Abstract
Objective:
We examined whether individual neuronal architecture obtained from the brain connectome can be used to estimate the surgical success of anterior temporal lobectomy (ATL) in patients with temporal lobe epilepsy (TLE).
Methods:
We retrospectively studied 35 consecutive patients with TLE who underwent ATL. The structural brain connectome was reconstructed from all patients using presurgical diffusion MRI. Network links in patients were standardized as Z scores based on connectomes reconstructed from healthy controls. The topography of abnormalities in linkwise elements of the connectome was assessed on subnetworks linking ipsilateral temporal with extratemporal regions. Predictive models were constructed based on the individual prevalence of linkwise Z scores >2 and based on presurgical clinical data.
Results:
Patients were more likely to achieve postsurgical seizure freedom if they exhibited fewer abnormalities within a subnetwork composed of the ipsilateral hippocampus, amygdala, thalamus, superior frontal region, lateral temporal gyri, insula, orbitofrontal cortex, cingulate, and lateral occipital gyrus. Seizure-free surgical outcome was predicted by neural architecture alone with 90% specificity (83% accuracy), and by neural architecture combined with clinical data with 94% specificity (88% accuracy).
Conclusions:
Individual variations in connectome topography, combined with presurgical clinical data, may be used as biomarkers to better estimate surgical outcomes in patients with TLE.
Temporal lobe epilepsy (TLE) is one of the most common forms of medication-refractory epilepsy.1–4 Uncontrolled epilepsy is associated with significant psychosocial disability5 and, for this reason, the American Academy of Neurology recommends that patients should be referred for surgery within 1 year of the diagnosis of refractory epilepsy.6 However, in the United States, patients are typically referred for surgery only after 10–20 years of recurrent seizures,7 when it is too late to prevent the long-term consequences of epilepsy.7
A possible reason underlying the reluctance toward surgery is the unpredictability of its results: even patients who are expected to achieve the best outcome continue to endure disabling seizures in up to 30%–40% of cases.5,8,9 The inability to reliably predict surgical success has presented a major barrier to the promotion of early surgical intervention and access to a timely cure.10,11
A leading hypothesis suggests that seizures arise from aberrant neuronal connections not removed during surgery.12–17 Recent methodologic improvements in diffusion MRI (dMRI) have enabled the detailed mapping of brain neural architecture at an individual level, i.e., the brain connectome,18,19 allowing for a direct test of the relationship between the topographical organization of aberrant networks and surgical outcome.
In this study, we evaluated methods to scrutinize and abridge individual connectome abnormalities, assessing whether connectome data would lead to improvement in anterior temporal lobectomy (ATL) outcome prediction beyond or in combination with presurgical clinical variables.
METHODS
Participants.
We retrospectively studied a consecutive cohort comprised of 35 patients with refractory TLE who were treated at the Comprehensive Epilepsy Center at the Medical University of South Carolina (MUSC) (mean age 41.8 ± 10.9 years, 11 male). This group of patients constitutes a large expansion on a cohort of patients with TLE previously described by our group.20 In this study, we only included patients with medication-refractory TLE due to hippocampal sclerosis, or with medical refractory nonlesional TLE. All patients were diagnosed according to the criteria defined by the International League Against Epilepsy,21 including a comprehensive neurologic evaluation, ictal EEG recordings, diagnostic MRI, and, when appropriate, nuclear medicine studies. All cases exhibited unilateral temporal lobe seizure onset during ictal EEG monitoring. All patients had routine diagnostic MRI revealing unilateral hippocampal atrophy (concordant with the side of ictal EEG seizure onset) or a normal study. Patients with structural abnormalities on MRI other than hippocampal atrophy or T2 signal hyperintensity were excluded from this study.
All patients were refractory to at least 2 first-line antiepileptic medications and the indication for surgical treatment was achieved by consensus during review of each case at the Refractory Epilepsy Conference at MUSC. All patients underwent anterior temporal lobectomy performed by the same surgeon, employing the same operative techniques. None of the patients had intraoperative or perioperative complications, and the preoperative antiepileptic regimen was continued for all patients in the postoperative period.
We assessed surgical outcome based on the Engel Surgical Outcome scale22 defined at least 1 year after surgery. Patients were classified into 2 groups: (1) free of disabling of seizures (i.e., seizure-free), equivalent to Engel Class I (including Class 1b patients with auras only) (18 patients); or (2) not seizure-free, equivalent to Engel Classes II, III, or IV (17 patients). We also collected presurgical clinical information to evaluate possible clinical predictors of seizure outcome.
As a control group, we studied 18 healthy individuals (mean age 40.5 ± 5.3 years, 8 male) recruited from the local community, with no significant medical history of neurologic or psychiatric problems. The control group was similar to the patient group in age (t[51] = 0.98, p = 0.33) and sex distribution (Yates χ2 = 1.96, p = 0.16). The data from the control group were used only to provide a normative basis for the connectomes from patients, as described below.
Standard protocol approvals, registrations, and patient consents.
The MUSC Institutional Review Board approved this study. Written informed consent was obtained from all control participants. Data from patients were obtained retrospectively through chart review and MRI analyses. Patient data were obtained as standard of care for medication-refractory epilepsy and were reviewed under the waiver of consent category.
MRI acquisition, data preprocessing, and connectome calculation.
In order to assess the individual connectome, we obtained T1-weighted and dMRI data from all participants. The methodologic details regarding MRI acquisition, data preprocessing, and connectome calculation are described in the e-Methods on the Neurology® Web site at Neurology.org. After the whole brain connectome was reconstructed, we restricted our analyses to links connecting the ipsilateral temporal lobe to extratemporal regions, as they likely represent the pathways for seizure propagation and epileptogenic changes. Even though our previous work demonstrates that connectome abnormalities may also extend to links within the contralateral hemisphere,20 we restricted our analysis here in order to concentrate on the most clinically meaningful regions and to avoid model overfitting due to overly abundant parameters.
Selection of subnetwork with the highest predictive value toward surgical outcome.
The main purpose of this study was to define a method to scrutinize and abridge the individual connectome in order to derive a personalized score of network abnormalities to be used toward outcome prediction. In this context, this goal and methodology is different from regional abnormalities demonstrated from previous groupwise studies from our group and others, indicating groupwise white matter loss,23,24 connectivity abnormalities,25–30 and neural network rearrangement in patients with TLE.20,31,32
We assessed the area under the curve (AUC) for the receiver operating characteristic (ROC) curve for each possible connectome link (including only connections from the temporal to extratemporal regions, as described in e-Methods). This was performed to compare seizure-free vs not seizure-free patients, using linkwise Z scores representing the relative distance of the patient's link weight, compared with the weight distribution in controls for that same link. Links with high AUC demonstrated a small overlap between patients with seizure-free outcome vs patients who were not seizure-free.
All links were sorted in descending order based on the AUC. Each patient received a score composed of the number of links with a Z score higher than Z = 2. This score was derived as follows: for each patient, a vector was obtained composed of links 1 to n representing the Z scores of links sorted by AUC, as described above. This vector was then binarized (if Z > 2, = 1, otherwise = 0), and the sum of all elements in each vector was obtained, yielding a score per patient. Note that, by generating 1 score for patient, we attempted to minimize model overfitting.
We repeated this process iteratively to assess the classifier accuracy across a variable range of subnetwork sizes. These steps are described in detail in the e-Methods (Assessment of subnetwork sizes).
Model cross-validation.
We performed a model cross-validation to test the predictive values of the subnetwork models, and to assess if feature selection obtained from the independent cross-validation would confirm the anatomical location of the network with the highest discrimination power.
Cross-validation was performed through a k-fold approach, where the overall data (35 patients) were split into k separate groups. Each group contained randomly selected patients (i.e., a mixture of patients seizure-free and not seizure-free). Model training was performed in the group composed of all participants allocated to k − 1 groups, and tested in the remaining group. We applied folding values composed of one participant, which is equivalent to a leave-one-out approach, when the model is trained based on all participants but one, and the model is tested on the excluded participant.
For each cross-validation step, model training involved sorting in descending order all possible links based on the T score obtained by comparing seizure-free vs non-seizure-free patients; only links with the highest T score were maintained (repeatedly with subnetworks ranging from 1 to 50 links) and each patient's score was achieved by summing the number of links with a Z score >2. This model was then applied to the test group, with documentation of accuracy, sensitivity, specificity, and predictive values. Of note, this model provided a single measure of network architecture abnormalities per participant, thus limiting model overfitting.
Predictive value of subnetwork model combined with clinical data.
We assessed the predictive value of clinical variables toward surgical outcome by evaluating clinical features commonly investigated as potential determinants of outcome9,16,33,34: age at onset of epilepsy, age at surgery, seizure frequency, duration of epilepsy, seizure burden, side of TLE, presence of hippocampal atrophy on diagnostic presurgical MRI, presurgical interictal epileptiform EEG findings, and epilepsy risk factors. The construction of the model representing clinical data is described in detail in the e-Methods (Model from clinical data). We also tested the combined predictive value of the network model combined with clinical data, where, for each participant, a single score was computed corresponding to the sum of network architecture abnormalities and the composite binary clinical variables described above. Similarly, the sensitivity, specificity, and predictive values of this model were computed for the point in the ROC curve with maximal accuracy. For each model (network data, clinical data, and combined data) we also performed a formal statistical assessment of surgical outcome prediction, based on R2 (proportional reduction in error), with the explanatory clinical factors of interest entered into the regression analysis.
RESULTS
Patient demographics.
The clinical information obtained from all patients is shown in table e-1. As explained in the Methods, this cohort constitutes an expansion on a surgical cohort previously described by our group.20 There were no significant differences in clinical variables between seizure-free patients vs patients who were not seizure-free after surgery.
Predictive value of temporal subnetwork toward surgical outcome.
Linkwise Z scores in patients are demonstrated in figure e-1 and in figure 1. Patients who were not seizure-free exhibited more links with higher Z score values compared with seizure-free patients. We observed a relatively high discrimination between the outcome groups by assessing models composed of subnetworks ranging from 1 to 50 links. The AUCs and predictive values obtained from the model constructed from these links are demonstrated in figure 2, A and B. We did not observe a significant correlation between the number of Z scores above 2 and disease duration (p = 0.17), frequency of seizures (p = 0.4), or lifetime seizure burden (p = 0.3).
Figure 1. Distribution of linkwise Z scores in seizure-free patients vs patients who were not seizure-free.

(A) Cumulative distribution function (CDF) of Z scores across the entire connectome for patients (separated by surgical outcome). The shaded areas demonstrate the range of CDFs per group with 50% confidence interval (CI). (B) Distribution of linkwise median Z scores (across all links, per patient) is different between outcome groups.
Figure 2. Results from the model constructed from individual connectome data.
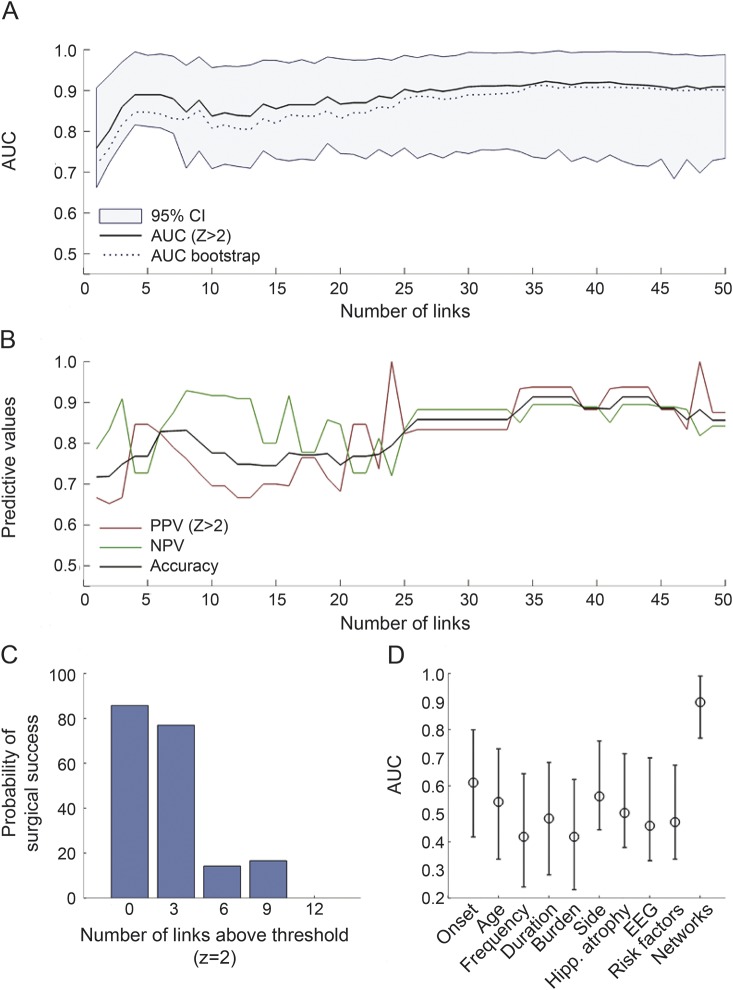
(A) The range of areas under the curve (AUCs) (y-axis) obtained from the model constructed from a subnetwork involving an ascending number of links (x-axis). The shaded area demonstrates the 95% confidence interval (CI) when resampling the linkwise data. (B) Corresponding predictive values. (C) The probability of surgical success as a function of the number of links above the critical Z threshold (Z = 2), when a subnetwork composed of 30 links is assessed. (D) The AUC obtained from the networks model (also including 30 links), in comparison with the AUCs from clinical variables. The error bars represent the AUCs obtained from bootstrapping. NPV = negative predictive value; PPV = positive predictive value.
Considering a model constructed based on 30 links, a clear decrement in the probability of surgical seizure freedom was noted when the number of links above Z >2 threshold increased (figure 2C).
Cross-validation of the temporal subnetwork model.
The model described above achieved excellent predictive values toward surgical outcome, but this is not surprising since the model was based on links with the highest discrimination between groups, even though resampling permitted a better appreciation of the confidence intervals associated with classification. However, the purpose of the approach described above was to provide a first pass assessment of the anatomy of the temporal subnetwork model, and to establish a basis for a more rigorous testing of the classification algorithm, which was then performed using a k-fold cross-validation approach.
By applying multiple k-folding cross-validation steps, we observed that feature selection during training (i.e., which links to choose as part of the model subnetwork) was fairly consistent. The subnetwork with the highest overlap between methods is shown in table 1 and figure 3. With a k-fold level set at leave one out, the model constructed with these 10 links yielded a negative predictive value (NPV) of 0.89 (with 0.28% of errors, mostly related to classifying patients with poor outcome, hence yielding a positive predictive value [PPV] of 0.65). The results below are in reference to this subnetwork.
Table 1.
Links composing the subnetwork with the highest ability to predict surgical outcome
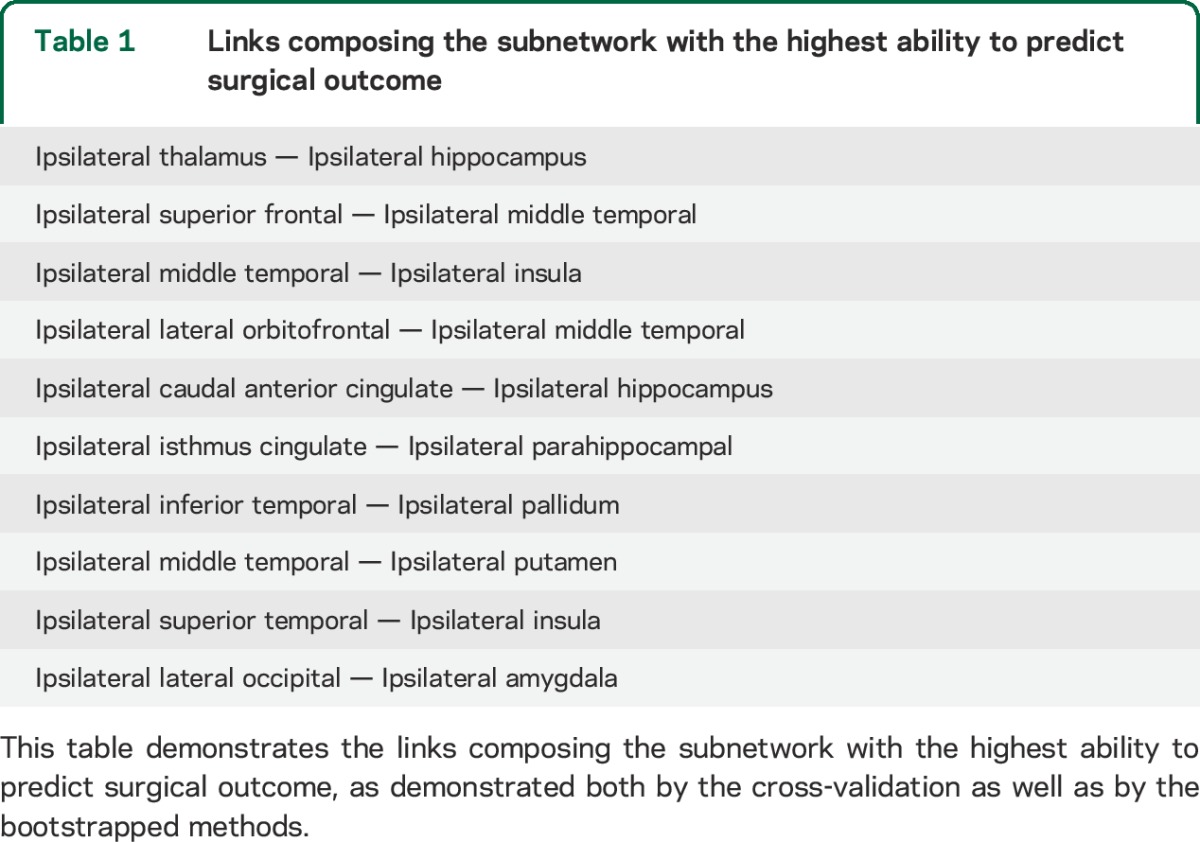
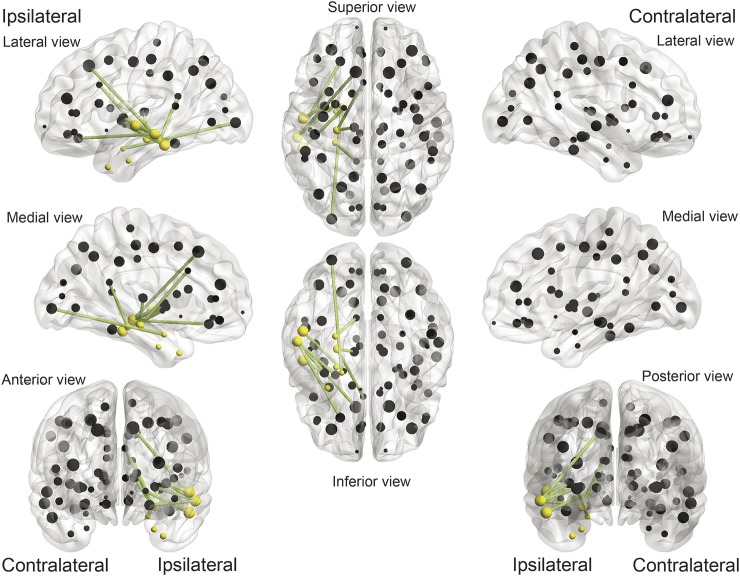
Figure 3. Connectome links were more commonly associated with surgical outcome, taking into account individual topographical variability.
Connectome links that were repeatedly chosen by the cross-validation model (in green), in relationship with cortical regions of interest (ROIs) (represented by spheres located in the ROI center of mass). Spheres in yellow represent the 8 ROIs defined as pertaining to the temporal region. The links in this figure correspond to the links outlined in table 1. In general, patients who exhibited a cumulative number of weights Z >2 among these links were less likely to become seizure-free after surgery.
Predictive values of the network architecture, clinical, and combined models.
The neural network model ability to discriminate between surgical outcome groups (as defined by the AUC) was noticeably higher than the ability of routine clinical data (figure 2D). The clinical variables with the highest predictive value toward a not seizure-free outcome (PPV) were higher seizure burden (0.79%) and higher seizure frequency (0.73%). Conversely, the clinical variables with the highest predictive values toward a seizure-free outcome (NPV) were absence of contralateral EEG abnormalities (0.72%) and presence of hippocampal atrophy (0.65%).
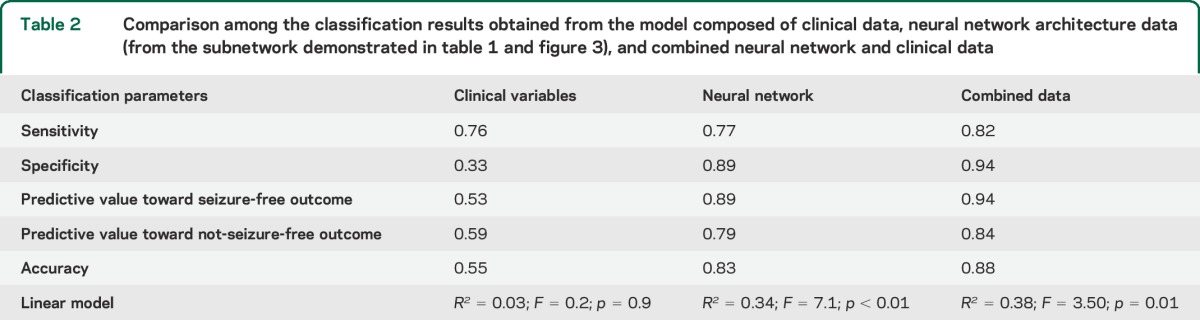
The clinical data model composed of composite scores based on the sum of the clinical scores demonstrated a sensitivity of 76%, with a relatively low specificity (33%), toward seizure freedom. This model of outcome prediction, based on R2 (proportional reduction in error), did not demonstrate a significant association between this mode of clinical variables and surgical outcome (F-statistic = 0.2, p = 0.9; R2 = 0.03).
In contrast, the model composed of the neural network data were associated with a sensitivity of 77% and specificity of 89% toward seizure freedom (F = 7.1, p < 0.01; R2 = 0.34).
Importantly, a model combining clinical data and neural network architecture demonstrated a sensitivity of 82%, with specificity of 94% toward seizure freedom. This model of outcome prediction based on R2 was also statistically significant (F = 3.50 p = 0.01; R2 = 0.38). The sensitivity, specificity, accuracy, predictive values, and linear regression statistics of these models are summarized in table 2.
Table 2.
Comparison among the classification results obtained from the model composed of clinical data, neural network architecture data (from the subnetwork demonstrated in table 1 and figure 3), and combined neural network and clinical data
DISCUSSION
We investigated whether individualized measures of neural network architecture derived from the structural brain connectome could be used as biomarkers to predict the outcome of ATL at an individual level with higher accuracy compared with clinical parameters. We observed that a seizure-free surgical outcome could be predicted with specificity of approximately 90% (with 83% accuracy) using neural network architecture data alone. The predictive power of this model was further enhanced by combining it with clinical data, yielding a specificity of 94% (with 88% accuracy) toward postoperative seizure freedom.
While the reproducibility of this approach should be confirmed by further tests of this model in a prospective and independent cohort, we suggest that the current results provide initial evidence for an innovative method that may become instrumental in the treatment and evaluation of epilepsy, as well as in understanding the mechanisms supporting epileptogenesis. Specifically, these results suggest that (1) neural architecture based on the connectome can be assessed on an individualized basis using widely available diagnostic imaging; (2) patterns of neural architecture involving the medial temporal lobe are associated with surgical outcome, albeit with individual differences in topography; and (3) neural architecture, particularly when combined with clinical data, can be used to predict surgical results with higher accuracy compared with current standards of surgical outcome prediction.
Our observations of abnormal connectivity in patients with TLE with worse surgical outcome may be an indicator of (1) a broader epileptogenic area outside the medial temporal lobe, either due to dual pathology or due to a network that is contiguous with the medial temporal lobe; (2) a biomarker of epilepsy severity; or (3) both. They also support that a critical mass of abnormal connections should exist outside the medial temporal lobe to maintain seizures, since, in some cases, the disruption of the network through surgery, either ATL or more selective approaches, can abolish epilepsy.
The results from this study should be interpreted in the context of its limitations. This study employed a model constructed based on a small cohort of patients with TLE. Even though we attempted to investigate a representative sample of patients with typical and characteristic clinical features of TLE, it is obviously possible that biases in our population may influence the model. Furthermore, the key aspect of this study is the evaluation of a personalized measure of outcome; hence individual biases are more likely to affect small studies. In this context, a number of elements such as sex, handedness, and hemispheric dominance for language may also influence individual architectural changes. These variables may play an important role when defining whether architectural changes are associated with epilepsy or simply represent physiologic variability. Therefore, larger cohorts may explore these variables and further refine the accuracy of the model. Likewise, it is possible that the model could be improved by a more fine-grained cortical segmentation or segmentations based on or combined with neurophysiology data. Finally, we did not observe a significant relationship between the number of abnormal links and disease duration or seizure frequency, possibly suggesting an inherent association between network abnormalities and the later development of surgical refractoriness. Nonetheless, lack of statistical power to detect a more subtle relationship could also explain these results. Larger and prospective studies could more thoroughly assess those accompanying issues related to the causes for the connectome abnormalities.
Some of the patients studied were also partly examined in a previous report from our group.20 Our previous study20 examined differences at a group level in relationship with (1) having epilepsy and (2) epilepsy surgical outcomes. Conversely, this current study addresses the same topic (epilepsy surgery and brain connectomes) but it is unique since it investigates the personalized biomarker potential of connectome abnormalities in TLE, describing a method to scrutinize and abridge the connectome in search for abnormalities, demonstrating that connectome data have a powerful synergistic effect with clinical data to predict surgical results significantly better than the current standards.
Our findings provide initial evidence for a promising new avenue in the clinical care of epilepsy, namely the evaluation of the clinical trajectory based on the person's unique neural architecture. This study provides proof of concept that the brain connectome can have a direct role in important clinical care decisions. These findings suggest that patients with epilepsy could be assessed in accordance with their unique individual connectome characteristics, combined with presurgical clinical data.
This model and these results should be further tested and refined on a larger cohort of patients, preferably recruited from multiple sites. If the results from this study are corroborated by larger prospective studies, they can lead to improved communication between patients and caregivers, and importantly, by reducing the unpredictability of surgical results, reduce the reluctance toward surgical treatment and promote earlier access to potentially definitive treatment for patients who would otherwise lack timely access to cure.
Supplementary Material
GLOSSARY
- ATL
anterior temporal lobectomy
- AUC
area under the curve
- dMRI
diffusion MRI
- MUSC
Medical University of South Carolina
- NPV
negative predictive value
- PPV
positive predictive value
- ROC
receiver operating characteristic
- TLE
temporal lobe epilepsy
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Leonardo Bonilha: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, contribution of vital reagents/tools/patients, acquisition of data, statistical analysis, study supervision. Jens H. Jensen: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval. Nathaniel Baker: drafting/revising the manuscript, accepts responsibility for conduct of research and final approval, statistical analysis. Jesse L. Breedlove: analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data. Travis Nesland: analysis or interpretation of data, accepts responsibility for conduct of research and final approval, statistical analysis. Jack J. Lin: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval. Daniel L. Drane: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and final approval.
STUDY FUNDING
Supported by the South Carolina Clinical & Translational Research Institute, with an academic home at the Medical University of South Carolina, through NIH grant numbers UL1 RR029882 and UL1 TR000062.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Margerison JH, Corsellis JA. Epilepsy and the temporal lobes: a clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain 1966;89:499–530. [DOI] [PubMed] [Google Scholar]
- 2.Wiebe S. Epidemiology of temporal lobe epilepsy. Can J Neurol Sci 2000;27(suppl 1):S6–S10; discussion S20–S21. [DOI] [PubMed] [Google Scholar]
- 3.Cahan LD, Engel J., Jr Surgery for epilepsy: a review. Acta Neurol Scand 1986;73:551–560. [DOI] [PubMed] [Google Scholar]
- 4.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001;345:311–318. [DOI] [PubMed] [Google Scholar]
- 5.Tellez-Zenteno JF, Wiebe S. Long-term seizure and psychosocial outcomes of epilepsy surgery. Curr Treat Options Neurol 2008;10:253–259. [DOI] [PubMed] [Google Scholar]
- 6.Engel J, Jr, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy. Epilepsia 2003;44:741–751. [DOI] [PubMed] [Google Scholar]
- 7.Haneef Z, Stern J, Dewar S, Engel J., Jr Referral pattern for epilepsy surgery after evidence-based recommendations: a retrospective study. Neurology 2010;75:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol 2008;7:525–537. [DOI] [PubMed] [Google Scholar]
- 9.Aull-Watschinger S, Pataraia E, Czech T, Baumgartner C. Outcome predictors for surgical treatment of temporal lobe epilepsy with hippocampal sclerosis. Epilepsia 2008;49:1308–1316. [DOI] [PubMed] [Google Scholar]
- 10.Langfitt JT, Wiebe S. Early surgical treatment for epilepsy. Curr Opin Neurol 2008;21:179–183. [DOI] [PubMed] [Google Scholar]
- 11.Wiebe S. Early epilepsy surgery. Curr Neurol Neurosci Rep 2004;4:315–320. [DOI] [PubMed] [Google Scholar]
- 12.Richardson MP. Large scale brain models of epilepsy: dynamics meets connectomics. J Neurol Neurosurg Psychiatry 2012;83:1238–1248. [DOI] [PubMed] [Google Scholar]
- 13.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010;51:676–685. [DOI] [PubMed] [Google Scholar]
- 14.Bonilha L, Martz GU, Glazier SS, Edwards JC. Subtypes of medial temporal lobe epilepsy: influence on temporal lobectomy outcomes? Epilepsia 2012;53:1–6. [DOI] [PubMed] [Google Scholar]
- 15.Coan AC, Appenzeller S, Bonilha L, Li LM, Cendes F. Seizure frequency and lateralization affect progression of atrophy in temporal lobe epilepsy. Neurology 2009;73:834–842. [DOI] [PubMed] [Google Scholar]
- 16.Cohen-Gadol AA, Wilhelmi BG, Collignon F, et al. Long-term outcome of epilepsy surgery among 399 patients with nonlesional seizure foci including mesial temporal lobe sclerosis. J Neurosurg 2006;104:513–524. [DOI] [PubMed] [Google Scholar]
- 17.Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia 2002;43:219–227. [DOI] [PubMed] [Google Scholar]
- 18.Sporns O. The human connectome: a complex network. Ann NY Acad Sci 2011;1224:109–125. [DOI] [PubMed] [Google Scholar]
- 19.Sporns O, Tononi G, Kotter R. The human connectome: a structural description of the human brain. PLoS Comput Biol 2005;1:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonilha L, Helpern JA, Sainju R, et al. Presurgical connectome and postsurgical seizure control in temporal lobe epilepsy. Neurology 2013;81:1704–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989;30:389–399. [DOI] [PubMed] [Google Scholar]
- 22.Engel J, Jr, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology 2003;60:538–547. [DOI] [PubMed] [Google Scholar]
- 23.Focke NK, Yogarajah M, Bonelli SB, Bartlett PA, Symms MR, Duncan JS. Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Neuroimage 2008;40:728–737. [DOI] [PubMed] [Google Scholar]
- 24.Bonilha L, Edwards JC, Kinsman SL, et al. Extrahippocampal gray matter loss and hippocampal deafferentation in patients with temporal lobe epilepsy. Epilepsia 2010;51:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonilha L, Nesland T, Martz GU, et al. Medial temporal lobe epilepsy is associated with neuronal fibre loss and paradoxical increase in structural connectivity of limbic structures. J Neurol Neurosurg Psychiatry 2012;83:903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernhardt BC, Chen Z, He Y, Evans AC, Bernasconi N. Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb Cortex 2011;21:2147–2157. [DOI] [PubMed] [Google Scholar]
- 27.Concha L, Kim H, Bernasconi A, Bernhardt BC, Bernasconi N. Spatial patterns of water diffusion along white matter tracts in temporal lobe epilepsy. Neurology 2012;79:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Concha L, Beaulieu C, Gross DW. Bilateral limbic diffusion abnormalities in unilateral temporal lobe epilepsy. Ann Neurol 2005;57:188–196. [DOI] [PubMed] [Google Scholar]
- 29.Gross DW, Concha L, Beaulieu C. Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia 2006;47:1360–1363. [DOI] [PubMed] [Google Scholar]
- 30.Concha L, Beaulieu C, Collins DL, Gross DW. White-matter diffusion abnormalities in temporal-lobe epilepsy with and without mesial temporal sclerosis. J Neurol Neurosurg Psychiatry 2009;80:312–319. [DOI] [PubMed] [Google Scholar]
- 31.DeSalvo MN, Douw L, Tanaka N, Reinsberger C, Stufflebeam SM. Altered structural connectome in temporal lobe epilepsy. Radiology 2014;270:842–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu M, Chen Z, Beaulieu C, Gross DW. Disrupted anatomic white matter network in left mesial temporal lobe epilepsy. Epilepsia 2014;55:674–682. [DOI] [PubMed] [Google Scholar]
- 33.Spencer SS, Berg AT, Vickrey BG, et al. Predicting long-term seizure outcome after resective epilepsy surgery: the multicenter study. Neurology 2005;65:912–918. [DOI] [PubMed] [Google Scholar]
- 34.Jeong SW, Lee SK, Hong KS, Kim KK, Chung CK, Kim H. Prognostic factors for the surgery for mesial temporal lobe epilepsy: longitudinal analysis. Epilepsia 2005;46:1273–1279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.