Abstract
BACKGROUND AND PURPOSE
The goal of this study was to explore the structural correlates of functional language dominance by directly comparing the brain morphology of healthy subjects with left- and right-hemisphere language dominance.
METHODS
Twenty participants were selected based on their language dominance from a cohort of subjects with known language lateralization. Structural differences between both groups were assessed by voxel-based morphometry, a technique that automatically identifies differences in the local gray matter volume between groups using high-resolution T1-weighted magnetic resonance images.
RESULTS
The main findings can be summarized as follows: (1) Subjects with right-hemisphere language dominance had significantly larger gray matter volume in the right hippocampus than subjects with left-hemisphere language dominance. (2) Leftward structural asymmetries in the posterior superior temporal cortex, including the planum temporale (PT), were observed in both groups.
CONCLUSIONS
Our study does not support the still prevalent view that asymmetries of the PT are related in a direct way to functional language lateralization. The structural differences found in the hippocampus underline the importance of the medial temporal lobe in the neural language network. They are discussed in the context of recent findings attributing a critical role of the hippocampus in the development of language lateralization.
Keywords: Hippocampus, language, lateralization, planum temporale, VBM
Introduction
Although the human cerebral hemispheres share the same overall structure, they are functionally different. For instance, in most humans the left hemisphere is dominant for language, whereas the right one is dominant for some visuospatial and attentional functions.1,2 The relationship between structure and function in the human brain, at either a macro- or microscopic level, is complex and so far poorly understood.3-5
In the past, anatomical explanations of left-hemisphere language dominance mostly focused on the leftward structural asymmetry of the planum temporale (PT). The PT is a roughly triangular area on the posterior surface of the temporal lobe, posterior to the primary auditory cortex that covers the first (anterior) transverse temporal gyrus (Heschl’s gyrus).6 Left-ward structural asymmetries of the PT were already observed in the 1920s,7,8 but only quantitatively confirmed half a century later by Geschwind and Levitzky in a postmortem study of 100 brains.9 The early reports on PT asymmetry were based on length or area measurements (for example, see References 9-11; for a review cf. Reference 12). Anatomically, however, the PT is not a plane but a three-dimensional convoluted structure. Brain imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) also allowed the in vivo assessment of PT surface and volumes. These studies confirmed the overall left–right asymmetry, but also showed the existence of large interindividual differences.6,13
Based on the known left-hemisphere language dominance in the general population, Geschwind and Lewitzky proposed that the leftward structural PT asymmetry is related to functional language dominance.9 Ever since then, the role of the PT has been of interest in the neurosciences. (cf. the reviews of Beaton14 and Galaburda et al15). Although nowadays most researchers agree on the general left–right asymmetry of the PT,16,17 the functional implication of this anatomical asymmetry still remains unknown. There is no general consensus whether or not reduced PT asymmetry is related to functional language dominance. One reason for the diversity of results is the absence of clearly identifiable anatomical borders of the PT. There is, for instance, no unanimously accepted boundary indicating where the PT ends and its parietal extension, the planum parietale, begins (for an overview of methodological problems cf. References 6 and 18-20). Even more importantly, PT asymmetry indices might be biased by the decision of whether or not to include additional, more posterior, transverse gyri (after the first transverse gyrus) into the PT region, since they are more likely to occur on the left than the right side.6 Another reason is that most studies related PT asymmetry only indirectly to functional language dominance, by using handedness as a surrogate marker of language lateralization. However, even those studies that directly compared language lateralization and PT asymmetry yielded conflicting results. While some studies claim a clear relationship between PT asymmetry and language dominance, either by a reversal of structural PT asymmetry21 or by a less consistent leftward PT asymmetry,22 other studies did not find a direct correlation between PT asymmetry and language dominance18,23-27
Other authors have associated anatomical asymmetries in frontal brain regions with language lateralization.18,28 Foundas et al were among the first to relate structural asymmetries in frontal brain areas directly to language lateralization.28 The authors used MR morphometry to compare the brain structure of epilepsy patients. Nine of 10 patients with lefthemisphere language dominance had a leftward asymmetry of the pars triangularis (a portion of Broca’s area); one patient who was right lateralized for language had a right-ward asymmetry of the pars triangularis. However, since only one subject was included in the right-hemisphere language dominance group, it is difficult to generalize these findings. Dorsaint-Pierre and colleagues report similar findings.18 The authors compared the brain morphometry of epilepsy patients with left- and right-hemisphere language dominance using MRI. They detected gray matter differences in the posterior part of the inferior frontal gyrus (corresponding functionally to the classical Broca’s area), which favored the left hemisphere in subjects with left-hemisphere language dominance and the right hemisphere in the right-hemisphere language group.
Overall, the existing data support the existence of structural differences between the hemispheres. However, evidence for a clear structure-function relationship with regard to language dominance remains scarce. This study was conducted to further explore structural correlates of functional language dominance by directly comparing the brain morphology of healthy subjects with left- and right-hemisphere language dominance. Structural differences between both language dominance groups were assessed by voxel-based morphometry (VBM), an exploratory whole-brain technique.29 Based on structural MR images, VBM identifies differences in the local composition of brain tissue while discounting large-scale differences in gross anatomy and position.16,29,30
Methods
Subjects
Twenty healthy volunteers were selected from a cohort of subjects with known language lateralization. Half of the subjects were left-hemisphere dominant for language, the other half right-hemisphere dominant. Both language dominance groups were matched for age, sex, and handedness (Table 1; left-hemisphere dominance group: 28 ± 4 years, four right-handers, four women; right-hemisphere dominance group: 28 ± 6 years, four right-handers, five women). None of the participants ± had a serious history of medical, neurological, or psychiatric illnesses, brain pathology, or abnormal brain morphology on T1-weighted MR images. All participants had at least completed the equivalent of a high-school degree (“Realschulabschluss” or “Abitur”) and were native German speakers. All subjects gave their written informed consent prior to participation, according to the declaration of Helsinki.
Table 1.
Study Participants’ Characteristics: Age, Sex, Handedness, and Language Lateralization
| Subject ID | Age | Sex | Handedness | LI (SDT) | LI (WGT) |
|---|---|---|---|---|---|
| Subjects with left-hemisphere language dominance | |||||
| L1 | 22 | M | −45 | 0.4 | 0.5 |
| L2 | 30 | W | 100 | 0.6 | 0.9 |
| L3 | 28 | W | 73 | – | 0.8 |
| L4 | 27 | W | −45 | 0.7 | – |
| L5 | 25 | W | −82 | 0.6 | 0.8 |
| L6 | 27 | M | −76 | – | 0.6 |
| L7 | 28 | M | 86 | – | 0.5 |
| L8 | 23 | M | 42 | 0.8 | – |
| L9 | 36 | M | −79 | 0.8 | – |
| L10 | 34 | M | −80 | 0.7 | 0.8 |
| Subjects with right-hemisphere language dominance | |||||
| R1 | 27 | M | −100 | – | −0.9 |
| R2 | 30 | W | −35 | – | −0.7 |
| R3 | 35 | M | 100 | −0.8 | −0.8 |
| R4 | 24 | W | 79 | −0.5 | −0.8 |
| R5 | 25 | W | −85 | −0.4 | −0.7 |
| R6 | 25 | M | 40 | −0.7 | −0.6 |
| R7 | 24 | W | −100 | −0.7 | −0.8 |
| R8 | 23 | M | −75 | −0.8 | – |
| R9 | 23 | M | −80 | −0.6 | – |
| R10 | 41 | W | 90 | −0.5 | – |
Handedness was assessed by the Edinburgh handedness inventory, language lateralization by the fMRI activation pattern obtained during a word generation task (WGT) and/or a synonym detection task (SDT).
In the following, we describe how language dominance and handedness was determined:
Determination of language dominance
For this study, language lateralization was determined by the brain activity in Broca’s area, as measured by functional magnetic resonance imaging (fMRI) during a word generation31 and/or a synonym detection task.32 Both tasks are known to determine hemispheric language dominance reliably33 and are validated by the Wada test.34,35
In fMRI studies, language lateralization is typically described by a lateralization index (LI). The LI is defined as
where AL and AR refer to measures of brain activity in homologous regions of interest (ROIs) within the left (L) and right (R) hemisphere. A positive value of LI represents left-hemispheric dominance; a negative value indicates right-hemispheric dominance. In the last decade, several approaches have been established to describe the brain activity AL/R and to choose suitable ROIs (for an overview, see References 33 and 36). In this study, we chose the following procedure:
Choice of ROI
In a first step, “activation regions” were derived from those voxels that were active at P < .01 (uncorrected) in at least 80% of subjects with left-hemisphere language dominance. These activation regions were located predominantly in the left hemisphere. Corresponding homologous regions in the right hemisphere were generated by reflection through the midline. In a second step, the activation regions were divided into ROI (ie, Broca’s area) and regions-of-not-interest (ie, all other regions, specifically the remaining prefrontal cortex).32-34,37
Choice of activity measure
The activity measure was based on the summed t-values within the left and right ROI, respectively. In a first step, a maximum t-value was calculated for each subject and ROI defined as the mean of those 5% of voxels showing the highest level of activation in the ROI. In a second step, the threshold for inclusion in the calculation of the LI was set at 50% of this mean maximum activation value. Only t-values of those voxels that exceeded the threshold were used to calculate the LI.32-34,37
Individuals were considered to be left-hemisphere dominant for language if they had an LI of more than +30 and right-hemisphere dominant if they had an LI of less than −30.33,38 Only subjects with clear language dominance were included; people with “bilateral” language lateralization were excluded (Table 1).
Determination of handedness
Handedness was determined by the Edinburgh handedness inventory,39 which ranges from −100 for strong left-handedness and +100 for strong right-handedness. In this questionnaire, subjects are asked which hand they prefer to write, to draw, to throw a ball, to use a pair of scissors, a toothbrush, a knife, a spoon, or a broom (upper hand), to strike a match or to open a box. Individuals were considered to be right-handed if they had an Oldfield score of more than +30 and left-handed if they had an Oldfield score of less than −30.40,41 Only subjects with clear handedness were included; ambidextrous people were excluded (Table 1).
MRI Acquisition Parameters
MRI data were acquired at the University of Magdeburg, Germany, on a neuro-optimized GE 1.5 T whole body scanner (GE Healthcare, Milwaukee, WI) equipped with a standard circular polarized head coil. High-resolution 3-D-datasets of T1-weighted anatomical images were acquired using a spoiled gradient echo sequence (echo time [TE] = 8 ms, repetition time [TR] = 24 ms, flip angle 30°, matrix 256 × 256 × 124, voxel size .977 × .977 × 1.5 mm3).
VBM Analysis
Data were processed according to the “optimized” VBM protocol analogous to the procedure described in Luders et al42 using the SPM2 software package (Wellcome Department of Cognitive Neurology).
Creation of templates–whole brain template and symmetrical gray matter template
A whole-brain template was created from all 20 T1-weighted images. These 20 images were normalized applying a 12-parameter affine transformation to match the Montreal Neurology Institute (MNI) template. After the normalizing step, one average image was created and smoothed with an 8-mm FWHM isotropic Gaussian kernel.
All 20 normalized, T1-weighted images were segmented, then flipped vertically in the midsagittal plane (x = 0) to create a symmetrical gray matter template. Finally, one average image from all gray matter images (normal and flipped) was created and smoothed with an 8-mm FWHM isotropic Gaussian kernel. In addition, we created symmetrical a priori maps by averaging the gray matter, white matter, and cerebrospinal fluid a priori maps provided with the SPM2 package with their flipped counterparts.
The symmetrical gray matter template and the symmetrical a priori maps were used in the subsequent spatial normalization and segmentation procedures described below.
VBM analysis
All 20 T1-weighted MR images were linearly transformed into MNI standard space using our whole-brain template. We then segmented the normalized images into gray matter, white matter, and cerebrospinal fluid by applying the SPM2 segmentation algorithm. The gray matter volumes were then used to estimate the spatial normalization parameters by matching them to our self-created symmetrical gray matter template using linear (12 parameter affine transformation) and non-linear components (7 × 8 × 7 basis functions). These transformations were used to spatially normalize the original T1-weighted images. After reslicing image volumes into isotropic voxels (1 mm3), a further preprocessing step was introduced by multiplying the voxel values in the segmented images by the Jacobian determinants to preserve the original volume (“modulation”). At last, the gray matter images were smoothed with an 8-mm Gaussian Kernel.
Statistical analysis
Statistical analysis was performed on the gray matter images using a 2 × 2 factorial design with the factors language dominance (left-hemisphere dominant and right-hemisphere dominant, respectively) and hemisphere (left hemisphere and right hemisphere, respectively). We were interested in (1) the comparison between both hemispheres (presented in the results section as post hoc analyses separately for both language dominance groups), and (2) the direct comparison between the language dominance groups. The first analysis probed, for instance, whether the PT is a structural marker of functional language dominance, while the second analysis explored structural correlates of functional language dominance, directly comparing two groups of individuals with each other on a voxel-by-voxel basis with the two groups differing in their functional language lateralization.
Anatomical differences are reported at P < .05, corrected for multiple comparisons using family-wise error rate (FWE). A more permissive statistical threshold of P < .001 uncorrected was applied for the classical language regions (Broca’s area, Wernicke’s area), the cerebellum, the inferior parietal lobule, and the hippocampal region. These brain regions are known to be functionally asymmetrical (ie, lateralized) involved in the neural language network43-45 and were therefore a priori defined as regions of interest. Only volume differences with a cluster size of at least 200 mm3 are reported. The anatomical localization of regional differences in local gray matter volume was determined using the software package Automated Anatomical Labeling46 and the Münster T2T converter.47
Results
Statistical analysis was performed on the gray matter images using a 2 × 2 factorial design with the factors language dominance (left-hemisphere dominant and right-hemisphere dominant, respectively) and hemisphere (left hemisphere and right hemisphere, respectively). We were interested in (1) the comparison between both hemispheres (presented as post hoc analyses separately for both language dominance groups), and (2) the direct comparison between the language dominance groups.
Comparison between hemispheres
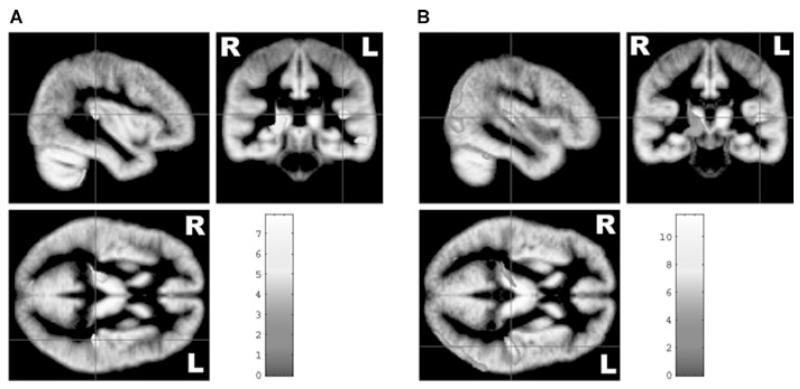
First, we assessed morphometric differences between the left and right hemispheres. Subjects with left-hemisphere language dominance showed relatively increased gray matter volume in the left temporal lobe (middle and superior temporal gyrus, incl. PT), the right parietal lobe (Precuneus), and the left and right cerebellum (Table 2, a1). Subjects with right-hemisphere language dominance had relatively increased gray matter volume in the left temporal lobe (middle and superior temporal gyrus, incl. PT) and the left frontal lobe (inferior frontal gyrus). No brain regions with relatively increased right-hemispheric volume were found (Table 2, a2). Of greatest interest is that leftward structural asymmetries in the PT region are present in both groups, independent on language dominance (Fig 1). This asymmetry is slightly more pronounced in the group of right-hemisphere language dominant subjects (cf. Table 2a).
Table 2.
(a) Differences in Gray Matter Volume between the Left and Right Hemisphere Presented Separately for Subjects with Left- and Right-Hemisphere Language Dominance, (b) Differences in Gray Matter Volume between Subjects with Left- and Right-Hemisphere Language Dominance
| Hemisphere | Cluster Location | MNI-Coordinates (X, Y, Z) | Cluster Size (mm3) | T-Values |
|---|---|---|---|---|
| (a1) Morphometric differences between left and right hemisphere for left-hemisphere dominant subjects | ||||
| L > R | Superior temporal gyrus | −65 −18 −2 | 1,194 | 5.13 |
| L > R | Cerebellum | −35 −43 −54 | 2,978 | 4.89 |
| L > R | Middle temporal gyrus | −64 −28 −15 | 1,479 | 4.70 |
| L > R | Superior temporal gyrus/Heschl’s gyrus | −41 −31 11 | 783 | 4.40 |
| L > R | Cerebellum | −35 −41 −27 | 244 | 3.93 |
| R > L | Precuneus | 15 −63 38 | 852 | 4.60 |
| R > L | Cerebellum | 15 −64 37 | 3,184 | 3.97 |
|
| ||||
| (a2) Morphometric differences between left and right hemisphere for right-hemisphere dominant subjects | ||||
| L > R | Superior temporal gyrus/Heschl’s gyrus | −47 −26 8 | 3,719 | 6.12 |
| L > R | Inferior frontal gyrus | −47 24 29 | 270 | 4.58 |
| L > R | Middle temporal gyrus | −65 −34 −3 | 603 | 4.30 |
|
| ||||
| (b1) Volume differences between subjects with left- and right-hemisphere dominance (LHD > RHD) | ||||
| No suprathreshold cluster | ||||
|
| ||||
| (b2) Volume differences between subjects with left- and right-hemisphere dominance (RHD > LHD) | ||||
| R | Hippocampus | 27 −33 −5 | 207 | 3.55 |
Fig 1.
Statistical parametric maps showing leftward PT asymmetry for subjects with (A) left- as well as those with (B) right-hemisphere language dominance. Structural differences are superimposed on the study-specific symmetrical gray matter template. Color scales bars indicate the T score. Please note that all results are presented in radiological convention (left = right).
Comparison between language dominance groups
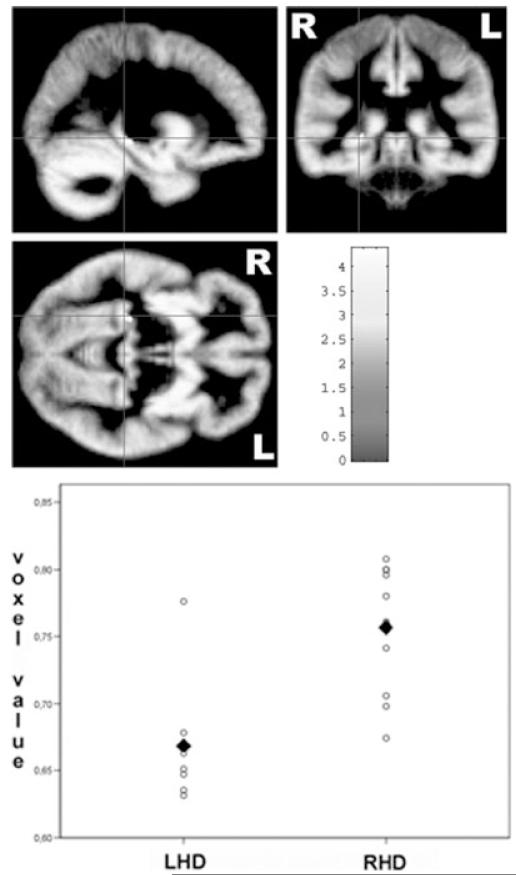
Second, we directly assessed the effects of functional language dominance on structural brain morphometry by comparing both groups with each other on a voxel-by-voxel basis. Subjects with righthemisphere language dominance had relatively increased gray matter volume in the right hippocampus (Table 2b, Fig 2). No suprathreshold clusters were observed in the reverse contrast.
Fig 2.
Top: Statistical parametric map showing morphometric differences between subjects with left- and right-hemisphere language dominance, superimposed on the study-specific symmetrical gray matter template. Subjects with right-hemisphere language dominance have increased gray matter volume in the right hippocampus compared to subjects with left-hemisphere language dominance. Color scales bar indicates the T score. Please note that all results are presented in radiological convention (left = right). Bottom: Distribution of the voxel values extracted at the statistical peak in the right hippocampus (MNI coordinates 27–33–5) for both language dominance groups. The mean voxel value for each group is marked with a bold diamant. The scatter plot demonstrates that the hippocampus effect is not driven by outliers in the right-hemisphere dominance group. LHD = subjects with left hemisphere language dominance; RHD = subjects with right-hemisphere language dominance.
It is noteworthy that the interhemispheric anatomical differences (resulting from the comparison of the left versus the right hemisphere within in one language dominance group) are more pronounced (in either language dominance group) than the differences between the language dominance groups (resulting from the comparison of the same hemisphere of subjects with left- and right-hemisphere language dominance). This is denoted by the height of the respective t-values. For instance, the morphometric difference in the hippocampal region (Table 2b) has a lower t-statistic than any of the inter-hemispheric differences (Table 2a).
Discussion
This study explored structural correlates of functional language dominance by comparing the brain morphometry of subjects with left- and right-hemisphere language dominance. The main findings can be summarized as follows:
-
(1)
We detected gray matter volume differences between both groups in the hippocampus, that is, subjects with right-hemisphere language dominance had relatively increased gray matter volume in the right hippocampus.
-
(2)
Leftward structural asymmetries in the PT region were observed in both groups, with a slightly more pronounced asymmetry in the group of subjects with right-hemisphere language dominance. This indicates that language dominance as a single factor does not explain the posterior superior temporal asymmetry in the PT region.
Methodological Issues
Selection of participants
The main strength of the study is the careful characterization of subjects. They were selected based on their language dominance and not by other surrogate markers of functional brain asymmetry such as handedness, as in many other lateralization studies. We ensured that both groups were matched for handedness, sex, and age to exclude any potential bias (Table 1). It might have been possible to also include subjects with “bilateral” language dominance. However, we decided to recruit only subjects with clear right- or left-hemisphere language dominance, since the classification “bilateral language dominance” is difficult to reproduce on the basis of fMRI data.33
One limiting factor in previous studies on structural-functional relationships was that measures were taken in epileptic patients who may have atypical brain morphology secondary to brain lesions.18,21 In our study, we specifically included healthy subjects without any evidence for brain lesions.
VBM analysis
Analysis of MRI data was performed by VBM. Compared to traditional ROI-based approaches (Luders et al42), VBM has the advantage that it circumvents the problem of delineating exact macroscopic boundaries of a particular brain structure. Although the method does not measure the absolute size of specific brain structures (eg, the PT), it provides an objective quantification of structural cerebral asymmetries. A further advantage of VBM is that the method can be performed across the entire brain, that is, the technique is not a priori restricted to specific brain regions. It is, however, not fully unbiased. For instance, both the choice of the template image and the smoothing filter applied prior to statistical analysis can affect the results. We used a symmetrical template since the standard asymmetrical template image might have further enhanced asymmetries in the original data. To smooth the data prior to statistical analysis, we applied an 8-mm Gaussian Kernel. To further confirm the robustness of our results, we also reanalyzed the data in a series of post hoc analyses using smoothing kernels of 6 mm, 10 mm, and 12 mm. The principal results, however, did not change.
Since VBM does not delineate macroscopic boundaries, we did not expect an exact match of the structural asymmetry in the temporal cortex detected by our VBM analysis and the macroscopic definition of the PT. Nevertheless, the structural asymmetry of the temporal lobe revealed by our VBM analysis has to be an equivalent of the macroscopically defined asymmetry of the PT. To check this equivalence, we proceeded in two steps. First, we compared our findings with the results of other VBM studies that investigated structural hemispheric asymmetries of the human brain.17,18 The authors found, just like us, a pronounced structural asymmetry in the temporal cortex that they identified as PT. The local maximum of the structural asymmetry in their studies matched the MNI coordinates of the temporal asymmetry of our study (see the Appendix). In a second step, we checked on the canonical MNI brain (supplied by SPM) that the local maxima of the structural temporal asymmetry obtained in the VBM analysis is well within the anatomical boundaries of the PT (using the definition given in Sequeira et al6).
There are also some other studies that compared manual measures of PT asymmetry and hemispheric structural asymmetries as revealed by VBM, but that did not report exact MNI coordinates. Luders et al found a positive correlation between manually and automatically obtained asymmetry scores in the temporal cortex.42 Similarly, Eckert and colleagues report a high correlation between a VBM measure in the posterior superior temporal gyrus and a manual measure of PT asymmetry.48
Neuroscientific Implications and Comparison with the Literature
Analysis 1: Structural Differences between the Hemispheres
Planum temporale
Explanations of left-hemisphere language dominance in the past focused mostly on the PT. Geschwind’s hypothesis that leftward structural PT asymmetry is related to functional language dominance9 received indirect support from different studies, in particular by the less pronounced leftward PT asymmetry in left-handers.17,49-52 (For reviews see References 12 and 53 but also see 20 and 26) Since the left PT comprises the major part of Wernicke’s area, its left-sided asymmetry may be a structural correspondence to the functional asymmetry found in Wernicke aphasics who lose the ability to understand spoken language. The asymmetry of the PT appears already at the fetal stage11,54 and does not change with increasing age or brain volume.55 Therefore, abnormalities in this brain region were interpreted as an early disruption of neurodevelopmental processes involved in the establishment of functional hemispheric lateralization. Also, fMRI studies showed that the PT is functionally involved in language processing, for example, in auditory processing.27
The exact nature of the PT asymmetry remained nevertheless controversial because only a few studies assessed the relation between language dominance and structural brain morphometry directly. Even those studies that directly compared language lateralization and PT asymmetry yielded conflicting results.18,21-27
In our study, we found a leftward asymmetry of the posterior superior temporal cortex within the PT region in both language dominance groups. This structural asymmetry therefore seems to be independent of language lateralization. These results are consistent with more recent studies showing no direct association between PT asymmetry and language dominance in epileptic patients18 as well as in healthy subjects.6,23,26 Rather, the degree of leftward asymmetry of the PT might be determined by a complex interaction of different factors such sex, language dominance, and handedness.6 Taken together, our findings confirm that structural asymmetries in the PT region (albeit clearly evident in both language dominance groups) have no direct relationship to language dominance.
Recently, it has been discussed that the PT is not a dedicated language processor, but rather involved as a computational hub in the analysis of complex sounds (Griffith et al 2002). PT asymmetry could therefore be related to more low-level auditory processing. Future research therefore has to combine structural assessment of PT asymmetry with a more complex battery of functional activations paradigms that not only cover higher-order language lateralization, but also simple auditory processing tasks.
Frontal brain regions
In a similar study to our’s, Dorsaint-Pierre and colleagues compared the brain structure of epileptic patients with left- and right-hemisphere language lateralization.18 They reported interhemispheric differences between the language dominance groups in frontal brain regions corresponding functionally to the classical Broca’s area (MNI coordinates −49, 8, 18). Left-hemisphere dominant subjects showed a leftward structural asymmetry, right-hemisphere dominant subjects a rightward asymmetry. In this study, we did not find interhemispheric differences between the language dominance groups in Broca’s area. Although the number of subjects included in our study is comparable to the study of Dorsain-Pierre et al, it is possible that in a larger study we might have also detected differences in the classical language regions.
Analysis 2: Structural Differences between Subjects with Left- and Right-Hemisphere Dominance
We found differences between both language dominance groups in the hippocampus. Subjects with right-hemisphere language dominance had significantly larger gray matter volume in the right hippocampus than subjects with left-hemisphere language dominance. This finding is novel (since no comparable study directly assessed the structure-function relationship with regard to language dominance).
At first glance, it might be surprising that the most prominent differences in brain structure related to language lateralization are found in the hippocampus and not, for instance, in “classical” language regions. However, growing evidence indicates a central role of the hippocampal formation in the learning of semantic information. The involvement of the left hippocampus in subjects with typical language dominance has been shown, for example, in learning a new vocabulary.44 In case of left-hemisphere language dominance, the left hippocampus seems to be interacting with neocortical language areas in the left hemisphere during language development.56 Similarly, the right hippocampus might play a comparable role in subjects with right-hemisphere language dominance.
Recently, it has been proposed that the hippocampus might even be the driving force for the development of language lateralization.57-59 Liegeois and coworkers showed in a study of 10 children that interhemispheric shifts in language dominance are typically not associated with lesion in close proximity to classical language-related areas, but rather in the temporal lobe.58 These findings have been confirmed by a larger study of Weber et al who investigated 84 adult epilepsy patients with structural lesions.59 Patients with left hippocampal sclerosis showed significantly less left lateralized language dominance than subjects with structural lesions in other parts of the brain. Particularly, patients with left frontal or temporal-lateral lesions displayed the same degree of left-hemispheric dominance as control subjects.
Our findings were not symmetrical, that is, we did not observe that subjects with left-hemisphere language dominance had a larger left hippocampus than subjects with right-hemisphere language dominance. One explanation might be that subjects with right-hemisphere language dominance do not only host language in the right hemisphere, but also other cognitive functions that are typically implemented there.60 Thus, in subjects with right-hemisphere language dominance the right hippocampus might not only adopt language learning, but more functions than in subjects with the typical brain organization.
At last, some limitations of this study should be mentioned:
First, since subjects with atypical language dominance are difficult to recruit, the sample size is relatively small for a VBM study. Subjects with atypical right-hemisphere language dominance were selected from a cohort of 326 healthy volunteers previously assessed for language dominance by functional transcranial Doppler sonography (“Münster Functional Imaging Study on the Variability of Hemispheric Specialization in Health and Disease”).41,61,62 Despite the limited sample size, we nevertheless believe that this study allows valuable insights into the neuroanatomy of language. However, some of our findings, that is, especially the results regarding the hippocampus, should be seen as preliminary until they have been reproduced in further studies.
Second, due to the limited sample size we were not able to investigate potential interactions between handedness, gender, and hemispheric dominance. Future studies with larger sample sizes might tackle this question by including handedness as a separate factor in the statistical analysis. These studies will in particular be able to compare subjects with atypical language dominance with the most typical control group, that is, right-handed subjects with left-hemisphere language dominance.
Third, we considered language dominance as a categorical variable (ie, we classified subjects as being either left- or right-hemisphere dominant). The classification left/right was based on the brain activation pattern obtained during a word generation and/or a semantic decision task. Both tasks are known to determine language dominance reliably and are validated by the Wada test.33 Since some subjects performed only one language task (see Table 1), we refrained from using also the degree of language lateralization for further analyses (eg, to correlate functional asymmetry values and individual gray matter maps) because, with regard to the degree of language dominance, both tasks yield slightly different results (see Table 1).
In summary, the results of this study do not support the view that asymmetries in the PT region are related in a direct way to functional language lateralization. Structural differences between both language dominance groups were instead found in the hippocampus. This finding underlines the importance of the hippocampcal formation in the neural language network. Further studies are now needed to entangle the influence of sex, handedness, and language dominance on brain structure, combining functional imaging with structural assessments of brain morphology in gray and white matter (eg, by diffusion tensor imaging).
Supplementary Material
Acknowledgments
This work was supported by the Innovative Medizinische Forschung Münster (JA110607), the Interdisciplinary Center of Clinical Research Münster (IZKF Projects FG2 and Kne3/074/04), and the Center of Advanced Imaging Magdeburg (CAI, BMBF – grant 01GO0404). We thank N. Fritz, Burbacher Institute of Advanced-Neuroscience, for helpful discussions on lateralization phenomena.
Footnotes
Additional supporting information may be found in the online version of this article:
Appendix. MNI coordinates of structural hemispheric asymmetries in the temporal cortex as assessed by VBM. Please note that there exist also several other studies that investigated cerebral asymmetry by VBM, but do not report exact MNI coordinates (eg, Luders et al,42 Eckert et al,48 and Good et al).16
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Stephan KE, Marshall JC, Friston KJ, et al. Lateralized cognitive processes and lateralized task control in the human brain. Science. 2003;301:384–386. doi: 10.1126/science.1086025. [DOI] [PubMed] [Google Scholar]
- 2.Hugdahl K. Lateralization of cognitive processes in the brain. Acta Psychol (Amst) 2000;105:211–235. doi: 10.1016/s0001-6918(00)00062-7. [DOI] [PubMed] [Google Scholar]
- 3.Amunts K, Schleicher A, Burgel U, et al. Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 4.Amunts K, Schleicher A, Ditterich A, et al. Broca’s region: cytoarchitectonic asymmetry and developmental changes. J Comp Neurol. 2003;465:72–89. doi: 10.1002/cne.10829. [DOI] [PubMed] [Google Scholar]
- 5.Josse G, Tzourio-Mazoyer N. Hemispheric specialization for language. Brain Res Brain Res Rev. 2004;44:1–12. doi: 10.1016/j.brainresrev.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Sequeira SDS, Woerner W, Walter C, et al. Handedness, dichoticlistening ear advantage, and gender effects on planum temporale asymmetry – A volumetric investigation using structural magnetic resonance imaging. Neuropsychologia. 2006;44:622–636. doi: 10.1016/j.neuropsychologia.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Pfeifer RA. Myelogenetisch -anatomische Untersuchungen über das kortikale Ende der Hörleitung. Abhandlungen der Mathematisch-Physischen Klasse der Sȧchsischen Akademie der Wissenschaften. 1920;37:1–54. [Google Scholar]
- 8.von Economo C, Horn L. Über Windungsrelief, Maße und Rindenarchitektonik der Supratemporalfläche, ihre individuellen und ihre Seitenunterschiede. Zeitschrift für die Gesamteund Neurologieund Psychiatrie. 1930;130:678–757. [Google Scholar]
- 9.Geschwind N, Levitsky W. Human brain: left-right asymmetries in temporal speech region. Science. 1968;261:186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- 10.Wada JA, Clarck R, Hamm A. Cerebral hemispheric asymmetry in humans: cortical speech zones in 100 adults and infant brains. Arch Neurol. 1975;32:239–246. doi: 10.1001/archneur.1975.00490460055007. [DOI] [PubMed] [Google Scholar]
- 11.Witelson SF, Pallie W. Left hemisphere specialization for language in the newborn. Neuroanatomical evidence of asymmetry. Brain. 1973;96:641–646. doi: 10.1093/brain/96.3.641. [DOI] [PubMed] [Google Scholar]
- 12.Shapleske J, Rossell SL, Woodruff PW, et al. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res Brain Res Rev. 1999;29:26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- 13.Watkins KE, Paus T, Lerch JP, et al. Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans. Cereb Cortex. 2001;11:868–877. doi: 10.1093/cercor/11.9.868. [DOI] [PubMed] [Google Scholar]
- 14.Beaton AA. The relation of planum temporale asymmetry and morphology of the corpus callosum to handedness, gender, and dyslexia: a review of the evidence. Brain Lang. 1997;60:255–322. doi: 10.1006/brln.1997.1825. [DOI] [PubMed] [Google Scholar]
- 15.Galaburda AM, LeMay M, Kemper TL, et al. Right-left asymmetrics in the brain. Science. 1978;199:852–856. doi: 10.1126/science.341314. [DOI] [PubMed] [Google Scholar]
- 16.Good CD, Johnsrude I, Ashburner J, et al. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. NeuroImage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- 17.Herve PY, Crivello F, Perchey G, et al. Handedness and cerebral anatomical asymmetries in young adult males. NeuroImage. 2006;29:1066–1079. doi: 10.1016/j.neuroimage.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 18.Dorsaint-Pierre R, Penhune VB, Watkins KE, et al. Asymmetries of the planum temporale and Heschl’s gyrus: relationship to language lateralization. Brain. 2006;129:1164–1176. doi: 10.1093/brain/awl055. [DOI] [PubMed] [Google Scholar]
- 19.Honeycutt NA, Musick A, Barta PE, et al. Measurement of the planum temporale (PT) on magnetic resonance imaging scans: temporal PT alone and with parietal extension. Psychiatry Res-Neuroimag. 2000;98:103–116. doi: 10.1016/s0925-4927(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 20.Zetzsche T, Meisenzahl EM, Preuss UW, et al. In-vivo analysis of the human planum temporale (PT): does the definition of PT borders influence the results with regard to cerebral asymmetry and correlation with handedness? Psychiatry Res. 2001;107:99–115. doi: 10.1016/s0925-4927(01)00087-7. [DOI] [PubMed] [Google Scholar]
- 21.Foundas AL, Leonard CM, Gilmore R, et al. Planum temporale asymmetry and language dominance. Neuropsychologia. 1994;32:1225–1231. doi: 10.1016/0028-3932(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 22.Moffat SD, Hampson E, Lee DH. Morphology of the planum temporale and corpus callosum in left handers with evidence of left and right hemisphere speech representation. Brain. 1998;121(Part 12):2369–2379. doi: 10.1093/brain/121.12.2369. [DOI] [PubMed] [Google Scholar]
- 23.Eckert MA, Leonard CM, Possing ET, et al. Uncoupled leftward asymmetries for planum morphology and functional language processing. Brain Lang. 2006;98:102–111. doi: 10.1016/j.bandl.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hellige JB, Taylor KB, Lesmes L, et al. Relationships between brain morphology and behavioral measures of hemispheric asymmetry and interhemispheric interaction. Brain Cogn. 1998;36:158–192. doi: 10.1006/brcg.1997.0951. [DOI] [PubMed] [Google Scholar]
- 25.Jancke L, Steinmetz H. Auditory lateralization and planum temporale asymmetry. Neuroreport. 1993;5:169–172. doi: 10.1097/00001756-199311180-00019. [DOI] [PubMed] [Google Scholar]
- 26.Josse G, Mazoyer B, Crivello F, et al. Left planum temporale: an anatomical marker of left hemispheric specialization for language comprehension. Brain Res Cogn Brain Res. 2003;18:1–14. doi: 10.1016/j.cogbrainres.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Tzourio N, Nkanga Ngila B, Mazoyer B. Left planum temporale surface correlates with functional dominance during story listening. Neuroreport. 1998;9:829–833. doi: 10.1097/00001756-199803300-00012. [DOI] [PubMed] [Google Scholar]
- 28.Foundas AL, Leonard CM, Gilmore RL, et al. Pars triangularis asymmetry and language dominance. Proc Natl Acad Sci USA. 1996;93:719–722. doi: 10.1073/pnas.93.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 30.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 31.Knecht S, Jansen A, Frank A, et al. How atypical is atypical language dominance? NeuroImage. 2003;18:917–927. doi: 10.1016/s1053-8119(03)00039-9. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez G, de Greiff A, von Oertzen J, et al. Language mapping in less than 15 minutes: real-time functional MRI during routine clinical investigation. NeuroImage. 2001;14:585–594. doi: 10.1006/nimg.2001.0854. [DOI] [PubMed] [Google Scholar]
- 33.Jansen A, Menke R, Sommer J, et al. The assessment of hemispheric lateralization in functional MRI-robustness and reproducibility. NeuroImage. 2006;33:204–217. doi: 10.1016/j.neuroimage.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 34.Adcock JE, Wise RG, Oxbury JM, et al. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. NeuroImage. 2003;18:423–438. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez G, Specht K, Weis S, et al. Intrasubject reproducibility of presurgical language lateralization and mapping using fMRI. Neurology. 2003;60:969–975. doi: 10.1212/01.wnl.0000049934.34209.2e. [DOI] [PubMed] [Google Scholar]
- 36.Wilke M, Lidzba K. LI-tool: a new toolbox to assess lateralization in functional MR-data. J Neurosci Methods. 2007;163:128–136. doi: 10.1016/j.jneumeth.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 37.Jansen A, Floel A, Deppe M, et al. Determining the hemispheric dominance of spatial attention: a comparison between fTCD and fMRI. Hum Brain Mapp. 2004;23:168–180. doi: 10.1002/hbm.20055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Springer JA, Binder JR, Hammeke TA, et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study [see comments] Brain. 1999;122:2033–2046. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- 39.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 40.Jansen A, Lohmann H, Scharfe S, et al. The association between scalp hair-whorl direction, handedness and hemispheric language dominance: is there a common genetic basis of lateralization? NeuroImage. 2007;35:853–861. doi: 10.1016/j.neuroimage.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 41.Knecht S, Drager B, Deppe M, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- 42.Luders E, Gaser C, Jancke L, et al. A voxel-based approach to gray matter asymmetries. NeuroImage. 2004;22:656–664. doi: 10.1016/j.neuroimage.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 43.Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- 44.Breitenstein C, Jansen A, Deppe M, et al. Hippocampus activity differentiates good from poor learners of a novel lexicon. NeuroImage. 2005;25:958–968. doi: 10.1016/j.neuroimage.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 45.Jansen A, Floel A, Van Randenborgh J, et al. Crossed cerebro-cerebellar language dominance. Hum Brain Mapp. 2005;24:165–172. doi: 10.1002/hbm.20077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 47.Deppe M, Steinstrater O, Sommer J, et al. The T2T-database java applet. NeuroImage. 2003;19:S989. [Google Scholar]
- 48.Eckert MA, Lombardino LJ, Walczak AR, et al. Manual and automated measures of superior temporal gyrus asymmetry: concordant structural predictors of verbal ability in children. Neuroimage. 2008;41:813–822. doi: 10.1016/j.neuroimage.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foundas AL, Leonard CM, Hanna-Pladdy B. Variability in the anatomy of the planum temporale and posterior ascending ramus: do right- and left handers differ? Brain Lang. 2002;83:403–424. doi: 10.1016/s0093-934x(02)00509-6. [DOI] [PubMed] [Google Scholar]
- 50.Habib M, Robichon F, Levrier O, et al. Diverging asymmetries of temporo-parietal cortical areas: a reappraisal of Geschwind/Galaburda theory. Brain Lang. 1995;48:238–258. doi: 10.1006/brln.1995.1011. [DOI] [PubMed] [Google Scholar]
- 51.Kertesz A, Black SE, Polk M, et al. Cerebral asymmetries on magnetic-resonance-imaging. Cortex. 1986;22:117–127. doi: 10.1016/s0010-9452(86)80036-3. [DOI] [PubMed] [Google Scholar]
- 52.Steinmetz H, Volkmann J, Jancke L, et al. Anatomical left-right asymmetry of language-related temporal cortex is different in left- and right-handers. Ann Neurol. 1991;29:315–319. doi: 10.1002/ana.410290314. [DOI] [PubMed] [Google Scholar]
- 53.Steinmetz H. Structure, functional and cerebral asymmetry: in vivo morphometry of the planum temporale. Neurosci Biobehav Rev. 1996;20:587–591. doi: 10.1016/0149-7634(95)00071-2. [DOI] [PubMed] [Google Scholar]
- 54.Chi JG, Dooling EC, Gilles FH. Left-right asymmetries of temporal speech areas of human fetus. Arch Neurol. 1977;34:346–348. doi: 10.1001/archneur.1977.00500180040008. [DOI] [PubMed] [Google Scholar]
- 55.Preis S, Jancke L, Schmitz-Hillebrecht J, et al. Child age and planum temporale asymmetry. Brain Cogn. 1999;40:441–452. doi: 10.1006/brcg.1998.1072. [DOI] [PubMed] [Google Scholar]
- 56.Opitz B, Friederici AD. Interactions of the hippocampal system and the prefrontal cortex in learning language-like rules. NeuroImage. 2003;19:1730–1737. doi: 10.1016/s1053-8119(03)00170-8. [DOI] [PubMed] [Google Scholar]
- 57.Knecht S. Does language lateralization depend on the hippocampus? Brain. 2004;127:1217–1218. doi: 10.1093/brain/awh202. [DOI] [PubMed] [Google Scholar]
- 58.Liegeois F, Connelly A, Cross JH, et al. Language reorganization in children with early onset lesions of the left hemisphere: an fMRI study. Brain. 2004;127:1229–1236. doi: 10.1093/brain/awh159. [DOI] [PubMed] [Google Scholar]
- 59.Weber B, Wellmer J, Reuber M, et al. Left hippocampal pathology is associated with atypical language lateralization in patients with focal epilepsy. Brain. 2006;129:346–351. doi: 10.1093/brain/awh694. [DOI] [PubMed] [Google Scholar]
- 60.Jansen A, Floel A, Menke R, et al. Dominance for language and spatial processing: limited capacity of a single hemisphere. Neuroreport. 2005;16:1017–1021. doi: 10.1097/00001756-200506210-00027. [DOI] [PubMed] [Google Scholar]
- 61.Knecht S, Deppe M, Dräger B, et al. Language lateralization in healthy right-handers. Brain. 2000;123:74–81. doi: 10.1093/brain/123.1.74. [DOI] [PubMed] [Google Scholar]
- 62.Knecht S, Drager B, Floel A, et al. Behavioural relevance of atypical language lateralization in healthy subjects. Brain. 2001;124:1657–1665. doi: 10.1093/brain/124.8.1657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.