Abstract
Objective:
To assess the practices and perceptions of brain death determination worldwide and analyze the extent and nature of variations among countries.
Methods:
An electronic survey was distributed globally to physicians with expertise in neurocritical care, neurology, or related disciplines who would encounter patients at risk of brain death.
Results:
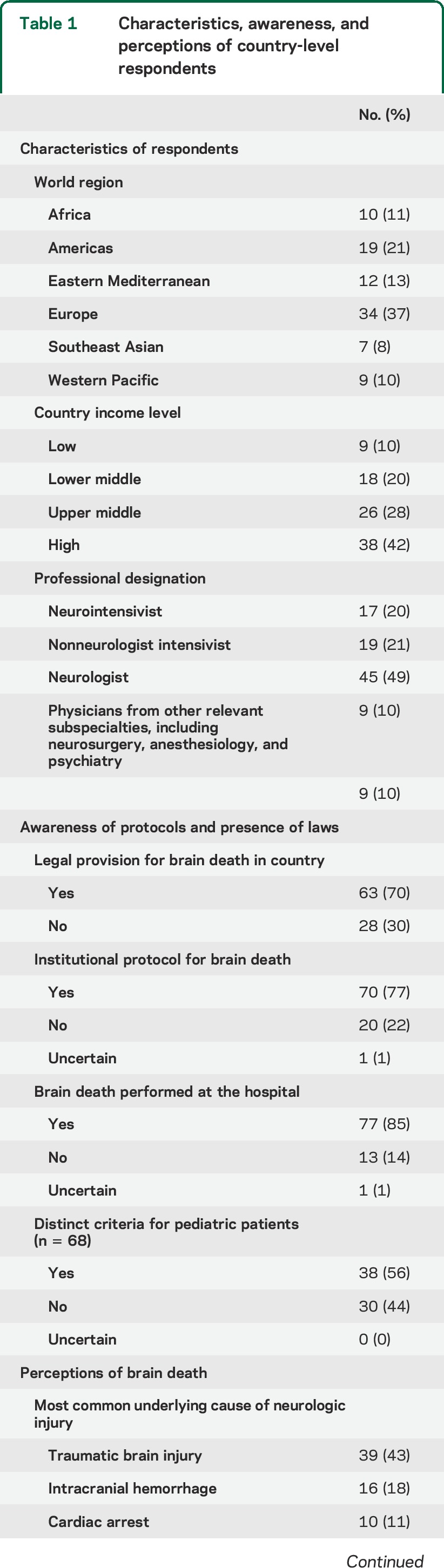
Most countries (n = 91, response rate 76%) reported a legal provision (n = 63, 70%) and an institutional protocol (n = 70, 77%) for brain death. Institutional protocols were less common in lower-income countries (2/9 of low [22%], 9/18 lower-middle [50%], 22/26 upper-middle [85%], and 37/38 high-income countries [97%], p < 0.001). Countries with an organized transplant network were more likely to have a brain death provision compared with countries without one (53/64 [83%] vs 6/25 [24%], p < 0.001). Among institutions with a formalized brain death protocol, marked variability occurred in requisite examination findings (n = 37, 53% of respondents deviated from the American Academy of Neurology criteria), apnea testing, necessity and type of ancillary testing (most commonly required test: EEG [n = 37, 53%]), time to declaration, number and qualifications of physicians present, and criteria in children (distinct pediatric criteria: n = 38, 56%).
Conclusions:
Substantial differences in perceptions and practices of brain death exist worldwide. The identification of discrepancies, improvement of gaps in medical education, and formalization of protocols in lower-income countries provide first pragmatic steps to reconciling these variations. Whether a harmonized, uniform standard for brain death worldwide can be achieved remains questionable.
The concept of brain death (“coma dépassé”) first emerged in the 1950s,1,2 driven by advances in critical care medicine such as cardiopulmonary resuscitation and mechanical ventilation. In 1995, the American Academy of Neurology (AAN) published evidence-based practice parameters,3 updated in 2010,4 providing an algorithmic, unified, step-by-step approach to brain death determination in adults. An international review of legal documents and protocols in 2002 found significant variations in the conduct of the apnea test, time to declaration, number of required examiners, and the necessity of ancillary testing.5 There may be a considerable separation between legal codes and the actual practice of brain death declaration.6 Limited information is available from low- and middle-income countries to date, although the burden of the major causes of brain death is increasing globally.7–11
The goal of the present study was to assess the perceptions and actual practices of brain death declaration in order to identify differences among physicians who perform it on a routine basis. A survey was distributed worldwide to address the key components outlined in the 2010 AAN practice parameters. This report comprehensively summarizes variations in brain death declaration in 91 countries with the goal of identifying areas that require international consensus.
METHODS
This study was reviewed by The Johns Hopkins University's institutional review board.
Survey design.
The authors (S.W., P.V.P., D.M.G., F.J.M.) designed a 40-question survey (see appendix e-1 on the Neurology® Web site at Neurology.org) on the opinions and usual practices in the setting of potential brain death declaration with the aims to: (1) assess international variations, (2) ascertain awareness of legal provisions and institutional protocols, (3) gather information about specific practices, and (4) evaluate the necessity to contact the local organ procurement organization before determination of brain death. The response goal was to include an expert informant from each country in which specialized neurologic practice occurs.
Respondents were asked their subjective understanding of brain death as well as awareness and knowledge of brain death protocols and procedures at the institutional and country levels. The medical qualifications of the respondents were sought, and only respondents whose qualifications included interaction with patients who could become brain dead were invited and included in the final analyses.
Certain questions were displayed contingent on preceding responses. For instance, questions about the specific practice of brain death declaration were only shown to a respondent if he/she confirmed that their hospital had an institutional protocol and if he/she had previously declared a patient brain dead before at his/her institution. Respondents who indicated that no patient had been declared brain dead at their hospital were asked about the reasons for the lack of brain death determination. For any question assessing knowledge or facts, respondents were given the option to indicate that they were uncertain about the answer.
Pilot testing.
The survey was pilot tested on a group of physicians in 6 countries, including neurologists and intensive care physicians. Based on the responses of the pilot survey and comments about the content, the final survey was modified, including rephrasing, removing, and adding questions. These test results were not included in the final analyses.
Survey distribution.
The survey was distributed electronically, utilizing the Qualtrics Survey Software (Harvard eCommons portal), in English. Participants were reached in 4 ways: (1) a survey link was e-mailed to the Neurocritical Care Society membership outside of the United States and Canada; (2) country representatives of the World Federation of Neurology were contacted and asked to either complete the survey or forward it to a colleague with expertise on the topic; (3) corresponding authors from relevant clinical publications on brain death or related topics were e-mailed, based on a literature search in PubMed using the key terms “brain death,” “organ donation,” “intracranial hemorrhage,” “traumatic brain injury” and the country name of interest; and (4) personal contacts of the authors in various countries with known expertise on brain death were invited to participate. Respondents were invited once by e-mail between January and November 2013, and a reminder was sent twice at 4-week intervals.
Statistical analysis.
All respondents who completed >50% of the survey were included in the analysis. Responses were reported descriptively using sample proportions, stratified by World Health Organization region12 and 2012 World Bank country income level (http://data.worldbank.org). Fisher exact test was used to determine whether 2 categorical variables were associated. All tests of hypothesis were 2-sided and conducted at significance level 0.05. Computations were performed in the R statistical computing environment (R Core Team [2014]).13
RESULTS
Survey respondents.
Responses were received from 94 of 123 countries in which potential respondents were identified (76%). Respondents from 89 countries completed all questions, and those from 2 additional countries completed >50% of the survey (final response rate for analyses 91/123, 74%).
Awareness and presence of laws and protocols related to brain death.
By country income level grouping, an institutional protocol was present in 2/9 of low-income (22%), 9/18 lower-middle (50%), 22/26 upper-middle (85%), and 37/38 high-income countries (97%) (test for trend, p < 0.001) (table 1). Approximately 33% of countries without an institutional brain death protocol (7/21) were in Africa, representing 70% of total respondents from that region. By comparison, only 2 of 34 European region countries (6%) reported no institutional protocol (7/21 Africa vs 2/34 Europe, p = 0.02). Respondents from 63 countries (70%) were aware of a legal provision for brain death. Respondents in 2 countries, United States and Australia, specified that the laws varied within the country.
Table 1.
Characteristics, awareness, and perceptions of country-level respondents
Practice of brain death declaration.
Thirteen respondents (14%, 6 low-income and 5 lower-middle-income countries, 6 African countries) stated that brain death declaration had not been performed in their hospital to date. The most frequent reasons were “no intensive care unit or advanced technology available” (n = 9, 70%) and “no physician expertise in brain death declaration” (n = 7, 53%), followed by “uncertainties about the concept of brain death” (n = 4, 31%). Nearly all physicians (95%, 72/76) from institutions in which brain death is an established concept reported that at least one patient was declared brain dead at their institution; by comparison, 33% (5/15) stated brain death was ever declared when the concept was not established (5/15) (p < 0.0001).
When asked, “Do you think brain death equates to cardiac death?” 57% responded no (n = 52; 6/9 [67%] low-income, 8/18 [44%] lower-middle-income, 17/26 [65%] upper-middle-income, 21/38 [55%] high-income, and 8/10 [80%] African countries). The income level of a country was not associated with the response of whether or not brain death equates to cardiopulmonary death (p = 0.77).
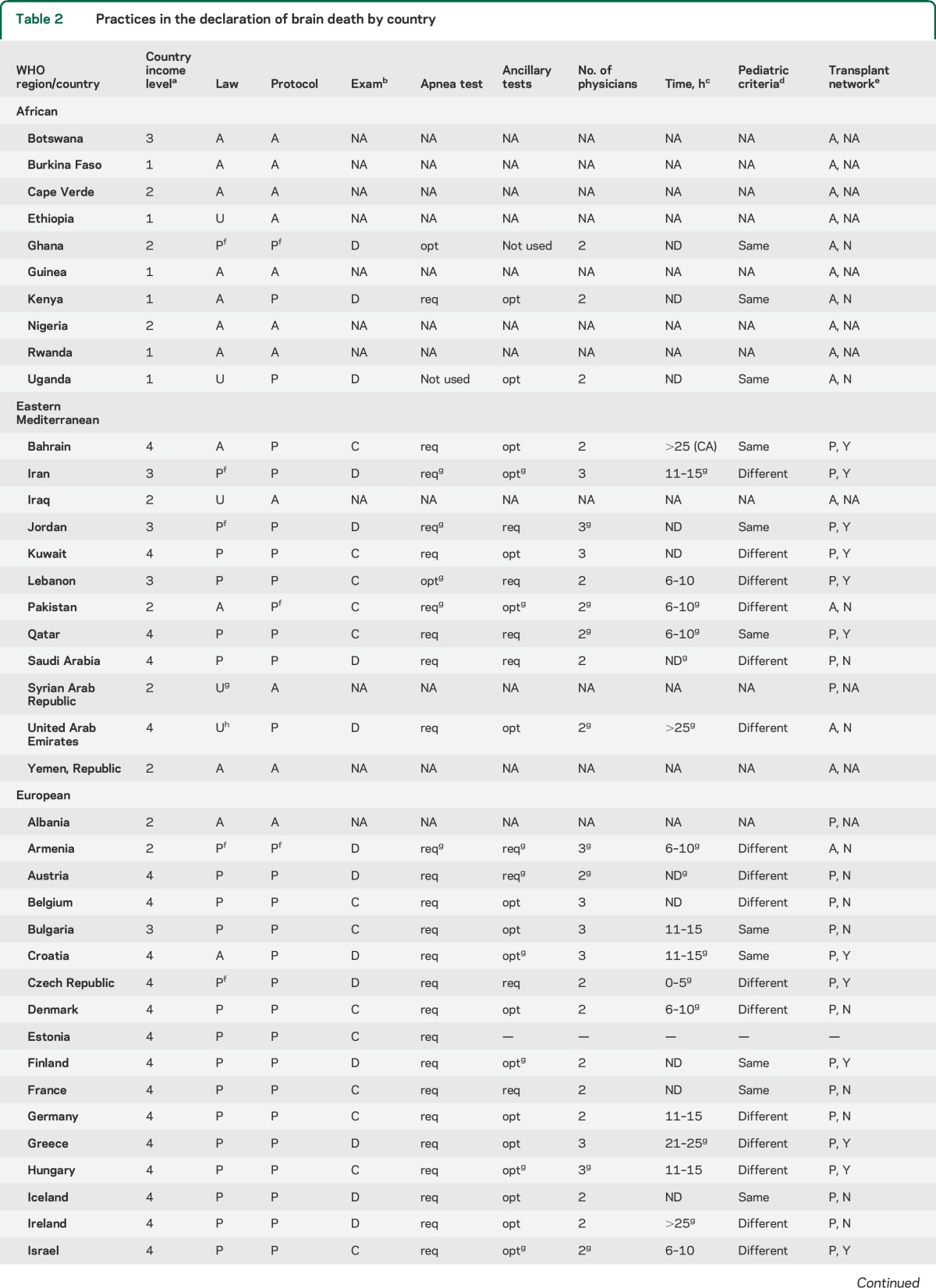
Among physicians who reported brain death as an established concept in their country and institution, marked variability was noted in requisite examination findings, process of apnea testing, necessity and type of ancillary testing, time to brain death declaration, as well as the number and minimum qualifications of physicians required for declaration (table 2). Only 10% (n = 7) reported that one physician alone was sufficient to declare brain death, while the majority reported that 2 (n = 40, 59%) or more (n = 21, 31%) physicians were needed to make the determination.
Table 2.
Practices in the declaration of brain death by country
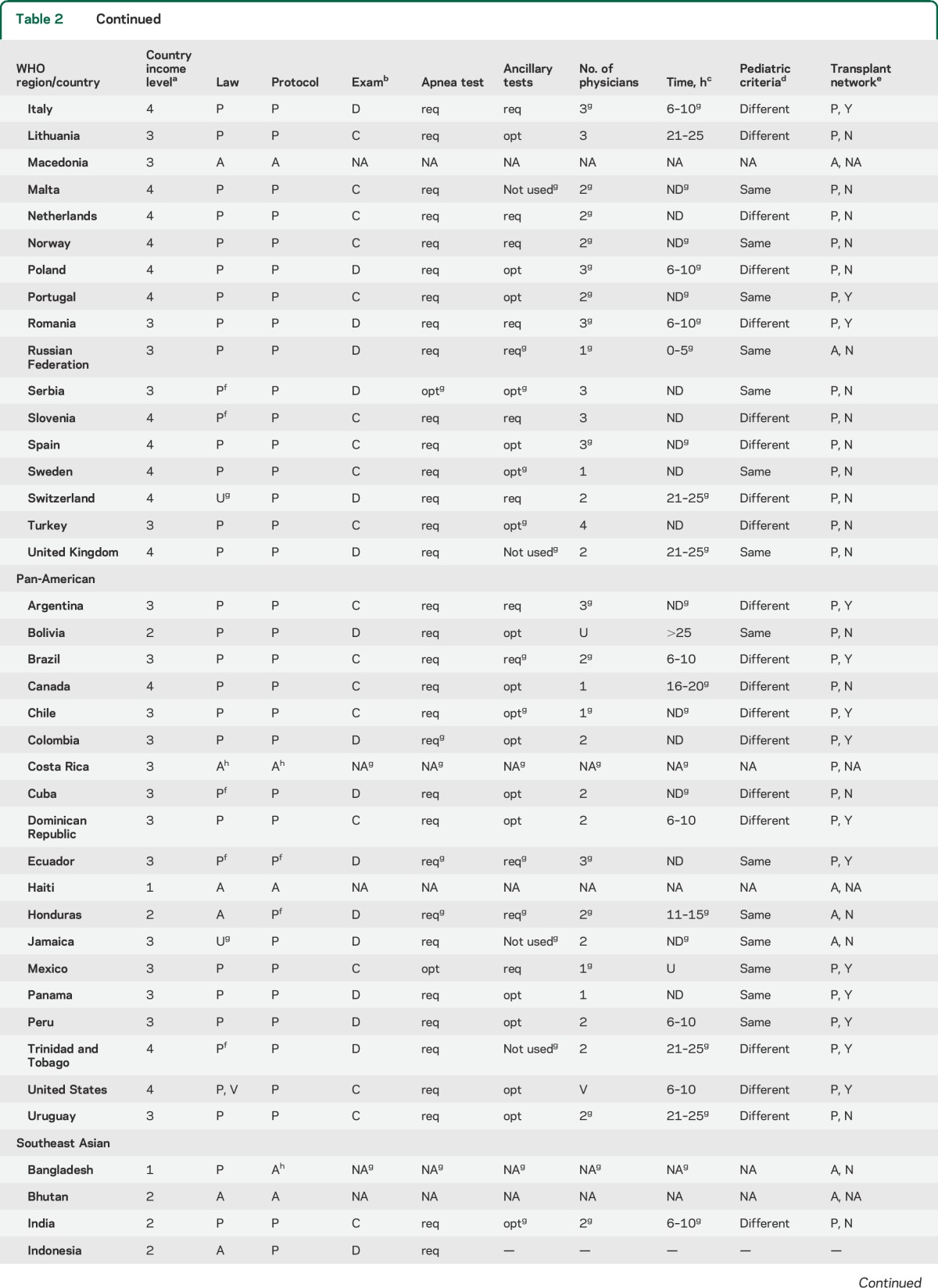
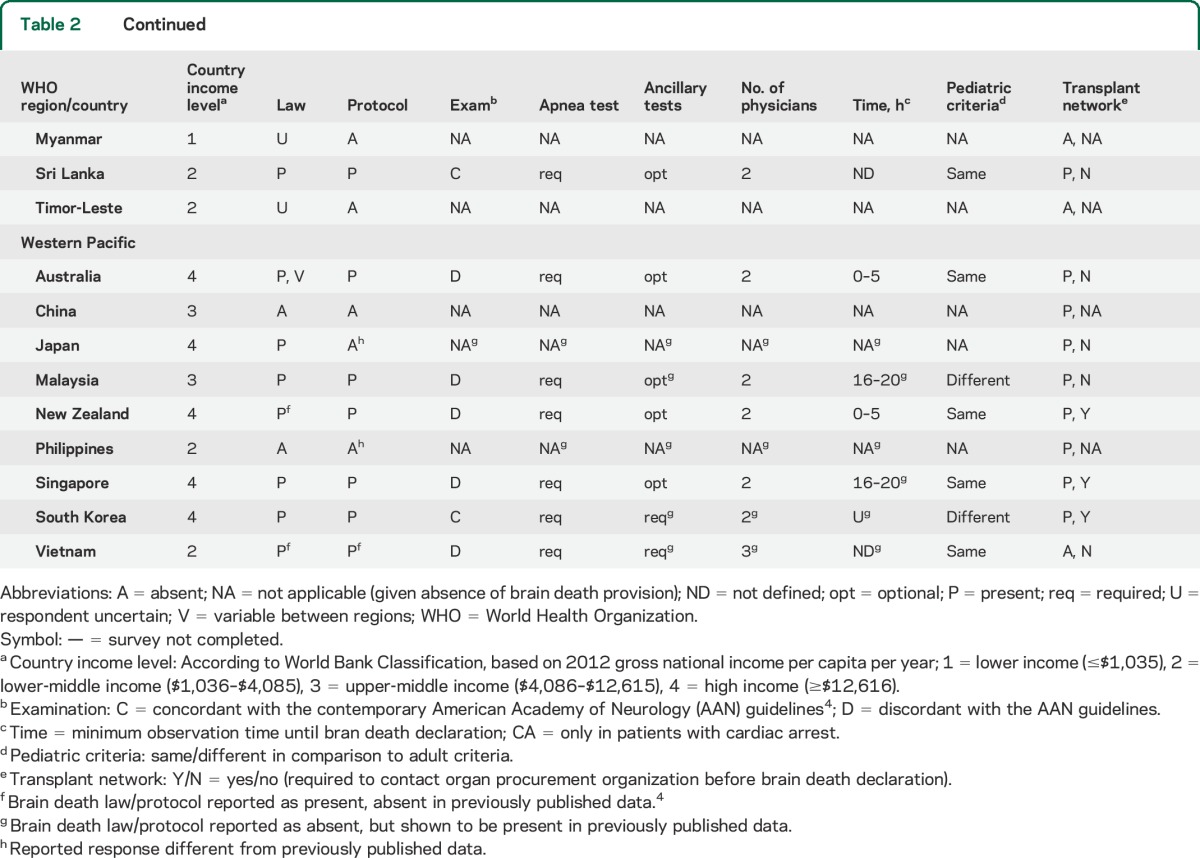
Two-thirds of respondents (66%, n = 45) stated that the determination of brain death can only be performed by an attending physician trained in neurology, neurosurgery, or intensive care medicine at their institution; an additional 30% (n = 20) of respondents reported that an attending physician from other medical or surgical specialties could also be credentialed. A quarter of respondents (n = 17, 25%) reported that resident-level trainees were permitted to declare patients independently of faculty-level physicians.
In most countries (65%, n = 44), respondents indicated that a specific time period was mandated between the initial neurologic deterioration until declaration; in 3 of these countries, this rule was only applicable to patients with global anoxic brain injury (i.e., from cardiac arrest) as the suspected underlying cause. Waiting periods ranged from <5 to >25 hours, most commonly between 6 and 10 hours (n = 15, 34%). There was consensus among 97% of respondents that there were specific requirements to consider sedatives medications and profound metabolic derangement as potential confounding factors to a patient's examination.
Clinical examination.
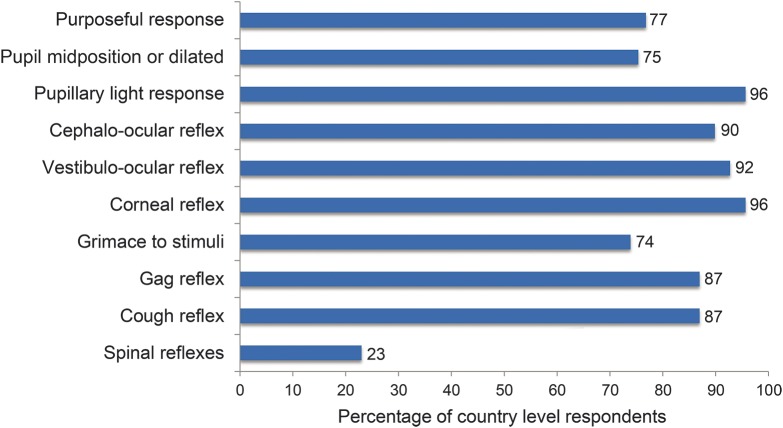
Many (53%, 37/70) reported the presence of an institutional protocol with an examination discordant with 2010 AAN criteria in at least one component. As one example, 23% (n = 16) stated that the absence of spinal reflexes was required (figure 1). There was no statistically significant association between country income level and adherence to the AAN criteria (p < 0.4396).
Figure 1. Required absences of neurologic function for the declaration of brain death, by percentage of respondents.
Apnea testing.
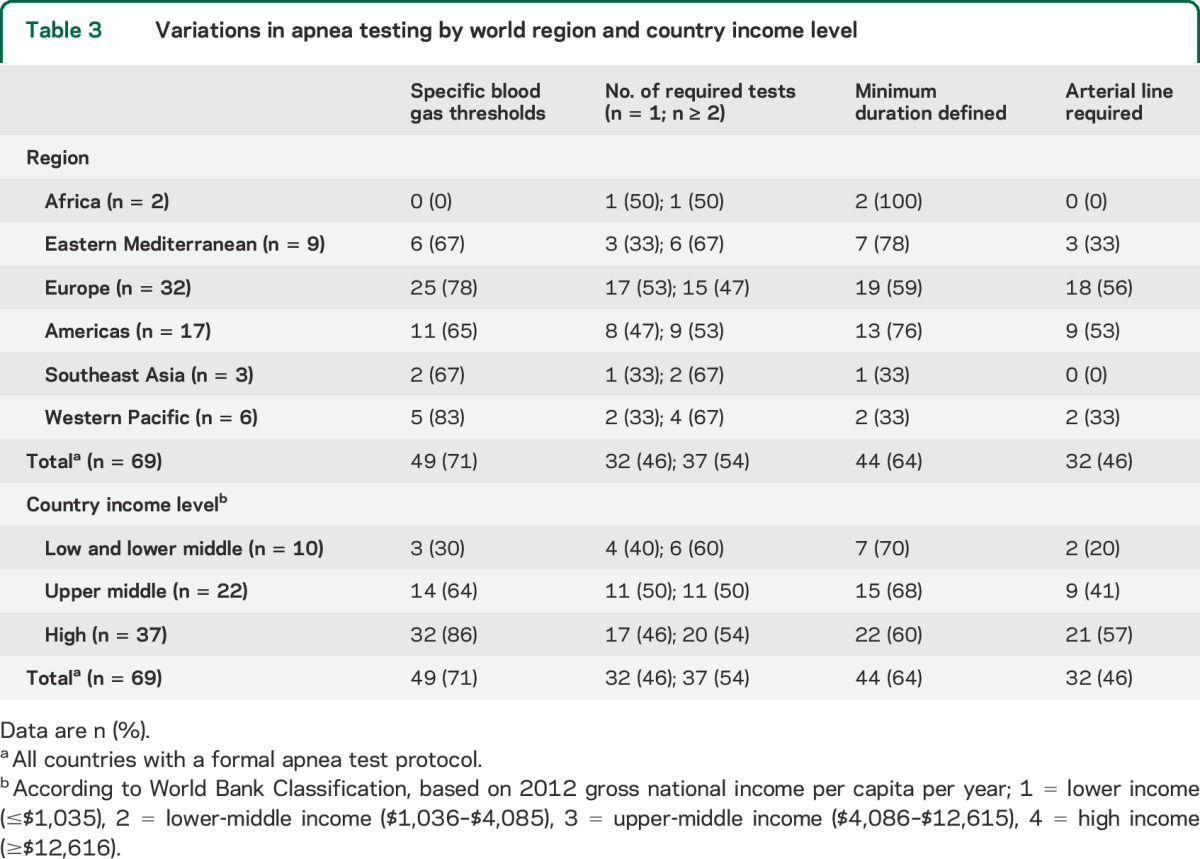
There were several discrepancies regarding the conduct of apnea testing, including specific blood gas requirements (baseline Pco2 before testing, Pco2 level required for declaration, change in Pco2 from initial Pco2), number of tests required (54% would perform the apnea test at least twice), the defined minimum duration (defined in 64% of countries), and whether an arterial line was required (46% indicated that it was) (table 3).
Table 3.
Variations in apnea testing by world region and country income level
Ancillary testing.
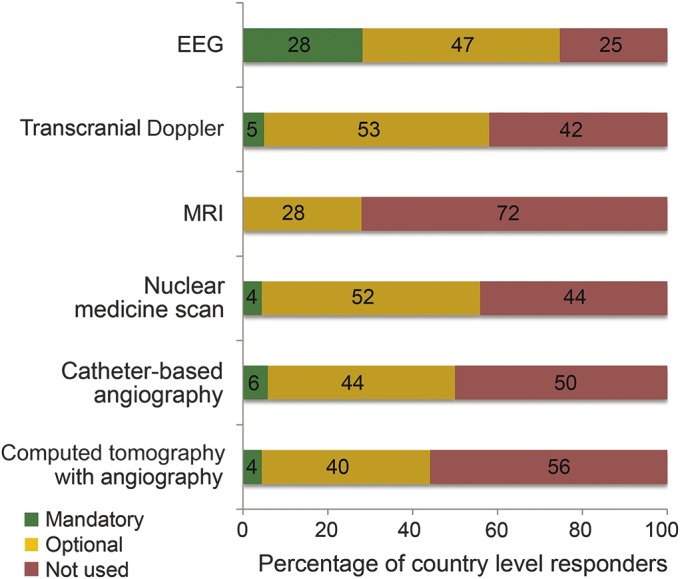
The most frequently required test was the EEG (n = 20, 29%), followed by transcranial Doppler ultrasound of the cerebral vasculature and catheter-based cerebral angiography (n = 4, 6% for each test) (table e-1, figure 2). There was no association between the use of ancillary testing and the country income level or a specific geographic region (p = 0.52). When asked whether a patient could be declared brain dead in the setting of an examination consistent with brain death but simultaneously equivocal ancillary testing, the majority of respondents answered no (56%, n = 38), with another 18% (n = 12) being uncertain, while 26% (n = 18) stated that they would proceed with brain death declaration.
Figure 2. Use of ancillary testing in the declaration of brain death worldwide.
Pediatric criteria.
Distinct criteria for declaration in children were common (n = 38, 56%; 0/2 [0%] low income, 3/9 [33%] lower-middle income, 13/22 [59%] upper-middle income; 22/36 [61%] high income), and did not appear to be related to the country income level (test of association, p = 0.304). The parameters reported to be different (multiple answers possible) included the minimum observation time before declaration (n = 33, 87%), number of clinical examinations required (n = 17, 45%), additional ancillary tests required (n = 14, 37%), required qualifications of providers (n = 6, 16%), number of providers required for declaration (n = 4, 11%), and time between examinations (n = 3, 8%).
Organ transplantation.
Countries with an organized transplant network were more likely to have a brain death provision compared with countries without (53/64 [83%] vs 6/25 [24%], p < 0.001). Among the countries with a reported law defining brain death, 29 (42%) required contacting the organ procurement organization before declaration. Nearly all (95%) of physicians from countries with an organized transplant network (61/64) reported that brain death was an established concept at their institution, compared with 56% of physicians from countries without an organized transplant network (14/25) (p < 0.0001). When adjusting for country income (by dichotomizing income level into lower- vs upper-income countries), this difference persisted (odds ratio 10.4, 95% confidence interval 1.7–62.3, p = 0.01).
DISCUSSION
We provide an initial assessment of the perceptions and daily practices surrounding the determination of brain death in 91 countries. Our study captures the largest number of countries to date in order to characterize variations in the practice of brain death determination. Previous studies have focused on high-income regions, such as Europe6 or the legal codes of a country.5 However, the actual practice of brain death declaration, particularly in countries with emerging markets, has not been studied comprehensively. In the past decade, medical technologies in middle- and some low-income countries have advanced, leading to wider attention to transplantation in these regions.14
We identified 70% of surveyed countries with recognized legal provisions for brain death, and 77% of countries in which informants reported institutional protocols. While it is possible that some countries may have protocols that the key informant was unaware of, we argue that it would be of little clinical utility to have provisions that expert providers are unaware of in a country. In practice, 22% of low-income vs 97% of high-income countries had an institutional protocol for brain death determination. The major reasons for the absence of brain death policies were the lack of either expertise or technology.
There was consensus that apnea testing was required for brain death declaration; however, extensive variations were noted in the conduct of the test. While some of these differences might be explained by the availability of resources such as arterial lines and reliable blood gas measurements, most variations appear to be driven by institutional preference.
The use of ancillary testing remains controversial globally. The frequent, and at times mandatory, use of EEG may derive from the earlier definitions of brain death.2 Moreover, some countries base declaration on brainstem death as opposed to whole brain death, including higher cortical function.5 Our results did not demonstrate that ancillary testing was used more frequently in higher-income countries. Possibly, in locations in which experts on brain death are rare, an increased number of tests and practitioners are engaged to make the determination. There was a sharp divide regarding the question of how to interpret equivocal testing in the setting of an examination positive for brain death, with half of the respondents expressing reluctance to declare a patient in such a scenario.
Slightly more than half of the respondents (56%) indicated that there were special criteria for brain death determination in children. It is unclear whether these differences have a valid physiologic basis, such as drug pharmacokinetics.
The number of respondents who described an examination discordant with the AAN criteria was surprisingly high (53%). Since the brain death examination does not require excessive resources, this trend is most likely explained by gaps in medical education and awareness. A relatively large proportion (23%) of respondents stated that they would require absent spinal reflexes to determine brain death, a criterion that was proposed in the 1969 definition but has since been revised.
The presence of a transplantation network was more important than the per-capita income level of a country when determining the establishment of a brain death protocol. Although this is not surprising, the influence was particularly strong even after adjusting for income level. Nearly all countries with an organized transplant network had an established concept of brain death (95%). This suggests that as transplantation networks become increasingly internationalized, the need for brain death education and resources will concomitantly increase.
Our study had several limitations. Since our intent was to study perceptions and practices, we are dependent on the expertise and awareness of our respondents through self-report. We compared the results of our survey with the previously published review of laws and protocols worldwide5 and highlighted differences between those protocols and our respondents' answers (table 2). Some of these differences might reflect updates of protocols since the 2002 study, variations among protocols within a country, or an inaccurate response to the survey question. Since the survey was distributed in English only, limited understanding of some questions for providers who practice in primarily non-English-speaking countries may have occurred. Our response rate, although high, may have increased if the survey was available in other languages.
In some cases, we asked the respondent his or her individuated perception. It is not clear in those cases how representative the respondent's answer would be of the country or institution as a whole. In some countries, laws and protocols regarding brain death determination also vary based on the region, city, or even among institutions within the same city. Likely, urban-rural differences exist, with the respondent, in many cases, commenting on the perceptions and practices in academic and urban centers. By contrast, most medical care in lower-income countries is outside of tertiary care centers. Therefore, our results cannot easily be extrapolated to most parts of lower-income countries where health services remain scarce or unavailable for neurologic care. We recognize that in some cases, the declaration of brain death may be completely irrelevant to low-income populations who have several more pressing neurologic and public health needs.
Overall, these findings underscore the importance of international partnerships between institutions to improve medical education and alleviate critical human resource needs in lower-income settings.15 A recent study demonstrated much-improved adherence to the AAN criteria following a didactic course and simulation exercise.16 Organ donation after brain death has high potential in many developing countries where consensus among the public and health care providers may be particularly high. For example, in Iran, 78% of medical staff and students had favorable attitudes to donation after brain death,17 and 91% of health care professionals in Karachi, Pakistan, identified brain death correctly.18 In Uruguay, donation after brain death is increasing, while in the United Kingdom, it remains stable.19
Populous countries, including India, China, and Egypt, experience legal barriers to the practice of brain death determination.20–22 A prior study5 demonstrated variations in legal codes worldwide, which may partially explain differences in practice.
Therefore, the physicians' experiences reflect both a combination of extant sociocultural forces and scientific recommendations. To promulgate a unified stance on brain death, valuable for practitioners everywhere, consensus among leading experts in the field is urgently required. Our intention for this survey is to identify the areas in which we could focus efforts for a harmonized international approach. Future efforts will need to include physicians with neurologic and critical care expertise, representatives of national and international major medical organizations (such as the World Health Organization or World Federation of Neurology), and scientific and medical advisors of government agencies. Given the tremendous variation among countries and lack of progress since a survey a decade ago, it remains unclear whether a worldwide consensus on brain death determination can be achieved.
Supplementary Material
GLOSSARY
- AAN
American Academy of Neurology
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
S.W. was involved in study conception, survey design, data collection, data analysis and interpretation, writing, and editing. E.F.M.W. was involved in study conception, data interpretation, and editing. P.V.P. was involved in survey design, data collection, interpretation, and editing. D.M.G. was involved in survey design, data collection, interpretation, and editing. J.C.H. was involved in data interpretation and editing. M.C. was involved in data analysis, writing, and editing. F.J.M. was involved in study conception, data interpretation and analysis, writing, editing, and study supervision.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Mollaret P, Goulon M. The depassed coma (preliminary memoir) [in French]. Rev Neurol 1959;101:3–15. [PubMed] [Google Scholar]
- 2.A definition of irreversible coma. Report of the Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death. JAMA 1968;205:337–340. [PubMed] [Google Scholar]
- 3.Wijdicks EF. Determining brain death in adults. Neurology 1995;45:1003–1011. [DOI] [PubMed] [Google Scholar]
- 4.Wijdicks EF, Varelas PN, Gronseth GS, Greer DM; American Academy of Neurology. Evidence-based guideline update: determining brain death in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2010;74:1911–1918. [DOI] [PubMed] [Google Scholar]
- 5.Wijdicks EF. Brain death worldwide: accepted fact but no global consensus in diagnostic criteria. Neurology 2002;58:20–25. [DOI] [PubMed] [Google Scholar]
- 6.Citerio G, Crippa IA, Bronco A, Vargiolu A, Smith M. Variability in brain death determination in Europe: looking for a solution. Neurocrit Care 2014;21:376–382. [DOI] [PubMed] [Google Scholar]
- 7.Kannus P, Niemi S, Parkkari J, Palvanen M, Sievanen H. Alarming rise in fall-induced severe head injuries among elderly people. Injury 2007;38:81–83. [DOI] [PubMed] [Google Scholar]
- 8.Hartholt KA, Van Lieshout EM, Polinder S, Panneman MJ, Van der Cammen TJ, Patka P. Rapid increase in hospitalizations resulting from fall-related traumatic head injury in older adults in the Netherlands 1986–2008. J Neurotrauma 2011;28:739–744. [DOI] [PubMed] [Google Scholar]
- 9.Ramanathan DM, McWilliams N, Schatz P, Hillary FG. Epidemiological shifts in elderly traumatic brain injury: 18-year trends in Pennsylvania. J Neurotrauma 2012;29:1371–1378. [DOI] [PubMed] [Google Scholar]
- 10.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009;8:355–369. [DOI] [PubMed] [Google Scholar]
- 11.Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol 2003;2:43–53. [DOI] [PubMed] [Google Scholar]
- 12.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean CB, Nielsen JD. Generalized linear mixed models: a review and some extensions. Lifetime Data Anal 2007;13:497–512. [DOI] [PubMed] [Google Scholar]
- 14.Spearman CW, McCulloch MI. Challenges for paediatric transplantation in Africa. Pediatr Transplant 2014;18:668–674. [DOI] [PubMed] [Google Scholar]
- 15.Mateen FJ. Neurocritical care in developing countries. Neurocrit Care 2011;15:593–598. [DOI] [PubMed] [Google Scholar]
- 16.Macdougall BJ, Robinson JD, Kappus L, Sudikoff SN, Greer DM. Simulation-based training in brain death determination. Neurocrit Care 2014;21:383–391. [DOI] [PubMed] [Google Scholar]
- 17.Zahmatkeshan M, Fallahzadeh E, Moghtaderi M, Najib KS, Farjadian S. Attitudes of medical students and staff toward organ donation in cases of brain death: a survey at Shiraz University of Medical Sciences. Prog Transplant 2014;24:91–96. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqui OT, Nizami S, Raza E, et al. Deceased-donor organ transplantation: knowledge and attitudes among health care professionals managing critically ill patients in Karachi. Exp Clin Transplant 2012;10:544–550. [DOI] [PubMed] [Google Scholar]
- 19.Mizraji R, Perez-Protto S, Etchegaray A, et al. Brain death epidemiology in Uruguay and utilization of the Glasgow coma score in acute brain injured patients as a predictor of brain death. Transplant Proc 2009;41:3489–3491. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal SK, Srivastava RK, Gupta S, Tripathi S. Evolution of the Transplantation of Human Organ Act and law in India. Transplantation 2012;94:110–113. [DOI] [PubMed] [Google Scholar]
- 21.Johnson RJ, Bradbury LL, Martin K, Neuberger J; UK Transplant Registry. Organ donation and transplantation in the UK—the last decade: a report from the UK National Transplant Registry. Transplantation 2014;97(suppl 1):S1–S27. [DOI] [PubMed] [Google Scholar]
- 22.Sui WG, Yan Q, Xie SP, et al. Successful organ donation from brain dead donors in a Chinese organ transplantation center. Am J Transplant 2011;11:2247–2249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.