Abstract
Objective:
To examine whether the presence of sleep-disordered breathing (SDB) is associated with an earlier age at mild cognitive impairment (MCI) or Alzheimer disease (AD)-dementia onset in participants from the Alzheimer's Disease Neuroimaging Initiative (ADNI) cohort. We also examined whether continuous positive airway pressure (CPAP) use is associated with delayed onset of cognitive decline.
Methods:
From the ADNI cohort, 3 subsets with progressively stringent criteria were created in a step-wise manner. Age at MCI or AD-dementia onset was the main outcome variable. Analyses were performed separately for each subset in untreated SDB+ vs SDB− and untreated SDB+ vs CPAP+ groups. Chi-square and t tests were performed to examine between-group differences. Survival analyses were performed using the Kaplan–Meier method, compared by the log-rank test, and assessed by multivariate Cox regression adjusting for potential confounders.
Results:
SDB+ patients had a younger age at MCI onset in all subsets (MC1: 72.63 vs 83.67; MC2: 72.15 vs 83.45; MC3: 77.40 vs 89.89; p < 0.01). SDB+ patients had a younger age at AD-dementia onset only in our most conservative subset (AC3: 83.46 vs 88.13; p < 0.05). In a combined outcome analysis, SDB+ patients had a younger age at onset to MCI or AD-dementia in all subsets. In subsets 1 and 2, CPAP use delayed the age at MCI onset (CMC1: 72.63 vs 82.10; CMC2: 72.11 vs 82.10; p < 0.01).
Conclusions:
Consistent with our hypothesis, the presence of SDB was associated with an earlier age at cognitive decline. Our findings in CPAP+ participants suggest that CPAP treatment of SDB may delay progression of cognitive impairment.
Obstructive sleep-disordered breathing (SDB) is characterized by abnormalities of respiratory pattern during sleep that range from significant snoring to frequent episodes of complete airway obstruction.1 SDB has a high prevalence in the elderly, affecting 52.6% of men and 26.3% of women.2 The relationship between SDB and cognitive impairment is conflicting.3 Several studies have shown an indirect association between excessive daytime sleepiness and the development of cognitive decline,4–7 but only recently did a study of 298 community-dwelling women find direct evidence that those with SDB were more likely to develop mild cognitive impairment (MCI) or dementia at the 5-year follow-up.8
In the present study, we hypothesized that among community-dwelling elderly participating in the longitudinal Alzheimer's Disease Neuroimaging Initiative (ADNI), (1) the presence of SDB would be independently associated with an earlier age at MCI onset (or at Alzheimer disease [AD]-dementia in patients with MCI), and (2) as an exploratory analysis, treatment with continuous positive airway pressure (CPAP) would be associated with a delay of the age at onset of cognitive decline. For this purpose, we analyzed the ADNI cohort for clinical information regarding the presence or absence of SDB and/or treatment with CPAP and self-reported and objective clinical information on age at MCI onset and/or age at AD-dementia onset.
METHODS
Data used were obtained from the ADNI database (adni.loni.usc.edu). The primary goal of ADNI is to test whether serial MRI, PET, other biomarkers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD-dementia. To date, ADNI has recruited more than 2,000 adults, aged 55 to 90 years, consisting of cognitively normal (NL) older individuals, people with early or late MCI, and people with early AD-dementia. The follow-up duration of each group is specified in the protocols for ADNI-1, ADNI-2, and ADNI-GO, ranges from 2 to 3 years, and is performed at 6-month intervals.
All analyses for the present study were based on recorded medical history data from baseline and follow-up visits downloaded from the ADNI Web site on May 7, 2014, which includes NL, patients with MCI, and patients with AD-dementia. Age at MCI onset or age at AD-dementia onset were the only variables of interest in all analyses and were defined as: (1) the reported best estimate of onset of MCI or AD-dementia symptoms by the subject (or informant) at baseline; (2) the first follow-up visit with an MCI (or AD) diagnosis after being diagnosed as NL (or MCI) at baseline by an ADNI study clinician. Presence of SDB was self-reported. Patients with reported “sleep apnea” or “obstructive sleep apnea (OSA)” were labeled SDB+ provided that they did not report any treatment (such as CPAP or upper airway surgery). Those participants who reported having “sleep apnea” and using “CPAP” or “Bi-PAP” treatment were labeled CPAP+. The remaining participants were considered SDB−. Two physicians (R.O. and A.V.) reviewed all medical history descriptions for SDB+ and CPAP+ patients to ensure they were allocated into the correct groups. Medical history data on 2,470 participants were initially downloaded from ADNI. See appendix e-1 on the Neurology® Web site at Neurology.org for a detailed listing of all ADNI variables used in this study.
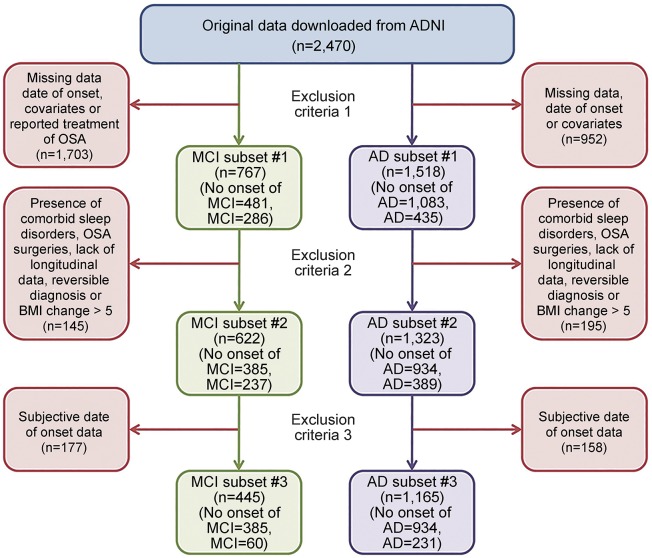
Because of the differing methods used to ascertain cognitive decline, the existence of missing data, and potential confounders in the ADNI cohort, as well as the generally low number of participants who reported being SDB+, we performed analyses on 3 subsets defined by progressively stringent criteria for inclusion, to establish the effect in a more inclusive sample with a larger number, as well as in smaller samples with more stringent criteria: the first subset (C1) only excluded participants with missing data; the second subset (C2) excluded participants with ambiguous group allocation; and the third subset was limited to participants with incident MCI or AD-dementia as documented by clinical assessment during the follow-up period (C3). The exact process of exclusion for both the subsets that were examined for the “age at MCI onset,” the subsets that were examined for the “age at AD-dementia onset,” and the subsets that were examined for the “age at MCI or AD-dementia onset” were as follows (see figure 1).
Figure 1. Stepwise exclusion process for MCI and AD subsets.
Survival analysis on age at MCI/AD onset was performed on 3 subsets defined by progressively stringent criteria for inclusion: the first subset (C1) excluded participants with missing data; the second subset (C2) excluded participants with ambiguous group allocation; and the third subset only contained participants with incident MCI or AD (C3). AD = Alzheimer disease; ADNI = Alzheimer's Disease Neuroimaging Initiative; BMI = body mass index; MCI = mild cognitive impairment; OSA = obstructive sleep apnea.
Participants were excluded from the first subset (C1) if: (1) it was not possible to establish the year of onset of MCI or AD-dementia symptoms (i.e., if they were missing a diagnosis at any visit or were without a reported age at MCI or AD-dementia onset); (2) they were missing data on body mass index (BMI), because being overweight is one of the primary risk factors for SDB and (at moderate levels) may have a protective effect on AD risk9; (3) they were missing APO ε4 status, because the APO ε4 allele is the strongest genetic risk factor for sporadic AD and may have additive effects with SDB10,11; and (4) they reported CPAP use or “surgery for sleep apnea,” because our analyses intended to isolate the effect of SDB on cognitive decline irrespective of treatment status.
Participants were excluded from the second subset (C2) if: (1) they reported comorbid sleep disturbances, such as “insomnia” and other nonspecified “sleep disturbances” or “sleep problems,” because of the high prevalence of insomnia symptoms and comorbid sleep disturbances usually reported by patients with SDB, which could affect group allocation, and because insomnia has been described as an independent risk factor for cognitive decline12; (2) they had a BMI change greater than 5 between any follow-up visit, because of the previously noted independent associations of BMI with SDB and AD-dementia; (3) they had an MCI or AD-dementia diagnosis at any time point but an NL diagnosis or MCI diagnosis at their last visit (MCI/AD-reversible impairment), to control for mis- or unspecified diagnoses; (4) they were without longitudinal follow-ups, in order to retain for analysis only the more robust longitudinal diagnoses.
Participants were excluded from our final subset (C3) if: (1) they had a “reported” age at onset of cognitive decline event, to control for recall bias and retain only those participants who objectively declined (certified by a clinician) from normal cognition to MCI or AD-dementia during the clinical follow-up period of the study. Thus, C3, our most “conservative” subset, included only cases of incident MCI or AD-dementia who declined to MCI or AD-dementia (or MCI first and later to AD) after being diagnosed as NL or MCI on their baseline evaluation. See figure 1 for a detailed flowchart of subset selection for the age at MCI and AD-dementia onset groups.
The same reliability steps were applied to the small sample of participants with data on CPAP treatment, creating 3 age at MCI onset and 3 age at AD-dementia onset subsets with CPAP+ and SDB+ participants. Those who reported surgery for sleep apnea were not included in these analyses.
Statistical analyses.
Statistical analyses were performed on all 3 of the resulting subsets independently by 2 statisticians (E.P. and S.L.). Differences in patient demographic and clinical characteristics between SDB groups were examined by the χ2 test for categorical variables and t tests for continuous variables. Survival curves of age at MCI or AD-dementia onset were calculated using the Kaplan–Meier method and compared by the log-rank test. Cox regression model analysis was applied to assess the age at onset of cognitive decline adjusting for APO ε4, sex, education, BMI, depression, cardiovascular disease, hypertension, diabetes, and age at baseline. Both censored (NL participants who did not decline to MCI and MCI participants who did not decline to AD-dementia by their last visit) and uncensored (MCI/AD decliners) participants were included in all analyses. Sensitivity analysis was performed on subsets 1 using multiple imputation of data to generate and pool 30 imputed datasets for participants missing data for BMI, APO ε4 status, or Geriatric Depression Scale. IVEware software, available at the University of Michigan Web site, was used for the imputation. After imputation, the same survival analyses were performed in the imputed datasets. For subsets 2, participants excluded for sleep disturbances were included into the datasets and survival analyses were performed again. The confidence level for statistical inference was 95% (p < 0.05). Statistical analysis was performed using SPSS version 21.0 (IBM Corp., Armonk, NY) and SAS/STAT software (SAS Institute, Cary, NC).
RESULTS
For the most inclusive age at MCI onset subset (MC1), at baseline, 63 participants were NL and diagnosed with MCI at follow-up, 441 participants were NL and did not develop MCI, 40 participants had MCI and diagnosed as NL at follow-up, 217 participants had MCI with a reported age at onset of MCI, and 6 participants had AD-dementia with a reported age at onset of MCI. See appendix e-2 for demographics and composition for other subsets.
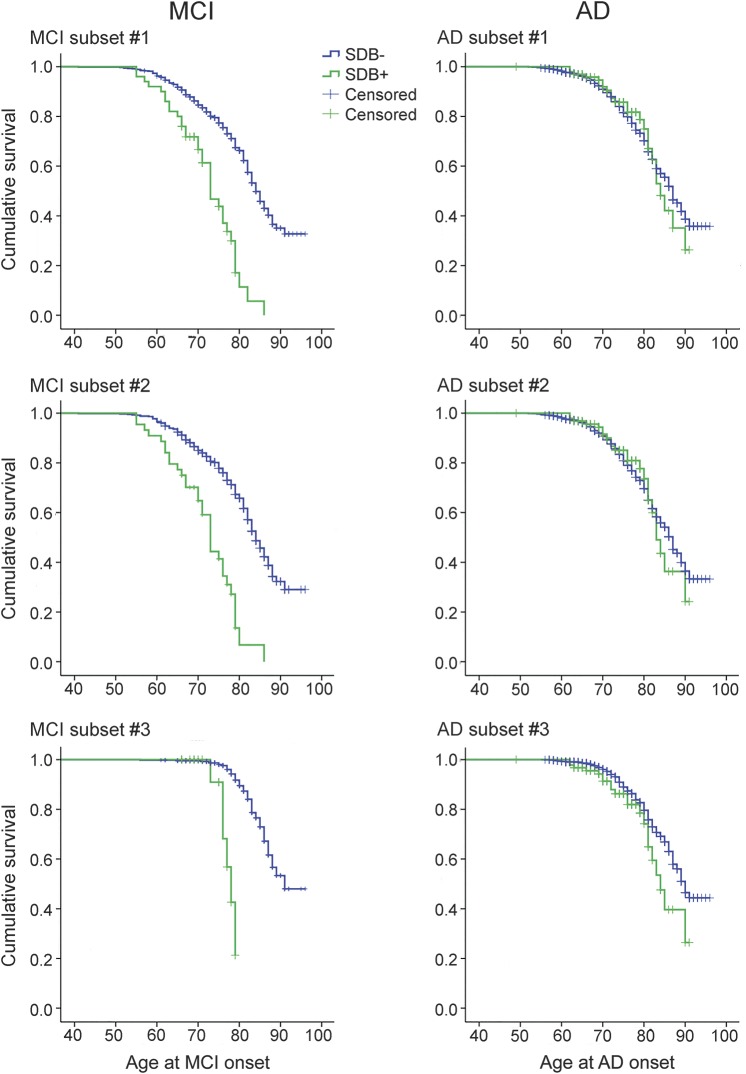
Figure 2 shows the survival curve with the age at MCI onset for SDB+ and SDB− participants in all 3 subsets. SDB+ patients had a significantly younger age at MCI onset than SDB− in all subsets (MC1: 72.63 vs 83.67, χ2 = 49.57, p < 0.01; MC2: 72.15 vs 83.45, χ2 = 52.81, p < 0.01; MC3: 77.40 vs 89.89, χ2 = 46.65, p < 0.01) (see table e-1). There was no change in the significance of differences between SDB+ and SDB− groups when controlling for APO ε4, sex, education, depression, cardiovascular disease, hypertension, diabetes, age at baseline visit, and BMI as covariates in the Cox regression analyses (see table e-2). Results remained the same when sensitivity analyses were performed imputing missing data for MC1 and when including participants with sleep disturbances in MC2 (see table 1 for full demographics).
Figure 2. Survival analysis comparing patients who were SDB− and SDB+ for all MCI and AD subsets.
Survival curves of age at MCI or AD-dementia onset using the Kaplan–Meier method showing patients who were SDB+ to have a significantly younger age at MCI onset than SDB− in all subsets and to have a significantly younger age at AD-dementia onset than SDB− in our most conservative subset. AD = Alzheimer disease; MCI = mild cognitive impairment; SDB = sleep-disordered breathing.
Table 1.
Patient demographic characteristics for MCI and AD subsets 1
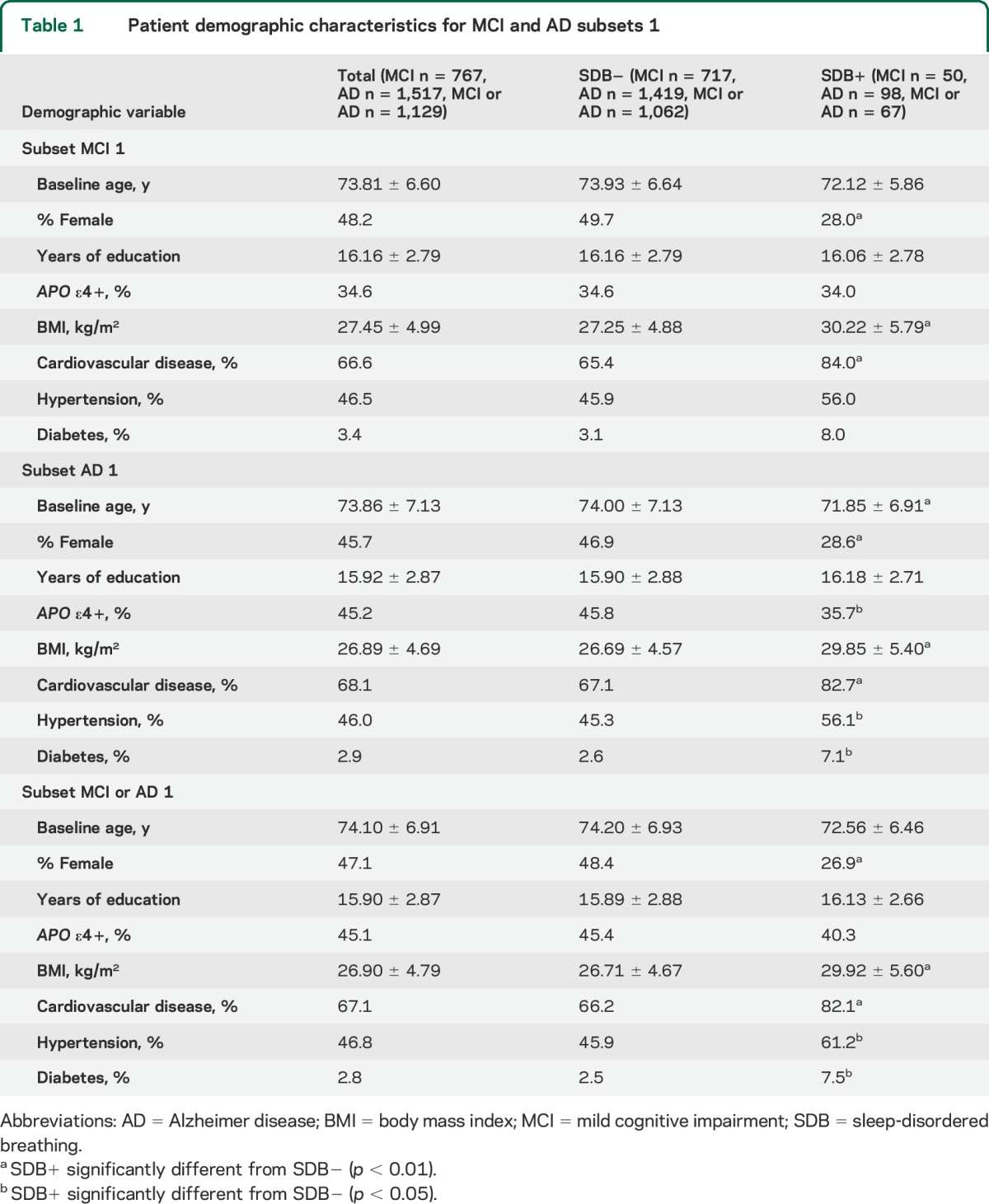
For the most inclusive AD subset (AC1), at baseline, 13 participants were NL and diagnosed with AD-dementia at follow-up, 491 participants were NL and did not develop AD-dementia, 40 participants had MCI and diagnosed as NL at follow-up, 550 participants had MCI and did not develop AD-dementia, 244 participants had MCI and developed AD-dementia at follow-up, and 179 participants had AD-dementia with a reported age at onset of AD-dementia (see table 1 for full demographics).
Figure 2 shows the survival curve with the age at AD-dementia onset for SDB+ and SDB− participants in the 3 AD subsets. SDB+ patients had a significantly younger age at AD-dementia onset than SDB− patients only in our most conservative subset (AC3: 83.46 vs 88.13, χ2 = 4.43, p < 0.05). In AC1 and AC2, the age at decline between SDB+ and SDB− participants was not significantly different, although there was a trend toward younger ages of decline in the SDB+ participants (see table e-1). When we controlled for APO ε4, sex, education, depression, cardiovascular disease, hypertension, diabetes, age at baseline, and BMI as covariates in the Cox regression analysis, the effect in AC3 became a trend (see table e-2). Results remained negative when sensitivity analyses were performed imputing missing data.
For the most inclusive age at MCI or AD-dementia onset (MAC1), at baseline, 63 participants were NL and diagnosed with MCI or AD-dementia at follow-up, 441 participants were NL and did not develop MCI or AD-dementia, 40 participants had MCI and diagnosed as NL at follow-up, 156 participants had MCI with a reported age at onset of MCI, 244 participants had MCI and developed AD-dementia at follow-up, and 185 participants had AD-dementia with a reported age at onset of cognitive decline (see table 1 for full demographics). Patients who were SDB+ had a significantly younger age at MCI or AD-dementia onset than SDB− in all subsets (MAC1: 73.48 vs 79.08, χ2 = 22.45, p < 0.01; MAC2: 73.06 vs 79.15, χ2 = 25.58, p < 0.01; MAC3: 75.21 vs 83.45, χ2 = 35.03, p < 0.01). There was no change in the significance of differences between SDB+ and SDB− groups when controlling for APO ε4, sex, education, depression, cardiovascular disease, hypertension, diabetes, age at baseline, and BMI as covariates in the Cox regression analyses. Results remained the same when sensitivity analyses were performed imputing missing data for MAC1 and when including participants with sleep disturbances in MAC2.
As an exploratory analysis, we compared the small number of participants with sleep apnea on CPAP (CPAP+) with those without reported treatment for sleep apnea (SDB+). For the most inclusive CPAP MCI subset (CMC1), at baseline, 9 participants were NL and declined to MCI at follow-up, 20 participants were NL and did not decline to MCI, 3 participants had MCI and diagnosed as NL at follow-up, and 30 participants had MCI with a reported age at onset of MCI (see table 2 for full demographics).
Table 2.
Patient demographic characteristics for CPAP MCI and AD subsets 1
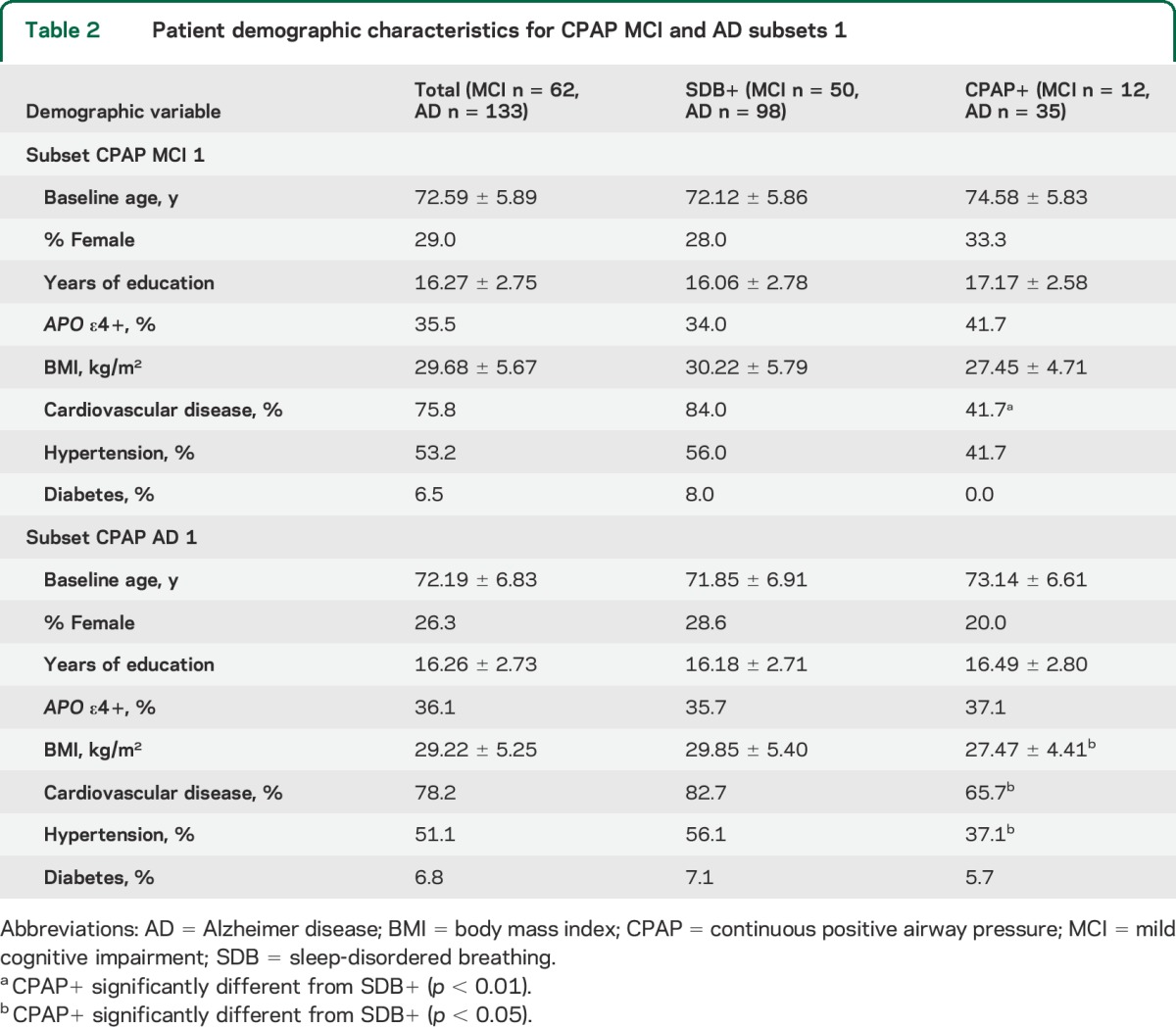
Figure e-1 shows the survival curves with the age at MCI or AD-dementia onset for SDB+ and CPAP+ participants in the 3 MCI subsets and the 3 AD subsets. Patients who were SDB+ not treated with CPAP had a significantly younger age at MCI onset than CPAP+, which was almost identical to the SDB− group (CMC1: 72.63 vs 82.10, χ2 = 8.53, p < 0.01; CMC2: 72.11 vs 82.10, χ2 = 8.68, p < 0.01). Although in CMC3 the age at decline between CPAP+ and untreated SDB+ participants was not significant, there was a trend toward younger ages of decline in the SDB+ group. The lack of significance may be attributable to a small sample size (10 CPAP+ and 18 SDB+) (see table e-3).
For the most inclusive CPAP AD subset (CAC1), 98 participants were SDB+ and 35 CPAP+ at baseline, 29 participants were NL and did not develop AD-dementia, 3 participants had MCI and diagnosed as NL at follow-up, 67 participants had MCI and did not develop AD-dementia, 31 participants had MCI and were diagnosed with AD-dementia at follow-up, and 3 participants had AD-dementia with a reported onset of AD-dementia (see table 2 for full demographics). There were no significant differences in age at AD-dementia onset in any of the 3 CPAP subsets (see figure e-1).
DISCUSSION
In this study, reported SDB was associated with an earlier age at MCI onset even when analyzing only incident cases of MCI or AD-dementia and adjusting for potential confounders. Prior work from our group has shown that there is an association between SDB and CSF AD-biomarker changes, including elevated CSF phosphorylated tau and β-amyloid 42 in NL elderly with the APO ε3/3 alleles.13 Both our CSF study and these findings from the ADNI cohort would be consistent with our hypothesis that SDB may accelerate cognitive decline in late life. To our knowledge, the present study is unique in reporting a potential treatment benefit of CPAP use in delaying age at MCI onset. Given the high prevalence of SDB and cognitive impairment among older adults, and the fact that CPAP is a currently available therapy for SDB, our findings suggest the need to directly examine the prospective benefit of therapy with CPAP for prevention or delay of cognitive decline in the elderly with SDB. Similar findings were reported in a subset of the Study of Osteoporotic Fractures (SOF) cohort,8 which found a higher risk of developing cognitive impairment among community-dwelling older women with SDB. An important strength of the SOF cohort was the use of in-lab objective sleep measurements. In contrast to our study, however, they included women who were older on average at baseline (mean age: 82.3 ± 3.2 years), and therefore were unable to observe the approximately 10-year difference in age at MCI onset that we observed in the ADNI cohort. In addition, cognitive impairment was determined in their cohort by a panel of clinical experts and based on cognitive scores, informant questionnaires, and use of medication, whereas ADNI utilizes a more standardized criteria for clinical characterization of participants14 and a different subject population (mostly community-dwelling elderly from research centers and memory clinics).
Recent work has shown that patients with AD-dementia have significantly slower cognitive decline over a 3-year follow-up period when treated for SDB.15 In contrast to the findings for MCI, for AD-dementia we did not find an association between CPAP use and delay in age at AD-dementia onset. However, our sample size was small and the years of active treatment exposure to CPAP previous to cognitive decline were unknown in most cases.
The prevalence of SDB in our sample from ADNI was low (7%) compared with the prevalence usually observed among the elderly of approximately 50% in men and 25% in women. Because SDB was self-reported in the ADNI cohort and not objectively measured, it is plausible that some of the participants who did not report SDB may have had undiagnosed SDB. With more objective in-lab methods used to confirm SDB, we would expect the observed difference in age at decline between SDB+ and SDB− patients to be even larger.
The lack of a relationship of SDB treatment status to age at AD-dementia onset, in contrast to the apparent presence of a relationship to age at MCI onset, may have been attributable to a more stringent definition of cognitive impairment implied by a diagnosis of AD-dementia than for a diagnosis of MCI, since symptoms of untreated SDB might have contributed to the diagnosis of MCI.16 It is also possible that our finding of a relationship of SDB treatment to delayed age at MCI onset may have been influenced by a generally greater compliance with health advice (balanced diet, exercise, treatment of comorbidities such as hypertension, diabetes, etc.) in participants with sleep apnea who adhered to and reported CPAP use than in those who remained untreated. In addition, ADNI did not specify a minimum threshold of treatment adherence for participants to be recorded as being treated with CPAP; therefore, it is feasible that the observed relationship between cognitive decline onset and SDB treatment status is influenced by a relatively wide range of CPAP compliance. This likely had the effect of reducing the magnitude of the relationship as participants with ineffectually low compliance may have been included in the analyses as receiving treatment. It should be noted that these findings were made in an observational study and as such do not indicate a causal relationship.
Strengths of our study were the use of a well-characterized longitudinal cohort. Limitations include the use of secondary analyses, retrospective and self-reported data, as well as the absence of objective measurements for severity of SDB or CPAP compliance. We are currently performing a 6-month pilot treatment study to aid in interpreting the current results by prospectively examining the effects of CPAP on longitudinal cognitive decline and CSF markers of neurodegeneration.
Supplementary Material
GLOSSARY
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- BMI
body mass index
- CPAP
continuous positive airway pressure
- MCI
mild cognitive impairment
- NL
cognitively normal
- SDB
sleep-disordered breathing
Footnotes
Supplemental data at Neurology.org
Contributor Information
For the Alzheimer's Disease Neuroimaging Initiative:
Michael W Weiner, Paul Aisen, Ronald Petersen, Clifford Jack, William Jagust, John C Morris, Andrew J Saykin, John Q Trojanowski, Arthur W Toga, and Laurel Beckett
AUTHOR CONTRIBUTIONS
R.O., T.G., E.P., A.V., I.A., D.R., M.L. participated in study conception and design. R.O., V.K., I.A., D.R., and M.L. supervised the study. R.O., E.P., S.L., J.L., M.W., E.D., and V.K. performed data acquisition. R.O., T.G., E.P., A.V., S.L., J.L., M.W., E.D., V.K., L.G., and L.M. performed data analysis and interpretation. R.O., T.G., E.P., and S.L. were involved in the statistical analysis. R.O., T.G., E.P., A.V., S.L., I.A., D.R., and M.L. drafted the report. R.O., T.G., and E.P. prepared the figures and tables.
STUDY FUNDING
Supported by grants from the Foundation for Research in Sleep Disorders, R01 HL118624-01 and CTSI UL1TR000038. Additional support is acknowledged from the philanthropy of Mr. James B. Kuhn. Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (NIH grant U01 AG024904) and DOD ADNI (Department of Defense award W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer's Association; Alzheimer's Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the NIH (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
DISCLOSURE
R. Osorio, T. Gumb, E. Pirraglia, A. Varga, S. Lu, J. Lim, M. Wohlleber, E. Ducca, V. Koushyk, L. Glodzik, and L. Mosconi report no disclosures relevant to the manuscript. I. Ayappa has received support for research from the industry in the past 24 months: grants and clinical trials from Fisher & Paykel Healthcare, Ventus Medical. I.A. holds multiple US and foreign patents covering techniques and analysis algorithms for the diagnosis of OSAHS and techniques for administering CPAP. Several of these have been licensed to Fisher & Paykel Healthcare and Advanced Brain Monitoring. D. Rapoport has received support for research from the industry in the past 24 months: grants and clinical trials from Fisher & Paykel Healthcare, Ventus Medical, and speaking and consulting engagements for Fisher & Paykel Healthcare. D.R. holds multiple US and foreign patents covering techniques and analysis algorithms for the diagnosis of OSAHS and techniques for administering CPAP. Several of these have been licensed to Biologics, Fisher & Paykel Healthcare, Advanced Brain Monitoring, and Tyco (Health C'Aire). M. de Leon serves on the external advisory board of Roche Pharmaceuticals and holds patents issued through NYU related to the image analysis of PET and MRI scans. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009;5:263–276. [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SD, Kang SH, Ju G, et al. The prevalence of and risk factors for sleep-disordered breathing in an elderly Korean population. Respiration 2014;87:372–378. [DOI] [PubMed] [Google Scholar]
- 3.Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 2012;35:1593–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaussent I, Bouyer J, Ancelin ML, et al. Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep 2012;35:1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foley D, Monjan A, Masaki K, et al. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. J Am Geriatr Soc 2001;49:1628–1632. [DOI] [PubMed] [Google Scholar]
- 6.Elwood PC, Bayer AJ, Fish M, et al. Sleep disturbance and daytime sleepiness predict vascular dementia. J Epidemiol Community Health 2011;65:820–824. [DOI] [PubMed] [Google Scholar]
- 7.Keage HA, Banks S, Yang KL, et al. What sleep characteristics predict cognitive decline in the elderly? Sleep Med 2012;13:886–892. [DOI] [PubMed] [Google Scholar]
- 8.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011;306:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: Cardiovascular Health Study. Arch Neurol 2009;66:336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 1993;261:921–923. [DOI] [PubMed] [Google Scholar]
- 11.O'Hara R, Schroder CM, Kraemer HC, et al. Nocturnal sleep apnea/hypopnea is associated with lower memory performance in APOE epsilon4 carriers. Neurology 2005;65:642–644. [DOI] [PubMed] [Google Scholar]
- 12.Osorio RS, Pirraglia E, Aguera-Ortiz LF, et al. Greater risk of Alzheimer's disease in older adults with insomnia. J Am Geriatr Soc 2011;59:559–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osorio RS, Ayappa I, Mantua J, et al. The interaction between sleep-disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer's disease in cognitively normal elderly individuals. Neurobiol Aging 2013;35:1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 2010;74:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Troussiere AC, Monaca CC, Salleron J, et al. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer's disease. J Neurol Neurosurg Psychiatry 2014;85:1405–1408. 10.1136/jnnp-2013-307544. [DOI] [PubMed] [Google Scholar]
- 16.Bombois S, Derambure P, Pasquier F, et al. Sleep disorders in aging and dementia. J Nutr Health Aging 2010;14:212–217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.