Abstract
Objective:
To evaluate maximal isometric muscle force at 18 years of age in relation to Parkinson disease (PD) later in life.
Methods:
The cohort consisted of 1,317,713 men who had their muscle strength measured during conscription (1969–1996). Associations between participants' muscle strength at conscription and PD diagnoses, also in their parents, were examined using multivariate statistical models.
Results:
After adjustment for confounders, the lowest compared to the highest fifth of handgrip strength (hazard ratio [HR] 1.38, 95% confidence interval [CI] 1.06–1.79), elbow flexion strength (HR 1.34, 95% CI 1.02–1.76), but not knee extension strength (HR 1.24, 95% CI 0.94–1.62) was associated with an increased risk of PD during follow-up. Furthermore, men whose parents were diagnosed with PD had reduced handgrip (fathers: mean difference [MD] −5.7 N [95% CI −7.3 to −4.0]; mothers: MD −5.0 N [95% CI −7.0 to −2.9]) and elbow flexion (fathers: MD −4.3 N [95% CI −5.7 to −2.9]; mothers: MD −3.9 N [95% CI −5.7 to −2.2]) strength, but not knee extension strength (fathers: MD −1.1 N [95% CI −2.9 to 0.8]; mothers: MD −0.7 N [95% CI −3.1 to 1.6]), than those with no such familial history.
Conclusions:
Maximal upper extremity voluntary muscle force was reduced in late adolescence in men diagnosed with PD 30 years later. The findings suggest the presence of subclinical motor deficits 3 decades before the clinical onset of PD.
Parkinson disease (PD) is a progressive neurodegenerative disease resulting primarily from the cell death of dopaminergic neurons in the substantia nigra, which produces the cardinal symptoms of bradykinesia, rigidity, and tremor.1
At the time of clinical diagnosis of PD, only 40%–60% of dopaminergic striatal neurons may remain.2,3 The clinical onset of PD is thus preceded by a prodromal phase of uncertain duration; estimates based on pathologic, imaging, and epidemiologic data have ranged from a few years to a couple of decades.3,4 Large epidemiologic studies have found associations with several nonspecific symptoms, including an increased incidence of constipation,5,6 depression,7,8 sleep disorders,9,10 and olfactory impairment,11,12 up to 2 decades before diagnosis. This phase has often been referred to as premotor, but later studies of patient-reported symptoms13,14 and objective prospective data from participants at high risk of PD15 indicated that subtle impacts on motor function may appear at least 2–5 years before PD diagnosis. Furthermore, in 1986, Koller and Kase16 first reported objective findings of reduced muscle strength in early PD.
We evaluated muscle strength in relation to PD later in life in a nationwide cohort of 1,300,000 Swedish men aged 18 years at baseline. Objective data for maximal voluntary muscle force, collected during military conscription tests, were analyzed in relation to the occurrence of PD during a mean follow-up period of about 30 years. In addition, associations between participants' muscle strength at conscription and PD diagnoses in their parents were examined.
METHODS
Study population.
The cohort considered for inclusion in this study comprised all Swedish men conscripted at age 18 years for compulsory military service between 1969 and 1996 (n = 1,346,746). Until recently, exemptions from military conscription for men in Sweden were given only to those who could provide documentation of a severe chronic medical condition or disability, representing only 2%–3% of Swedish men.
To identify participants who had died or emigrated during the follow-up period, the National Cause of Death Register, which is administered by the National Board of Health and Welfare, and the Statistics Sweden database were searched using the unique personal identification numbers given to all Swedish citizens. Registry errors (date of death or emigration falling before date of conscription) were found for 458 participants, who were excluded from the study. Participants (n = 28,573) with unknown or extreme values for body weight (<40 or >170 kg) or height (<150 or >215 cm) at conscription were also excluded, as were 2 participants diagnosed with PD before conscription. A total of 29,033 participants were excluded, leaving a final cohort of 1,317,713 men available for further analyses.
All conscripts underwent highly standardized, 2-day-long physical examinations at regional conscription centers before receiving assignments in the Swedish Armed Forces. The present study used the measurements of muscle strength and physical fitness obtained during these examinations. A physician examined each conscript and diagnosed any disorder according to the Swedish version of the ICD (8th edition). Weight was measured to the nearest kilogram and height was measured to the nearest centimeter using standardized equipment. Information on education level (categorized into 4 groups: ≤9 years of primary school; 2 and 3 years of secondary school, respectively; university education), early disability pension, and annual income 15 years after conscription was obtained from the Statistics Sweden database. Data on smoking habit (yes/no) at baseline were available for a subcohort of 24,185 participants conscripted predominantly in 1969–1970.
Standard protocol approvals, registrations, and patient consents.
Ethics statement.
The local ethics committee of Umeå University and the National Board of Health and Welfare in Sweden approved the study protocol of the present study.
Muscular strength tests.
Isometric maximal muscle force was measured by knee extension, elbow flexion, and handgrip tests. Each muscle group was tested 3 times and measured in Newtons (N). If the third value was highest, the subject was tested until the value stopped increasing. All apparatus were calibrated daily. The handgrip strength of each subject's strongest hand was measured using a dynamometer while the subject was standing with the upper arm held vertically along the body and the elbow flexed at 90°. Right elbow flexion strength was measured while the subject was strapped in a seated position with the elbow flexed at 90° and the forearm held vertically. The left hand was placed on the left knee to ensure that the shoulders remained straight during the test. The dynamometer strap was placed at the level of the radial styloid process and the subject flexed the elbow maximally. To ensure reproducibility, each subject was instructed to hold the thumb upward during the test. The strength of right knee extension was measured while the subject was in a seated position with the arms crossed over the chest and the right knee flexed at 90°. The dynamometer strap was placed at the malleolus of the right ankle and the subject extended the leg maximally.
Handgrip, elbow flexion, and knee extension strength measurements were missing for 86,855, 86,831, and 86,914 participants in the study cohort, respectively. We also excluded muscle strength values <100 N; at least one muscle strength test value was excluded for 109 participants.
Physical fitness test.
Physical working capacity was assessed using an electrically braked bicycle ergometer test. In short, all participants underwent a resting electrocardiogram and, if the results were normal, proceeded with a 5-minute submaximal bicycling regimen at work rates of 75–175 watts (W), depending on body weight. The workload was increased by 25 W/min until exhaustion and final work rates were recorded in W (Wmax). Wmax values <100 N (n = 20) were excluded.
Of the total cohort, 1,151,018 men completed the physical fitness test between 1972 and 1996. Physical fitness test results were available for 675 of the men who later developed PD.
Diagnoses of PD.
PD diagnoses in the study cohort through December 31, 2012, were acquired using the participants' personal identification numbers from the National Patient Register (NPR), which provides records of all public specialist care in Sweden. Diagnoses of PD were coded as 332A in records using the Swedish version of the ICD-9 (1987–1997) and as G209 in records using the ICD-10 (1998–2012). Diagnoses of PD in participants' parents were also tracked using the Statistics Sweden database and the NPR by linkage with the participants' personal identification numbers. The NPR is administered by the Center for Epidemiology of Sweden's National Board of Health and Welfare. In general, diagnoses recorded in the NPR have shown a high degree of validity, with positive predictive values of 85%–97%.17
In total, 977 men in the cohort were diagnosed with PD during follow-up. A total of 9,672 (median 6 [range 1–154] per subject) medical consultations were recorded for these 977 men. The most common were carried out in a neurology unit (48%), in internal medicine (30%), and in a rehabilitation medicine unit (5%).
Statistical analyses.
Data are presented as valid percents and means ± SDs unless otherwise indicated. Unadjusted differences between participants according to PD status during follow-up were analyzed using Student t tests and χ2 tests. Linear regression models were used to test whether men diagnosed with PD during follow-up had lower muscle strength at baseline than the rest of the cohort after adjustment for confounders. In initial models, the associations were adjusted for age, year of conscription, weight, and height. In later models, other covariates were included according to table 1. The results of these models are presented in table 2. Cox proportional hazard models were used to investigate associations between variables assessed at baseline and the later risk of PD. In these models, muscle strength and physical fitness were treated as continuous variables (per SD decrease) and by fifths, with the highest fifth serving as a reference. The associations were adjusted for covariates as presented in tables 3 and 4. The study endpoint for these analyses was the date of PD diagnosis, death or emigration, or December 31, 2012, whichever came first. The proportional hazards assumption was checked graphically by studying Kaplan-Meier curves and log minus log plots. To evaluate whether the association between muscle strength and PD was dependent on age at diagnosis, product interaction terms were constructed between the different estimates of muscle strength and age at diagnosis of PD. These interaction terms were then evaluated in Cox regression models only in participants diagnosed with PD during follow-up. Statistical analyses were performed using SPSS for Windows (version 21.0; SPSS, Chicago, IL). p Values < 0.05 were considered significant.
Table 1.
Baseline characteristics of participants and frequency of Parkinson disease diagnoses during follow-up in the participants and in their parents, for the total cohort and according to fifths of handgrip strength
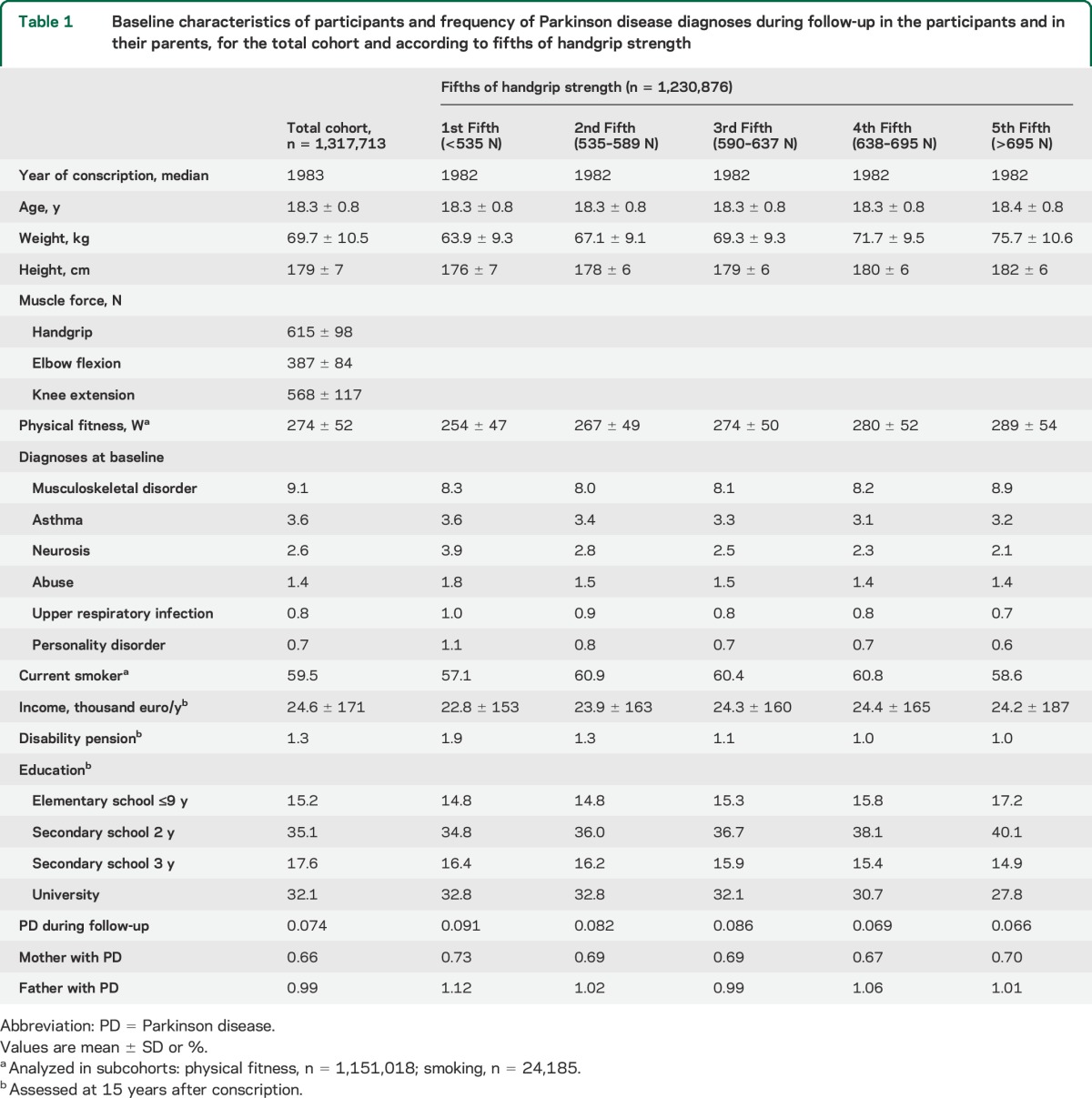
Table 2.
Differences in maximal voluntary muscle force at baseline, according to PD diagnoses in participants during follow-up and in their parents, investigated by multivariate linear regression models
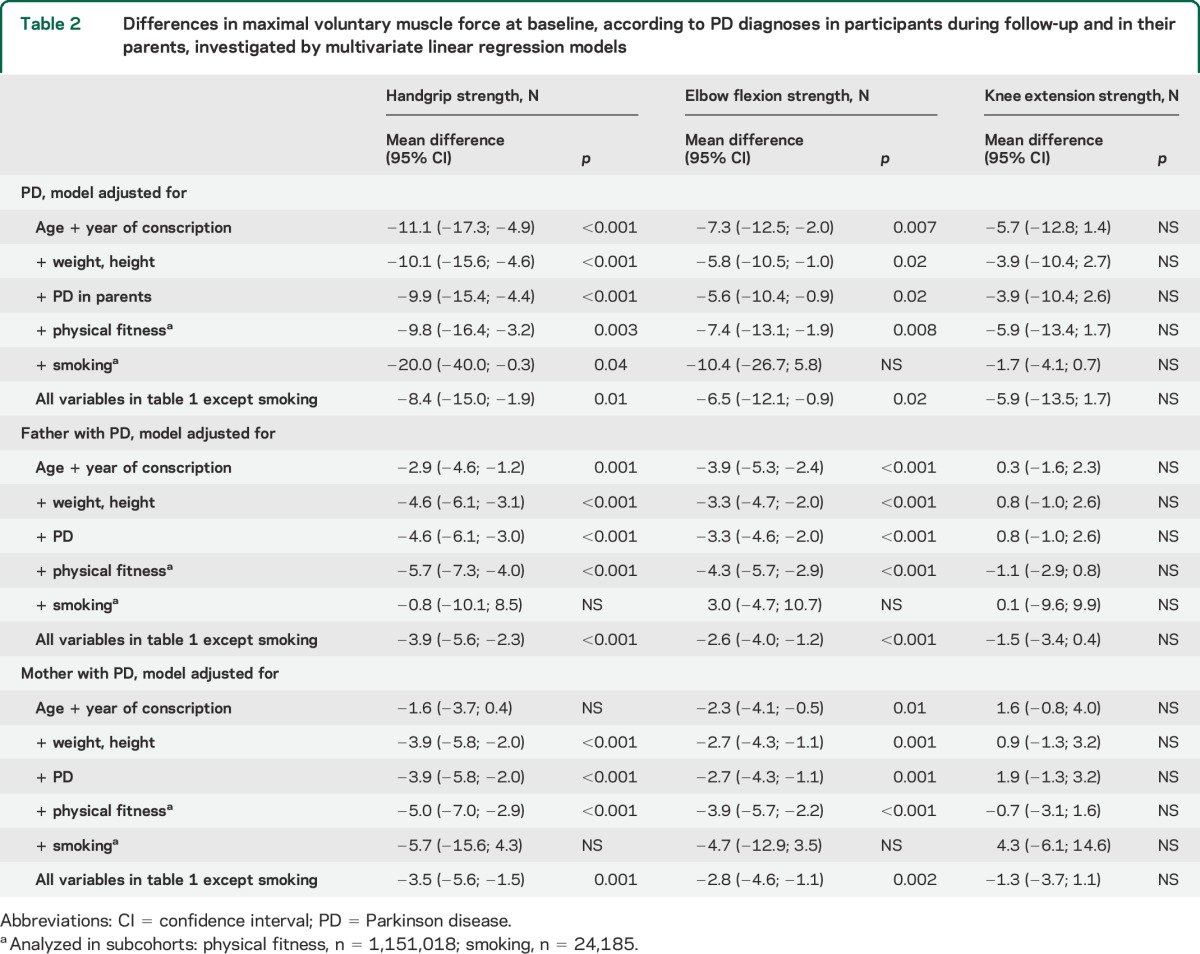
Table 3.
Associations between muscle strength and the risk of PD, investigated using Cox proportional hazard models
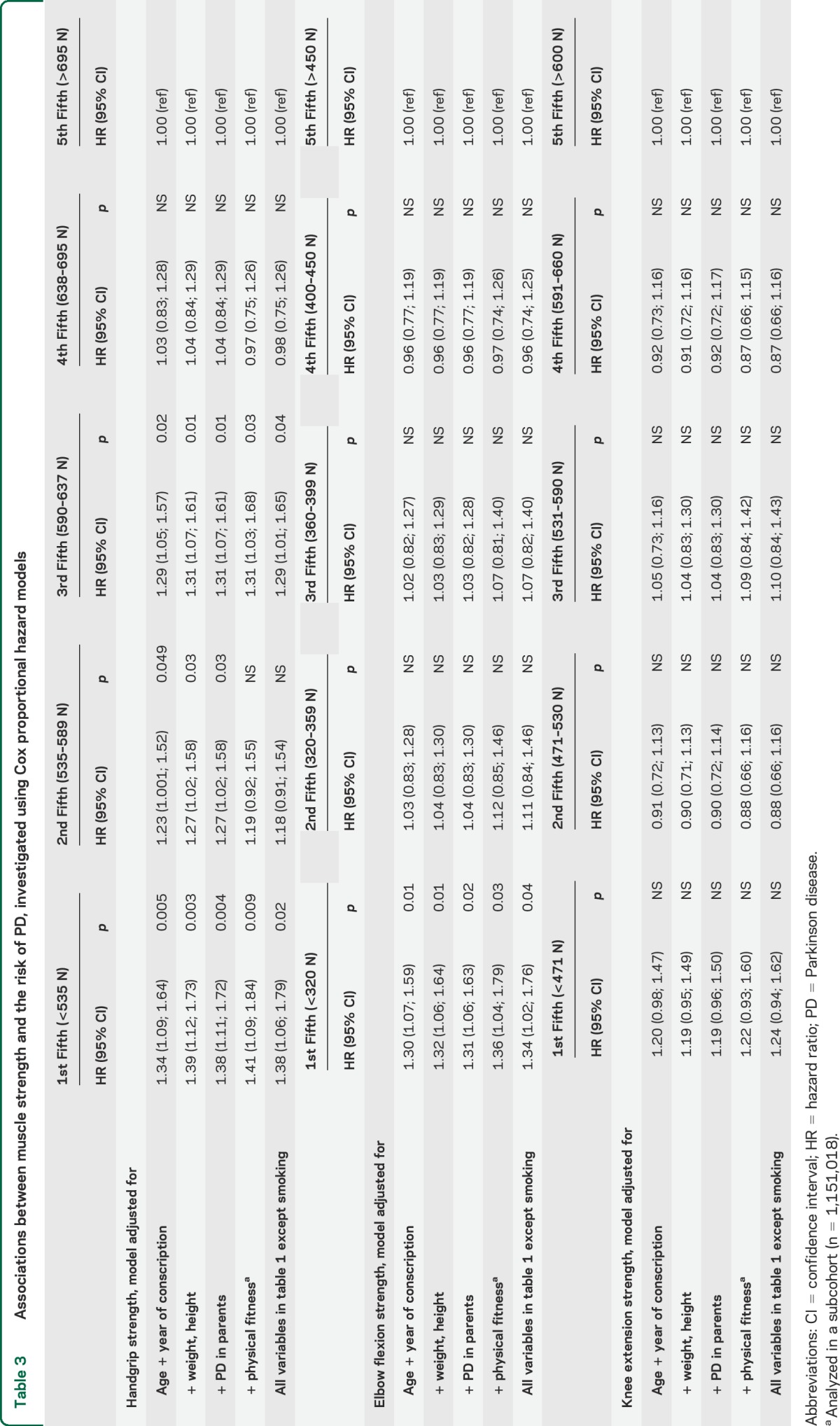
Table 4.
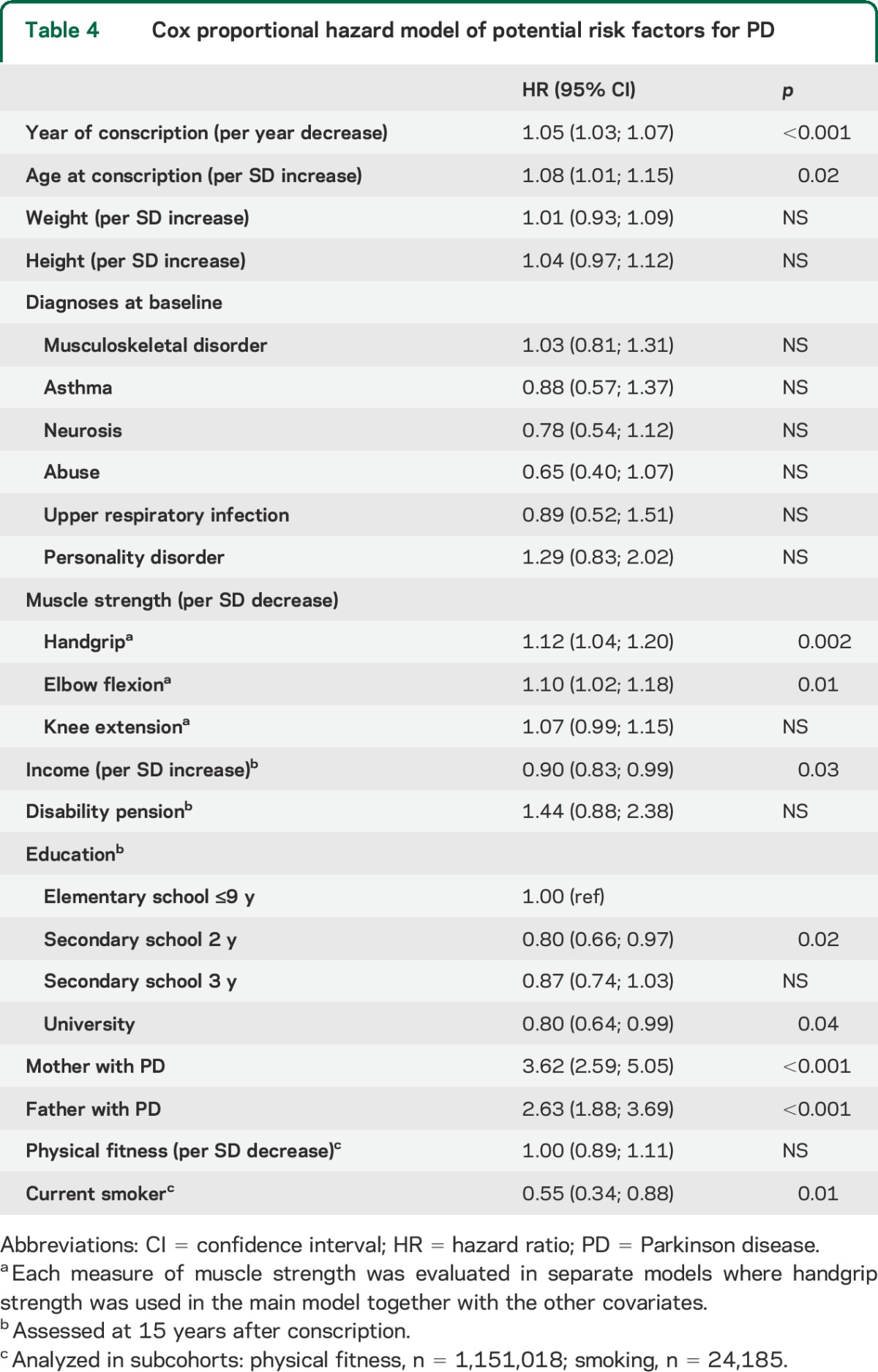
Cox proportional hazard model of potential risk factors for PD
RESULTS
The final cohort comprised 1,317,713 participants with a mean age of 18.3 ± 0.8 years at conscription and a mean follow-up period of 29.0 ± 8.4 years. Table 1 presents baseline characteristics and socioeconomic characteristics 15 years after conscription for the total cohort and by fifths of handgrip strength. In general, participants with greater handgrip strength were heavier, taller, and more physically fit at conscription, and less educated 15 years later, than were those with less handgrip strength.
During follow-up, 977 participants were diagnosed with PD at a mean age of 50.0 ± 7.1 years (median 51.2; interquartile range 45.9–55.3). A larger proportion of participants with than without PD diagnoses during follow-up had a parent with PD (6.9% vs 1.6%; p < 0.001).
In linear regression models adjusted for age, year of conscription, weight, and height, participants diagnosed with PD during follow-up had less handgrip strength (mean difference [MD] −10.1 N; 95% confidence interval [CI] −15.6 to −4.6) and elbow flexion strength (MD −5.8 N; 95% CI −10.5 to −1.0) than those with no PD diagnoses, but the MD in knee extension strength (−3.9 N; 95% CI −10.4 to 2.7) was not significant (table 2). Further adjustments for all variables listed in table 1 except smoking did not change the significance of the results, with only marginal changes in the MDs between participants with and without PD during follow-up.
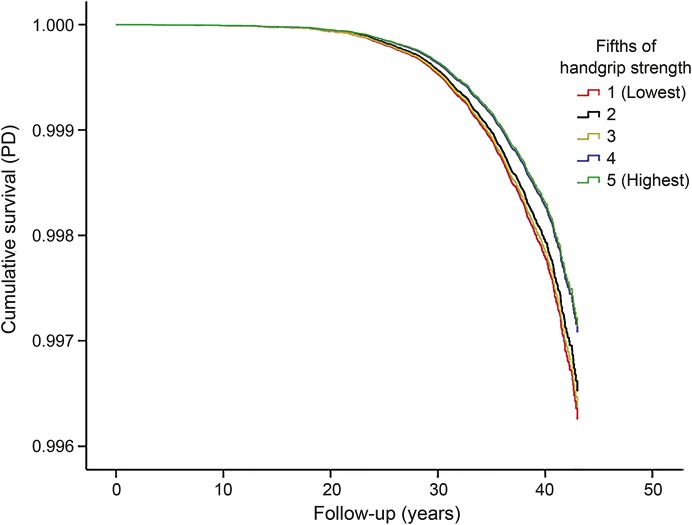
The cumulative survival with respect to PD, for fifths of handgrip strength after adjustment for age and year of conscription, weight, and height, is shown in the figure. In Cox regression models adjusted for all variables in table 1 except smoking, the lowest fifths of handgrip strength (hazard ratio [HR] 1.38; 95% CI 1.06–1.79) and elbow flexion strength (HR 1.34; 95% CI 1.02–1.76), but not knee extension strength (HR 1.24; 95% CI 0.94–1.62), were associated with an increased risk of PD diagnosis during follow-up in comparison with the highest fifth (table 3).
Figure. Handgrip strength and Parkinson disease.
Cumulative survival with respect to Parkinson disease (PD) for fifths of handgrip strength.
To test the consistency of the results, we evaluated whether PD in the participants' parents was associated with the muscle strength of men in the cohort using linear regression. After adjusting for age, year of conscription, weight, and height, men whose mothers had been diagnosed with PD had less handgrip strength (MD −3.9 N; 95% CI −5.8 to −2.0) and elbow flexion strength (MD −2.7 N; 95% CI −4.3 to −1.1), but not knee extension strength (MD 0.9 N; 95% CI −1.3 to 3.2), than those whose mothers had not been diagnosed with PD (table 2). Similarly, handgrip strength (MD −4.6 N; 95% CI −6.1 to −3.1) and elbow flexion strength (MD −3.3 N; 95% CI −4.7 to −2.0) values, but not knee extension strength values (MD 0.8 N; 95% CI −1.0 to 2.6), were lower in men whose fathers had been diagnosed with PD than in men whose fathers received no such diagnosis. We also evaluated any heritable component of PD in the present sample. In models adjusted for all covariates in table 1 except smoking and physical fitness, the risk of PD was greater for men whose mothers (HR 3.62; 95% CI 2.59–5.05) or fathers (HR 2.63; 95% CI 1.88–3.69) had PD than for men whose mothers and fathers had not received a PD diagnosis (table 4). In a subcohort of 24,185 men, smoking and the risk of PD was evaluated, including 77 men diagnosed with PD during follow-up. After adjusting for all other potential confounders except physical fitness, smoking at conscription was associated with a decreased risk of PD during follow-up (HR 0.55; 95% CI 0.34–0.88) (table 4). Finally, we tested whether interaction terms between muscle strength and age at diagnosis of PD was associated with the risk of PD adjusting for all covariates except smoking and physical fitness. None of the production interaction terms for age at diagnosis of PD and elbow flexion strength (p = 0.36), handgrip strength (p = 0.66), or knee extension strength (p = 0.84) were significant.
DISCUSSION
In the present study, we found that participants who developed PD a mean of 32 years later had less voluntary maximal isometric muscle force in late adolescence than those who did not develop this disease. The differences found were small (≤2%), but highly significant, and the results remained consistent after adjustment for potential confounders. The pattern of reduced muscle strength only in the upper extremities is of interest, as motor symptoms of PD debut most commonly at these sites18; e.g., tremor of one hand and reduced arm swing while walking.
When Koller and Kase16 first reported reduced muscle strength in early PD, weakness was detected by quantitative measurements but not by manual muscle testing. Subsequent similar investigations testing the hypothesis of muscle weakness in PD have produced inconsistent results,19 which may be related to differences in study design and, in light of the findings of the present study, limited statistical power. The differences found in the present cohort were highly significant but evident only for the upper extremities, and of great interest with respect to the initiation of the neurodegenerative processes of PD. To further test the consistency of the results, we evaluated muscle strength in the men investigated in relation to PD diagnoses in their parents. The results showed a high degree of consistency with other analyses, as less handgrip and elbow flexion strength, but not knee extension strength, at 18 years of age was associated with diagnoses of PD in the participants' parents. These associations were similar for PD in mothers and fathers, suggesting a genetic link between low muscle strength in young men and later risk of PD. As the differences in muscle strength were detected in late adolescence, it would be of interest, although difficult, to study whether these deficits are present already in childhood or perhaps even at birth.
In the present study, PD in the participants' parents was also a strong independent risk factor for PD development. The HRs obtained were similar to those from previous epidemiologic studies, with PD diagnosis in a first-degree relative associated with an estimated 2- to 7-fold increased risk of PD development.20 Familial PD was previously considered to be a specific subform of the disease comprising a minority of PD cases, but recent genetic discoveries have suggested that low-penetrant gene variants underlie a substantial share of the total incidence of the disease.20 The results of the present study of a nationwide cohort of men may support this hypothesis.
Other than genetics, physical exercise is an important factor affecting muscle strength. This factor has also been suggested to influence the risk of PD, and this hypothesis has been supported by findings of inverse relationships between midlife physical activity levels and the later risk of PD; the possibility of reversed causality has been discussed as prodromal symptoms of PD may affect exercise habits long before diagnosis.21–23 The present study found substantial relationships between physical fitness and muscle strength, but the associations between muscle strength and PD were independent of physical fitness, and physical fitness was not associated with the risk of PD. Thus, exercise habits are unlikely to underlie the associations between PD and muscle strength observed in this study.
Some limitations of this study should be considered. PD diagnoses were acquired from registries, and not clinically confirmed. However, the accuracy of diagnoses listed in Swedish registries is expected to be high,17 and the registry of PD diagnoses for the study participants was based on repeated consultations in specialist care settings. Furthermore, the results of the sensitivity analysis supported the accuracy of the diagnosis, as smoking at conscription and PD in the participants' parents were significant risk factors for the diagnosis of PD in the study cohort. Given that the cohort comprised only men, the findings may not apply to women. Finally, the results may apply predominantly to participants with young-onset PD given the relative young mean age at diagnosis in the present study. However, our final sensitivity analysis indicates that the associations found between low muscle strength and PD were independent of age at diagnosis of PD. The main strengths of this study include the use of objective, highly standardized measurements of muscle strength and the uniform age of participants at baseline, which provided reliable baseline data. Access to registry data for PD diagnoses in the participants' parents provided the opportunity to confirm the main associations observed and evaluate the role of any genetic component of the relationship between PD and muscle strength. Finally, the examination of a large nationwide cohort that included almost 1,000 cases of PD, together with the use of Swedish registry data, which resulted in almost no loss to follow-up, increased the external validity of the results.
In the present study, low muscle strength of the upper extremities in late adolescent men was associated with an increased risk of PD more than 30 years later. The observed differences were subtle, but significant and consistent, and seemed to have a genetic component. Low muscle strength may indicate the presence of subclinical motor deficits in late adolescence in people later diagnosed with PD.
Supplementary Material
GLOSSARY
- CI
confidence interval
- HR
hazard ratio
- ICD
International Classification of Diseases
- MD
mean difference
- NPR
National Patient Register
- PD
Parkinson disease
AUTHOR CONTRIBUTIONS
P.N. and A.N. conceived the idea for the study. H.G. compiled and analyzed the data and made initial drafts of tables and figures with input from A.N., P.N., S.S., and J.A., H.G. led the writing of the article with contribution from all other authors.
STUDY FUNDING
Supported by the Swedish Research Council. The funding body did not play a role in the design, writing, or decision to publish this manuscript.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Rodriguez-Oroz MC, Jahanshahi M, Krack P, et al. Initial clinical manifestations of Parkinson's disease: features and pathophysiological mechanisms. Lancet Neurol 2009;8:1128–1139. [DOI] [PubMed] [Google Scholar]
- 2.Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain 1991;114:2283–2301. [DOI] [PubMed] [Google Scholar]
- 3.Marek K, Jennings D. Can we image premotor Parkinson disease? Neurology 2009;72:S21–S26. [DOI] [PubMed] [Google Scholar]
- 4.Hawkes CH, Del Tredici K, Braak H. A timeline for Parkinson's disease. Parkinsonism Relat Disord 2010;16:79–84. [DOI] [PubMed] [Google Scholar]
- 5.Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson's disease. Neurology 2001;57:456–462. [DOI] [PubMed] [Google Scholar]
- 6.Savica R, Carlin JM, Grossardt BR, et al. Medical records documentation of constipation preceding Parkinson disease: a case-control study. Neurology 2009;73:1752–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishihara-Paul L, Wainwright NW, Khaw KT, et al. Prospective association between emotional health and clinical evidence of Parkinson's disease. Eur J Neurol 2008;15:1148–1154. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson FM, Kessing LV, Bolwig TG. Increased risk of developing Parkinson's disease for patients with major affective disorder: a register study. Acta Psychiatr Scand 2001;104:380–386. [DOI] [PubMed] [Google Scholar]
- 9.Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol 2006;5:572–577. [DOI] [PubMed] [Google Scholar]
- 10.Abbott RD, Ross GW, White LR, et al. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology 2005;65:1442–1446. [DOI] [PubMed] [Google Scholar]
- 11.Haehner A, Hummel T, Hummel C, Sommer U, Junghanns S, Reichmann H. Olfactory loss may be a first sign of idiopathic Parkinson's disease. Mov Disord 2007;22:839–842. [DOI] [PubMed] [Google Scholar]
- 12.Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, Wolters E, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann Neurol 2004;56:173–181. [DOI] [PubMed] [Google Scholar]
- 13.de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Subjective complaints precede Parkinson disease: the Rotterdam Study. Arch Neurol 2006;63:362–365. [DOI] [PubMed] [Google Scholar]
- 14.Gaenslen A, Swid I, Liepelt-Scarfone I, Godau J, Berg D. The patients' perception of prodromal symptoms before the initial diagnosis of Parkinson's disease. Mov Disord 2011;26:653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postuma RB, Lang AE, Gagnon JF, Pelletier A, Montplaisir JY. How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behaviour disorder. Brain 2012;135:1860–1870. [DOI] [PubMed] [Google Scholar]
- 16.Koller W, Kase S. Muscle strength testing in Parkinson's disease. Eur Neurol 1986;25:130–133. [DOI] [PubMed] [Google Scholar]
- 17.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schelosky L, Poewe W. Topographical onset and progression of motor symptoms in idiopathic Parkinson's disease. Mov Disord 1990;5:S13. [Google Scholar]
- 19.Cano-de-la-Cuerda R, Perez-de-Heredia M, Miangolarra-Page JC, Munoz-Hellin E, Fernandez-de-Las-Penas C. Is there muscular weakness in Parkinson's disease? Am J Phys Med Rehabil 2010;89:70–76. [DOI] [PubMed] [Google Scholar]
- 20.Shulman JM, De Jager PL, Feany MB. Parkinson's disease: genetics and pathogenesis. Annu Rev Pathol 2011;6:193–222. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology 2005;64:664–669. [DOI] [PubMed] [Google Scholar]
- 22.Thacker EL, Chen H, Patel AV, et al. Recreational physical activity and risk of Parkinson's disease. Mov Disord 2008;23:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Q, Park Y, Huang X, et al. Physical activities and future risk of Parkinson disease. Neurology 2010;75:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.