulence of Cryptococcus neoformans. mBio. 2012;3:e00285–e00212. doi: 10.1128/mBio.00285-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickes BL, Edman JC. The Cryptococcus neoformans GAL7 gene and its use as an inducible promoter. Molecular microbiology. 1995;16:1099–1109. doi: 10.1111/j.1365-2958.1995.tb02335.x. [DOI] [PubMed] [Google Scholar]
- Wolf J, Espadas-Moreno J, Luque-Garcia JL, Casadevall A. Interaction of Cryptococcus neoformans extracellular vesicles with the cell wall. Eukaryotic cell. 2014 doi: 10.1128/EC.00111-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue C, Bahn YS, Cox GM, Heitman J. G Protein-coupled Receptor Gpr4 Senses Amino Acids and Activates the cAMP-PKA Pathway in Cryptococcus neoformans. Mol Biol Cell. 2006;17:667–679. doi: 10.1091/mbc.E05-07-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O, Alvarez M, Telzak A, Rivera J, Casadevall A. The relative susceptibility of mouse strains to pulmonary Cryptococcus neoformans infection is associated with pleiotropic differences in the immune response. Infection and immunity. 2007;75:2729–2739. doi: 10.1128/IAI.00094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Fanning ML, Lane T. Efficient RNAi-based gene family knockdown via set cover optimization. Artificial intelligence in medicine. 2005;35:61–73. doi: 10.1016/j.artmed.2005.01.009. [DOI] [PubMed] [Google Scholar]