Abstract
Objective
Muscle spasticity is one of the major impairments that limits recovery in hemispheric stroke survivors. One potential contributing mechanism is hyperexcitability of motoneurons. Previously, the response latency of the surface electromyogram (EMG) record evoked by joint rotation has been used to characterize motoneuron excitability. Given the limitations of this method, the objective of the current study was to reexamine the excitability of motoneurons in chronic stroke survivors by estimating reflex latency using single motor unit discharge.
Methods
We quantified the excitability of spastic motoneurons using the response latency of a single motor unit discharge elicited by a position controlled tap on the biceps brachii tendon. We applied tendon taps of different amplitudes on the biceps tendons of both arms of the stroke survivors. Unitary reflex responses were recorded using intramuscular EMG recordings.
Results
Our results showed that the latency of unitary discharge was systematically shorter in the spastic muscle compared with the contralateral muscle, and this effect was consistent across multiple tap amplitudes.
Conclusions
This method allowed us to quantify latencies more accurately, potentially enabling a more rigorous analysis of contributing mechanisms.
Significance
The findings provide evidence supporting a contribution of hyperexcitable motoneurons to muscle spasticity.
Keywords: Spasticity, motoneuron excitability, hyperexcitability, reflex latency, tendon tap
Introduction
Spasticity, affecting up to 43% of chronic stroke survivors (Wissel et al., 2013), is diagnosed clinically as muscular hypertonia, coupled with other reflex disturbances. This hypertonia is defined as a velocity-dependent resistance to stretching due to exaggerated reflex responses (Lance, 1980). There are also concurrent mechanical changes of the muscular-tendon complex, which also contribute to increased muscle tone. Although spasticity can sometimes be beneficial for certain functional movements (e.g., making locomotion and body weight support possible), it is still a major neurological impairment that frequently limits motor functions of many stroke survivors. For example, it can lead to abnormal muscle activation patterns and to disabling body and joint postures in both upper and lower extremities (Knutsson et al., 1979, Knutsson et al., 1980, Finley et al., 2008, Trumbower et al., 2010). These postures, called contractures, can hinder normal functional output and induce disability in daily activities.
Spasticity arises primarily because of increased tonic stretch reflex responses (hyperreflexia), but it can also trigger changes in the mechanical properties of the muscle and connective tissues, as quantified by an increase in the mechanical stiffness of the spastic muscle (Dietz et al., 1981, Lee et al., 1987, O’Dwyer et al., 1996, Dietz et al., 2007). Independently, hyperexcitability of the reflex arc, manifested by an increased stretch reflex response has also been recognized as one major contributor to hypertonia (Gottlieb et al., 1978, Powers et al., 1988, Powers et al., 1989, Dietz et al., 2007). One possible mechanism that can contribute to the overall reflex response is increased motoneuron excitability (Katz et al., 1989). This is the focus of our current study.
One standard test of motoneuron excitability is mediated through the evaluation of stretch reflex or H-reflex latency, often combined with measurements of the ratio between the maximum H-reflex and M-wave magnitudes, where a shorter latency and a larger (H/M) ratio represent signs of more excitable motoneurons. However, there have been inconsistent observations regarding the actual reflex latency in the spastic muscles of stroke survivors. In several reports, stretching the soleus muscle via transient ankle joint rotations, or using electrical stimulation of the posterior tibial nerve, studies have reported that both the stretch reflex latency and H-reflex latency were shorter in the spastic muscle compared with the contralateral one (Hui-Chan et al., 1993, Levin et al., 1993, Bakheit et al., 2003). In contrast, others have observed that the H-reflex latency in the spastic gastrocnemius muscle was not different from the contralateral one (Pisano et al., 2000, Bakheit et al., 2005).
These inconsistent findings may arise in part from unreliable estimates of reflex latency from the surface electromyogram (EMG). For example, the surface EMG is typically used to record the reflex responses, but the rise time of the reflex response is typically slow, due to progressive recruitment of different motor units, many with very small size. As a result, the reflex latency is highly sensitive to the reflex onset criterion, and this criterion is often set differently in different studies. Additionally, the non-selectivity of the stimulus input may also bias the latency estimates. During either joint rotations or nerve stimulations, multiple non-targeted muscles are activated inevitably (Perry, 1993), and the reciprocal excitatory and inhibitory projections between muscles may further bias the estimates of motoneuron excitability of the targeted muscle. Therefore, these approaches may provide inaccurate estimates of the physiological status of the spastic spinal motoneuron.
To overcome these limitations, we estimated the reflex latency of single motor unit discharges elicited by precisely controlled tendon taps, delivered to a single muscle. Specifically, we examined the reflex latency in passive spastic biceps brachii muscle, and we compared it with the contralateral muscle of chronic stroke survivors. We applied tendon taps with amplitudes that were small enough (i.e., 0.5, 1, and 2 mm) to only elicit single motor unit discharges. These single motor unit recordings provided a very sharp rise time of the unitary reflex response, and allowed us to derive a highly reliable estimate of the reflex latency. The specificity of the single muscle stimulation using a precisely controlled tapper ensured the consistency of the stimulus input, and eliminated the potential activation of non-targeted muscles.
Using this technique, we compared the reflex latency differences between the spastic and contralateral biceps of ten stroke survivors. Our results showed that the reflex latency in the spastic muscle was significantly shorter compared with the contralateral muscle in seven stroke subjects, and this latency difference was reversed in two stroke subjects. It was not significantly different in one subject. The findings provide evidence for the existence of hyperexcitable motoneurons, as one of the potential neural mechanisms that can contribute to spasticity in stroke survivors.
The relevance of these latency observations to our understanding of the mechanisms of spasticity will also be discussed. In particular, the relative contributions of sustained depolarization of hyperexcitable motoneurons, as compared with enhancement of the size or rise time of stretch-evoked excitatory postsynaptic potentials (EPSPs) will be explored. The findings provide evidence and a potential tool to identify the reflex contributions to spasticity, which can further inform decision making for spasticity management, including physical or pharmacological therapies, botulinum toxin injections, or surgery. Additionally, our results further revealed limitations of current clinical assessment techniques (e.g., Modified Ashworth Scores), that are unable to distinguish neural (e.g., hyperreflexia) from mechanical contributions (e.g., contracture) of muscle hypertonia. More quantitative assessment approaches are needed for better diagnosis and clinical decision making.
Methods
Participants
Ten chronic hemispheric stroke survivors (8 male, 2 female) volunteered to participate in this study. Inclusion criteria for the stroke subjects were: spasticity present in the upper extremity (Modified Ashworth Scale ≥ 1), stroke onset longer than 6 months, medically stable, no concurrent medical illnesses, no significant cardiorespiratory, metabolic, orthopedic, or other neurological disease, and no history of multiple or recurrent vascular episodes. The demographic profiles of the stroke subjects are summarized in Table 1. The participants gave informed consent via protocols approved by the Institutional Review Board under the Office for the Protection of Human Subjects at Northwestern University.
Table 1.
The demographic profile of the stroke subjects
| Subject | Age (years) | Sex | Chronicity | MAS | DTR | FMA | Chedoke | No. of sequences | |
|---|---|---|---|---|---|---|---|---|---|
| Spastic | Contra | ||||||||
| 1 | 43 | M | 37 | 2 | 2+ | 26 | 3 | 6 | 9 |
| 2 | 57 | M | 303 | 2 | 3+ | 19 | 3 | 9 | 18 |
| 3 | 67 | M | 204 | 2 | 2+ | 10 | 3 | 5 | 5 |
| 4 | 66 | M | 47 | 1+ | 2+ | 42 | 5 | 14 | 5 |
| 5 | 71 | M | 146 | 1 | 2+ | 16 | 3 | 10 | 8 |
| 6 | 48 | M | 185 | 1 | 2+ | 29 | 4 | 8 | 6 |
| 7 | 52 | F | 40 | 1 | 3+ | 18 | 3 | 8 | 21 |
| 8 | 60 | M | 78 | 1+ | 2+ | 24 | 4 | 10 | 5 |
| 9 | 53 | F | 14 | 1 | 3+ | 20 | 3 | 19 | 10 |
| 10 | 68 | M | 130 | 2 | 3+ | 19 | 4 | 9 | 13 |
Note: Chronicity: months post stroke; MAS: Modified Ashworth Scale; DTR: Deep Tendon Reflex score; FMA: Upper extremity Fugl-Meyer Assessment with a maximum score of 66 signifying no impairment; Chedoke: Chedoke-McMaster Stroke Assessment with a maximum score of 7 signifying no impairment; No. of sequences: number of action potential sequences identified; Spastic: spastic side; Contra: contralateral side.
A research physical therapist performed the clinical evaluation prior to the experimental testing. This included:
Spasticity: an assessment of spasticity at the elbow, using the Modified Ashworth Scale (Bohannon et al., 1987), and an estimate of the magnitude of the biceps deep tendon reflex, elicited with a clinical hammer, using a 4 point scale (Walker, 1990). The lower boundary for inclusion of the stroke subjects was a Modified Ashworth score of 1 and a deep tendon reflex score of 2+.
Motor impairment: the physical therapist further assessed the upper arm impairment with the Fugl-Meyer Assessment Scale (Fugl-Meyer et al., 1975) and the Chedoke-McMaster Stroke Assessment Scale (Gowland et al., 1993).
Experimental Setup
Linear Motor for Tendon Tap
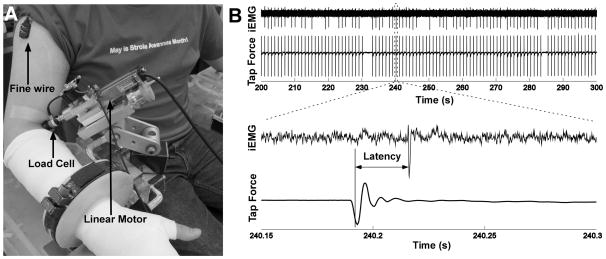
Participants were seated upright in a Biodex chair with their shoulder placed in 45° of abduction and neutral rotation, with the elbow in 120° of extension, and the wrist in 45° of supination and 0° of flexion/extension. The forearm was cast from just below the elbow to the most distal point of the finger and attached to a ring-mount interface to standardize arm position, and minimize activity of muscles. A position controlled linear actuator (Linmot Inc.), as shown in Figure 1A, was positioned perpendicular to the biceps tendon. To ensure that the probe was placed consistently in contact with the tendon across testing sessions, the anatomical location of the muscular-tendon junction was marked on the skin guided by the ultrasound imaging (Supersonic Imagine Inc.) of the biceps muscle-tendon complex. The probe was then placed just below the muscular-tendon junction. A 6-D load cell (Nano17, ATI, Inc.) was mounted at the end of the probe to measure the static indentation forces and the transient tap forces. The force and moment signals were low-pass filtered at 400 Hz, and sampled at 1 kHz.
Figure 1.
Experimental setup, force and EMG recordings, and response latency measurement. A: Experimental setup with intramuscular EMG and force recordings. B: Exemplar recordings of intramuscular EMG and tap forces during a tendon tap sequence. The zoomed window at the bottom shows the tap force, the evoked reflex response, and the reflex latency calculation.
Intramuscular EMG Recordings
Intramuscular EMG data from the biceps muscle were recorded with Teflon-coated double-stranded wires (bifilar 50 μm, California Fine Wire). The fine-wires were cut to expose only the cross section to increase the recording selectivity. The wire was then inserted into a 30-gauge hypodermic needle and the wire tip was bent to form a barb. The bipolar intramuscular EMG signals were amplified, band-pass filtered (20 Hz to 2 kHz), and sampled at 10 kHz using the Bagnoli sEMG system (Delsys, Inc.). Surface EMG signals were also recorded from the short and long heads of the biceps to ensure that the muscle was in a quiescent state.
Procedures
The linear motor was lowered close to the biceps tendon using a positioning micrometer (Velmex, Bloomfield, NY) until the end of the probe was in contact with the skin surface This position was then recorded and the load cell reading was set at zero newtons. The probe was then advanced progressively to induce a static indentation force to the tendon, which imposed a static stretch of the biceps muscle. The micrometer provided sub millimeter (1/10 mm) positioning accuracy of the probe, which was stable and repeatable throughout the experiment.
At each static indentation depth, a series of 10 transient taps was applied. If no single motor unit responses were observed, the probe was further indented by a 1 mm step, and taps were applied again until one motor unit discharge response was recorded over 10 taps. Depending on the frequency of motor unit response, a series of 400–800 taps was then applied to the biceps tendon with an inter-tap interval of 1 s. The number of taps was determined through pilot testing, so that a sufficient number of reflex responses could provide a sharp distribution of the reflex latency (a standard deviation less than 0.5 ms). Three tap amplitudes (0.5, 1, and 2 mm), with one tap amplitude at each series were applied to the tendon at each indentation depth. The two sides of each stroke subject were tested in separate sessions, approximately one week apart.
Data Analysis
The tap force data at each tap series were selected and the timing of the peak force was estimated from a peak detector custom written in Matlab (The Mathworks). The timing of the tap was then specified as arising at 80% of the peak tap force during the pushing-in phase, where the slope of the force profile was approximately the steepest, such that the timing estimate would be most sensitive to the force profile of the tap.
The intramuscular EMG signals were high-pass filtered at 400 Hz to remove the motion artifact induced by the tap. Similar to the tap timing estimation, the timing of the motor unit discharge was estimated as arising at 80% of the rising edge for a positive peak or 80% of the falling edge for a negative peak. The latency of the single motor unit discharge relative to the timing of the tap was then calculated (Figure 1B). The distribution of the latency at each tap series was constructed, and the mean value of the distribution was calculated as the latency estimate of the motor unit discharge, given that the latency followed a normal distribution based on the Lilliefors test.
In order to quantify possible latency differences between the spastic and contralateral sides of the stroke subjects, a two-sided Wilcoxon rank sum test was used to test the difference of latency (from the same tap amplitude) between the two sides of the stroke subjects. Additionally, the relative difference in the latency from the same tap amplitude was compared between the two sides based on Equation 1.
| (1) |
where LatencyS and LatencyC represent the latency on the spastic and contralateral sides, respectively.
The relative difference in latency was also correlated with the clinical assessment scales, using Spearman’s rank correlation given that the assessment scores are discrete ordinal data. The association between the latency and the tap amplitude was also examined within the same testing session, using the Spearman’s rank correlation test, given that the tap amplitudes were discrete ordinal data, but are not interval scaled; namely, the output effect in the 2 mm tap input was not considered two time of the 1 mm tap and 4 times of the 0.5 mm tap, considering the nonlinear input-output properties of the reflex response.
Results
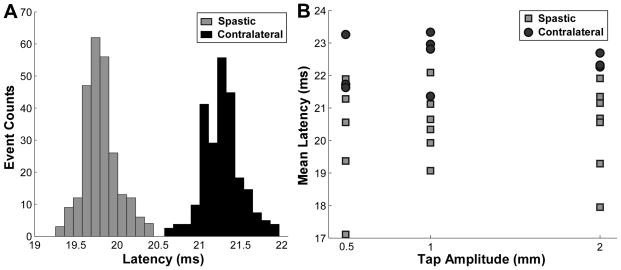
The distribution of the unitary reflex latency in each tap sequence was calculated, and the mean value of the distribution was then compared between the spastic and contralateral side of the same subject. Figure 2A shows two examples of single unit latency distributions with the same tap amplitude (1 mm). Each latency distribution in Figure 2A came from one tap sequence and the SD of the distribution was typically less than 0.5 ms. Here two examples (one from each side) are shown for illustration purposes. The latency was discernibly shorter in the spastic muscle compared with the contralateral one. The mean latencies of different tap sequences at different tap amplitudes from one stroke subject are further depicted in Figure 2B. For this stroke survivor, the mean latency ranged from 17.11 to 22.09 ms for the spastic biceps, and from 21.42 to 23.33 ms for the contralateral biceps.
Figure 2.
Latency distribution and the mean latency comparison between the spastic and contralateral sides of a stroke subject. A: Exemplar distributions of the reflex latency from the spastic and contralateral sides during a 1 mm tap sequence. B: Average latency of individual tap sequences as a function of tap amplitude. Each symbol represents the mean latency of a single motor unit response during each tap sequence.
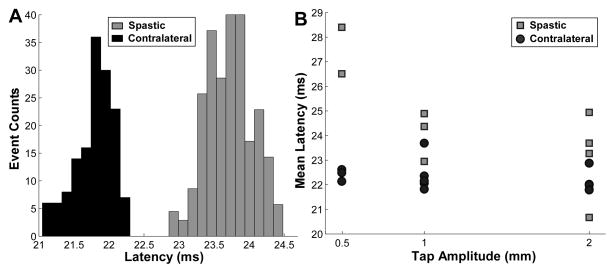
An exemplar subject with a different latency distribution pattern is shown in Figure 3. Here, the latency was longer in the unitary reflex response of the spastic muscle compared with the contralateral one during the 2-mm tap amplitude (Figure 3A). The mean latency of each tap sequence as a function of the different tap amplitudes is shown in Figure 3B. These values include all the mean latencies from the recording session with different tap amplitudes and indentation depths. The mean latency ranged from 20.62 to 28.40 ms for the spastic biceps, and 21.79 to 23.69 ms from the contralateral biceps.
Figure 3.
Latency distribution and the mean latency comparison between the spastic and contralateral sides of a second stroke subject. A: Exemplar distributions of the reflex latency from the spastic and contralateral sides during a 2 mm tap sequence. B: Average latency of individual tap sequences as a function of tap amplitude. The latency in spastic muscle was longer than in the contralateral muscle.
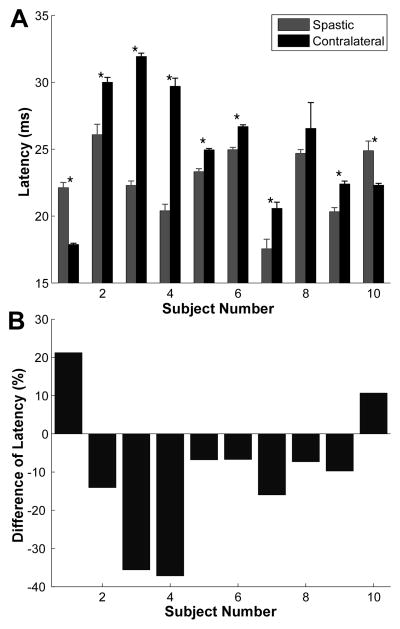
Using the rank correlation test, the latency was associated with the tap amplitude, which likely modulates the size of the afferent input. However, no significant correlation was found between the unit latency and the tap amplitude within the same testing session for the same subject (p > 0.05). The difference in latency from the same tap amplitude between the spastic and contralateral sides was calculated. Given that the effect of tap amplitude was not significant, an average of the latency from the three tap amplitudes was calculated for both sides of the stroke subjects (Figure 4A). Similarly, a difference in latency from the three tap amplitudes was also calculated using Equation 1, and the results from individual subjects are shown in Figure 4B.
Figure 4.
Latency and normalized difference of latency of 10 stroke subjects. A: Latency comparison between the two sides of the stroke subjects. Asterisk represents significant difference. Error bars represent the standard error of latency. B: Relative difference of latency (spastic side – contralateral side) of the stroke subjects.
Among the ten stroke subjects, seven subjects showed a significantly shorter reflex latency in the spastic muscle compared with the contralateral one (p < 0.05), two subjects showed that the reflex latency was significantly longer in the spastic muscle than in the contralateral one (p < 0.05), and the latency was not significantly different between the two sides in one subject (p > 0.05).
The difference of latency was also correlated with the severity of spasticity (Modified Ashworth Scale and Deep Tendon Reflex) and the degree of motor impairment (Fugl-Meyer Assessment and Chedoke-McMaster Assessment). The correlation between the difference of latency and the spasticity scores was not significant (p > 0.05). Similarly, the correlation with the motor impairment assessment was also not significant (p > 0.05). There were no discernable differences regarding the clinical assessment scores in the two subjects that showed reversal of latency differences between the two sides. However, the potential factors that influenced the latency measures are further discussed in the Discussion section.
Discussion
By recording single motor unit responses evoked by a precisely controlled tendon tapper, we were able to accurately estimate the reflex latency of motor unit discharges in passive biceps muscles of chronic stroke survivors. This technique allows us to estimate the reflex latency more rigorously than previous methods that depend on relatively insensitive surface EMG recordings, and that were derived during angular joint rotations or electrical stimulations that typically involve multiple muscles. Using this tapper-based technique, we compared the difference of reflex latency between the spastic and contralateral sides of chronic stroke survivors at different tendon tap amplitudes. Our results revealed that the latency in the spastic muscle was significantly shorter compared with the contralateral muscle in 7 out of 10 stroke subjects, and this effect was reversed in two stroke subjects. (The latency was not statistically different in one stroke subject). These findings provide evidence for increased motoneuron excitability in our subject cohort. The results also provide support for earlier findings that show a substantially reduced reflex threshold in spastic muscles after stroke (Powers et al., 1988, Musampa et al., 2007).
In contrast to a shorter latency in the spastic biceps of 7 subjects, two subjects showed shorter latency in the contralateral sides. One common feature of these two subjects was the thick fat layer above the muscular-tendon junction. Specifically, the fat layer was 17 mm above the biceps tendon of subject 1, and the fat layer was 26 mm for subject 10 based on our ultrasound data. The thick fat layer may have limited the effect of tendon taps at initial indentation. Indeed, in order to apply effective tendon taps to trigger motor unit discharges from these two subjects, we had to apply large static tendon indentions (20 mm in the spastic and 23 mm in the contralateral sides of subject 1, and 18mm in the spastic and 22 mm in the contralateral sides of subject 10, whereas most of other subjects had initial indentations below 10 mm). However, we did not observe differences in the thickness of fat layer between the two sides based on our ultrasound data, and the tendon taper was position controlled to deliver accurate tap amplitudes even with large indentation forces Therefore, it is unlikely that the fat layer would account for the reversed latency effect.
It is possible that the reverse latency effect can come from a potential sample bias towards high threshold units from the intramuscular recordings as described in the subsequent ‘Limitation’ section. Namely, much higher threshold motor units were recorded in the spastic compared with the contralateral biceps. However, we believe that this possibility is unlikely, since 2–3 electrodes were inserted at different locations of the biceps muscle during each session, and two recording sessions were performed at each side to confirm the latency results. Therefore the probability of consistently recording high threshold motor units in the spastic muscle seems very low.
The latency was not significantly different between the two sides of subject 8. The lack of difference may be due to the fact that the motoneurons in the contralateral biceps of subject 8 were also hyperexcitable (Deep Tendon Reflex was 2+ in the contralateral side compared with 2+ in the spastic side). We are uncertain about the origins of such changes in the contralateral muscle. Nevertheless, our current latency measure was able to capture potential abnormalities in the contralateral muscles.
Mechanisms of shorter latency
The observed shorter reflex latency in the spastic muscle in most chronic stroke survivors could potentially be attributed to one of two major factors or to their combination: a sustained depolarization of the resting membrane potential of the spinal motoneuron, or a faster rise of the stretch-evoked excitatory postsynaptic potential (EPSP), as illustrated in Figure 5. There is indirect evidence that both mechanisms can exist in “spastic” motoneurons.
Figure 5.
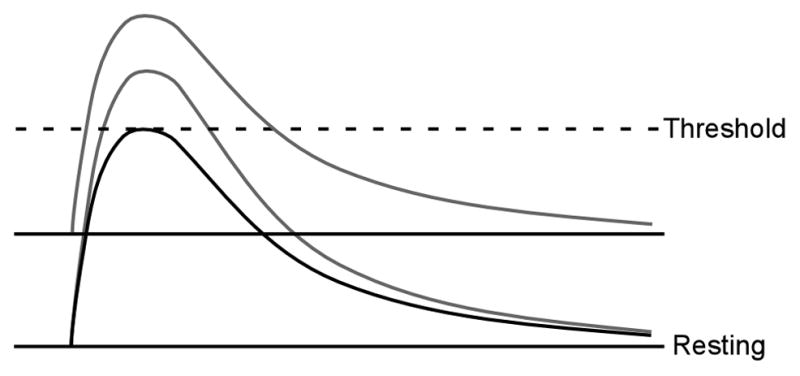
Diagram of the mechanisms of reduced reflex latency. A larger and faster rise of EPSP, or a sustained depolarization of the membrane potential.
A sustained depolarization brings the motoneuron closer to the threshold for excitation. Therefore, an action potential can be triggered earlier, before the composite EPSP reaches its peak (i.e., effective rise time less than the actual time-to-peak of EPSP), which essentially reduces the latency between an afferent input and a motoneuron discharge. A depolarized membrane potential can arise from an enhanced tonic input from excitatory spinal interneurons, or from tonically active descending pathways such as the lateral vestibulospinal tracts (Miller et al., 2014). There could also be a reduced inhibitory input from segmental inhibitory interneurons (such as Renshaw cell recurrent inhibition, or Ia reciprocal inhibition). Finally, there could be a change in the intrinsic excitability of motoneurons, mediated by neuromodulator increases in a voltage-gated persistent inward current (PIC), for example. Both voltage gated sodium and calcium channels can lead to prolonged depolarization of the membrane potential (Li et al., 2004, Heckman et al., 2005)). Although sodium or calcium PICs could increase depolarization, and extend its duration, these currents are unlikely to increase the slope of the initial EPSP measurably, because they do not turn on immediately.
In the second alternative (EPSP properties), action potential latency could be shorter because the tap-evoked EPSP has a faster rise time, a larger peak amplitude, or both. The rise time is most critical, because motoneuron action potential threshold is highly sensitive to the time derivative of the membrane voltage, especially for large amplitude EPSPs. We begin with an analysis of the potential origins of sustained depolarization in hyperexcitable motoneurons.
In our current study, an initial static indentation was applied to the tendon before the tap, and this pre-stretch may lead to sustained discharge of muscle spindle afferents and, therefore, may depolarize the membrane potential of the motoneuron to a degree. In 9 out of 10 stroke survivors, larger indentation depths on the contralateral biceps were needed to elicit consistent action potentials during the tendon tap. As a result, the larger depolarizing current imposed from a larger pre-stretch in the contralateral muscle may balance out, or at least reduce, the possible depolarization differences in the spastic motoneuron. Further investigation is required, possibly in animal models, to confirm the effect of tendon indentation on motoneuron membrane potential.
To return to EPSP contributions, the transient tendon tap elicits a composite EPSP with a rise time of approximately 10 ms, as estimated in human motoneurons (Burke et al., 1984). A larger or steeper rising EPSP could generate a slightly shorter reflex latency, because the action potential threshold is reached more quickly. Several mechanisms could contribute to the change of EPSP properties. A reduction in the membrane conductance can influence the input-output gain, such that a given afferent input can lead to a larger and faster than normal voltage change in the motoneuron membrane, resulting in a faster rise of EPSP. Recent studies have shown that both sodium and calcium PICs, especially the fast sodium PIC, can amplify the motoneuron responses to synaptic input (Manuel et al., 2007, Powers et al., 2012), although probably not for the first few milliseconds.
It is also possible that reduced presynaptic inhibition at the Ia afferent terminals, leading to a more effective afferent synaptic input, could induce a steeper rise of EPSP. A change in the level of presynaptic inhibition can influence the release of neurotransmitters by the afferent fiber, which can possibly generate a larger and steeper rise of EPSP. Typically, a suppression of the H-reflex from tendon vibration of the same muscle is regarded as evidence of presynaptic inhibition. Some studies have shown that the tendon vibration is less effective in suppressing the H-reflex in spastic stroke patients (Milanov, 1992, Stein et al., 1993), while others have found that the effectiveness of brief vibration is not changed (Faist et al., 1994). Therefore, it is necessary to examine the possibility of reduced presynaptic inhibition in spastic stroke using alternative methods that can avoid potential pitfalls (e.g., activation depression of afferent neurons from tendon vibration (Hultborn et al., 1987)).
Association with clinical assessment
When the estimated change of reflex latency was associated with the clinical assessment scales, there was no correlation between the difference in latency and the assessed severity of spasticity, and similarly no correlation was observed between the latency difference and the motor impairment assessment. This lack of correlation could be due to several factors.
First, the severity of spasticity based on the Modified Ashworth Scale ranged from 1 to 2 (with 4 being most spastic) for our recruited stroke survivors; namely, only mild to moderate spastic subjects were recruited in the current study. It is possible that an inclusion of more severely spastic subjects could improve the correlation between the change of reflex latency and the clinical spasticity assessment. However, the existing results already showed a wide range of differences in reflex latency for the subjects with the same Modified Ashworth score. For example, the subjects with a score of 2 had difference of latency ranging from −35% (a strongly shorter latency in the spastic muscle) to 21% (a longer latency in the spastic muscle). Therefore, increasing the range of spasticity severity may not have improved the correlation with clinical scores.
Second, the lack of correlation can also arise from the different characteristics between the reflex latency measure and the clinical assessment scale. The latency measure quantifies motoneuron excitability, whereas the Modified Ashworth assessment represents a resistance to passive stretch that is felt by the clinical examiner. This resistance can come from hyperexcitable stretch reflex or the mechanical property changes of the muscle-tendon complex (e.g., stiffened and shortened muscle fibers partly due to disuse). Studies have shown that muscle contracture can contribute to high muscle tone in the absence of overt reflex hyperexcitability (O’Dwyer et al., 1996). Therefore, the Modified Ashworth scale was insufficient to distinguish these two mechanisms (Pandyan et al., 2003). Additionally, although the inter-rater reliability of assessment score has been tested previously (Pandyan et al., 1999), the score is still dependent on the subjective judgment of the degree of stretch resistance felt by the examiner, and the accuracy and consistency of the assessment may not be guaranteed across patients (Kumar et al., 2006). Therefore, it is not entirely surprising that we did not find a correlation between the reflex latency measure and the clinical assessment scores.
Although the Modified Ashworth score is still widely used in the clinic, our current study further points out the limitation of the clinical scale, which is the inability to distinguish reflex component from mechanical property changes in the muscle. More quantitative assessment approaches that are capable of distinguishing the different components of spasticity are needed for quantifying and managing spasticity in the clinical setting. Recently, different approaches have been proposed to better quantify different aspects of spasticity, including reflex threshold and gain as well as mechanical measures (Levin et al., 1993, Condliffe et al., 2005, Calota et al., 2008, Chardon et al., 2014); however, these approaches typically require lengthy setup time and specialized technical knowledge for clinicians. More simplified and user friendly quantitative approaches may be necessary for wide application in the clinic.
Limitations
Although the current study provides a more accurate estimate of the reflex latency using single unit reflex responses evoked by precisely controlled tendon taps, the limited sample of single motor unit action potential trains could potentially bias the latency estimates comparing with the population estimates obtained using surface EMG recordings. During the experiment, the tap was delivered over a period of 1.5–6 ms depending on the tap amplitude, and the rise time of the composite EPSP would then last approximately 10 ms or even longer. Therefore, depending on the location of the intramuscular recording electrode, the latency estimate derived from the recorded single unit action potential represents just one sample of the motor unit population. However, during each session multiple (5–21) motor unit action potentials were recorded at different tap sequences, which indeed represent a random sample of the population latency. Therefore, the averaged latency measure as shown in Figure 4A is likely a representative estimate of the reflex latency of the biceps muscle.
Given that the reflex activation threshold may differ between the two sides of the stroke subjects, we have to indent the tendon of the contralateral biceps deeper, leading to a greater degree of muscle stretch, in order to record consistent action potentials using the comparable tap amplitudes between two sides. This larger pre-stretch may have produced more sustained depolarizing current to the contralateral motoneuron than to the spastic motoneuron, which may bias the latency estimates. However, this bias would make our results even stronger, because the higher depolarizing current presumably reduces the estimated reflex latency in the contralateral muscle. Therefore, we may get an even higher degree of asymmetry in the reflex latency between the two sides of the stroke subjects, if the pre-stretch level was matched between two sides. There may also be differences in fusimotor drive to muscle spindles on both sides, affecting muscle spindle responsiveness, although there is no clear evidence supporting this possibility (Wilson et al., 1999).
In the current study, the contralateral limb was used as the ‘control’ limb. It is possible that the contralateral limb may also be affected by the stroke. This was especially the case for subject 8, who showed increased tendon tap reflex on both limbs. However, as shown in Figure 4, there was large variability in the latency measures between subjects, and a between-subject comparison may be potentially problematic. Because muscle sizes, limb length, and other anatomical features are more likely to be similar between sides, we still prefer within subject comparisons to large scale comparisons against normal controls, where between subject variability is certainly considerable greater.
Conclusions
Overall, the current study offers a more accurate method to estimate the stretch reflex latency compared with the method using surface EMG detection of reflex through joint rotations, and provides evidence for increased motoneuron excitability that can contribute to spasticity after stroke. Our findings provide direct evidence for the contribution of the major neural component to spasticity in chronic stroke survivors. At present, we cannot separate the effects of sustained depolarization from changes in EPSP magnitude and time course, although both mechanisms may well contribute. Clearly, additional studies are necessary to further understand the physiological mechanisms that contribute to hyperexcitable motoneurons in stroke.
Highlights.
Hyperexcitability of motoneurons is one potential contributing mechanism towards muscle spasticity in stroke.
We quantified the reflex latency of single motor units evoked from a precisely controlled tendon tap on the biceps muscle, as an estimate of motoneuron hyperexcitability.
The latency of the unitary discharge was systematically shorter in the spastic muscle compared with the contralateral muscle.
Acknowledgments
This study was supported by NIH R24 HD50821-07 to WZR.
Footnotes
Conflict of Interest Statement
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakheit AM, Maynard V, Shaw S. The effects of isotonic and isokinetic muscle stretch on the excitability of the spinal alpha motor neurones in patients with muscle spasticity. Eur J Neurol. 2005;12:719–24. doi: 10.1111/j.1468-1331.2005.01068.x. [DOI] [PubMed] [Google Scholar]
- Bakheit AM, Maynard VA, Curnow J, Hudson N, Kodapala S. The relation between Ashworth scale scores and the excitability of the alpha motor neurones in patients with post-stroke muscle spasticity. J Neurol Neurosurg Psychiatry. 2003;74:646–8. doi: 10.1136/jnnp.74.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–7. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, McKeon B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. J Neurophysiol. 1984;52:435–48. doi: 10.1152/jn.1984.52.3.435. [DOI] [PubMed] [Google Scholar]
- Calota A, Feldman AG, Levin MF. Spasticity measurement based on tonic stretch reflex threshold in stroke using a portable device. Clin Neurophysiol. 2008;119:2329–37. doi: 10.1016/j.clinph.2008.07.215. [DOI] [PubMed] [Google Scholar]
- Chardon MK, Rymer WZ, Suresh NL. Quantifying the deep tendon reflex using varying tendon indentation depths: applications to spasticity. IEEE Trans Neural Syst Rehabil Eng. 2014;22:280–9. doi: 10.1109/TNSRE.2014.2299753. [DOI] [PubMed] [Google Scholar]
- Condliffe EG, Clark DJ, Patten C. Reliability of elbow stretch reflex assessment in chronic post-stroke hemiparesis. Clin Neurophysiol. 2005;116:1870–8. doi: 10.1016/j.clinph.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Dietz V, Quintern J, Berger W. Electrophysiological studies of gait in spasticity and rigidity. Evidence that altered mechanical properties of muscle contribute to hypertonia. Brain. 1981;104:431–49. doi: 10.1093/brain/104.3.431. [DOI] [PubMed] [Google Scholar]
- Dietz V, Sinkjaer T. Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol. 2007;6:725–33. doi: 10.1016/S1474-4422(07)70193-X. [DOI] [PubMed] [Google Scholar]
- Faist M, Mazevet D, Dietz V, Pierrot-Deseilligny E. A quantitative assessment of presynaptic inhibition of Ia afferents in spastics. Differences in hemiplegics and paraplegics. Brain. 1994;117:1449–55. doi: 10.1093/brain/117.6.1449. [DOI] [PubMed] [Google Scholar]
- Finley JM, Perreault EJ, Dhaher YY. Stretch reflex coupling between the hip and knee: implications for impaired gait following stroke. Exp Brain Res. 2008;188:529–40. doi: 10.1007/s00221-008-1383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Gottlieb GL, Agarwal GC, Penn R. Sinusoidal oscillation of the ankle as a means of evaluating the spastic patient. J Neurol Neurosurg Psychiatry. 1978;41:32–9. doi: 10.1136/jnnp.41.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowland C, Stratford P, Ward M, Moreland J, Torresin W, Van Hullenaar S, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993;24:58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve. 2005;31:135–56. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Hui-Chan CW, Levin MF. Stretch reflex latencies in spastic hemiparetic subjects are prolonged after transcutaneous electrical nerve stimulation. Can J Neurol Sci. 1993;20:97–106. doi: 10.1017/s0317167100047636. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Pierrot-Deseilligny E, Shindo M. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol. 1987;389:757–72. doi: 10.1113/jphysiol.1987.sp016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz RT, Rymer WZ. Spastic hypertonia: mechanisms and measurement. Arch Phys Med Rehabil. 1989;70:144–55. [PubMed] [Google Scholar]
- Knutsson E, Martensson A. Dynamic motor capacity in spastic paresis and its relation to prime mover dysfunction, spastic reflexes and antagonist co-activation. Scand J Rehabil Med. 1980;12:93–106. [PubMed] [Google Scholar]
- Knutsson E, Richards C. Different types of disturbed motor control in gait of hemiparetic patients. Brain. 1979;102:405–30. doi: 10.1093/brain/102.2.405. [DOI] [PubMed] [Google Scholar]
- Kumar RT, Pandyan AD, Sharma AK. Biomechanical measurement of post-stroke spasticity. Age Ageing. 2006;35:371–5. doi: 10.1093/ageing/afj084. [DOI] [PubMed] [Google Scholar]
- Lance JW. Symposium synopsis. In: GFR, RYR, Koella WP, editors. Spasticity: disordered motor control. Chicago: Year Book Medical Publishers; 1980. pp. 485–95. [Google Scholar]
- Lee WA, Boughton A, Rymer WZ. Absence of stretch reflex gain enhancement in voluntarily activated spastic muscle. Exp Neurol. 1987;98:317–35. doi: 10.1016/0014-4886(87)90245-7. [DOI] [PubMed] [Google Scholar]
- Levin MF, Hui-Chan C. Are H and stretch reflexes in hemiparesis reproducible and correlated with spasticity? J Neurol. 1993;240:63–71. doi: 10.1007/BF00858718. [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol. 2004;91:767–83. doi: 10.1152/jn.00788.2003. [DOI] [PubMed] [Google Scholar]
- Manuel M, Meunier C, Donnet M, Zytnicki D. Resonant or not, two amplification modes of proprioceptive inputs by persistent inward currents in spinal motoneurons. J Neurosci. 2007;27:12977–88. doi: 10.1523/JNEUROSCI.3299-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanov I. A comparative study of methods for estimation of presynaptic inhibition. J Neurol. 1992;239:287–92. doi: 10.1007/BF00810355. [DOI] [PubMed] [Google Scholar]
- Miller DM, Klein CS, Suresh NL, Rymer WZ. Asymmetries in vestibular evoked myogenic potentials in chronic stroke survivors with spastic hypertonia: evidence for a vestibulospinal role. Clin Neurophysiol. 2014 doi: 10.1016/j.clinph.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musampa NK, Mathieu PA, Levin MF. Relationship between stretch reflex thresholds and voluntary arm muscle activation in patients with spasticity. Exp Brain Res. 2007;181:579–93. doi: 10.1007/s00221-007-0956-6. [DOI] [PubMed] [Google Scholar]
- O’Dwyer NJ, Ada L, Neilson PD. Spasticity and muscle contracture following stroke. Brain. 1996;119:1737–49. doi: 10.1093/brain/119.5.1737. [DOI] [PubMed] [Google Scholar]
- Pandyan AD, Johnson GR, Price CI, Curless RH, Barnes MP, Rodgers H. A review of the properties and limitations of the Ashworth and modified Ashworth Scales as measures of spasticity. Clin Rehabil. 1999;13:373–83. doi: 10.1191/026921599677595404. [DOI] [PubMed] [Google Scholar]
- Pandyan AD, Price CI, Barnes MP, Johnson GR. A biomechanical investigation into the validity of the modified Ashworth Scale as a measure of elbow spasticity. Clin Rehabil. 2003;17:290–3. doi: 10.1191/0269215503cr610oa. [DOI] [PubMed] [Google Scholar]
- Perry J. Determinants of muscle function in the spastic lower extremity. Clin Orthop Relat Res. 1993;288:10–26. [PubMed] [Google Scholar]
- Pisano F, Miscio G, Del Conte C, Pianca D, Candeloro E, Colombo R. Quantitative measures of spasticity in post-stroke patients. Clin Neurophysiol. 2000;111:1015–22. doi: 10.1016/s1388-2457(00)00289-3. [DOI] [PubMed] [Google Scholar]
- Powers RK, Campbell DL, Rymer WZ. Stretch reflex dynamics in spastic elbow flexor muscles. Ann Neurol. 1989;25:32–42. doi: 10.1002/ana.410250106. [DOI] [PubMed] [Google Scholar]
- Powers RK, Marder-Meyer J, Rymer WZ. Quantitative relations between hypertonia and stretch reflex threshold in spastic hemiparesis. Ann Neurol. 1988;23:115–24. doi: 10.1002/ana.410230203. [DOI] [PubMed] [Google Scholar]
- Powers RK, Nardelli P, Cope TC. Frequency-dependent amplification of stretch-evoked excitatory input in spinal motoneurons. J Neurophysiol. 2012;108:753–9. doi: 10.1152/jn.00313.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RB, Yang JF, Belanger M, Pearson KG. Modification of reflexes in normal and abnormal movements. Prog Brain Res. 1993;97:189–96. doi: 10.1016/s0079-6123(08)62277-3. [DOI] [PubMed] [Google Scholar]
- Trumbower RD, Ravichandran VJ, Krutky MA, Perreault EJ. Contributions of altered stretch reflex coordination to arm impairments following stroke. J Neurophysiol. 2010;104:3612–24. doi: 10.1152/jn.00804.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker HK. Deep Tendon Reflexes. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3. Boston: 1990. [PubMed] [Google Scholar]
- Wilson LR, Gandevia SC, Inglis JT, Gracies J, Burke D. Muscle spindle activity in the affected upper limb after a unilateral stroke. Brain. 1999;122:2079–88. doi: 10.1093/brain/122.11.2079. [DOI] [PubMed] [Google Scholar]
- Wissel J, Manack A, Brainin M. Toward an epidemiology of poststroke spasticity. Neurology. 2013;80:S13–9. doi: 10.1212/WNL.0b013e3182762448. [DOI] [PubMed] [Google Scholar]