Abstract
Purpose:
To investigate constancy, within a treatment session, of the time lag relationship between implanted markers in abdominal tumors and an external motion surrogate.
Methods:
Six gastroesophageal junction and three pancreatic cancer patients (IRB-approved protocol) received two cone-beam CTs (CBCT), one before and one after treatment. Time between scans was less than 30 min. Each patient had at least one implanted fiducial marker near the tumor. In all scans, abdominal displacement (Varian RPM) was recorded as the external motion signal. Purpose-built software tracked fiducials, representing internal signal, in CBCT projection images. Time lag between superior–inferior (SI) internal and anterior–posterior external signals was found by maximizing the correlation coefficient in each breathing cycle and averaging over all cycles. Time-lag-induced discrepancy between internal SI position and that predicted from the external signal (external prediction error) was also calculated.
Results:
Mean ± standard deviation time lag, over all scans and patients, was 0.10 ± 0.07 s (range 0.01–0.36 s). External signal lagged the internal in 17/18 scans. Change in time lag between pre- and post-treatment CBCT was 0.06 ± 0.07 s (range 0.01–0.22 s), corresponding to 3.1% ± 3.7% (range 0.6%–10.8%) of gate width (range 1.6–3.1 s). In only one patient, change in time lag exceeded 10% of the gate width. External prediction error over all scans of all patients varied from 0.1 ± 0.1 to 1.6 ± 0.4 mm.
Conclusions:
Time lag between internal motion along SI and external signals is small compared to the treatment gate width of abdominal patients examined in this study. Change in time lag within a treatment session, inferred from pre- to post-treatment measurements is also small, suggesting that a single measurement of time lag at the session start is adequate. These findings require confirmation in a larger number of patients.
Keywords: gated radiation therapy, respiratory motion
1. INTRODUCTION
In gated radiation therapy, the beam is enabled within a certain portion of the breathing cycle, which is called a gate.1 In systems which use a signal external to the patient to gate the Linac, it is assumed that there is a strong correlation between internal and external signal.2,3 The external signal is obtained by tracking an external breathing motion surrogate placed on the abdomen while the internal signal is often obtained by radiographically tracking an internal breathing motion surrogate implanted near the tumor.4–6 There are several studies about the correlation between internal and external signal. Some of the studies suggest that it is acceptable to use external motion surrogate to predict tumor motion.7–10 Schweikard et al.7 found that respiratory motion of internal organs can be correlated with an external motion surrogate in pancreatic cancer patients, which allows the margin placed around tumor to be reduced by a substantial amount. Ionascu et al.8 found good correlation between an external motion surrogate and internal motion along superior–inferior (SI) direction in lung cancer patients. Vedam et al.9 and Mageras et al.10 showed strong correlation between diaphragm motion and respiration signal obtained from the abdominal wall in lung cancer patients. However, other studies show lesser correlation in excursion and phase relationship between internal and external motion surrogates.11–14 Hoisak et al.11 found geographical miss of the target is possible while using the surrogate to guide treatment in lung cancer patients and recommended caution and direct tumor imaging to verify the accuracy of tumor-surrogate relationship. Tsunashima et al.12 found that the external respiratory motion waveform did not always accurately correspond to the tumor motion in lung, liver, and esophagus cancer patients. Koch et al.13 found that an external fiducial might not be sufficient to predict complex movement of lung for effective respiratory gating. Liu et al.14 also found that motion of a large lung tumor along its edge may be complex, making it more difficult to track using exterior markers and reduce the treatment margin. Ge et al.15 reported that inaccurate respiratory gating may occur within a treatment session in pancreatic and liver cancer patients, and one of the main causes identified was change of external–internal correlation over the treatment session. Gierga et al.16 observed variability in pancreatic and liver tumor positions of up to 9 mm for a given external marker position; the variability was dependent on the location of the external marker on the patient.
Under conditions of gating with external surrogates, interfraction variation in tumor and internal organ positions has been reported in various studies, requiring daily imaging for target localization and correction. Redmond et al.17 used repeated respiration correlated CT (RCCT) scans to study variation in internal/external time shift over the treatment course in lung cancer patients. A study of 20 patients found mean changes in time shift between lung tumor and xyphoid process of 14% of the breathing period and up to 50% change in one patient. The findings indicate that using external motion surrogates and assuming constancy of time shift, as established at simulation, introduces errors in tumor position at treatment.
Interfraction variation in time shift implies that either internal/external correlation needs to be re-established at each treatment fraction or an appropriate PTV margin should be used in lung cancer patients.18 Intrafraction variation would require much more frequent correction. Therefore, the objective of our study is to investigate the change in time lag relationship between internal and external signal within a treatment session in gated radiation therapy.
2. METHODS AND MATERIALS
On one treatment day, six gastroesophageal junction (GEJ) and three pancreatic cancer patients received cone-beam CTs (CBCT), one immediately before (pre-RT) and one immediately after (post-RT) the treatment. The time between the two scans varied from 13 to 29 min. All the patients were enrolled in an Institutional Review Board (IRB) approved imaging protocol and were treated on a Varian TrueBeam Linac. Approximately a week before the simulation, one to three Visicoil™ markers were implanted in or near the tumor of each patient. Visicoils were 10 mm long and 0.75 mm in diameter. The CBCT acquisition settings were 125 kV, 1469–1986 mA s, a CBCT arc of 360° in half-fan mode, at a rate of 11 frames/s, resulting in 656–657 projection images per scan. In the half-fan mode scans, the image detector was laterally offset, resulting in markers outside the field of view (FOV) in some of the images.
The external breathing signal was obtained from the Varian RPM system in which an integrated Polaris camera images a block with infrared reflective markers placed on the patient’s abdomen. The RPM system records the anterior–posterior (AP) motion of the block. The internal breathing signal was obtained using a purpose-built computer program19 to track the implanted markers visualized in the projection images acquired for the CBCT. The program tracked the centroid of the markers assuming rigid body motion, rather than tracking the differential motion of individual markers. To enable recording of the RPM signal during CBCT acquisition, the following procedure was followed. In the ARIA treatment management system, a CBCT setup field was defined and assigned to a mock treatment plan, which was designated as gated but not used for treatment. This setup field was selected for acquiring the CBCT in treatment mode on the TrueBeam. During CBCT acquisition, the RPM signal was simultaneously recorded into the header information of the projection images. Since these images were used to obtain the internal signal, corresponding internal and external signals had the same time stamp. The superior–inferior motion of the internal signal was compared with the one-dimensional (anterior–posterior block motion) external signal. The time lag between internal and external signal (described below) was computed separately for each breathing cycle, then averaged over cycles to obtain the time lag for the CBCT scan. Approximately eight breathing cycles (range 4–14) were analyzed per scan. To obtain the time lag for each cycle, the time stamps of the external signal were shifted back and forth in time with respect to internal signal in steps of 0.09 s, and the correlation coefficient was calculated at each step. In this way, the external signal was moved against the internal signal at least two time steps beyond the maximum correlation coefficient point on both sides. The correlation coefficient curve was fitted with a fourth order polynomial, and the shift in external time stamp at the peak of the fit interpreted as the time lag. The change in time lag within a treatment session was calculated as the difference in time lag of the CBCT scans immediately before and after the treatment.
3. RESULTS
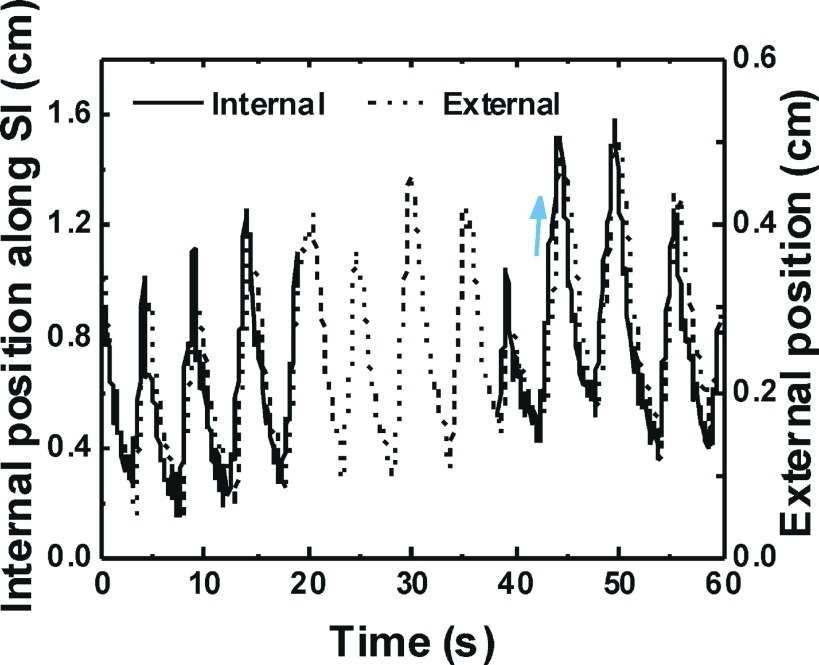
The internal and external signal of the patient with maximum time lag is shown in Fig. 1. There is no internal signal from about 19–38 s for this patient because implanted Visicoil markers were out of the field of view during that time. Similar breathing traces were observed in other patients except in two for whom the markers were always inside field of view. The time lag between the internal and external signals varied from 0.02 to 0.36 s with mean and standard deviation of 0.11 ± 0.08 s in eighteen CBCT scans of the nine patients.
FIG. 1.
Internal and external signal of a patient with maximum time lag. Arrow shows direction of inhalation.
From the time lag between internal and external signals, the discrepancy between the internal SI position and that predicted from the external signal (referred to here as the external prediction error) was calculated from each breath cycle by multiplying the mean speed of the internal signal (i.e., distance traveled along the SI direction divided by breathing period) by the time lag and averaging over cycles. The average external prediction error varied from 0.1 ± 0.1 to 1.6 ± 0.4 mm in 18 CBCT scans of the nine patients. The average pre-RT and post-RT time lags and corresponding average external prediction error for all patients are shown in Fig. 2. The change in average time lag from pre-RT to post-RT scan varied from 0.01 to 0.22 s (mean and standard deviation of 0.06 ± 0.07 s); this is shown in Fig. 3. A typical gate width for gated treatments in our clinic is 40% of the breathing period from RCCT scan obtained at simulation. For these patients, the breathing period varied from 4.1 to 7.9 s from RCCT scan and the calculated gate width varied from 1.6 to 3.1 s.
FIG. 2.
Time lag and external prediction error along the SI direction in each cone-beam CT scan of nine patients. Positive time lag indicates that external signal is lagging internal signal. Bars indicate mean of values over breathing cycles in the scan and error bars indicate standard deviations. Patients labeled P1–P3 correspond to pancreas, G1–G6 correspond to GEJ.
FIG. 3.
Change in time lag and external prediction error within a treatment session for each patient.
The change in time lag varied from 0.6% to 10.8% of gate width (mean and standard deviation of 3.1% ± 3.7%). From the external prediction error computed in the pre-RT and post-RT CBCT scans, we calculated the change in external prediction error within a treatment session, which varied across patients from 0.0 to 1.0 mm. Change in external prediction error within a treatment session is also shown in Fig. 3. The change in time lag varied from 0.4% to 6.7% of the breathing period (mean and standard deviation of 2.1% ± 1.4%).
We also separated the inhalation and exhalation portions of the internal and external signal of all the patients to see the correlation in scatter plot, and the range of R2 correlation coefficient in inhalation and exhalation phase of the patients was found to be 0.58–0.99 with the median value of 0.93. A scatter plot of exhalation and inhalation of internal and external signal for one of the patients with maximum time lag of 0.36 s is shown in Fig. 4. R2 correlation coefficient of the patient with maximum time lag, in inhalation and exhalation phase, is 0.73 and 0.83, respectively. Note that hysteresis is evident owing to the time lag, that is, internal positions corresponding to the same external position are different between inhalation and exhalation.
FIG. 4.
Scatter plot of internal vs external position for a patient (Patient P1) with maximum time lag.
4. DISCUSSION
The observation that the time lag between internal and external signal remains small over a single treatment session implies that there is a strong correlation between internal and external signals. As seen in Fig. 1, the external signal does not consistently lag or lead the internal signal during a given scan. However, when averaged over all the breathing cycles for each patient, the external signal lagged internal in 17 out of 18 scans. Since we used the same time stamp and image to extract both internal and external signals, the observed time lag is not due to the system error. Since the tumor motion inside the body occurs slightly before the motion of RPM block placed on the abdomen due to respiration, it may partly explain why external signal lags internal signal. However, determining whether there is a biomechanical basis for this is beyond the scope of this study.
Our study demonstrates that the change in time lag over the duration of a single treatment is small relative to both gate width and breathing period, which implies that the time lag between internal and external signal is almost constant over a treatment session of less than 30 min. We have studied only pancreatic and GEJ cancer patients, whereas results may be different for other tumor sites. In gated radiation therapy, which assumes that there is a good external–internal correlation and predicts tumor position from external signal, it is essential to establish a reliable temporal relationship between internal and external signal. Since the time lag is almost constant within a treatment session, our study indicates that measurement of the time lag at the beginning of the treatment session, such as using a short fluoroscopic exposure in the treatment room at the start of each treatment, is sufficient in gated radiation therapy in the abdomen. In contrast to other studies that examined interfractional changes in time lag,17,18 we have investigated the change in time lag between internal and external signal within a treatment session and found minimal effect of changes in time lag on the external prediction error. It was also observed that larger time lag does not necessarily lead to larger external prediction error since the latter is the product of both time lag and breathing velocity. For example, in Fig. 2, time lag in patient G1 is larger than in G5 (0.23 vs 0.09 s), but because breathing velocity in G1 is smaller (0.19 vs 1.73 cm/s), the resultant external prediction error is smaller (0.44 vs 1.6 mm). Thus, considering time lag alone introduces small (∼1 mm) uncertainties in predicting tumor position in our data sample. Since our study examined only a single fraction for each patient, it provides no information on how frequently time lag should be measured to monitor interfractional changes.
Other factors can contribute to errors in external prediction of tumor position. Ge et al.15 reported that intrafraction changes in external–internal correlation affected gating accuracy in approximately 19% of treatment sessions of abdominal patients, but they did not specifically investigate time lag as a contributing source of such changes. In one case, they identified that change in correlation was caused by drift of fiducial position relative to the external signal. In our study, patient setup was often corrected using couch adjustments after the pretreatment CBCT scan. As a result, couch positions differed between pre- and post-treatment CT scans, which precluded measurement of internal-external positional drift. Time lag in combination with tumor motion in the AP and left–right (LR) directions can also contribute to external prediction error. We can estimate this contribution by measuring the AP and LR motion extent (end expiration to end inspiration) from the RCCT scans acquired at simulation of the patients in this study. We find that the 3D motion extent (quadrature sum of SI, AP, and LR) is between 4% and 23.8% larger relative to the SI component alone in this patient cohort. Thus, we estimate that external prediction error for 3D internal motion is larger than SI motion by similar amounts.
5. CONCLUSION
The findings of a study of nine abdominal cancer patients shows that within a single treatment session, the time lag between internal fiducial motion in the superior–inferior direction, and a one-dimensional external motion signal of abdominal displacement, is small relative to the gate widths used in radiation treatments of these patients. The intrafractional change in time lag is also small compared to the gate width in all patients. This study suggests that a single measurement is adequate to establish internal–external correlation at the start of a gated treatment session in abdominal tumors. These findings require confirmation in a larger number of patients and in different tumor sites.
ACKNOWLEDGMENTS
This research was supported in part by Award No. R01 CA126993 from the National Cancer Institute, and by a research grant from Varian Medical Systems. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
REFERENCES
- 1.Keall P. J., Mageras G. S., Balter J. M., Emery R. S., Forster K. M., Jiang S. B., Kapatoes J. M., Kubo H. D., Low D. A., Murphy M. J., Murray B. R., Ramsey C. R., van Herk M. B., Vedam S. S., Wong J. W., and Yorke E., “The management of respiratory motion in radiation oncology, Report of AAPM Task Group 76,” Med. Phys. 33, 3874–3900 (2006). 10.1118/1.2349696 [DOI] [PubMed] [Google Scholar]
- 2.Kubo H. D. and Hill B. C., “Respiration gated radiotherapy treatment: A technical study,” Phys. Med. Biol. 41, 83–91 (1996). 10.1088/0031-9155/41/1/007 [DOI] [PubMed] [Google Scholar]
- 3.Vedam S. S., Keall P. J., Kini V. R., and Mohan R., “Determining parameters for respiration-gated radiotherapy,” Med. Phys. 28, 2139–2146 (2011). 10.1118/1.1406524 [DOI] [PubMed] [Google Scholar]
- 4.Ford E. C., Mageras G. S., Yorke E., Rosenzweig K. E., Wagman R., and Ling C. C., “Evaluation of respiratory movement during gated radiotherapy using film and electronic portal imaging,” Int. J. Radiat. Oncol., Biol., Phys. 52, 522–531 (2002). 10.1016/S0360-3016(01)02681-5 [DOI] [PubMed] [Google Scholar]
- 5.Shirato H., Shimizu S., Kunieda T., Kitamura K., van Herk M., Kagei K., Nishioka T., Hashimoto S., Fujita K., Aoyama H., Tsuchiya K., Kudo K., and Miyasaka K., “Physical aspects of a real-time tumor-tracking system for gated radiotherapy,” Int. J. Radiat. Oncol., Biol., Phys. 48, 1187–1195 (2000). 10.1016/s0360-3016(00)00748-3 [DOI] [PubMed] [Google Scholar]
- 6.Tang X., Sharp G. C., and Jiang S. B., “Fluoroscopic tracking of multiple implanted fiducial markers using multiple object tracking,” Phys. Med. Biol. 52, 4081–4098 (2007). 10.1088/0031-9155/52/14/005 [DOI] [PubMed] [Google Scholar]
- 7.Schweikard A., Glosser G., Bodduluri M., Murphy M. J., and Adler J. R., “Robotic motion compensation for respiratory movement during radiosurgery,” Comput. Aided Surg. 5, 263–277 (2000). 10.3109/10929080009148894 [DOI] [PubMed] [Google Scholar]
- 8.Ionascu D., Jiang S. B., Nishioka S., Shirato H., and Berbeco R. I., “Internal-external correlation investigations of respiratory induced motion of lung tumors,” Med. Phys. 34, 3893–3903 (2007). 10.1118/1.2779941 [DOI] [PubMed] [Google Scholar]
- 9.Vedam S. S., Kini V. R., Keall P. J., Ramakrishnan V., Mostafavi H., and Mohan R., “Quantifying the predictability of diaphragm motion during respiration with a noninvasive external marker,” Med. Phys. 30, 505–513 (2003). 10.1118/1.1558675 [DOI] [PubMed] [Google Scholar]
- 10.Mageras G., Yorke E., Rosenzweig K., Braban L., Keatley E., Ford E., Leibel S. A., and Ling C. C., “Fluoroscopic evaluation of diaphragmatic motion reduction with a respiratory gated radiotherapy system,” J. Appl. Clin. Med. Phys. 2, 191–200 (2001). 10.1120/1.1409235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoisak J. D. P., Sixel K. E., Tirona R., Cheung P. C. F., and Pignol J. P., “Correlation of lung tumor motion with external surrogate indicators of respiration,” Int. J. Radiat. Oncol., Biol., Phys. 60, 1298–1306 (2004). 10.1016/j.ijrobp.2004.07.681 [DOI] [PubMed] [Google Scholar]
- 12.Tsunashima Y., Sakae T., Shioyama Y., Kagei K., Terunuma T., Nohtomi A., and Akine Y., “Correlation between the respiratory waveform measured using a respiratory sensor and 3D tumor motion in gated radiotherapy,” Int. J. Radiat. Oncol., Biol., Phys. 60, 951–958 (2004). 10.1016/j.ijrobp.2004.06.026 [DOI] [PubMed] [Google Scholar]
- 13.Koch N., Liu H. H., Starkschall G., Jacobson M., Forster K., Liao Z., Komaki R., and Stevens C. W., “Evaluation of internal lung motion for respiratory-gated radiotherapy using MRI: Part I–Correlating internal lung motion with skin fiducial motion,” Int. J. Radiat. Oncol., Biol., Phys. 60, 1459–1472 (2004). 10.1016/j.ijrobp.2004.05.055 [DOI] [PubMed] [Google Scholar]
- 14.Liu H. H., Koch N., Starkchall G., Jacobson M., Forster K., Liao Z., Komaki R., and Stevens C. W., “Evaluation of internal lung motion for respiratory-gated radiotherapy using MRI: Part II–Margin reduction of internal target volume,” Int. J. Radiat. Oncol., Biol., Phys. 60, 1473–1483 (2004). 10.1016/j.ijrobp.2004.05.054 [DOI] [PubMed] [Google Scholar]
- 15.Ge J., Santanam L., Yang D., and Parikh P. J., “Accuracy and consistency of respiratory gating in abdominal cancer patients,” Int. J. Radiat. Oncol., Biol., Phys. 85, 854–861 (2013). 10.1016/j.ijrobp.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 16.Gierga D. P., Brewer J., Sharp G. C., Betke M., Willett C. G., and Chen G. T. Y., “The correlation between internal and external markers for abdominal tumors: Implication for respiratory gating,” Int. J. Radiat. Oncol., Biol., Phys. 61, 1551–1558 (2005). 10.1016/j.ijrobp.2004.12.013 [DOI] [PubMed] [Google Scholar]
- 17.Redmond K. J., Song D. Y., Fox J. L., Zhou J., Rosenzweig C. N., and Ford E., “Respiratory motion changes of lung tumors over the course of radiation therapy based on respiration-correlated four-dimensional computed tomography scans,” Int. J. Radiat. Oncol., Biol., Phys. 75, 1605–1612 (2009). 10.1016/j.ijrobp.2009.05.024 [DOI] [PubMed] [Google Scholar]
- 18.Korreman S. S., Nøttrup T. J., and Boyer A. L., “Respiratory gated beam delivery cannot facilitate margin reduction, unless combined with respiratory correlated image guidance,” Radiother. Oncol. 86, 61–68 (2008). 10.1016/j.radonc.2007.10.038 [DOI] [PubMed] [Google Scholar]
- 19.Regmi R., Lovelock D. M., Hunt M., Zhang P., Pham H., Xiong J., Yorke E. D., Goodman K. A., Rimner A., Mostafavi H., and Mageras G. S., “Automatic tracking of arbitrarily shaped implanted markers using kilovoltage projection images: A feasibility study,” Med. Phys. 41, 071906 (11pp.) (2014). 10.1118/1.4881335 [DOI] [PMC free article] [PubMed] [Google Scholar]