Abstract
Objectives:
We hypothesized that integrated motor-visual functions measured by manipulative manual dexterity are affected by white matter lesion (WML) burden as measured on cranial MRI across relevant brain regions in subjects at risk of preclinical occult vascular disease.
Methods:
A real-time cross-sectional study of healthy subjects aged 29 to 74 years with a family history of early-onset coronary artery disease (n = 714; mean age, 51 ± 11 years; mean education, 14 ± 3 years; 42% male; 38% black) were identified from probands with coronary artery disease diagnosed before age 60 years. WMLs on 3-tesla brain MRI and Grooved Pegboard scores were measured.
Results:
WMLs were observed at all ages. Mean pegboard scores were 108 ± 18, similar to normal populations. In unadjusted analysis, WMLs and pegboard scores were significantly correlated by region (total WMLs, r = 0.34, p = 0.0001; frontal [r = 0.34, p < 0.0001], insula [r = 0.31, p < 0.0001], parietal [r = 0.31, p < 0.0001], and temporal [r = 0.17, p < 0.0001]). In multivariate analysis predicting (log) pegboard score adjusted for age, sex, race, education, regional or total volumes, and familial nonindependence, total WML volume (p = 5.79E − 05) and regional WML volumes (p < 0.01) retained statistical significance in all but the youngest age quartile (29–43 years).
Conclusions:
Greater WML volumes in different brain regions are associated with higher pegboard scores (worse performance) independent of age, sex, race, education, and total or regional volumes. This suggests that small vessel cerebrovascular disease may be present in healthy individuals in a preclinical state with measurable impact on complex integrative functions in individuals with excess risk of clinical vascular disease.
White matter lesions (WMLs) appearing as hyperintense regions on fluid-attenuated inversion recovery (FLAIR) and T2 MRIs are thought to be caused by small vessel cerebrovascular disease.1 WML volume and number are associated with age and are most notable in older age groups.1,2 Overall, WML burden is an independent predictor of clinical dementia and of decrements in motor function, including gait, psychomotor speed, and motor apraxia.3–6
We have published data that show a high prevalence of occult coronary artery disease (CAD) and cerebral small vessel lesions in younger and middle-aged people from families with a history of early-onset CAD.7–9 High-risk individuals may also be at increased risk of functional consequences of occult small vessel cerebrovascular disease.9 To date, most studies of WMLs have addressed older populations and persons with cognitive functional decline or clinical dementia.2 The Northern Manhattan Study, which was conducted in a healthy community population, found that patients with a WML volumetric threshold at the 75th percentile performed significantly worse than those in the lowest quartile on a manual dexterity test. WML volumes across the continuum were not evaluated, and the population was elderly.6
Few studies have addressed task-specific regions of the brain, WMLs, and a measure of complex psychomotor function in apparently healthy people. Because manipulative manual dexterity involves many integrative functions in the brain, it serves as an excellent measure to examine the functional impact of WML burden in task-specific regions of the brain. Functional imaging studies show that corticospinal tracts originating from the cerebral cortex are involved in manual dexterity in humans.10
The neuronal tracts controlling manual dexterity originate from cortical regions in the frontal lobe and include the supplementary motor area, anterior cingulate, and postarcuate gyrus, as well as the parietal and insular cortices.11 However, the extent to which incremental WML burden causes variation within the normal ranges of manipulative manual dexterity in task-specific regions of the brain in a healthy younger and primarily middle-aged population remains unknown. Therefore, we examined asymptomatic healthy individuals from high-risk families in a real-time cross-sectional analysis to test the hypothesis that decrements in function on a test of manipulative manual dexterity, even within normal ranges, are associated with increasing WML burden in multiple task-relevant areas of the brain.
METHODS
Sample and recruitment.
Participants in this cross-sectional study were healthy family members of persons with known early-onset CAD. Healthy subjects were randomly selected and recruited (2008–2013) from the Genetic Study of Atherosclerosis Risk (GeneSTAR),7 an ongoing prospective study of vascular disease risk factors and occult disease conducted at the Johns Hopkins medical campus in Baltimore, MD. The parent study was designed to characterize the genetic and biological factors associated with incident cardiovascular and cerebrovascular disease in 3,533 family members of 891 probands with documented early-onset CAD. A total of 1,943 individuals were eligible; 186 refused, 1,757 were randomly assigned. We explored eligibility for 1,293 participants; 126 were ineligible. The first 808 agreeable, eligible participants contacted were included. The sample for this cross-sectional MRI substudy consisted of 714 apparently healthy individuals with complete data at the time of analysis; 86 participants missing MRI volumetric analysis, 5 missing the Grooved Pegboard Test, and 3 missing lipid data were excluded. These 714 participants were identified from 374 families (one index case per family); on average, we screened 1.9 ± 1.3 relatives per family (range 1–8).
Probands were identified upon hospitalization for acute myocardial infarction or an acute coronary syndrome with angiographic evidence of a flow-limiting stenosis of >50% diameter in at least one coronary artery before 60 years of age. Probands did not participate in this study. Apparently healthy asymptomatic siblings, their offspring, and the offspring of the probands were eligible for the study if they were 29 to 75 years of age, had no known personal history of CAD, stroke, TIAs, or disfigurement of their hands affecting Grooved Pegboard Test performance. Siblings and offspring were excluded if they used chronic corticosteroids or had life-threatening diseases, neurologic diseases impairing accurate MRI interpretation, and implanted metals prohibiting MRI scans. Participants with atrial fibrillation or symptomatic cardiovascular disease were excluded from the study.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Johns Hopkins Medicine Institutional Review Board (NA_00002856). All participants provided written informed consent before screening. This was not a clinical trial.
Participant screening.
A physician examined each participant and took a complete medical history, including medications, a history of arthritis, and evaluation of alcohol and other drug use. All participants were screened for coronary disease and stroke risk factors, including hypertension, diabetes, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, body mass index, and smoking.12 Race was self-reported. Height and weight (in pounds) were measured with participants wearing indoor clothing and no shoes. Body mass index was calculated as weight in kilograms/(height in meters)2.13 Current cigarette smoking was assessed by self-report of any smoking within the past month and/or 2 expired carbon monoxide levels of ≥8 ppm. Blood pressure was measured 3 times over the course of 1 day according to guidelines of the American Heart Association.14 The average systolic and diastolic blood pressures were used to characterize blood pressure. Hypertension was defined as ≥140 mm Hg systolic or 90 mm Hg diastolic, and/or use of an antihypertensive drug. After participants had fasted for 12 hours overnight, blood was taken for measurement of cholesterol and glucose levels. Type 2 diabetes was defined as a physician-diagnosed history, a fasting glucose level of ≥126 mg/dL, and/or use of hypoglycemic antidiabetic medications. Total cholesterol, high-density lipoprotein cholesterol, and triglyceride levels were measured according to the United States Centers for Disease Control standardized methods,15 and low-density lipoprotein cholesterol was estimated by using the Friedewald formula.16 For persons with triglyceride levels >400 mg/dL, standard ultracentrifugation methods were used to determine low-density lipoprotein cholesterol. Alcohol use and substance use were determined from standardized questionnaires from the Statewide Behavioral Risk Factor Survey of the Centers for Disease Control. Heavy alcohol consumption was defined according to the National Institute of Alcohol Abuse and Alcoholism and the Centers for Disease Control as ≥8 drinks per week for women or 14 for men.
MRI.
All participants underwent MRI with a Philips 3T imaging unit according to standardized protocols. The series included axial T1-weighted MPRAGE (magnetization-prepared rapid-gradient echo) and axial turbo spin echo FLAIR as follows. (1) Axial T1-weighted MPRAGE: repetition time 10 milliseconds (ms), echo time 6 ms, voxel size 0.75 × 0.75 × 1.0 mm3, contiguous slices, with field of view imaging 240 mm, and matrix 256 × 256 × 160 mm. (2) Axial turbo spin echo FLAIR: repetition time 11,000 ms, inversion time 2,800 ms, echo time 68 ms, voxel size 0.47 × 0.47 × 3.0 mm3, contiguous slices, field of view 240 mm, and matrix 256 × 256 mm. A blinded trained nonstudy neuroradiologist and a study neuroradiologist (D.Y.) read all images using methods developed in the Atherosclerosis Risk in Communities Study.17 Initial discordances were reviewed and resolved by consensus. Silent infarcts were defined as those cystic lesions incorporating signal characteristics similar to CSF T1 dark, T2 bright, with a diameter >3 mm as identified by a human reader.
Volumetric assessment.
Anatomical volumes of discrete brain regions and tissue types were determined with MPRAGE images, while WML volumes were determined using FLAIR images coregistered into MNI (Montreal Neurological Institute) space.18 MPRAGE images were skull-stripped and coregistered to FLAIR images. Spatial normalization of the coregistered MPRAGE and FLAIR images into MNI space was performed via affine transformation. A trained rater manually delineated the WMLs on the normalized FLAIR images (with reference to the MPRAGE images for verification of apparent pathology) using an in-house–developed lesion segmentation protocol with MIPAV (Medical Image Processing, Analysis, and Visualization) software.19 WML volumes included those lesions that were T2 hyperintense based on an intrinsic threshold of lesion detection. We then separated the complete WML volumes from the healthy brain tissue in the FLAIR images and classified them with an automated probabilistic methodology using a topology-preserving algorithm (topology-preserving anatomy-driven segmentation [TOADS]).18 The WML volumes were found in periventricular, juxtacortical, and subcortical regions.
The TOADS algorithm was also used to identify each brain area of interest on MPRAGE images by applying an atlas from the International Consortium on Brain Mapping, which allowed for determination of regional volumes.20 We measured total brain, intracranial, cortical gray matter, and white matter volumes, as well as regional volumes including frontal, parietal, temporal, and occipital lobes, insula, and cerebellum. Total brain volume (in cubic millimeters) was identified as the sum of white matter, WML, and gray matter volumes from the vertex of the brain to the foramen magnum. Intracranial volume was defined (in cubic millimeters) as the sum of all meningeal material, soft tissue, and sulcal and ventricular cerebrospinal volumes inferior to bone from the vertex to the foramen magnum.21
Mini-Mental State Examination test.
On the day of examination, the Mini-Mental State Examination (MMSE) was administered by a trained professional nurse as a brief test for the quantitative assessment of cognitive function and impairment. The MMSE examines orientation, immediate and short-term memory, attention and calculation, language, and praxis. The National Institute for Health and Care Excellence classifies scores of 25–30 of 30 as normal, 21–24 as mild, 10–20 as moderate, and <10 as severe cognitive impairment.22
Manipulative manual dexterity test.
On the day of the MRI, we assessed manual dexterity using the Grooved Pegboard Test (Lafayette Instruments, Lafayette, IN).23 Each peg has a ridge that must be placed correctly to fit into its corresponding pegboard holes. This requires significant visual input, as well as sensory feedback and intention adjustments.24 The Grooved Pegboard Test has well established norms.25 Participants were given 25 pegs to place into the board as quickly as possible during one trial per hand. The sum of the time taken to complete the test (in seconds), the number of dropped pegs, and the total number of pegs placed was calculated for each hand.23 Because all subjects completed all 25 pegs, the scores were essentially determined by time and the number of dropped pegs. The scores of both hands were averaged, and the average was used in all analyses. A higher score indicated worse performance.
Statistical analysis.
We examined all distributions using standard descriptive analyses. We used Spearman correlation coefficients for nonnormally distributed variables. We used separate multivariate linear regression analysis to determine the relationship of total WML burden and also WML burden by task-specific brain regions, including frontal lobe, parietal lobe, occipital lobe, temporal lobe, insula, and cerebellum, to Grooved Pegboard performance. The Generalized Estimating Equations approach was used to adjust for nonindependence among families. The Grooved Pegboard Test score had a right-skewed distribution; hence, the scores were logarithmically transformed for regression analysis and the regression coefficients were exponentiated to the original scale (eβ − 1) for tabulation and expressed as the percent difference in the geometric mean of pegboard scores associated with greater WML volumes. Regression coefficients were tabulated per SD of total and regional WML volumes, respectively, as SD represents the typical interindividual difference in WML volumes in this sample. The original unit of volume measurement (cubic millimeter) is not directly interpretable because this small volume does not represent a meaningful difference between individuals, either in total or regional WML volumes. We also examined the age interaction of the association between total WML volume and pegboard score. We performed a series of Generalized Estimating Equation models dichotomizing age at every possible integer value and included an interaction term for association of total WML volume with pegboard score. For each model, we assessed the fit using the overall model χ2. We present a credible range of best fit age dichotomy as models with up to 2 units lower − (log [p value]) associated with the model χ2.
RESULTS
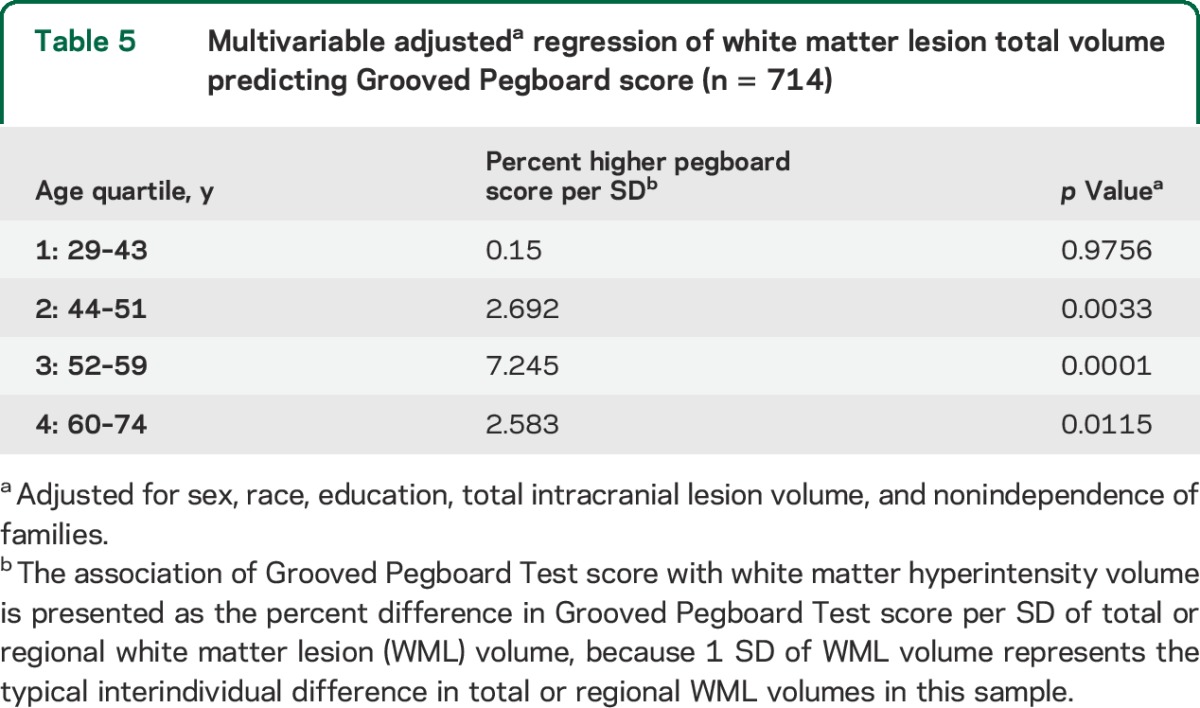
Sample characteristics are shown in table 1. Subjects comprised 714 individuals with an average age in “middle age.” Age ranged from 29 to 74 years, with mean age of 51 (±11) years. More than a third were African American, and slightly less than half were male. Alcohol and substance abuse were uncommon, as were physical disabilities obviating the ability to perform the manual testing. The raw Grooved Pegboard Test score was normalized by log transformation as described in the methods section. Most participants (91%) had WMLs, although volumes varied markedly. In unadjusted models of WMLs in cubic millimeters, a comparison of regional volumes to log Grooved Pegboard Test score showed that increasing WML volumes in all task-specific brain areas were significantly associated with increasing log score and worse performance on the test (table 2). No significant association was observed between WML volume in the occipital lobe and Grooved Pegboard Test score. Table 3 shows the unadjusted distributions of Grooved Pegboard Test score based on tertile of total brain WML volume within the 4 age quartiles. Although there was an increment across all ages, the association between WML volume and test performance is evident in all but the youngest age quartile. Table 4 shows separate multivariate regression models for the relationship between total WML volume for each region adjusted for sex, age, race, familial nonindependence, educational level, and the total volume of each region. Significant associations were observed between WML volume and log-normalized Grooved Pegboard Test score for all brain regions except the temporal and occipital lobes. Additional multivariable adjusted analysis demonstrated the relationship within age quartile for total WML volume and the Grooved Pegboard Test score (table 5). Again, the relationship was incremental and statistically significant within all but the youngest age quartile of subjects younger than 44 years of age. Only in the oldest age quartile did the Grooved Pegboard Test score reach levels beyond the age-specific reference norms.6 Additional multivariate regression analyses were also performed adding heavy alcohol consumption as described in the methods (n = 14), arthritis (n = 163), and scores from the MMSE (99% in the normal range of 25 or above). No silent strokes or lacunar strokes were observed in the study population. None of these variables were statistically significantly associated with the Grooved Pegboard scores in multivariate analyses, and when added to our final regression equations, did not alter the significance of the relationship between any WML volumes and the Grooved Pegboard Test scores (results not shown).
Table 1.
Sample characteristics (n = 714)
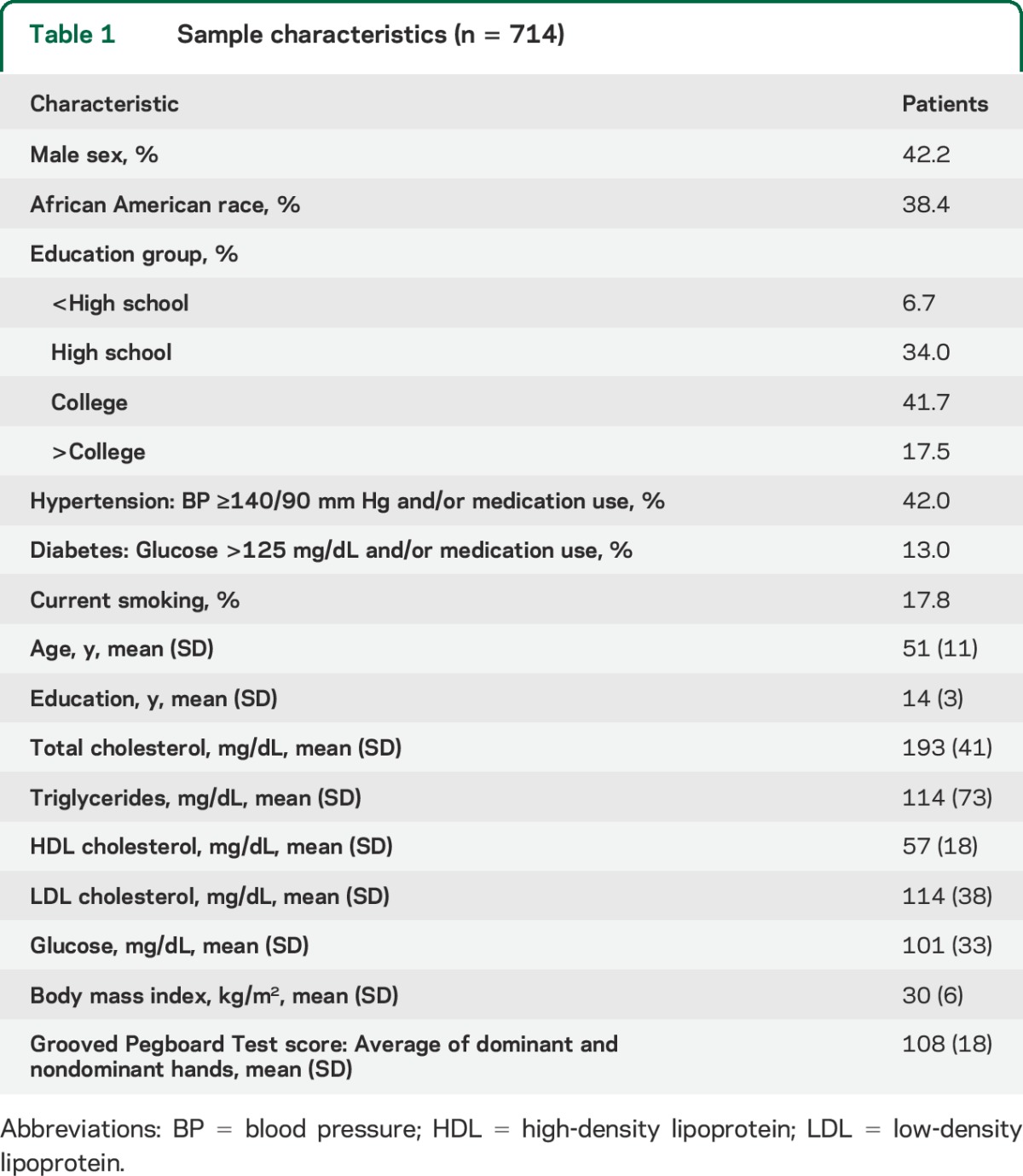
Table 2.
Bivariable associations of white matter lesion volumes by region with Grooved Pegboard Test scores (n = 714)
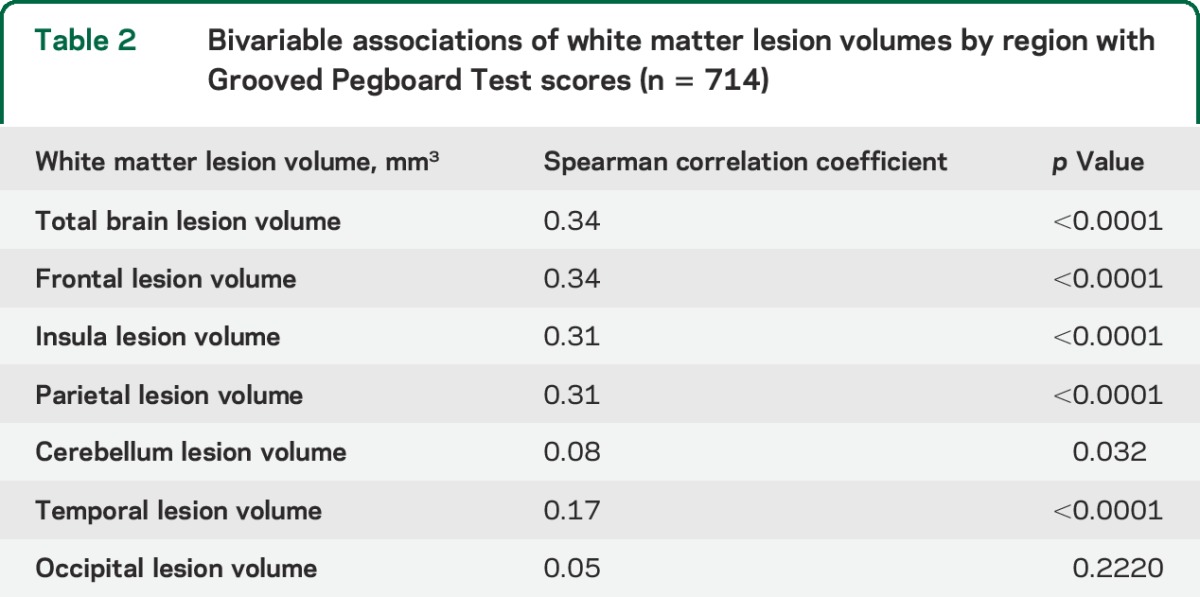
Table 3.
Grooved Pegboard Test score raw distributions by tertiles of white matter total lesion volume
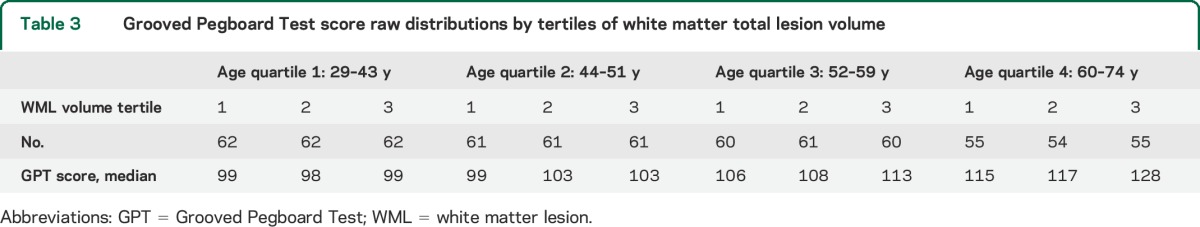
Table 4.
Separate multivariablea adjusted regression models for total and regional white matter lesion volumes predicting Grooved Pegboard Test score (n = 714)
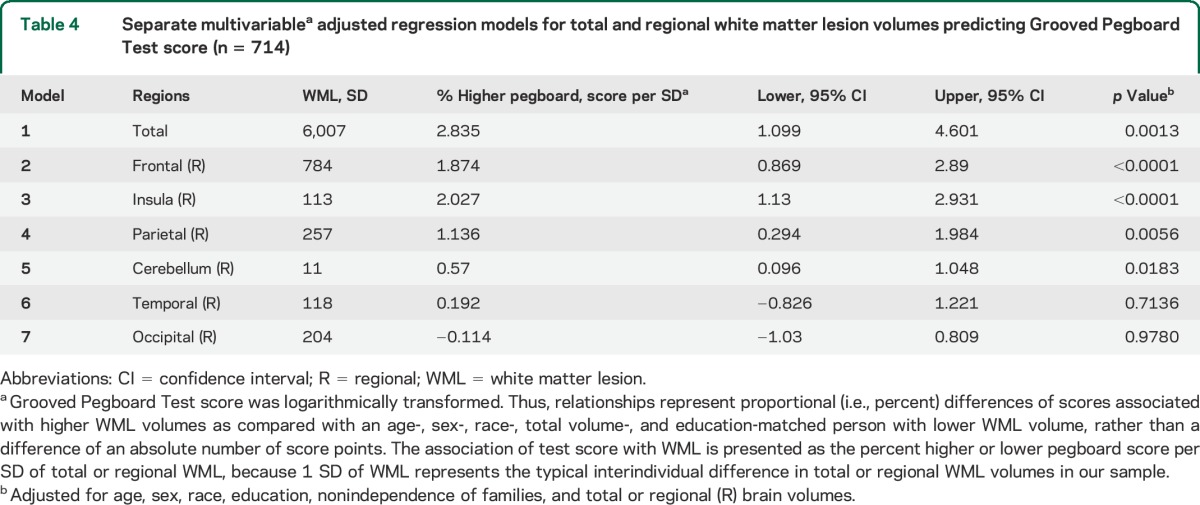
Table 5.
Multivariable adjusteda regression of white matter lesion total volume predicting Grooved Pegboard score (n = 714)
DISCUSSION
Manipulative manual dexterity, within normal performance ranges on the Grooved Pegboard Test, was significantly associated with increasing WML burden in our apparently healthy sample of individuals with increased susceptibility to subsequent cardiovascular and cerebrovascular events. Subjects overall were notably younger than in prior studies. Even after adjustment for age, sex, race, and educational level, and for total volumes of each region, the relationship was still detected in our age quartiles commencing at age 44 years. This association was observed across many different brain regions that are involved in manipulative manual dexterity. Furthermore, the findings were not altered by MMSE scores, the presence of arthritis, substance abuse, or heavy alcohol consumption. The findings suggest that in this population enriched for vascular disease susceptibility, WML burden has a significant impact on the manual dexterity performance of healthy people as young as 44 years of age, the youngest ages reported to date. These data support the important concept that WML burden at almost any time in life, regardless of symptomatic state, is associated with decrements in functioning.
WML burden has been associated with decreased capacity for gross motor function such as gait, as well as visuo-psychomotor function, in people older than 65 years.6,26–28 However, this is the first study to suggest that in a higher-risk population, the associations exist at much younger ages.
Scores on the Grooved Pegboard Test have been more strongly predictive of cognitive involvement than motor involvement in persons with Parkinson disease, suggesting that manual dexterity involves brain regions well beyond those that control merely motor function.29 These findings suggest that the disruption of cerebral white matter in key regions, such as the frontal, temporal, and insular lobes, interferes with the execution of complex integrative behaviors that operate across different sensory and motor modalities. Manipulative manual dexterity is a complex function that may be more sensitive to the effects of ischemic white matter disease than other less complex gross motor tasks such as gait.
Our study population is limited to families with a history of early-onset CAD.7 Unique characteristics predispose these families to preclinical vascular disease, and WMLs may not be as prevalent or deleterious in the general population. Our study population is generally enriched for traditional atherosclerotic risk factors as well as concomitant vascular disease genetic factors. Other longitudinal studies have reported increased rates of WMLs with aging and incremental disruption in cognitive function.30 Because our study was cross-sectional, it is limited in its ability to demonstrate lesion progression and the meaning of the association over time.30
Findings confirm that greater WML volumes in multiple brain locations are associated with higher pegboard scores (worse performance) across the normal ranges, independent of age, sex, race, and education or total volumes of each region examined. The results suggest that small vessel cerebrovascular disease is present in healthy individuals in an early preclinical state. WML volumes thus have a small but measurable impact on complex integrative functions as measured by manipulative manual dexterity in individuals with a known excess risk of clinical vascular disease. Additional longitudinal studies with serial measures of complex integrative psychomotor tasks, including manual dexterity, may prove critical in characterizing the nature of WML progression and declines in these performance-related cognitive-motor functions. The involvement of white matter in multiple brain areas suggests the possibility that specific combinations of WML locations may influence manual dexterity rather than a single WML burden in a single brain region. The effects of combined regions should be investigated in future studies using techniques such as functional connectivity or fMRI.
Because early but mild impairment in performance occurs with increasing WML burden, it would be important to determine the extent to which progression of WMLs can be delayed by traditional approaches to vascular disease prophylaxis.6,28,31
Supplementary Material
GLOSSARY
- CAD
coronary artery disease
- FLAIR
fluid-attenuated inversion recovery
- MMSE
Mini-Mental State Examination
- MPRAGE
magnetization-prepared rapid-gradient echo
- TOADS
topology-preserving anatomy-driven segmentation
- WML
white matter lesion
Footnotes
Editorial, page 1914
AUTHOR CONTRIBUTIONS
Paul A. Nyquist designed and conceptualized the study, aided in the analysis and interpretation of the data, wrote and drafted the final manuscript, and is responsible for its final content. Lisa R. Yanek aided in the analysis and interpretation of the data and helped draft the final manuscript. Murat Bilgel aided in the analysis and interpretation of the data and helped draft the final manuscript. Jennifer L. Cuzzocreo aided in the analysis and interpretation of the data. Lewis C. Becker helped conceptualize the study, aided in the analysis and interpretation of the data, and drafted the final manuscript. Karrine Chevalier-Davis aided in the collection of data, writing of the manuscript, and editing of the manuscript. David M. Yousem aided in interpretation of the data and drafted the final manuscript. Jerry Prince aided in the analysis and interpretation of the data. Brian G. Kral aided in the design, interpretation of the data, and helped draft the manuscript. Dhananjay Vaidya completed the analysis and interpretation of the data and helped draft the final manuscript. Diane M. Becker designed and conceptualized the study, aided in the analysis and interpretation of the data, and helped draft the final manuscript.
STUDY FUNDING
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the NIH under award R01NS062059. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURE
P. Nyquist is funded by RO1NS062059-01. L. Yanek, M. Bilgel, and J. Cuzzocreo report no disclosures relevant to the manuscript. L. Becker is funded by the following NIH grants: HL107446, HL112064, HL092165, NS062059, HL089474, NR010021, HL088215, and HL099747. K. Chevalier-Davis and D. Yousem report no disclosures relevant to the manuscript. J. Prince is a founder and stockholder of Diagnosoft, Inc. and was a consultant for Diagnosoft, Inc. His research is funded by NIH grants 5R21EB012765, 5R21EY022150, 5R01NS056307, R21NS082891, 5R01NS070906, 2R01nS055951, and 5R01CA133015. He received research support from Siemens Corporation. B. Kral reports no conflicts. D. Vaidya is a consultant for MBC Inc., and is funded by NIH grants HL092165, ES021366, and TR001079. D. Becker is funded by NIH grants HL107446, HL112064, HL092165, NS062059, HL089474, NR010021, HL088215, and HL099747. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology 2008;71:804–811. [DOI] [PubMed] [Google Scholar]
- 2.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM; Rotterdam Scan Study. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke 2003;34:1126–1129. [DOI] [PubMed] [Google Scholar]
- 3.Hannestad J, Taylor WD, McQuoid DR, et al. White matter lesion volumes and caudate volumes in late-life depression. Int J Geriatr Psychiatry 2006;21:1193–1198. [DOI] [PubMed] [Google Scholar]
- 4.Baezner H, Blahak C, Poggesi A, et al. Association of gait and balance disorders with age-related white matter changes: the LADIS Study. Neurology 2008;70:935–942. [DOI] [PubMed] [Google Scholar]
- 5.Blahak C, Baezner H, Pantoni L, et al. Deep frontal and periventricular age related white matter changes but not basal ganglia and infratentorial hyperintensities are associated with falls: cross sectional results from the LADIS Study. J Neurol Neurosurg Psychiatry 2009;80:608–613. [DOI] [PubMed] [Google Scholar]
- 6.Wright CB, Festa JR, Paik MC, et al. White matter hyperintensities and subclinical infarction: associations with psychomotor speed and cognitive flexibility. Stroke 2008;39:800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker DM, Yook RM, Moy TF, Blumenthal RS, Becker LC. Markedly high prevalence of coronary risk factors in apparently healthy African-American and white siblings of persons with premature coronary heart disease. Am J Cardiol 1998;82:1046–1051. [DOI] [PubMed] [Google Scholar]
- 8.Nyquist PA, Wityk R, Yanek LR, et al. Silent small-vessel cerebrovascular disease and silent myocardial ischemia in families with premature coronary disease. Neuroepidemiology 2009;33:66–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kral BG, Nyquist P, Vaidya D, et al. Relation of subclinical coronary artery atherosclerosis to cerebral white matter disease in healthy subjects from families with early-onset coronary artery disease. Am J Cardiol 2013;112:747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holtrop JL, Loucks TM, Sosnoff JJ, Sutton BP. Investigating age-related changes in fine motor control across different effectors and the impact of white matter integrity. Neuroimage 2014;96:81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darian-Smith I, Galea MP, Darian-Smith C. Manual dexterity: how does the cerebral cortex contribute? Clin Exp Pharmacol Physiol 1996;23:948–956. [DOI] [PubMed] [Google Scholar]
- 12.Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA 2003;290:1049–1056. [DOI] [PubMed] [Google Scholar]
- 13.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr 1998;68:899–917. [DOI] [PubMed] [Google Scholar]
- 14.Brook RD, Appel LJ, Rubenfire M, et al. Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the American Heart Association. Hypertension 2013;61:1360–1383. [DOI] [PubMed] [Google Scholar]
- 15.Myers GL, Kimberly MM, Waymack PP, Smith SJ, Cooper GR, Sampson EJ. A reference method laboratory network for cholesterol: a model for standardization and improvement of clinical laboratory measurements. Clin Chem 2000;46:1762–1772. [PubMed] [Google Scholar]
- 16.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 17.Manolio TA, Kronmal RA, Burke GL, et al. Magnetic resonance abnormalities and cardiovascular disease in older adults: the Cardiovascular Health Study. Stroke 1994;25:318–327. [DOI] [PubMed] [Google Scholar]
- 18.Shiee N, Bazin PL, Ozturk A, Reich DS, Calabresi PA, Pham DL. A topology-preserving approach to the segmentation of brain images with multiple sclerosis lesions. Neuroimage 2010;49:1524–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bazin PL, Cuzzocreo JL, Yassa MA, et al. Volumetric neuroimage analysis extensions for the MIPAV software package. J Neurosci Methods 2007;165:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazziotta J, Toga A, Evans A, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos Trans R Soc Lond B Biol Sci 2001;356:1293–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carass A, Cuzzocreo J, Wheeler MB, Bazin PL, Resnick SM, Prince JL. Simple paradigm for extra-cerebral tissue removal: algorithm and analysis. Neuroimage 2011;56:1982–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NICE. Donepezil, Galantamine, Rivastigmine and Memantine for the Treatment of Alzheimer's Disease. London: National Institute for Health and Clinical Excellence (NICE); 2011:84. [Google Scholar]
- 23.Lezak M. Neuropsychological Assessment. New York: Oxford University Press; 1983. [Google Scholar]
- 24.Yancosek KE, Howell D. A narrative review of dexterity assessments. J Hand Ther 2009;22:258–269. [DOI] [PubMed] [Google Scholar]
- 25.Lafayette Instruments. The Grooved Pegboard User Manual. 2002. Available at: www.lafayetteinstrument.com. Accessed September 1, 2014. [Google Scholar]
- 26.Tullberg M, Fletcher E, DeCarli C, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology 2004;63:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verdelho A, Madureira S, Moleiro C, et al. White matter changes and diabetes predict cognitive decline in the elderly: the LADIS Study. Neurology 2010;75:160–167. [DOI] [PubMed] [Google Scholar]
- 28.Viana-Baptista M, Bugalho P, Jordao C, Ribeiro O, Esperanca-Pina JA, Ferro J. Motor dysfunction correlates with frontal white matter ischemic changes in patients with leukoaraiosis. J Aging Res 2011;2011:950341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bezdicek O, Nikolai T, Hoskovcova M, et al. Grooved pegboard predicates more of cognitive than motor involvement in Parkinson's disease. Assessment 2014;21:723–730. [DOI] [PubMed] [Google Scholar]
- 30.Sachdev P, Wen W, Chen X, Brodaty H. Progression of white matter hyperintensities in elderly individuals over 3 years. Neurology 2007;68:214–222. [DOI] [PubMed] [Google Scholar]
- 31.Kochunov P, Coyle T, Lancaster J, et al. Processing speed is correlated with cerebral health markers in the frontal lobes as quantified by neuroimaging. Neuroimage 2010;49:1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


