Abstract
Objective:
Varicella-zoster virus (VZV) infection may trigger the inflammatory cascade that characterizes giant cell arteritis (GCA).
Methods:
Formalin-fixed, paraffin-embedded GCA-positive temporal artery (TA) biopsies (50 sections/TA) including adjacent skeletal muscle and normal TAs obtained postmortem from subjects >50 years of age were examined by immunohistochemistry for presence and distribution of VZV antigen and by ultrastructural examination for virions. Adjacent regions were examined by hematoxylin & eosin staining. VZV antigen–positive slides were analyzed by PCR for VZV DNA.
Results:
VZV antigen was found in 61/82 (74%) GCA-positive TAs compared with 1/13 (8%) normal TAs (p < 0.0001, relative risk 9.67, 95% confidence interval 1.46, 63.69). Most GCA-positive TAs contained viral antigen in skip areas. VZV antigen was present mostly in adventitia, followed by media and intima. VZV antigen was found in 12/32 (38%) skeletal muscles adjacent to VZV antigen–positive TAs. Despite formalin fixation, VZV DNA was detected in 18/45 (40%) GCA-positive VZV antigen–positive TAs, in 6/10 (60%) VZV antigen–positive skeletal muscles, and in one VZV antigen–positive normal TA. Varicella-zoster virions were found in a GCA-positive TA. In sections adjacent to those containing VZV, GCA pathology was seen in 89% of GCA-positive TAs but in none of 18 adjacent sections from normal TAs.
Conclusions:
Most GCA-positive TAs contained VZV in skip areas that correlated with adjacent GCA pathology, supporting the hypothesis that VZV triggers GCA immunopathology. Antiviral treatment may confer additional benefit to patients with GCA treated with corticosteroids, although the optimal antiviral regimen remains to be determined.
Giant cell arteritis (GCA) is a disease of the elderly characterized by severe headache/head pain and scalp tenderness. Many patients also have a history of jaw claudication, polymyalgia rheumatica, fever, night sweats, weight loss, fatigue, and elevated erythrocyte sedimentation rate and C-reactive protein. Vision loss occurs often, requiring immediate corticosteroid treatment. Temporal artery (TA) biopsy reveals vessel wall damage and inflammation, with multinucleated giant cells and/or epithelioid macrophages. Skip lesions are common. TA biopsies are pathologically negative in many clinically suspect cases.1
Recent correlative clinico-virologic studies of TA biopsies from patients with clinically suspect GCA but pathologically negative biopsies revealed infection with varicella-zoster virus (VZV).2–4 Further studies detected VZV antigen in 5/24 (21%) GCA-negative TAs.5 Subsequently, a GCA-negative TA was found to contain VZV antigen and VZV DNA in multiple regions (skip areas); examination of additional sections adjacent to those containing VZV antigen revealed classic GCA pathology, resulting in a change of diagnosis from GCA-negative to GCA-positive.6 Finally, VZV antigen and VZV DNA were found in multiple noncontiguous (skip) areas in the TA of a patient with clinical GCA and ipsilateral ophthalmic-distribution zoster, followed 2 weeks later by VZV encephalitis and 2 months later by ischemic optic neuropathy, but whose TA was pathologically negative for GCA.7 The repeated detection of VZV in multiple GCA-negative TAs as well as in a GCA-positive TA prompted virologic analysis of TA biopsies from patients with pathologically confirmed GCA and control TAs removed at autopsy from adults > age 50 to test for a causal link between VZV and GCA.
METHODS
Human temporal arteries.
A total of 86 de-identified formalin-fixed, paraffin-embedded (FFPE) TA biopsy specimens from patients >50 years of age with histopathologically confirmed GCA were obtained from 13 institutions: University of Colorado Hospital, Aurora; VA Boston Healthcare System, Jamaica Plain, MA; Hospital of the University of Pennsylvania, Philadelphia; Johns Hopkins Hospital, Baltimore, MD; University of North Carolina Hospital, Chapel Hill; Mount Sinai Medical Center, Miami Beach, FL; Center for Oculoplastic Surgery, Austin, TX; University Hospital, Essen, Germany; University Medical Center Göttingen, Germany; Landspitali University Hospital, Reykjavik, Iceland; Centre Hospitalier Universitaire d'Amiens, Paris, France; Vancouver General Hospital, British Columbia, Canada; and Assaf Harofeh Medical Center, Zerifin, Israel (table e-1 on the Neurology Web site at Neurology.org).
In addition, 16 control TAs were removed postmortem from age-matched subjects >50 years of age at the University of Colorado Hospital, Arapahoe County Coroner, and Denver Office of the Medical Examiner. None of the controls exhibited skin evidence of recent herpes zoster or had any recent history of diabetes, cancer, alcohol or other substance abuse, immunosuppression, or use of immunosuppressive or anti-inflammatory drugs, including corticosteroids. Control TAs were formalin-fixed and paraffin-embedded for virologic analysis.
Study oversight.
De-identified archived TAs (containing only noncoded specimen number, age, and sex) and control TAs obtained at autopsy are not considered human subject research by the Colorado Multiple Institutional Review Board and thus are deemed exempt from review as defined by its policies and regulations and in accordance with US Office for Human Research Protections and US Food and Drug Administration guidelines. Clinical information regarding a history of zoster, zoster vaccination, or medications was not available.
Immunohistochemical analysis of human temporal arteries for VZV antigen.
One hundred 5-μm sections of all FFPE TAs were cut and baked for 1 hour at 60°C. Every other section (50 slide sections/TA) was then deparaffinized and immunostained with a 1:500 dilution of mouse monoclonal anti-VZV glycoprotein E (gE) IgG1 antibody (Santa Cruz Biotechnology, Dallas, TX) for 2 hours, followed by a 1:1,000 dilution of secondary biotinylated goat anti-mouse antibody (Dako, Carpinteria, CA) for 1 hour and prediluted streptavidin-alkaline phosphatase (BD Biosciences, San Diego, CA) for 1 hour. All incubations were at room temperature. The color reaction was developed under a light microscope using the fresh fuchsin substrate system (Dako) with levamisole (Dako; 24 μg/mL), as described.5 Slides were counterstained with 1× hematoxylin for 2 minutes. When a section was found to contain VZV antigen, at least 2 adjacent sections were stained as above except that mouse anti-VZV gE IgG1 antibody was replaced with a 1:500 dilution of control mouse IgG1 antibody (Dako). To confirm the specificity of VZV antigen detection, some TAs that contained VZV antigen were also stained with 2 different anti-VZV antibodies: (1) 1:10,000 dilution rabbit anti-VZV IE63 antibody, and (2) a different mouse anti-VZV gE antibody directed against amino acids 150–162.8 Two negative controls for rabbit anti-VZV IE63 antibody were provided by replacing the rabbit anti-VZV IE63 antibody with either normal rabbit serum or rabbit anti–herpes simplex virus (HSV)-1 antibody (Dako). Positive controls consisted of VZV-infected cadaveric cerebral and temporal arteries maintained for 14 days in vitro and then stained with mouse anti-VZV gE IgG1 antibody as described above.
Using light microscopy, 2 readers (D.G. and M.A.N.) blinded to the diagnosis of GCA-positive or normal TA examined each section. A TA section was deemed positive or negative for VZV antigen only when both readers agreed; the readers disagreed about 4/86 GCA-positive TAs and 3/16 normal TAs, and those 7 TAs were excluded from the study. The distribution of VZV antigen and number of skip lesions, defined as at least 2 VZV antigen–positive regions flanked by VZV antigen–negative regions, were then assessed.
Histopathologic analysis of sections adjacent to those containing VZV antigen for pathologic features of GCA.
For each section that contained VZV antigen, an adjacent section (within 5 μm) was stained with hematoxylin and eosin. Slides were examined by standard light microscopy by the neuropathologist (P.J.B.) and neurovirologists (D.G. and M.A.N.) for GCA positivity, defined based on the presence of medial damage, medial inflammation, and giant or epithelioid cells. A TA section was deemed GCA-positive only when all readers agreed that all 3 criteria were met.
PCR amplification of VZV DNA in temporal artery sections containing VZV antigen.
Every section of 61 VZV antigen–positive TAs was scraped with a scalpel, pooled, placed into 200 µL lysis buffer with proteinase K, and incubated overnight at 56°C (DNeasy Blood and Tissue Kit; Qiagen, Germantown, MD). DNA was extracted per the manufacturer's protocol and eluted in 50 µL water. Quantitative PCR analysis with primers for VZV and for glyceraldehyde-3-phosphate-dehydrogenase (GAPdH) was performed on 5 µL, as described.7 Similarly, every VZV antigen–positive section of skeletal muscle adjacent to a TA was scraped and treated as described above to detect amplifiable VZV DNA by real-time quantitative PCR. Samples were considered positive for VZV DNA if (1) no VZV DNA amplification was detected in samples without added DNA, (2) GAPdH was detected in wells with TA DNA, and (3) at least 2 of 4 PCR replicates amplified at least 10 copies of target VZV DNA per 5 µL of DNA extracted. VZV DNA copy number was determined using known concentrations of VZV DNA as PCR standards.9
Ultrastructural examination.
Immunofluorescent staining with mouse monoclonal antibody (Mab 3B3) directed against VZV gE was used as described8 to locate foci of VZV infection. Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) were then used to identify viral particles, as described.10,11
RESULTS
Presence and distribution of VZV antigen in pathologically verified GCA-positive temporal arteries.
Immunohistochemical analysis of 4,100 sections from 82 pathologically verified GCA-positive TAs (50 slides/TA) detected VZV antigen in 61 (74%) TAs (figure 1) compared with 1/13 (8%) normal TAs (p < 0.0001, relative risk 9.67, 95% confidence interval [CI] 1.46, 63.629). In most (49/61 [80%]) GCA-positive TAs that contained virus, VZV was seen in multiple (2–20) regions (skip areas) (figure 1, M, O, and Q) with intervening VZV-negative regions (figure 1, N and P). A total of 347 VZV-positive skip areas were found in the 61 TAs containing VZV antigen; analysis of these skip areas from all arterial sections for distribution of virus revealed VZV antigen in the adventitia, media, and intima (339 [49%], 220 [32%], and 129 [19%] times, respectively).
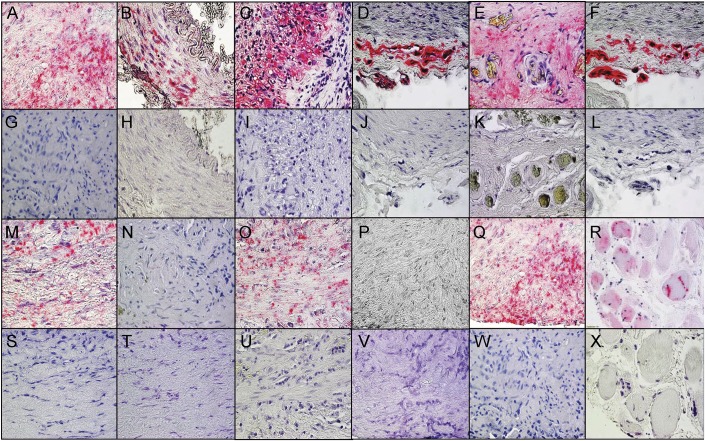
Figure 1. VZV antigen in temporal arteries pathologically positive for GCA and in skeletal muscle adjacent to the infected temporal artery.
Immunohistochemical analysis using mouse anti–varicella-zoster virus (VZV) glycoprotein E (gE) IgG1 antibody (see Methods) revealed VZV antigen in giant cell arteritis (GCA)-positive temporal arteries (TAs) in all arterial regions. VZV antigen (pink color) was seen in the adventitia and media (A), exclusively in the media (B), and in the media and intima (C) of 3 different GCA-positive TAs, as well as in the adventitia (D) of a positive control VZV-infected cadaveric cerebral artery 14 days after infection in vitro. The presence of VZV antigen was confirmed by immunostaining the same TA shown in panel A (E) and the same positive control VZV-infected cadaveric cerebral artery shown in panel D (F) with rabbit anti-VZV IE63 antibody. No staining was seen in sections adjacent to those containing VZV antigen when mouse isotype IgG1 antibody was substituted for mouse anti-VZV gE IgG1 antibody (G–J) or when normal rabbit serum was substituted for rabbit anti-VZV antibody (K, L). Discontinuous skip areas containing VZV antigen in a TA that was pathologically positive for GCA were shown by immunohistochemical staining with mouse anti-VZV gE IgG1 antibody as described in the Methods. VZV antigen was seen at positions 125–130 μm (M), 215–220 μm (O), and 255–260 μm (Q), but not at intervening regions at positions 131–214 μm (N) and 221–254 μm (P). Sections adjacent to those shown in panels M–Q were negative after immunostaining with mouse isotype IgG1 antibody (S–W). VZV antigen was often seen in skeletal muscle adjacent to a VZV-infected TA immunostained with mouse anti-VZV gE IgG1 antibody (R) that was not seen with mouse IgG1 antibody (X). 600× magnification.
Varicella-zoster virions in a temporal artery.
Ultrastructural examination was performed on a GCA-positive TA (figure 2A) in which both immunohistochemical (figure 2B) and immunofluorescent (figure 2D) staining revealed VZV antigen that was not seen with anti-HSV-1 antibody (figure 2C) or when primary antibody was omitted (figure 2E). TEM revealed an enveloped virus particle (figure 2F) and SEM showed a cluster of virus particles in the adventitia egressing through an outer cell wall (figure 2G).
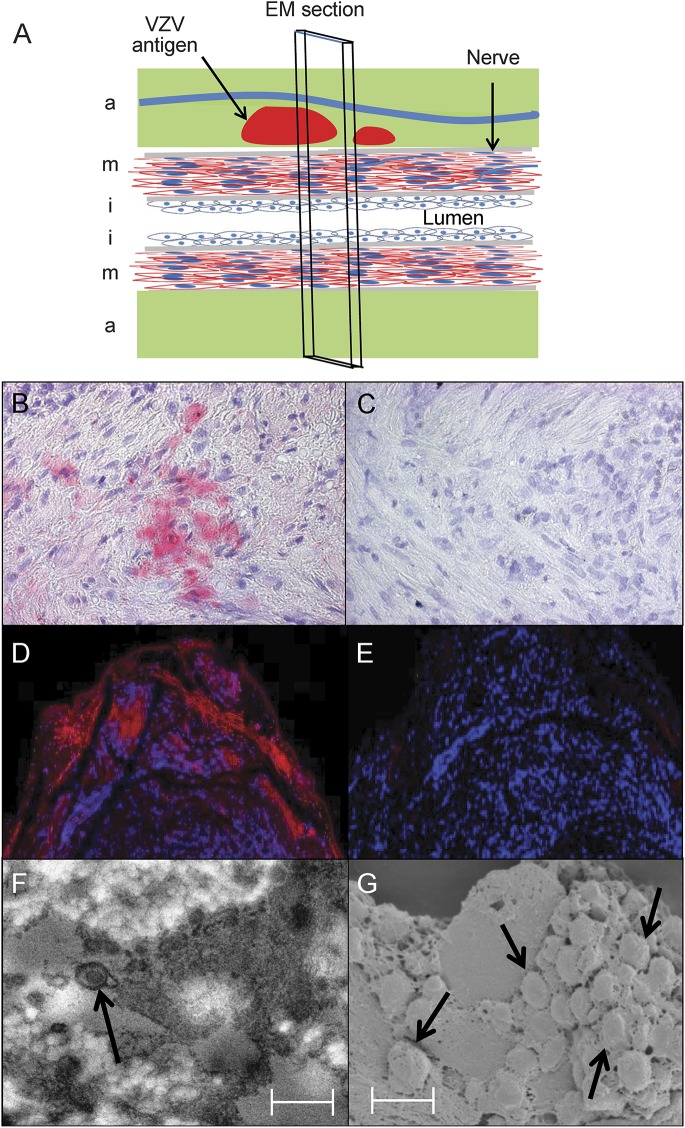
Figure 2. Immunofluorescent staining and ultrastructural imaging of VZV-infected temporal artery.
A giant cell arteritis–positive temporal artery (A) was examined for the presence of varicella-zoster virus (VZV) antigen in the adventitia (a), media (m), and intima (i). Immunohistochemical staining with rabbit anti-VZV IE63 antibody revealed VZV antigen in the media (B, pink color), but not after staining with rabbit anti–herpes simplex virus-1 antibody (C). Immunofluorescent staining with a different mouse anti-VZV IgG antibody than that used for immunohistochemistry in figure 1 revealed VZV antigen in the adventitia (D, red color), but not when primary antibody was omitted (E). Sections adjacent to those containing VZV antigen were prepared for transmission electron microscopy (TEM) and scanning electron microscopy (SEM) as described in the Methods. TEM revealed an enveloped virus particle (F, arrow), and SEM showed a cluster of virus particles in the adventitia egressing through an outer cell wall (G, arrows). Viral particles appear slightly larger than 200 nm because they were sputter coated with a gold alloy. In panels F and G, scale bars = 300 nm. EM = electron microscopy.
VZV antigen in skeletal muscle adjacent to infected temporal arteries.
Skeletal muscle was found in 39/82 (48%) GCA-positive TAs. Among the 61 GCA-positive TAs that contained VZV antigen, 32 (52%) also contained muscle, and VZV antigen was found in 12/32 (38%) of these samples (figure 1, R and X).
VZV DNA in temporal arteries.
Of the 61 GCA-positive TAs that contained VZV antigen, only 45 contained amplifiable cellular DNA. Of these 45 TAs, 18 (40%) contained VZV DNA. VZV DNA was also detected in one normal TA in which VZV antigen was found in 12 skip areas. Of the 12 skeletal muscles that contained VZV antigen, 10 contained amplifiable cellular DNA. Of these 10 skeletal muscles, 6 (60%) contained VZV DNA. In 27 GCA-positive TAs and 4 skeletal muscles, all of which contained VZV antigen and amplifiable cellular DNA, VZV DNA was not detected. This is most likely due to formalin fixation, which reduces the abundance of amplifiable DNA; furthermore, the number of VZV antigen–positive slides from which DNA was extracted was often low.
Association of histopathologic changes with VZV antigen in temporal arteries.
In GCA-positive TAs, histopathologic analysis of sections adjacent to those containing VZV antigen revealed GCA pathology in 248/279 (89%) sections (figure 3; 95% CI 86%, 93%), whereas the 18 sections adjacent to 12 skip areas in the one normal TA that contained VZV antigen showed no areas of GCA pathology (0%; 95% CI 0%, 19%). The difference in pathology proportions was highly significant (p < 0·0001, Fisher exact association test).
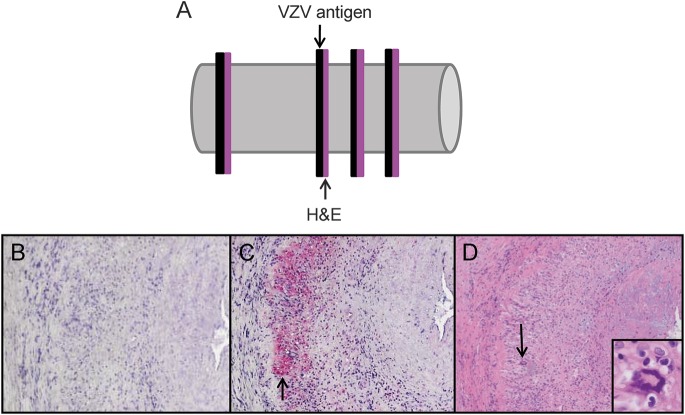
Figure 3. Pathologic analysis of sections adjacent to those containing VZV antigen from GCA-positive temporal arteries.
Temporal arteries (TAs) in which varicella-zoster virus (VZV) antigen was detected immunohistochemically were further analyzed pathologically. Sections adjacent to those containing VZV antigen were stained by hematoxylin & eosin (H&E) (A). No staining was seen with mouse isotype IgG1 antibody (B). VZV antigen was found in nearly all giant cell arteritis (GCA)-positive TAs (C, pink color, arrow). H&E of the adjacent section showed classic GCA pathology (D), with inflammation, necrosis of the media, and giant cells (arrow, inset, 600× magnification) corresponding to VZV antigen (C, arrow). 100× magnification.
DISCUSSION
Herein we report findings from the immunohistochemical analysis of 4,100 sections from 82 pathologically verified GCA-positive TAs (50 slides/TA). Using an anti-VZV IgG1 antibody directed against VZV gE that is found only in productively infected cells, immunohistochemical analysis detected VZV antigen in 61/82 (74%) GCA-positive TAs, while PCR amplified VZV DNA (despite formalin fixation) in 18/45 (40%) VZV antigen–positive TAs that contained amplifiable cellular DNA. Both TEM and SEM revealed varicella-zoster virions in VZV antigen–positive areas. The relevance of VZV in GCA-positive TAs was further shown by (1) the presence of VZV in skip areas, paralleling a characteristic feature of GCA pathology; (2) the close proximity of VZV antigen in TAs with adjacent GCA pathology; and (3) the presence of VZV antigen and VZV DNA in skeletal muscle adjacent to VZV antigen–positive TAs. Because all subjects were de-identified except for age and sex, no clinico-virologic correlations could be made regarding the presence of VZV in GCA-positive TAs and a history of zoster, zoster vaccination, or medication.
VZV antigen was found in cells in the adventitia, media, and intima of TAs, consistent with the well-documented ability of VZV to replicate in all organs during disseminated varicella and zoster as well as in all human non-neuronal cells in tissue culture. The similar distribution of VZV antigen predominantly in the arterial adventitia, slightly less in the media, and least in the intima in both GCA-positive TAs and intracerebral VZV vasculopathy12 indicates that the mechanism by which VZV infects arteries after reactivation from cranial nerve ganglia is transaxonal spread to arteries, initially infecting the adventitia followed by extension transmurally. Such a mechanism is further supported by our detection of varicella-zoster virions in the adventitia of a GCA-positive TA.
The presence of VZV antigen in GCA lesions is not likely to be a bystander effect due to subclinical reactivation induced by inflammation. After more than 3 decades of analysis of tissue sections for VZV from patients with inflammatory/infectious diseases, we found that while VZV causes inflammation, inflammation does not cause VZV to reactivate and infect the inflamed region. We have never found VZV in inflammatory brain tissue13 or in highly inflammatory CSF in patients with meningitis.14 VZV is only associated with inflammation when VZV causes the inflammatory response, as exemplified by our demonstrations of VZV in intracerebral arteries of patients with VZV vasculopathy15 and our recent demonstration of VZV antigen and VZV DNA in a patient with VZV pneumonitis, giant cells, and Cowdry A inclusions.16
A close association of GCA with intracerebral VZV vasculopathy was also revealed by in situ hybridization, which detected VZV DNA in a GCA-positive TA from a patient who developed bilateral internuclear ophthalmoplegia and right middle cerebral artery stroke 2 months after ophthalmic-distribution zoster,17 and by immunohistochemical detection of VZV antigen in a GCA-positive TA from a patient who developed choroidal infarction 4 months after ophthalmic-distribution zoster.18 Together, the collective clinical, pathologic, and virologic findings indicate that GCA is essentially a VZV vasculopathy primarily affecting the TA.
It is not surprising that skeletal muscle adjacent to VZV-infected TAs is infected with VZV, since the mammalian superficial TA is richly innervated19 and nociception in connective tissue of the temporalis muscle is relayed by afferent fibers with cell bodies in the trigeminal ganglia20,21 from which VZV reactivates.
A comparison of the presence of VZV in GCA-positive TAs and normal TAs obtained from individuals over age 50 (when GCA usually develops) revealed important differences. VZV was present in only 1/13 normal TAs, and even though VZV antigen was found in 12 skip areas, histopathologic analysis revealed no GCA pathology in any of 18 sections adjacent to those containing VZV antigen. In contrast, histopathologic analysis of 279 sections adjacent to those containing VZV antigen in GCA-positive TAs revealed GCA pathology in 248 (89%) sections, confirming the close relationship between GCA pathology and the presence of VZV antigen. The presence of VZV in one normal TA most likely indicated subclinical VZV reactivation since that individual had no symptoms of GCA before death and no histopathologic changes of GCA in the TA.
Previous studies to detect VZV in temporal artery biopsies.
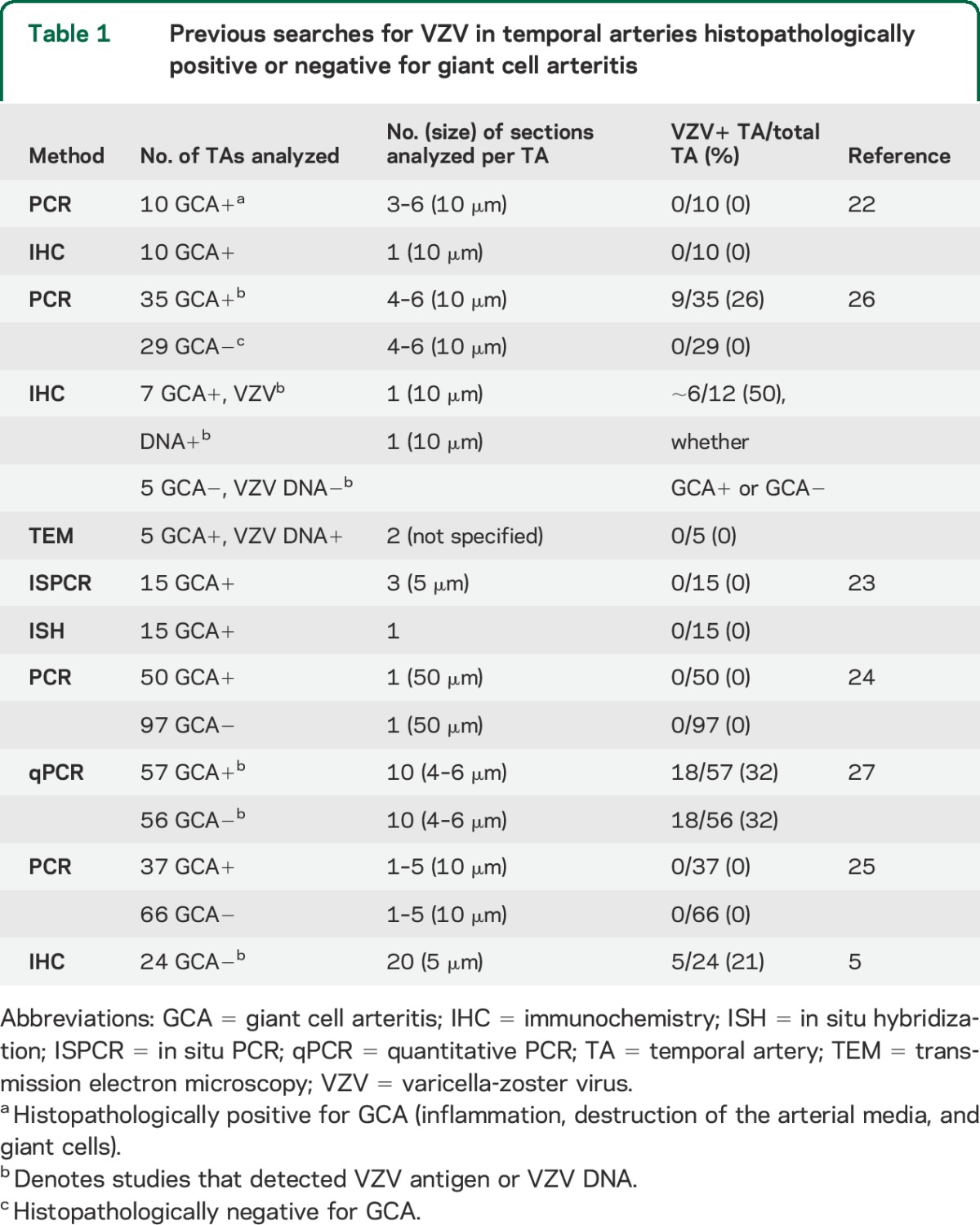
In earlier studies that examined FFPE GCA-positive and GCA-negative TAs for VZV, detection of VZV DNA and/or VZV antigen correlated positively with the number of sections examined (table 1). For example, PCR analyses of FFPE GCA-positive and GCA-negative TA biopsy specimens did not reveal VZV DNA when 1–6 sections per TA were examined.22–25 Furthermore, immunohistochemistry with VZV-specific antibodies did not detect VZV antigen when only one section from each TA was analyzed,22 and no herpesvirus particles were found in 2 sections of each TA examined by TEM.26 In contrast, examination of 4–6 sections detected VZV DNA in 9/35 (26%) GCA-positive TAs,26 and examination of 10 sections detected VZV DNA in 18/57 (32%) specimens from GCA-positive TAs and 18/56 (32%) from GCA-negative TAs.27 Furthermore, 25 sections of each FFPE TA biopsy specimen revealed VZV antigen in 5/24 (21%) GCA-negative TAs.5 While an apparent exception is the detection of VZV antigen in ∼50% of GCA-positive and GCA-negative TAs when only one section was studied,26 sampling was biased since more than half of the TAs were already known to contain VZV DNA. Overall, our detection of VZV antigen in 74% of GCA-positive TAs indicates the value of staining more than 10 sections. In fact, we predict that analysis of hundreds of sections of each TA will reveal VZV in nearly every GCA-positive artery.
Table 1.
Previous searches for VZV in temporal arteries histopathologically positive or negative for giant cell arteritis
Evidence that supports the notion that VZV causes GCA.
VZV is an exclusively human virus that does not produce disease in experimentally infected animals. Nevertheless, multiple findings strongly indicate that VZV is a cause of GCA: (1) VZV was found in 74% of TAs pathologically positive for GCA; (2) despite formalin fixation, VZV DNA was detected in many VZV antigen–positive TAs; (3) VZV antigen and VZV DNA were found in skeletal muscle adjacent to TAs containing VZV; (4) varicella-zoster virions were present in a GCA-positive TA; (5) VZV antigen predominated in the adventitia, indicating that after reactivation from cranial nerve ganglia VZV travels transaxonally to initially infect the arterial adventitia followed by transmural extension; (6) the pathology and distribution of VZV antigen in GCA-positive TAs are virtually identical to those in patients who died of intracerebral VZV vasculopathy; and (7) the presence of VZV antigen was significantly associated with GCA pathology. Because multiple agents underlie other diseases (e.g., pneumonitis and meningitis), it remains possible that agents in addition to VZV cause GCA.
Implications for treatment.
The collective findings above directly affect future treatment considerations for patients with GCA. The dramatic favorable response to treatment with corticosteroids in many patients with GCA indicates that the disorder is the result of an inflammatory response to VZV. Many patients require months to years of treatment to fully suppress inflammatory activity. Furthermore, despite corticosteroid therapy, many patients with GCA still experience loss of vision and stroke,28 and some develop more widespread vasculopathy.29 A possible explanation for these refractory cases is the long-term potentiation of VZV infection by corticosteroid treatment. Indeed, the apparent role of VZV in triggering the immunopathology of GCA warrants addition of antiviral treatment to corticosteroids. Because GCA seems to be a VZV vasculopathy primarily affecting the TA, treatment of GCA patients with corticosteroids and IV acyclovir, as recommended for intracerebral VZV vasculopathy, is likely to be the most effective therapy and may shorten the length of treatment with corticosteroids. It remains unclear whether standard oral antiviral agents (e.g., valacyclovir, 1 g, 3 times daily for 2–4 weeks) in conjunction with corticosteroids will be as effective as IV acyclovir and corticosteroids; dosage and duration of treatment also remain to be determined.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Stefan Sillau for statistical analysis, John Heinegg and Marina Hoffman for editorial review, and Cathy Allen for word processing and formatting.
GLOSSARY
- CI
confidence interval
- FFPE
formalin-fixed, paraffin-embedded
- GAPdH
glyceraldehyde-3-phosphate-dehydrogenase
- GCA
giant cell arteritis
- gE
glycoprotein E
- HSV
herpes simplex virus
- SEM
scanning electron microscopy
- TA
temporal artery
- TEM
transmission electron microscopy
- VZV
varicella-zoster virus
Footnotes
Editorial, page 1918
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Gilden: drafted and revised the manuscript for content; designed and supervised the study; collected, analyzed, and interpreted data. Ms. White: collected, analyzed, and interpreted data; revised the manuscript for content. Ms. Khmeleva: collected, analyzed, and interpreted data. Ms. Heintzman: collected, analyzed, and interpreted data. Mr. Choe: collected, analyzed, and interpreted data. Dr. Boyer: analyzed and interpreted the data. Dr. Grose: contributed to implementation of the study. Dr. Carpenter: contributed to the implementation of the study. Ms. Rempel: collected, analyzed, and interpreted data. Mr. Bos: collected, analyzed, and interpreted data. Dr. Kandasamy: supplied clinical material necessary for conducting the study. Dr. Lear-Kaul: supplied clinical material necessary for conducting the study. Dr. Holmes: supplied clinical material necessary for conducting the study. Dr. Bennett: supplied clinical material necessary for conducting the study; analyzed and interpreted the data. Dr. Cohrs: contributed to the implementation of the study. Dr. Mahalingam: contributed to the implementation of the study. Dr. Mandava: supplied clinical material necessary for conducting the study. Dr. Eberhard: supplied clinical material necessary for conducting the study. Dr. Bockelman: supplied clinical material necessary for conducting the study. Dr. Poppiti: supplied clinical material necessary for conducting the study. Dr. Tamhankar: supplied clinical material necessary for conducting the study. Dr. Fogt: supplied clinical material necessary for conducting the study. Dr. Amato: supplied clinical material necessary for conducting the study. Dr. Wood: supplied clinical material necessary for conducting the study. Dr. Durairaj: supplied clinical material necessary for conducting the study. Dr. Rasmussen: supplied clinical material necessary for conducting the study. Dr. Petursdottir: supplied clinical material necessary for conducting the study. Dr. Pollak: supplied clinical material necessary for conducting the study. Dr. Mendlovic: supplied clinical material necessary for conducting the study. Dr. Chatelain: supplied clinical material necessary for conducting the study. Dr. Keyvani: supplied clinical material necessary for conducting the study. Dr. Brueck: supplied clinical material necessary for conducting the study. Dr. Nagel: drafted and revised the manuscript for content; designed and supervised the study; collected, analyzed, and interpreted data.
STUDY FUNDING
This work was supported in part by NIH grants AG032958 to D.G. and NS 067070 to M.A.N.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Lenton J, Donnelly R, Nash JR. Does temporal artery biopsy influence the management of temporal arteritis? QJM 2006;99:33–36. [DOI] [PubMed] [Google Scholar]
- 2.Salazar R, Russman AN, Nagel MA, et al. Varicella zoster virus ischemic optic neuropathy and subclinical temporal artery involvement. Arch Neurol 2011;68:517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathias M, Nagel MA, Khmeleva N, et al. VZV multifocal vasculopathy with ischemic optic neuropathy, acute retinal necrosis and temporal artery infection in the absence of zoster rash. J Neurol Sci 2013;325:180–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagel MA, Russman AN, Feit H, et al. VZV ischemic optic neuropathy and subclinical temporal artery infection without rash. Neurology 2013;80:220–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagel MA, Bennett JL, Khmeleva N, et al. Multifocal VZV vasculopathy with temporal artery infection mimics giant cell arteritis. Neurology 2013;80:2017–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagel MA, Khmeleva N, Boyer PJ, Choe A, Bert R, Gilden D. Varicella zoster virus in the temporal artery of a patient with giant cell arteritis. J Neurol Sci 2013;335:228–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teodoro T, Nagel MA, Geraldes R, et al. Biopsy-negative, VZV-positive giant cell arteritis, zoster, VZV encephalitis and ischemic optic neuropathy, all in one. J Neurol Sci 2014;343:195–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grose C, Tyler S, Peters G, et al. Complete DNA sequence analyses of the first two varicella-zoster virus glycoprotein E (D150N) mutant viruses found in North America: evolution of genotypes with an accelerated cell spread phenotype. J Virol 2004;78:6799–6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baird NL, Bowlin JL, Yu X, et al. Varicella zoster virus DNA does not accumulate in infected human neurons. Virology 2014;458–459:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harson R, Grose C. Egress of varicella-zoster virus from the melanoma cell: a tropism for the melanocyte. J Virol 1995;69:4994–5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padilla JA, Nii S, Grose C. Imaging of the varicella zoster virion in the viral highways: comparison with herpes simplex viruses 1 and 2, cytomegalovirus, pseudorabies virus, and human herpes viruses 6 and 7. J Med Virol 2003;70(suppl 1):S103–S110. [DOI] [PubMed] [Google Scholar]
- 12.Nagel MA, Traktinskiy I, Azarkh Y, et al. Varicella zoster virus vasculopathy: analysis of virus-infected arteries. Neurology 2011;77:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgoon MP, Cohrs RJ, Bennett JL, et al. Varicella zoster virus is not a disease-relevant antigen in multiple sclerosis. Ann Neurol 2009;65:474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzales N, Tyler KL, Gilden DH. Recurrent dermatomal vesicular skin lesions: a clue to diagnosis of herpes simplex virus 2 meningitis. Arch Neurol 2003;60:868–869. [DOI] [PubMed] [Google Scholar]
- 15.Gilden DH, Kleinschmidt-DeMasters BK, Wellish M, Hedley-Whyte ET, Rentier B, Mahalingam R. Varicella zoster virus, a cause of waxing and waning vasculitis: the New England Journal of Medicine case 5-1995 revisited. Neurology 1996;47:1441–1446. [DOI] [PubMed] [Google Scholar]
- 16.Nandhagopal R, Khmeleva N, Jayakrishnan B, et al. Varicella zoster virus pneumonitis and brainstem encephalitis without skin rash in an immunocompetent adult. Open Forum Infect Dis 2014; (Summer) 1 10.1093/ofid/ofu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Abdulla NA, Rismondo V, Minkowski JS, Miller NR. Herpes zoster vasculitis presenting as giant cell arteritis with bilateral internuclear ophthalmoplegia. Am J Ophthalmol 2002;134:912–914. [DOI] [PubMed] [Google Scholar]
- 18.Al-Abdulla NA, Kelley JS, Green WR, Miller NR. Herpes zoster vasculitis presenting as giant cell arteritis with choroidal infarction. Retina 2003;23:567–569. [DOI] [PubMed] [Google Scholar]
- 19.Olesen IJ, Gulbenkian S, Valenca A, et al. The peptidergic innervation of the human superficial temporal artery: immunohistochemistry, ultrastructure, and vasomotility. Peptides 1995;16:275–287. [DOI] [PubMed] [Google Scholar]
- 20.Dong XD, Mann MK, Sessle BJ, Arendt-Nielsen L, Svensson P, Cairns BE. Sensitivity of rat temporalis muscle afferent fibers to peripheral N-methyl-D-aspartate receptor activation. Neuroscience 2006;141:939–945. [DOI] [PubMed] [Google Scholar]
- 21.Castrillon EE, Cairns BE, Wang K, Arendt-Nielsen L, Svensson P. Comparison of glutamate-evoked pain between the temporalities and masseter muscles in men and women. Pain 2012;153:823–829. [DOI] [PubMed] [Google Scholar]
- 22.Nordborg C, Nordborg E, Petursdottir V, et al. Search for varicella zoster virus in giant cell arteritis. Ann Neurol 1998;44:413–414. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy PG, Grinfeld E, Esiri MM. Absence of detection of varicella-zoster virus DNA in temporal artery biopsies obtained from patients with giant cell arteritis. J Neurol Sci 2003;215:27–29. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Pla A, Bosch-Gil JA, Echevarria-Mayo JE, et al. No detection of parvovirus B19 or herpesvirus DNA in giant cell arteritis. J Clin Virol 2004;31:11–15. [DOI] [PubMed] [Google Scholar]
- 25.Cooper RJ, D'Arcy S, Kirby M, et al. Infection and temporal arteritis: a PCR-based study to detect pathogens in temporal artery biopsy specimens. J Med Virol 2008;80:501–505. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell BM, Font RL. Detection of varicella zoster virus DNA in some patients with giant cell arteritis. Invest Ophthalmol Vis Sci 2001;42:2572–2577. [PubMed] [Google Scholar]
- 27.Alvarez-Lafuente R, Fernandez-Gutierrez B, Jover JA, et al. Human parvovirus B19, varicella zoster virus, and human herpes virus 6 in temporal artery biopsy specimens of patients with giant cell arteritis: analysis with quantitative real time polymerase chain reaction. Ann Rheum Dis 2005;64:780–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotter I, Henes JC, Wagner AD, Loock J, Gross WL. Does glucocorticosteroid-resistant large-vessel vasculitis (giant cell arteritis and Takayasu arteritis) exist and how can remission be achieved? A critical review of the literature. Clin Exp Rheumatol 2012;30(1 suppl 70):S114–S129. [PubMed] [Google Scholar]
- 29.Parra J, Domingues J, Sargento-Freitas J, Santana I. Extensive intracranial involvement with multiple dissections in a case of giant cell arteritis. BMJ Case Rep 2014. 10.1136/bcr-2014–204130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.