Abstract
Objective:
Evaluate the impact of concomitant enzyme (CYP3A4)-inducer antiepileptic drugs (EIAEDs) on the efficacy and safety of perampanel in patients from the 3 phase-III clinical trials.
Methods:
Patients with pharmacoresistant partial-onset seizures in the 3 phase-III clinical studies were aged 12 years and older and receiving 1 to 3 concomitant antiepileptic drugs. Following 6-week baseline, patients were randomized to once-daily, double-blind treatment with placebo or perampanel 8 or 12 mg (studies 304 and 305) or placebo or perampanel 2, 4, or 8 mg (study 306).
Results:
Treatment response assessed by median percent reduction in seizure frequency and responder rates improved with perampanel compared with placebo. However, at 8 and 12 mg, the treatment response was significantly greater in patients receiving non-EIAEDs. The treatment effect (perampanel–placebo) also demonstrated a dose-dependent increase in all patients. The overall incidence of treatment-emergent adverse events was similar regardless of the presence of EIAEDs. Occurrence of some adverse events, such as fatigue, somnolence, dizziness, irritability, was greater in patients receiving non-EIAEDs, as was discontinuation because of adverse events.
Conclusions:
Perampanel shows efficacy and safety in the presence and absence of EIAEDs. As systemic exposure to perampanel increases, so does efficacy. Given the extensive metabolism of perampanel, systemic exposure is clearly reduced with concomitant administration of CYP3A4 inducers. This supports the strategy of dosing perampanel to clinical effect. Recognition of these pharmacokinetic interactions will be important in the optimization of this novel medication.
Classification of evidence:
This study provides Class II evidence that 2 to 12 mg/d doses of perampanel reduced seizure frequency and improved responder rate in the presence and absence of EIAEDs.
Despite several new antiepileptic drugs (AEDs) emerging over the past 20 years, seizure freedom eludes many patients with epilepsy.1–4 Perampanel (FYCOMPA; Eisai Inc., Woodcliff Lake, NJ), first in a novel class of AEDs, is an orally active, noncompetitive, selective AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid)-receptor antagonist4,5 approved in more than 40 countries, including the United States and in Europe, for adjunctive treatment of partial seizures with or without secondarily generalized seizures, in patients with epilepsy aged ≥12 years, and in Canada in patients aged ≥18 years.6–8 Efficacy of perampanel was demonstrated in 3 multicenter, double-blind, randomized, parallel-group, placebo-controlled phase III trials of patients with treatment-resistant partial-onset seizures already taking 1 to 3 AEDs.9–11 These studies clearly demonstrated that, when given up to 12 mg/d as adjunctive treatment, perampanel significantly reduced seizure frequency and increased responder rates.9–11
Perampanel is mainly eliminated by oxidative metabolism, mediated primarily by CYP3A4.12 Population pharmacokinetic (PK) analyses have shown that 3 frequently used CYP3A4 enzyme-inducing AEDs (EIAEDs) (carbamazepine [CBZ], oxcarbazepine [OXC], phenytoin [PHT]) significantly increase perampanel apparent oral clearance.13,14
Pooled analysis of the phase III studies showed that perampanel plasma concentrations increase proportionally with doses 2 to 12 mg.15 In the presence of EIAEDs, albeit at lower concentrations, perampanel plasma concentrations were similarly increased.15 Of note, PK/pharmacodynamic analyses showed that increased steady-state perampanel plasma concentrations were related to decreased seizure frequency14 and increased probability of achieving ≥50% reduction in seizure frequency.16
Further evaluation of the phase III clinical data in these patients is essential to understand the potential impact of PK interactions on efficacy, tolerability, and dosing of this new AED.
METHODS
Classification of evidence.
This evaluation of the phase III clinical studies provides Class II evidence that once-daily perampanel (2–12 mg) reduced seizure frequency and improved responder rate in the presence and absence of EIAEDs in pharmacoresistant patients with partial-onset seizures.
Standard protocol approvals, registrations, and patient consents.
The 3 phase-III studies (304: NCT00699972; 305: NCT00699582; and 306: NCT00700310) were conducted in North and South America, Europe, Australia, India, Israel, Russia, South Africa, and Asia between April 2008 and January 2011.6–8 All studies were conducted in accordance with the Helsinki Declaration, European Medicines Agency requirements, and the US Code of Federal Regulations, as appropriate. National regulatory authorities in each country and independent ethics committees/institutional review boards for each site reviewed trial protocols, amendments, and informed consent.6–8
Patients.
Patients were aged 12 years and older with treatment-resistant partial-onset seizures (with or without secondarily generalized seizures) despite receiving stable doses of 1 to 3 approved AEDs.15 A detailed description of the inclusion/exclusion criteria, allocation method, and other details can be found in the individual published studies.9–11 Patients were permitted only one AED known to induce the metabolism of other AEDs. These were defined at the outset of the studies as CBZ, PHT, phenobarbital, or primidone.15 Subsequent population-PK analyses demonstrated that only CBZ, OXC, and PHT resulted in statistically significant increases in perampanel oral clearance.15 For analyses presented herein, EIAEDs include only CBZ, OXC, and PHT.
Study design.
The double-blind studies were conducted in 3 phases: baseline, the double-blind treatment phase (a 6-week dose-titration period followed by a 13-week maintenance period), and a follow-up phase of 4 weeks for patients who withdrew prematurely or did not elect to enter the ongoing extension study.9–11,17 Patients were randomized to once-daily, double-blind treatment with placebo, perampanel 8 mg, or perampanel 12 mg (1:1:1) in studies 304 and 305.9,10 In study 306, patients were randomized to placebo or perampanel 2, 4, or 8 mg (1:1:1:1).11 During the titration phase, perampanel doses were increased by 2 mg per week to the randomized dose, and dose reductions were permitted for intolerability.9–11,17 Patients treated with perampanel continued treatment with the dose achieved during titration throughout the maintenance period.9–11
Efficacy and safety assessments.
Efficacy assessments included the median percent reduction in seizure frequency from baseline (all partial-onset seizure types) per 28 days of treatment during the double-blind period and the responder rate (proportion of patients achieving a ≥50% reduction in seizure frequency per 28 days in the maintenance period vs baseline) in the presence and absence of concomitant EIAEDs.9–11,15
A large decrease in median percent change in seizure frequency per 28 days was observed in the perampanel 8-mg groups in all regions. However, an unusually high placebo responder rate of 33.3% was observed in study 304 among patients in the Central and South America region compared with North American sites, which showed a responder rate of 21.9%.9 Furthermore, the responder rate of placebo-treated patients in studies 305 and 306 was 14.7% and 17.9%, respectively.10,11 A significant (p = 0.042) treatment-by-region interaction for the Central and South America region was observed following an analysis of covariance (ANCOVA) using rank-transformed data from all regions and 8-mg treatment groups (common to all 3 studies). The overall safety, PK, and PK/pharmacodynamic profile were generally similar for the Central and South America region compared with the overall population, and the reasons for the high placebo response are yet unknown.
Safety assessments included the incidence rates of the most frequent treatment-emergent adverse events (TEAEs).9–11,15 The safety analysis set included all randomized patients who received the study drug and had at least one postdose safety assessment. The completers set included patients in the full intent-to-treat set who completed the double-blind study.
Dose-response analyses were also performed using the actual (last) dose taken by subjects rather than the randomized dose because randomized dose analyses may underestimate efficacy at higher doses in typical AED clinical trials.13
Statistical analysis.
To show differential effect of EIAEDs on dose, analyses presented here use actual (last) dose for the pooled phase III data in patients who completed the study. Statistical significance of efficacy for perampanel with EIAEDs and non-EIAEDs is noted by comparing perampanel doses with placebo only in studies in which those doses were included (2 and 4 mg, study 306; 8 mg, studies 304, 305, and 306; 12 mg, studies 304 and 305). Because of the skewed distribution, the baseline seizure frequency per 28 days and the percentage change per 28 days during treatment were rank-transformed separately before the analysis of median percent change from baseline in seizure frequency. An ANCOVA was then conducted on the rank-transformed data (rank ANCOVA) with treatment and region as factors and the ranked baseline seizure frequency per 28 days as a covariate. A p value ≤0.05 was considered statistically significant. Treatment effect is presented as median reduction in seizure frequency over the maintenance period. Robust nonparametric Hodges-Lehmann estimates of median placebo-corrected treatment effects and 95% confidence interval are provided. Rank ANCOVA was used to compare median percent change between EIAED and non-EIAED dose groups. Responder rates were analyzed over the maintenance period (last observation carried forward) using the χ2 test. Baseline characteristics, the incidence of patient discontinuation, and overall adverse events were compared between EIAED and non-EIAED perampanel dose groups using ANCOVA for continuous variables and the χ2 test for categorical variables.
RESULTS
Patients.
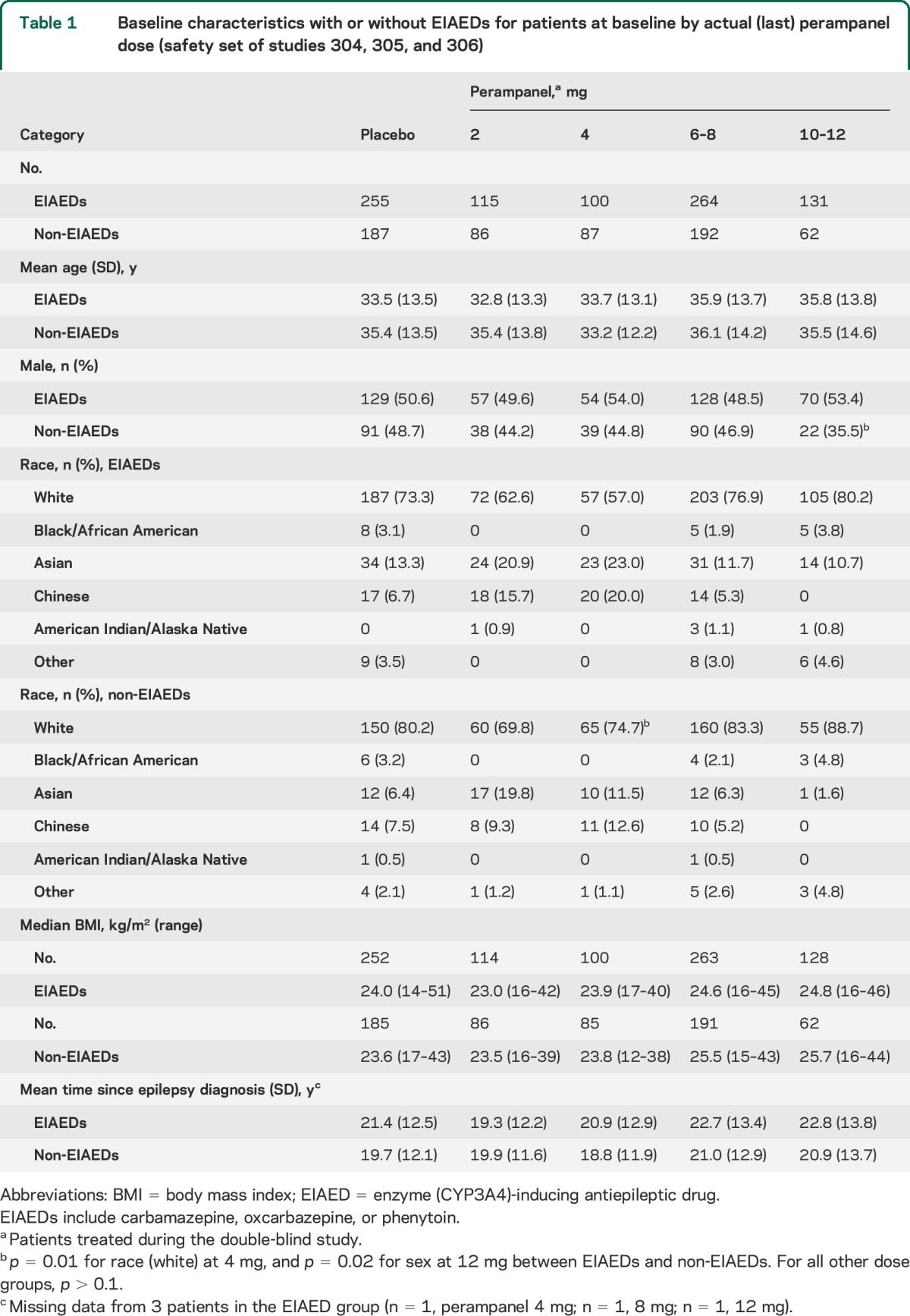
A total of 1,480 patients were randomized and treated in studies 304, 305, and 306.15,18 At baseline, age, body mass index, and time since epilepsy diagnosis were similar in patients who were receiving EIAEDs (CBZ, OXC, or PHT) and those receiving non-EIAEDs (table 1). Overall, there were slightly higher percentages of males, Hispanics, and Asians among the patients receiving EIAEDs at baseline compared with those receiving non-EIAEDs (table 1). Differences for race and sex were observed only for 4 mg and 10–12 mg, respectively (p ≤ 0.05; table 1). Of the total patients, 1,264 who completed the maintenance period of the phase III studies were included in the actual (last)-dose analysis.
Table 1.
Baseline characteristics with or without EIAEDs for patients at baseline by actual (last) perampanel dose (safety set of studies 304, 305, and 306)
Population PK analyses.
In the presence of EIAEDs, perampanel average steady-state plasma concentrations for all last (actual) doses at the end of the maintenance period were numerically lower compared with non-EIAEDs (table e-1 on the Neurology® Web site at Neurology.org).15 However, they also increased linearly in a dose-dependent manner in patients taking EIAEDs and non-EIAEDs.
In the population PK analysis, phenobarbital had no effect on perampanel clearance and resulting concentration while topiramate reduced the area under the curve (AUC) by 20% (not clinically relevant). In comparison, CBZ, OXC, and PHT increased perampanel clearance by 3-, 2-, and 2-fold, respectively (table e-2). This resulted in reduced perampanel AUC and is considered clinically important.
Efficacy endpoints.
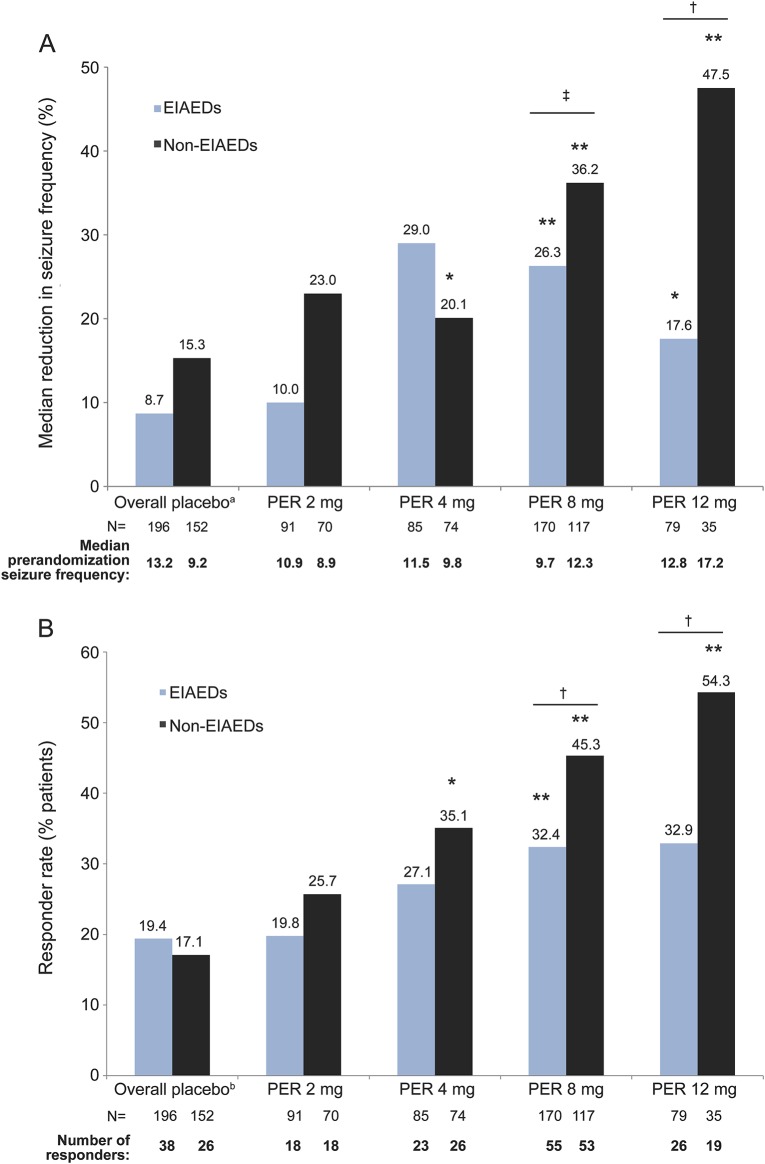
The data in figure e-1 show that treatment with perampanel increased the median percent change from baseline in seizure frequency and the responder rates (proportion of patients with ≥50% decrease in seizure frequency) of completers, in the presence and absence of EIAEDs, by actual (last) dose. In the absence of EIAEDs, the reduction in seizure frequency was observed for perampanel doses except for 4 mg (figure e-1). In addition, responder rates were numerically lower in the presence of EIAEDs across all dose groups (figure e-1). This analysis includes the population of Central and South America from study 304, where a high placebo-response effect was observed, resulting in the only treatment-by-region interaction of the phase III program.9 Because previous analysis has demonstrated a treatment-by-region interaction (p = 0.042) indicating that Central and South America differed from all other regions in the combined studies, additional analyses presented exclude data from sites in Central and South America (figure 1, table 2). As presented in figure 1, a dose response was observed with perampanel treatment by showing an improvement in seizure control, regardless of the presence or absence of EIAEDs.
Figure 1. Treatment response in completers by actual (last) perampanel dose (studies 304, 305, and 306).
(A) Median percent reduction in seizure frequency per 28 days from baseline over double-blind period. (B) Responder rate during maintenance period. Excludes patients from Central and South America and perampanel 6 and 10 mg. EIAEDs include carbamazepine, oxcarbazepine, or phenytoin. aOverall placebo is shown in graph; actual placebo median percent reduction in seizure frequency for each dose group was used for statistical analysis (EIAED placebo: 2 mg = 10.1, 4 mg = 10.1, 8 mg = 8.7, 12 mg = 5.8; non-EIAED placebo: 2 mg = 12.7, 4 mg = 12.7, 8 mg = 15.3, 12 mg = 15.9). bOverall placebo is shown in graph; actual placebo responder rate for each dose group was used for statistical analysis (EIAED placebo: 2 mg = 18.1, 4 mg = 18.1, 8 mg = 19.4, 12 mg = 20.6; non-EIAED placebo: 2 mg = 19.4, 4 mg = 19.4, 8 mg = 17.1, 12 mg = 15.0). *p < 0.05; **p < 0.01 vs placebo for each dose; †p < 0.05; ‡p < 0.005 EIAEDs vs non-EIAEDs for each dose based on rank analysis of covariance (for median percent reduction) and χ2 test (responder rate). EIAED = enzyme (CYP3A4)-inducing antiepileptic drug; N = number of patients in each group.
Table 2.
Median placebo-adjusted treatment effect by actual (last) perampanel dose based on the presence or absence of EIAEDs during the maintenance period (studies 304, 305, and 306)a

At 8- and 12-mg perampanel doses, treatment response (as assessed by median percent reduction in seizure frequency or responder rate) was more robust when perampanel was given concomitantly with non-EIAEDs compared with EIAEDs (p ≤ 0.05; figure 1). Perampanel doses of 4, 8, and 12 mg, when given concomitantly with non-EIAEDs, showed an improvement for the median percent reduction in seizure frequency (p = 0.018, p < 0.0001, p = 0.008, respectively) compared with placebo, whereas 8 and 12 mg perampanel given concomitantly with EIAEDs showed an improvement compared with placebo (p = 0.0001, p = 0.016, respectively; figure 1A). Accordingly, treatment effect (expressed as perampanel–placebo response rates) also demonstrated a similar dose-dependent increase in therapeutic response that was numerically greater in patients receiving non-EIAEDs (table 2). The data for the 6-mg (n = 23) and 10-mg (n = 4) doses are not presented, because very few patients were treated at these dose levels.
Adverse events.
The number of patients who completed the study is shown in table 3. Completion rates for patients who received EIAEDs were similar for each perampanel dose and placebo groups. Completion rates for patients who did not receive EIAEDs ranged from 74.2% to 87.4%. With the exception of the 10- to 12-mg dose group, the completion rates were similar for the 2 patient cohorts. A higher completion rate in the 10- to 12-mg dose for patients receiving EIAEDs suggests that this group may have been able to maintain a higher perampanel dose. The primary reason for discontinuation in the presence and absence of EIAEDs was the occurrence of TEAEs. The data show that, for perampanel doses greater than 4 mg/d, TEAEs leading to study discontinuation were more frequent in patients receiving non-EIAEDs (p ≤ 0.05; table 3).
Table 3.
Completion rates and incidence of TEAEs leading to discontinuation by actual (last) daily perampanel dose in the presence and absence of EIAEDs (safety set of studies 304, 305, and 306)

The overall incidence of TEAEs was slightly higher in patients receiving non-EIAEDs compared with those taking EIAEDs, with the greatest difference (8.3%) in the perampanel 6- to 8-mg group (table 4). However, the incidence of any TEAE did not show a difference between patients receiving concomitant EIAEDs and non-EIAEDs. The most frequently occurring TEAEs (≥10%) for any perampanel treatment group in the presence of EIAEDs were dizziness, somnolence, and headache (table 4). The most frequently occurring TEAEs (≥10%) for any perampanel treatment group in patients receiving non-EIAEDs were dizziness, somnolence, fatigue, headache, irritability, ataxia, and fall (table 4).
Table 4.
Rates of the most common TEAEs (≥10%) by actual (last) daily perampanel dose in the presence and absence of EIAEDs (safety set of studies 304, 305, and 306)

DISCUSSION
The aim of the present analysis was to demonstrate the impact of concomitant EIAEDs on the efficacy and safety of perampanel, and in turn provide a rationale for the perampanel dosing recommendations. Monotherapy is generally the preferred first-line treatment for epilepsy; however, a significant proportion of patients will require regimens consisting of multiple AEDs to achieve treatment success. PK interactions arising from polytherapy have the potential to complicate epilepsy management.19 Despite the availability of new AEDs that are devoid of drug interactions, comedication with EIAEDs is still commonplace.20,21 For drugs such as perampanel that are extensively metabolized via the CYP isozyme system, interactions that increase oral clearance (and consequently, decrease systemic exposure) have the potential to ultimately reduce clinical efficacy.22 Because perampanel has been approved as adjunctive therapy, it is important for clinicians to understand these potential PK interactions to maximize its therapeutic benefit and reduce the risk of adverse events.19
Our analysis demonstrates that perampanel is indeed efficacious in patients when given as adjunctive treatment; however, the expected magnitude of therapeutic response may be influenced by concomitant therapy. This finding does not appear to reflect a pharmacodynamic interaction. In agreement with a previous analysis, concomitant EIAED treatment does not alter the perampanel plasma concentration-response relationship for efficacy or tolerability.13 Rather, based on PK analysis showing that steady-state perampanel plasma concentrations can be reduced in patients receiving CYP3A4 enzyme-inducing medications, the most reasonable explanation is that the perampanel dose-response curve is shifted in patients receiving inducing AEDs.13 In other words, since the likelihood of efficacy of perampanel has been shown to increase with increasing perampanel systemic exposure (i.e., plasma concentration), then higher doses and a more frequent up-titration schedule may be required to maximize efficacy when using perampanel in patients receiving drugs such as CBZ, OXC, or PHT.13 In population PK analysis, phenobarbital had no significant effect on perampanel AUC and topiramate reduced the AUC by 20% (not clinically relevant). In comparison, CBZ, OXC, and PHT affected perampanel apparent oral clearance and subsequently reduced perampanel AUC by about 67%, 50%, and 50%, respectively, all of which were considered clinically important. In addition, results from population PK analyses demonstrated that 12 mg perampanel did not significantly affect the clearance of certain AEDs, including PHT, but did significantly increase the clearance of CBZ and other AEDs, although the increases were each less than 10%. Coadministration of OXC resulted in a 26% decrease in OXC clearance and increased its concentrations.
On a practical basis, regulatory agencies recommend 2 approaches to manage dosing of perampanel appropriately for patients who are also receiving EIAEDs. The US Food and Drug Administration–approved perampanel prescribing information recommends a starting dosage of 2 mg/d (given at bedtime) for patients who are taking non-EIAEDs and 4 mg/d for patients taking EIAEDs.6 The perampanel dose can be increased gradually in 2 mg/d weekly increments to a maximum dose of 4 to 12 mg/d based on clinical response and tolerability.6 However, the European Medicines Agency recommends initiation of treatment with perampanel at 2 mg/d, irrespective of concomitant EIAEDs.7 Because the half-life of perampanel will be markedly shortened by EIAEDs,6 patients receiving concomitant EIAEDs may be titrated weekly while patients receiving non-EIAEDs should be titrated no more frequently than at 2-week intervals.7 The dose may be increased based on clinical response and tolerability to a maintenance dose of 4 to 8 mg/d.7 Depending on an individual's clinical response and tolerability at a dose of 8 mg/d, the dose may then be increased to 12 mg/d.6,7 Each set of recommendations presents a different approach to addressing EIAED concerns, but both yield the same result: gradual dosage titration over time in patients with concomitant EIAEDs to compensate for enhanced CYP3A4-mediated perampanel elimination.
Regarding adverse events, particularly those leading to treatment discontinuation, the profile of perampanel in the presence and absence of EIAEDs was qualitatively comparable although quantitatively somewhat higher in the absence of EIAEDs. The most common adverse events (≥10%) in the presence and absence of EIAEDs were dizziness, somnolence, fatigue, headache, irritability, ataxia, and fall, which was consistent with adverse events in the overall phase III studies. The adverse event with the greatest incidence was dizziness, which was similar between EIAEDs and non-EIAEDs; however, at higher doses of perampanel, somnolence was moderately lower with EIAEDs, suggesting that reduced perampanel plasma concentrations with EIAEDs may reduce the incidence of somnolence and other adverse events. Overall, study discontinuation rates because of adverse events were greater in the patient group receiving non-EIAEDs. It is certainly plausible that, in these patients, perampanel plasma concentrations at each dosage level were greater than in those receiving EIAEDs, as shown previously.15 Although discontinuations because of adverse events were greater at a high 10- to 12-mg dose for the non-EIAEDs group (24.2%) compared with the group taking EIAEDs (7.6%), the responder rates were 39% vs 12%, respectively, for the 2 groups. This is in agreement with published results, suggesting that, for patients who are able to tolerate higher perampanel doses (resulting in higher PK concentrations in the non-EIAED group, in this case), there are additional benefits in seizure control.23
In addition to recognizing the potential for accelerated metabolism of perampanel when adding it to a regimen containing an EIAED, clinicians must also be cognizant of the potential for deinduction. When reducing or withdrawing EIAEDs from a patient's treatment regimen, plasma concentrations of perampanel are likely to increase, which can result in new or intensified adverse events. Because of the relatively long half-life of perampanel, potential changes in clinical response due to changes in plasma concentration might be expected to evolve slowly. Clinicians will need to monitor these patients closely for clinical response and tolerability when reducing or withdrawing an EIAED.
When perampanel is used as adjunctive therapy, clinicians can reasonably expect a favorable therapeutic response in pharmacoresistant patients with partial-onset seizures, irrespective of concomitant AEDs. Of note, this post hoc analysis suggests that enzyme-inducing PK interactions are important determinants in optimizing therapy with this new molecule. Clinicians will need to consider concomitant medications when initiating perampanel treatment and when determining optimal maintenance dosages as well as patient tolerability.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. David Squillacote, formerly an employee of Eisai Inc., who was involved in initial discussions about the concept of the manuscript.
GLOSSARY
- AED
antiepileptic drug
- ANCOVA
analysis of covariance
- AUC
area under the curve
- CBZ
carbamazepine
- EIAED
enzyme-inducing antiepileptic drug
- OXC
oxcarbazepine
- PHT
phenytoin
- PK
pharmacokinetic
- TEAE
treatment-emergent adverse event
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Gidal: study concept and design, acquisition of data, data analysis and interpretation, writing the manuscript, study supervision. Dr. Laurenza: data analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Hussein: data analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Yang: critical revision of the manuscript for important intellectual content. Dr. Fain: critical revision of the manuscript for important intellectual content. Dr. Edelstein: involved in initial discussions about the manuscript and helped develop the first draft. Mr. Kumar: statistical data analysis and interpretation. Dr. Ferry: study concept and design, acquisition of data, interpretation of data analyses, writing the manuscript, study supervision.
STUDY FUNDING
Editorial support was funded by Eisai Inc. and provided by Sui Generis Health, LLC, and Imprint Publication Science.
DISCLOSURE
B. Gidal receives honoraria from serving as a speaker for UCB and XenoPort and from consulting for Eisai and Upsher-Smith Labs. A. Laurenza is an employee of Eisai Inc. Z. Hussein is an employee of Eisai Ltd. H. Yang is an employee of Eisai Inc. R. Fain is an employee of Eisai Inc. J. Edelstein is a former employee of Sui Generis Health. D. Kumar is an employee of Eisai Inc. J. Ferry is an employee of Eisai Inc. Go to Neurology.org for full disclosures.
REFERENCES
- 1.French JA. Refractory epilepsy: clinical overview. Epilepsia 2007;48:3–7. [DOI] [PubMed] [Google Scholar]
- 2.Perucca E, French JA, Bialer M. Development of new antiepileptic drugs: challenges, incentives, and recent advances. Lancet Neurol 2007;6:793–804. [DOI] [PubMed] [Google Scholar]
- 3.Brodie MJ, Barry SJE, Bamagous GA, Norrie JD, Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology 2012;78:1548–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rektor I. Perampanel, a novel, non-competitive, selective AMPA receptor antagonist as adjunctive therapy for treatment-resistance partial-onset seizures. Expert Opin Pharmacother 2013;14:225–235. [DOI] [PubMed] [Google Scholar]
- 5.Rogawski MA, Hanada T. Preclinical pharmacology of perampanel, a selective non-competitive AMPA receptor antagonist. Acta Neurol Scand Suppl 2013;197:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fycompa [prescribing information]. Woodcliff Lake, NJ: Eisai Inc.; 2014. [Google Scholar]
- 7.Fycompa [summary of product characteristics]. Hatfield, UK: Eisai Europe Limited; 2012. [Google Scholar]
- 8.Fycompa [product monograph]. Mississauga, ON: Eisai Limited; 2013. [Google Scholar]
- 9.French JA, Krauss GL, Biton V, et al. Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304. Neurology 2012;79:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.French JA, Krauss GL, Steinhoff BJ, et al. Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305. Epilepsia 2012;54:117–125. [DOI] [PubMed] [Google Scholar]
- 11.Krauss GL, Serratosa JM, Villanueva V, et al. Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology 2012;78:1408–1415. [DOI] [PubMed] [Google Scholar]
- 12.Franco V, Crema F, Iudice A, Zaccarad G, Grillo E. Novel treatment options for epilepsy: focus on perampanel. Pharmacol Res 2013;70:35–40. [DOI] [PubMed] [Google Scholar]
- 13.Gidal BE, Ferry J, Majid O, Hussein Z. Concentration-effect relationships with perampanel in patients with pharmacoresistant partial-onset seizures. Epilepsia 2013;54:1490–1497. [DOI] [PubMed] [Google Scholar]
- 14.Hussein Z, Critchley D, Ferry J, Laurenza A. Population pharmacokinetics of perampanel, a selective, non-competitive AMPA receptor antagonist, in patients with refractory partial-onset seizures participating in a randomized, double-blind, placebo-controlled phase III study. Poster presented at the 29th International Epilepsy Congress; August 28–September 1, 2011; Rome.
- 15.Hussein Z, Ferry J, Krauss GL, Squillacote D, Laurenza A. Demographic factors and concomitant antiepileptic drugs have no effect on the pharmacodynamics of perampanel. Poster presented at the 64th Annual Meeting of the American Academy of Neurology; April 21–28, 2012; New Orleans.
- 16.Hsu WW, Sing CW, He Y, Worsley AJ, Wong ICK, Chan EW. Systematic review and meta-analysis of the efficacy and safety of perampanel in the treatment of partial-onset epilepsy CNS Drugs 2013;27:817–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurenza A, Gidal B, Hussein Z, et al. Evaluation of efficacy and safety of perampanel in the presence of concomitant CYP3A4-inducing AEDs: analyses from the perampanel phase 3 clinical trials. Poster presented at the 66th American Epilepsy Society Annual Meeting; November 30–December 4, 2012; San Diego.
- 18.Steinhoff BJ, Ben-Menachem E, Ryvlin P, et al. Efficacy and safety of adjunctive perampanel for the treatment of refractory partial seizure: a pooled analysis of three phase III studies. Epilepsia 2013;54:1481–1489. [DOI] [PubMed] [Google Scholar]
- 19.St. Louis EK. Truly “rational” polytherapy: maximizing efficacy and minimizing drug interactions, drug load, and adverse effects. Curr Neuropharmacol 2009;7:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gidal BE, French JA, Grossman P, Le Teuff G. Assessment of potential drug interactions in patients with epilepsy: impact of age and sex. Neurology 2009;72:419–425. [DOI] [PubMed] [Google Scholar]
- 21.Beyenburg S, Stavem K, Schmidt D. Placebo-corrected efficacy of modern nonenzyme-inducing AEDs for refractory focal epilepsy: systematic review and meta-analysis. Epilepsia 2012;53:512–520. [DOI] [PubMed] [Google Scholar]
- 22.Brodie MJ, Mintzer S, Pack AM, Gidal BE, Vecht CJ, Schmidt D. Enzyme induction with antiepileptic drugs: cause for concern? Epilepsia 2013;54:11–27. [DOI] [PubMed] [Google Scholar]
- 23.Kramer LD, Satlin A, Krauss GL, et al. Perampanel for adjunctive treatment of partial-onset seizures: a pooled dose-response analysis of phase III studies. Epilepsia 2014;55:423–431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.