Abstract
This study investigated the impact of infant maltreatment on juvenile rhesus monkeys’ behavioral reactivity to novel stimuli and its associations with amygdala volume. Behavioral reactivity to novel stimuli of varying threat intensity was measured using Approach/Avoidance (AA) and Human Intruder (HI) tasks. In vivo magnetic resonance imaging (MRI) was used to measure amygdala volume. Interestingly, group behavioral differences were context-dependent. When exposed to a human intruder, maltreated subjects displayed more anxious behaviors than controls; however, when presented with fear-evoking objects, maltreated animals exhibited increased aggression and a shorter latency to inspect the objects. Finally, under testing conditions with the lowest levels of threat (neutral novel objects) maltreated animals also showed shorter latencies to inspect objects, and reduced avoidance and increased exploration compared to controls. This suggests alterations in threat assessment and less behavioral inhibition in animals with early adverse experience compared to controls. Some of these behavioral responses were associated with amygdala volume, which was positively correlated with abuse rates received during infancy, particularly reflecting a relationship with exploration, consistent with previous studies.
Keywords: Nonhuman primates, rhesus monkey, early life stress, infant maltreatment, brain development, behavioral reactivity, amygdala
Childhood maltreatment is associated with psychopathology, including anxiety, mood and conduct disorders, impulsivity, and aggression (Cohen, Brown, & Smailes, 2001; Manly, Kim, Rogosch, & Cicchetti, 2001). Although internalizing symptoms (e.g. anxiety, negative mood, and social withdrawal) have been reported in children and adult victims of childhood maltreatment (Keiley, Howe, Dodge, Bates, & Pettit, 2001; Manly et al., 2001; Young, Abelson, Curtis, & Neese, 1997), other studies report that maltreatment is more likely associated with externalizing problems (e.g. aggression, impulsivity, conduct disorders, delinquency) or comorbid internalizing/externalizing behaviors (Cicchetti & Rogosch, 2001; Manly et al., 2001; Stouthamer-Loeber, Loeber, Horrish, & Wei, 2001; McMillen et al., 2005). Alterations in threat perception are hypothesized to underlie some of these externalizing symptoms. For example, physically abused children are hyper-vigilant to aggressive stimuli, more likely to believe others will behave aggressively toward them, and to respond aggressively when confronted with ambiguous social situations (Dodge, Lochman, Harnish, Bates, & Pettit, 1997; Price & Glad, 2003).
Infant maltreatment not only occurs in humans, but also in nonhuman primates (Brent, Koban, & Ramirez, 2002; Johnson, Kamilaris, Calogero, Gold, & Chousos, 1996; Maestripieri, 1998; Sanchez, 2006; Troisi & D’Amato, 1983), with prevalence rates of 2-5% reported in macaque species (Maestripieri, 1999). In these studies maternal physical abuse is operationalized as violent behaviors, such as crushing, dragging, or throwing the infant (Maestripieri, 1998; Troisi & D’Amato, 1983), that cause intense infant distress and are very different from both the aggressive repertoire of these species and the bites and slaps exhibited during conflicts related to weaning. Infant abuse is exhibited mostly during the first 3 postnatal months and repeated with successive offspring (Maestripieri, 1998; McCormack, Sanchez, Bardi, & Maestripieri, 2006; Sanchez, 2006). It has a higher prevalence among related females, suggesting intergenerational transmission along the maternal line (Maestripieri, Tomaszycki, & Carroll, 1999; Maestripieri et al., 2006). Therefore, the spontaneous occurrence of infant maltreatment in these nonhuman primate species provides a unique opportunity to use a naturalistic animal model to investigate the behavioral, physiological, and neurobiological effects of maternal maltreatment in longitudinal studies with strong experimental control.
Previous studies in rhesus monkeys have demonstrated that, comparable to human children, abusive parenting leads to infant socioemotional alterations, including increased emotional reactivity (tantrums and screams), delayed independence from the mother, and reduced levels of play (Maestripieri & Carroll, 1998; Maestripieri, Jovanovic, & Gouzoules, 2000; McCormack, et al., 2006; McCormack, Newman, Higley, Maestripieri, & Sanchez, 2009). The neurobiological underpinnings of these behavioral consequences are only beginning to be understood and are a focus of this study, which examined whether the emotional alterations reported during infancy persist during the juvenile, prepubertal period and are associated with developmental changes in amygdala volume, a brain structure important for emotional regulation (Phelps & LeDoux, 2005; Quirk & Beer, 2006) and very sensitive to early experiences and stress (Landers & Sullivan, 2012; McEwen, 2012; Pechtel & Pizzagalli, 2011; Tottenham et al., 2010). The study of the juvenile and adolescent periods is critical developmentally because of significant socioemotional changes in this species, including affiliative (Ehardt & Bernstein, 1987) and aggressive behaviors (Bernstein & Ehardt, 1985) that result in intense peer-directed activities that become increasingly gender-specific with age and involve sequences of behaviors that mimic adult social interactions (see Suomi, 2005 for review). The juvenile period includes the prepubertal period and onset of puberty (typically reached around 3 years in females and 4 years in males - Suomi, 2005; van Wagenen & Catchpole, 1956; Wilson, Walker, & Gordon, 1983), developmental transitions where an increased vulnerability to stressors has also been reported in humans (Spear, 2000), and the incidence of psychopathology increases (Forbes, Williamson, Ryan, & Dahl, 2004), particularly in children with previous exposure to adversity (Gunnar & Vazquez, 2006).
The neurobiological changes that underlie maltreatment-related behavioral alterations are not clearly understood. Limbic regions that play critical roles regulating socioemotional behavior impacted by early adversity, particularly the amygdala, are likely candidates. Magnetic resonance imaging (MRI) studies have shown both increases and decreases in the volumes of the amygdala, and other limbic brain regions including the hippocampus and prefrontal cortex (PFC) in adults with histories of maltreatment (Bremner, 2002, 2003, 2006; Dannlowski et al., 2012; Teicher, Anderson, & Polcari, 2012; van Harmelen et al., 2010). Studies in children and adolescents exposed to early adversity also report inconsistent alterations in amygdala volume, some reporting larger and others reporting smaller volumes (see Tottenham & Sheridan 2010 for review; Tottenham et al. 2010; Hanson, Nacewicz et al. 2014), as well as more diffuse neural alterations including reductions in temporal, frontal, and parietal cortical gray matter (GM) volumes and decreased corpus callosum (CC) and white matter (WM) volumes (De Brito et al., 2013; Edmiston et al., 2011; Hanson et al., 2010; Hanson et al., 2012; Teicher et al., 2003). These inconsistencies could be explained by differences between studies in developmental timing of the experience and of the measures, or to the possibility that early life stress-related effects on amygdala volume may only be detected later in life, after adolescence (Nelson, 2013; Tottenham & Sheridan, 2010).
Given these inconsistencies and questions in the human literature, particularly during development, the goal of this study was to examine the persistent effects of infant maltreatment on emotional reactivity and its potential relation to alterations in amygdala volume during the juvenile period using a nonhuman primate model where the adverse experience takes place specifically during the first few postnatal months. For this we used standardized laboratory tasks (the Human Intruder Paradigm - Kalin & Shelton, 1989- and the Approach/Avoidance Test - Machado, Kazama & Bachevalier, 2009; Meunier, Bachevalier, Murray, Malkova, & Mishkin, 1999) that evoke strong and distinct behavioral responses to novel stimuli of varying degrees of threat in rhesus monkeys, including defensive responses reflecting underlying states of fear and anxiety, as well as aggressive and submissive responses, and exploration. These are unconditioned responses present very early in life which are stable throughout development (Kalin, Shelton, & Takahashi, 1991). Furthermore, the behavioral responses to these laboratory tasks and the underlying neural circuits have been extensively studied and are regulated by amygdala circuits (Kalin & Shelton, 2001; Kalin, Shelton, Fox, Oakes, & Davidson, 2007; Meunier et al., 1999; Prather et al., 2001). We hypothesized that early adverse experience would result in persistent increased emotional reactivity during the juvenile period. Specifically, based on the human literature cited above, we predicted that abused subjects would show more impulsive and aggressive behavior, in parallel to higher levels of fear and anxiety, than controls. We also hypothesized that these behavioral effects would be related to alterations in amygdala volume, based on prior work supporting the role of the amygdala in emotional reactivity, particularly during these tasks (Machado et al., 2009; Meunier et al., 1999; Raper, Wilson, Sanchez, Machado & Bachevalier, 2013). Although preclinical studies of chronic and early life stress report alterations in amygdala structure and function (Sanchez, Ladd & Plotsky, 2001) often involving increases in amygdala volume (McEwen & Gianoros 2010), the inconsistent effects reported in human maltreated populations make it difficult to make directional volumetric predictions.
METHODS
Subjects and Housing
This study was conducted at the Yerkes National Primate Research Center (YNPRC) Field Station, Emory University (Lawrenceville, GA). The subjects were 20 juvenile rhesus macaques (Macaca mulatta) living in four large social groups consisting of 2-3 adult males and 18-49 adult females with their sub-adult and juvenile offspring. The groups were housed in outdoor compounds with adjacent indoor housing areas. Water was available ad libitum and monkey chow and fresh fruit/vegetables and enrichment items were provided twice daily. All studies were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Emory University Institutional Animal Care and Use Committee (IACUC).
The studies were conducted when subjects were juveniles (behavioral testing: at 2 years, ranging 26-28 months of age; MRI scans: at 4 years, ranging 48-55 months of age). None of the subjects had reached puberty prior to behavioral testing. Ten of the subjects (6 females, 4 males) experienced maternal physical abuse early in infancy (Abused group; see operational definition below and in McCormack et al., 2006). The other 10 subjects (6 females, 4 males) were non-abused controls. Controls were matched to abused subjects by sex, age, mother’s dominance rank and, whenever possible, social group of origin. All mothers were multiparous females (average of prior offspring: 6 for abusive group, range = 3-12, 6.2 for controls, range = 2-12). In order to avoid confounding effects of heritability on our measures, the adult females were selected from different matrilines (i.e. they were unrelated).
Following behavioral definitions, observation protocols and inclusion/exclusion criteria described in detail for this species in previous publications (e.g. Maestripieri, 1998; McCormack et al., 2006; McCormack et al., 2009; Troisi & D’Amato, 1983), infants were classified as abused if the mother was observed exhibiting at least 3 instances of the following violent, aberrant, behaviors towards the infant during its first 3 months of life: (1) dragging infant by its tail or leg while walking or running; (2) crushing infant against the ground with hands; (3) throwing infant with one hand while standing or walking; (4) stepping or sitting on infant; (5) rough grooming: restraining infant and pulling its hair causing distress calls; (6) abusive carry: carrying infant with one arm away from her body. As reviewed above, these behaviors caused distress in the infants, who experienced an average of 1.5 abuse events per hour during their first month of life (McCormack et al., 2006).
Experimental Procedures
Prior to this study, mother-infant pairs were captured once per month during the first 6 months of life and every 6 months thereafter for blood sampling and to undergo testing. All mothers were trained to move on command from the outdoor corral into the indoor area, where they were transferred to a squeeze cage via a transfer box carrying their infants until the infants were 12 months old. After 12 months, juveniles were trained to go from the outdoor corral to the indoor area without their mothers and to transfer into a transport box for transport to the testing room, in less than 10 minutes.
Prior to testing, subjects were habituated to the testing room and cage 3 times per week for 20-min sessions for two weeks. During habituation, a food reward (jelly bean) was placed on top of the plexiglass testing box (without objects inside it) so that subjects learned that there was a positive reward available for approaching the testing box. All subjects retrieved and consumed the food reward during habituation sessions. A digital camera was set up 2 meters from the testing cage to videotape the subjects during testing.
After habituation, subjects (26-28 mos old) were tested with the Approach/Avoidance Test and Human Intruder Paradigm in separate sessions, 3 days apart. The order of the tasks was counterbalanced to control for test order effects.
Approach/Avoidance Tests (AA: Neutral and Fear-Evoking Objects)
On the day of testing, each subject was transported to the testing room and placed in the testing cage with the plexiglass box (30×30 ×30 cm) attached to it. Subjects were able to reach through a small hole in the testing cage to manipulate and bite items in the box, but could not retrieve them. During two separate sessions, 3 days apart, and following a 2 min habituation to the testing room and apparatus, the subject was presented with either 6 neutral/positive or 6 fear-evoking objects placed inside the plexiglass box, for 5 min each, with a positive food reward (jelly bean) on the top of the box. Objects in each test were presented in the same order across subjects. The Approach/Avoidance task is designed to measure the conflict between exploratory behaviors and behavioral inhibition in response to novel objects of varying threat intensities (Machado et al., 2009; Meunier et al., 1999; Williamson et al., 2003). Objects were chosen based on characteristics previously shown to evoke fearful or defensive responses in this species in previous studies (Williamson et al., 2003). For the Neutral Object Test, a roll of construction tape, a rubber ball, a piece of kiwi, a plastic cup, a toy train, and a baby rattle were presented in the order listed, for 5 min each. During the Fear Object Test, subjects were presented with a toy frog with large eyes, a toy dinosaur with teeth, a battery-operated pig that moved and “oinked”, a plastic owl with large eyes presented with playback of predatory hawk calls, a rubber snake, and a mirror (to simulate the presence of a social intruder), in the order listed, for 5 min each.
Human Intruder Paradigm (HI)
The HI paradigm (Kalin & Shelton, 1989) was used to assess emotional reactivity (including fearful, anxious and aggressive behaviors) to an unfamiliar human under 3 different and consecutive conditions, 10 min each, that pose different degrees of threat to rhesus monkeys: (1) Alone condition (no human intruder), (2) moderately threatening Profile condition (no eye contact: NEC), and (3) a threatening Stare condition (human makes direct eye contact with the monkey). On the day of testing, the subject was transferred to the testing room and placed in the testing cage, where they first stayed alone; this Alone condition elicits distress responses (vocalizations, locomotion) and exploration. Next, for the NEC, the intruder entered the room and stood next to the digital camera, 3 m from the cage, presenting his/her profile to the monkey. During this condition rhesus monkeys typically stop vocalizing and become behaviorally inhibited (freeze) while scanning the environment/intruder. After this, the intruder left the room. Upon return (2 min after) the intruder stood in the same place as in the profile condition, but facing the monkey and making direct eye contact with the animal (Stare condition). Direct eye contact is a threatening behavior for rhesus macaques, which typically stop freezing and display aggressive or submissive behaviors towards intruder.
All sessions were videotaped for later scoring of the following behaviors by trained coders (>90% agreement): latency to eat food reward (jelly bean) and to inspect/touch/bite each object in the AA tests, fearful and anxious behaviors, aggressive and submissive behaviors, vocalization, exploration, and locomotion. See Suppplemental Material (Table 1) for the detailed ethogram, operational definitions of behaviors coded, and the scoring method.
MRI Acquisition and Analysis
T1-MRI scans were acquired when the animals were 4 years old (range: 48-55 mos; mean±SEM scan ages: maltreated animals=51.99±0.6 mos, controls=51.98±0.57 mos). The scanning age was not different between Control and Abused animals, as described in Results. Images were acquired on a 3T Siemens Trio scanner (Malvern, PA) at the YNPRC Imaging Center using a transmit and receive volume coil and a magnetization prepared rapid gradient echo (MPRAGE) sequence with the following parameters: TI/TR/TE=950/3000/3.3ms; 4 averages; voxel size: 0.6 mm3 isotropic (Howell et al., 2013).
Automatic segmentation of the amygdala was performed using an atlas-based approach in which a population-based atlas was first non-linearly registered into each subject’s native space using Advanced Normalization Tools (ANTs) and the AugoSeg software (Avants, Epstein, Grossman, & Gee, 2008; Styner et al., 2007). These warp fields were then applied to a manual segmentation of the amygdala. Automatic segmentations of the amygdala were manually adjusted to ensure neuroanatomical accuracy by a rater blind to experimental groups and guided by anatomical criteria in the Saleem and Logothetis macaque brain atlas (Saleem & Logothetis, 2012) and anatomical landmarks for the macaque amygdala (Amaral & Bassett, 1989; Price, Russcher, & Amaral, 1987), with the hippocampus as the posterior boundary, the rostral periamygdaloid cortex as the anterior boundary, the white matter and cerebral spinal fluid (CSF) as the ventrolateral boundaries, and the rhinal fissure defining the ventromedial border.
Data Analysis
For low occurrence behavioral categories, behavior that was not performed by all animals was transformed from durations and frequencies to categorical data (“yes” or “no”) based on whether or not each animal performed the behavior. Thus, latencies, exploration, locomotion, inspect, manipulate, avoid during Stare condition, and freeze during Profile condition were analyzed as frequencies (number of occurrences per minute) and durations (proportion of entire test), while all other behaviors were transformed to categorical data. The categorical data was analyzed using Chi-square tests and reported as likelihood ratios with Fisher’s exact p values for group effects and Breslow-Day values for group by sex effects, due to their higher stringency. Duration and frequency data were analyzed with repeated measures ANOVAs, with each AA test type (Neutral Object vs. Fear Object Test) and HI test condition (Alone vs. Profile vs. Stare) as the repeated factor, and group (Control vs. Abused) and sex as fixed factors. Tukey’s HSD post-hoc tests were used following significant F values.
A Student’s t-test was used to examine potential group differences in total brain volume to rule out its confounding effect on amygdala size. Amygdala volume was analyzed using a repeated measures MANOVA with hemisphere (left vs. right) as the repeated measure and group (Control vs. Abused) as fixed factor, and total brain volume as a covariate. Spearman Rank order correlations were conducted to determine the associations between behavioral data where group differences were detected (frequency & duration) and amygdala volume, after controlling for total brain size and group effects. Data was analyzed using SPSS. Significant effects were set at p<0.05 level for all analyses (two-tailed).
RESULTS
Behavioral Results (Laboratory Paradigms)
Approach/Avoidance Tests (Neutral and Fear-Evoking Objects)
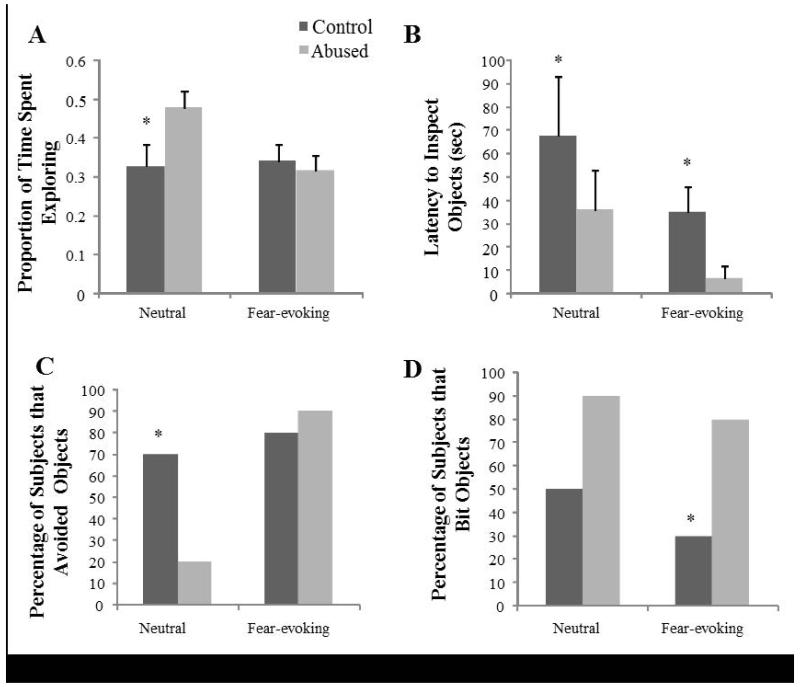
There was a significant test effect (F (1, 14) = 5.946, p = 0.028, ) and test by group interaction effect (F (1, 14) = 7.942, p = 0.014, ) for exploration during the Approach/Avoidance Tests. The proportion of time spent exploring was greater during the Neutral (M = 0.429, SE = 0.036) than during the Fear Object Test (M = 0.349, SE = 0.028). As shown in Figure 1A, abused subjects spent a greater proportion of time exploring (M = 0.478, SE = 0.045) than control subjects (M = 0.327, SE = 0.058) during the Neutral Object Test (t (1, 17) = 2.253, p = 0.040), but not during the Fear Test (Abused: M = 0.339, SE = 0.0444; controls: M = 0.3169, SE = 0.038; t (1, 17) = 0.109, p = 0.915). There was also a main group effect for the latency to visually inspect objects (F (1, 14) = 5.442, p = 0.033, ), so that abused subjects had a shorter latency (M = 21.457sec, SE = 10.897) compared to controls (M = 57.408sec, SE = 10.897), independently of whether they were neutral or fear-evoking (see Figure 1B). A group effect was also detected for proportion of time spent inspecting objects visually, independent of object valence, (F (1, 14) = 6.700, p = 0.021, ), with abused subjects spending a larger proportion of time (M = 0.058, SE = 0.006) visually inspecting objects than controls M = 0.035, SE = 0.006). There were also test effects for the latency to eat the food reward (F (1, 14) = 12.987, p = 0.002, ), latency to bite objects (F (1, 14) = 29.291, p < 0.001, ), and the proportion of time inspecting objects (F (1, 14) = 25.430, p < 0.001, ). Thus, the latencies to eat the food reward and bite object were shorter for neutral objects (M = 122.648sec, SE = 18.104; M = 170.160sec, SE = 21.572, respectively) than for fear-evoking objects (M = 178.425sec, SE = 23.944; M = 276.246sec, SE = 9.582, respectively). Despite their group, subjects also spent a greater proportion of time inspecting (visually, without touching) fear-evoking objects (M = 0.074, SE = 0.009) than neutral objects (M = 0.019, SE = 0.004).
Figure 1. Behavioral responses to the Approach/Avoidance Tests.
(A) Exploration: Abused subjects spent a greater proportion of time exploring during the Neutral Object Test (t (1, 17) = 2.253, p= 0.040). (B) Latency to inspect the objects: Abused subjects had a shorter latency to inspect objects (F (1, 14) = 5.442, p= 0.033). (C) Percentage of animals that avoided objects: A smaller percentage of abused subjects avoided the neutral objects (χ2 = 5.3, p = 0.04). (D) Percentage of animals that bit objects: A larger percentage of abused animals bit the fear-evoking objects (χ2 = 5.3, p = 0.04).
For the categorical data, there was a significant group effect for avoidance of neutral objects (χ2 = 5.3, p = 0.04; Figure 1C), with a smaller percentage of abused subjects (20%) avoiding the neutral objects compared to control subjects (70%). For the Fear Object Test, there was also a group effect for biting objects (χ2 = 5.3, p = 0.04; Figure 1D), such that a larger percentage of abused animals (80%) bit the fear-evoking objects compared to control animals (30%).
There were no group, group by sex, or group by sex by test effects for proportion of time spent visually inspecting and manipulating objects or for latencies to manipulate objects, bite objects, or eat the food reward (p > 0.05), or in the number of control and abused subjects that performed the following behaviors: freezing, withdrawal, grimace, coo, displacement behavior, agitation, threat, lipsmack, exploration of box, inspect/manipulate object, or locomotion (p > 0.05).
Human Intruder Paradigm
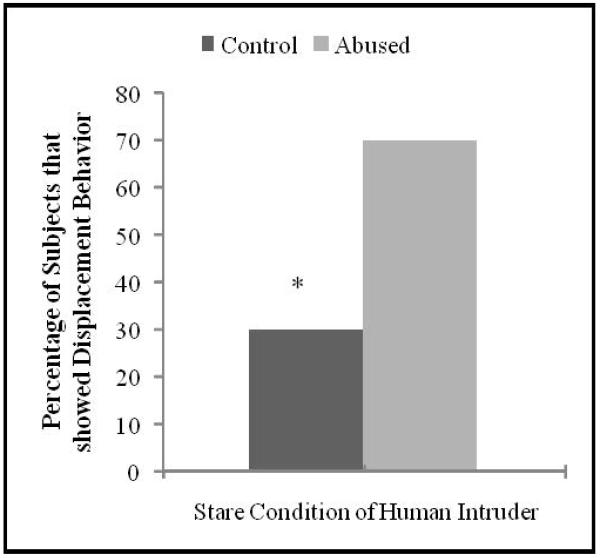
There was a group effect for displacement behavior during the Stare condition of the HI Paradigm (χ2 = 4.54, p = 0.05), with a larger percentage of abused subjects (77.78%) showing displacement/anxious behavior (yawn, scratch, and body shake) compared to controls (30%; see Figure 2). Of the abused animals 30% exhibited both anxious behavior during the Stare condition and aggressive behavior in response to the fear object, while none of the controls showed this co-occurrence.
Figure 2. Behavioral responses during the Human Intruder Paradigm.
A larger percentage of abused subjects showed displacement/anxious behavior (yawn, scratch, and body shake) during the Stare condition, compared to control subjects (χ 2 = 4.54, p = 0.05).
Condition effects, characteristic of the HI paradigm were also found. Thus, freezing and threatening behavior varied across conditions (F (2, 30) = 307.078, p <0.001, ; F (2, 30) = 36.616, p <0.001, , respectively). Subjects froze more during the NEC (M = 0.843, SE = 0.045) than during the Alone (M = 0.068, SE = 0.030) or Stare conditions (M = 0.028, SE = 0.019; t (1, 18) = 17.613, p < 0.001; t (1, 18) = 12.849, p < 0.001, respectively). Subjects also threatened more during the Stare (M = 8.482occurences/minute, SE = 1.946) than during the Alone or NEC conditions (M = 0.0 occurences/minute, SE = 0.0, for Alone and NEC; t (1, 18) = 6.854, p < 0.001).
There were no main group, interaction or condition effects for duration of freezing, avoiding, exploration, locomotion or inspection (p > 0.05). There were no group effects either, in agitation, lipsmacks, coos, or threats. No passive, grimace, or withdraw behaviors were observed.
Total Brain and Amygdala Volumes
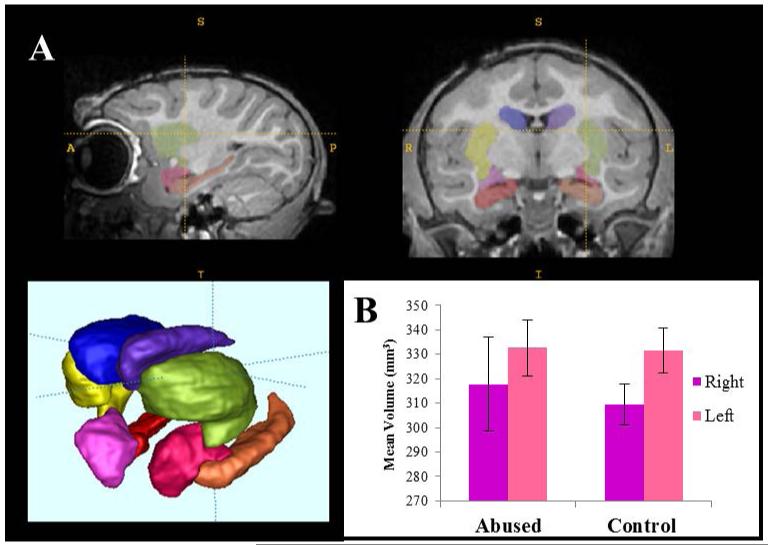
There were no differences in scanning age between abused and control animals (t(17)=0.007, p=0.99). No difference in total brain volume was detected, either, between abused (M=96766.48 mm3, SE=2484.83) and control subjects (M= 93031.43 mm3, SE=2676.89) (t(17)= −1.02, p=0.32). No significant effects of group (F (1,16)=0.002, p=0.961, ) or hemisphere (F(1,16)=0.241, p=0.63, ) were detected on amygdala volume (abused: right, M = 317.78 mm3, SE = 19.29, left M = 332.72 mm3, SE = 11.43; and control: right M = 309.43 mm3, SE = 8.56, left M = 331.60 mm3, SE = 9.25). And, finally, there was no group effect on the ratio of right/left amygdala volumes, either.
Correlations: Amygdala Volume, Behavioral Reactivity, and Infant Abuse Rates
Correlations were calculated controlling for both group (due to the group differences in behavioral responses during the AA and HI described above), and whole brain volume (as commonly done in the literature, see Tottenham and Sheridan 2010 for review) by adding them as covariates in the statistical models. A significant positive correlation between both right and left amygdala volumes and abuse rates received during the first three months of life was detected (right, r=0.70, p=0.01; left, r=0.748, p=0.005). A closer look at the subject plots identified an abused subject that received very high rates of abuse and also had the largest amygdalae volumes (both left and right), above 2 SD from the mean. When this subject was excluded from the analyses no significant correlations were detected.
Correlations between amygdala volume and behavioral reactivity were performed in each group separately. Thus, while in the control group a positive correlation was detected between right amygdala volume and duration of cage exploration –tactile, oral, or visual- during both the Neutral Object Test (r=0.79, p=0.03), and the Fear Object Test (r=0.75, p=0.05), in the abused group a negative correlation was detected instead between right amygdala volume and duration of cage exploration only during the Fear Object Test (r=−0.92, p=0.03).
DISCUSSION
Our findings suggest context-dependent alterations in behavioral reactivity to novel stimuli in juvenile rhesus macaques that were abused by their mothers as infants, and some interesting associations with amygdala volume. Thus, when exposed to a human intruder, maltreated subjects showed more displacement/anxious behaviors than controls; however, when presented with fear-evoking objects, maltreated animals exhibited increased aggression and vigilance (visual inspection) towards the objects. Finally, under testing conditions with the lowest levels of threat (neutral novel objects) maltreated animals showed shorter latencies to inspect objects, and reduced avoidance and increased exploration compared to controls. This suggests alterations in threat assessment and potentially less behavioral inhibition in animals with early adverse experience, in comparison to controls. The seemingly opposite behavioral effects of maltreatment (e.g., increased fear/vigilance in some animals and aggression in others, and co-occurrence of increased fear and aggression in 30% of abused subjects), is interesting because it shows some parallels with reports in human populations (Cicchetti & Rogosch 2001;Manly et al., 2001; McMillen et al., 2005). Some of these behavioral responses, in particular the levels of exploration, were associated with amygdala volume, which was positively correlated with abuse rates received during infancy.
The three laboratory tests elicited very specific and context-dependent behavioral patterns. Similarly to reports in abused children (e.g., Cicchetti & Rogosch 2001), the behavioral differences detected between abused and control subjects were also context-dependent, and support the manifestation of different alterations (increased anxiety/fearfulness or aggression than controls, or co-ocurrence of both), depending on the level of threat and the individual. Thus, when exposed to a low threat level (neutral novel objects), abused subjects spent more time exploring the environment visually, had a shorter latency to inspect the objects, and fewer abused animals avoided the objects than control animals. This could reflect alterations in threat assessment and potentially less behavioral inhibition/fearfulness than in controls under low levels of threat, at least based on reports showing that anxiogenic drugs decrease environmental exploration in this species (e.g., Kalin, Shelton & Turner 1992).
However, when exposed to a higher level of threat (Fear Object Test or HI), a different behavioral pattern emerged. During the Fear Object Test, when juveniles intensify the visual inspection of the objects in comparison to neutral stimuli (consistent with heightened vigilance and previous reports in macaques, Meunier et al., 1999), a greater number of abused subjects exhibited aggression (biting) toward the objects, as well as increased vigilance (i.e. longer visual inspection of objects and shorter latencies) as compared to controls. These results could reflect differences in threat assessment and ability to inhibit aggressive, possibly risky, responses towards potentially dangerous stimuli. They are also consistent with human literature showing that physically abused children are more likely to attend to aggressive cues and respond aggressively, especially in contexts where there is a perceived threat (Dodge et al., 1997; Price & Glad, 2003; Shields & Chicchetti, 1998), which has been partially attributed to these children’s selective attention towards threat-related cues (Pollak & Tolley-Shell, 2003). The behavioral response/strategy we detected is not incompatible, therefore, with an underlying higher fear state.
Consistent with previous studies using the HI Paradigm in this species (Kalin & Shelton, 1989), we found that the NEC condition elicited an increase in freezing behavior, while the Stare condition induced higher levels of aggressive threats across both groups of animals. Kalin and Shelton (1989) proposed that these different behavioral patterns/strategies have adaptive ethological significance in response to potential threats (such as predators). During the Stare condition though, a greater number of abused subjects exhibited displacement behaviors (scratch, yawn, body shake) than controls. Displacement behaviors index anxiety in rhesus monkeys and other primates (Brent et al., 2002; Maestripieri, 1993). They are exhibited by rhesus monkeys in naturalistic conflict situations, increase with stress/threat intensity (Troisi, 2002), and are potentiated by anxiogenic drugs and reduced by anxiolytics (Schino, Perretta,Taglioni, Monaco & Troisi, 1996). A common outcome of child maltreatment is internalizing disorders such as anxiety and depression (Bolger & Patterson, 2001; Cohen et al., 2001; Keiley et al., 2001; Manly et al., 2001; Young et al., 1997); thus, our findings of greater number of abused animals exhibiting anxious behaviors than controls, at least under high levels of threat, is consistent with reports in the human literature. Increased anxiety is, indeed, a common outcome of early life stress in other animal models that involve mother-infant relationship disruption (see Sanchez et al., 2001 for review; Sanchez, Noble, Lyon, Plotsky, Davis, Nemeroff & Winslow, 2005).
Our findings of context-dependent manifestations of behavioral alterations in juvenile abused macaques (higher aggression versus higher anxiety, sometimes co-occurring in the same individuals) support similar views in humans (Buss, Davidson, Kalin, & Goldsmith, 2004) and are consistent with the complex behavioral alterations reported in abused children (e.g., Cicchetti & Rogosch, 2001). Thus, while some studies report internalizing symptoms (e.g. anxiety, negative mood, social withdrawal) in neglected, physically and sexually abused children and adult victims of child abuse (Bolger & Patterson, 2001; Keiley et al., 2001; Manly et al., 2001; Young et al., 1997), other studies report externalizing problems, such as aggression, impulsivity, conduct disorders or comorbid internalizing and externalizing problems (Cicchetti & Rogosch, 2001;Manly et al., 2001; McMillen et al., 2005). Although factors such as type, severity, and timing of maltreatment likely contribute to different behavioral outcomes, it is also possible that specific behavioral manifestations of altered emotional regulation are context-dependent or involve plurifinality of psychopathologies. Interestingly, the seemingly paradoxical effects detected in our study (increased aggression versus increased anxiety/vigilance), and context-dependent increased versus decreased behavioral inhibition/fearfulness has also been reported in rhesus monkeys with amygdala lesions in similar novelty tests (Meunier et al., 1999).
Despite the lack of group differences in amygdala volume in the current study, a positive correlation was found between amygdala volume and abuse rates received during infancy, suggesting that a developmental impact of the early adverse experience on this limbic structure may be emerging during the juvenile period. Although we recognize the disagreement in the literature, this relationship seems consistent with prior reports of increased amygdala volume resulting from early adversity and chronic stress (Tottenham & Sheridan 2010; McEwen & Gianoros 2010). Our findings also support the view that the effects of early life stress on amygdala volume may be not be detected until later in life, after adolescence (Nelson, 2013; Tottenham & Sheridan, 2010). Our group has also reported alterations in white matter microstructural integrity in these same subjects (Howell et al., 2013), particularly in tracts involved in visual processing, emotional regulation and somatosensory and motor integration, similar to brain WM regions affected in maltreated children (Choi, Jeong, Polcari, Rohan, & Teicher, 2009; Jackowski et al., 2008; Paul et al., 2008), suggesting alterations in neural circuits that could underlie the alterations in emotional reactivity reported in the current study.
Interestingly, opposite relationships were found between amygdala volume and behavioral responses to the novel stimuli in the Approach/Avoidance tests for abused versus control subjects. The positive correlation between right amygdala volume and increased time spent exploring in controls in contrast to the negative correlation found in the abused animals could be interpreted as an inverted “U” shape relationship, where the animals with the highest amygdala volumes (i.e. highest abuse rates) show the lowest exploration, or highest behavioral inhibition, commonly interpreted as an underlying fear state (Fox, Henderson, Marshall, Nichols & Ghera, 2005) and consistent with reports of increased right amygdala volume in children with generalized anxiety disorder (De Bellis et al., 2000). Alternatively, this opposite relationship with amygdala volume may, reflect alterations in amygdala function, a view supported by findings in both humans and animal models (Cohen et al., 2013). In summary, these group differences in amygdala-behavior associations suggest that alterations in the right amygdala may underlie the behavioral alterations in threat assessment reported in the current study.
In summary, this study provides evidence that infant maltreatment has long-term, persistent, consequences on the behavioral reactivity of juvenile rhesus monkeys to novel stimuli. When exposed to a human intruder, maltreated subjects displayed more anxious behaviors than controls; however, when presented with fear-evoking objects, maltreated animals exhibited increased aggression and a shorter latency to inspect the objects. Finally, under testing conditions with the lowest levels of threat (neutral novel objects), maltreated animals also showed shorter latencies to inspect objects, and reduced avoidance and increased exploration of stimuli compared to controls. In addition, amygdala volume was associated with rates of abuse received during infancy, suggesting an effect of the early adverse experience on the structural development of this structure. Some of the behavioral responses to novel stimuli where group differences were detected were associated with amygdala volume in both groups of animals, particularly reflecting a relationship with exploration. Although the findings of this study are interesting and provide some explanations and support for contradictory reports in human studies, they also need to be interpreted with caution due to the limitations of our small sample size and the multiple comparisons performed, as well as the presence of outliers for the volumetric data. Further studies are needed to replicate these findings with larger sample sizes and to examine the neurodevelopmental time courses of alterations underlying behavioral effects.
Supplementary Material
Figure 3. Amygdala volumes measured using structural MRI.
(A) Example of automatic segmentation of subcortical structures, including the right (purple) and left (pink) amygdala. (B) No significant differences in amygdala volumes (right, left or right/left ratio) were detected between abused and control animals (uncorrected for brain volume, but similar results detected after correction).
Acknowledgements
We thank Anne Graff and Richelle Scales for technical assistance with experimental procedures and Irwin Bernstein for his help in developing the behavioral ethogram. This research was supported by grants MH62577 and MH63097 (DM) and MH065046 (MMS) from the National Institute of Mental Health (NIMH) and HD055255 from the National Institute of Child Health and Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH, NICHD or the National Institutes of Health. The project was also funded by a NARSAD Young Investigator Award (MMS) and the National Center for Research Resources P51RR165 (Yerkes National Primate Research Center –YNPRC-Base grant) and is currently supported by the Office of Research Infrastructure Programs/OD P51OD11132. The YNPRC is fully accredited by the American for the Assessment and Accreditation of Laboratory Care, International.
Footnotes
Disclosure/Conflict-of-Interest Statement: The authors declare no conflicting financial or other competing interests.
REFERENCES
- Amaral DG, Bassett JL. Cholinergic innervation of the monkey amygdala: an immunohistochemical analysis with antisera to choline acetyltransferase. Journal of comparative Neurology. 1989;281(3):337–361. doi: 10.1002/cne.902810303. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein IS, Ehardt CL. Age-sex differences in the expression of agonistic behavior in rhesus monkey (Macaca mulatta) groups. Journal of Comparative Psychology. 1985;99:115–132. [PubMed] [Google Scholar]
- Bolger KE, Patterson CJ. Pathways from child maltreatment to internalizing problems: Perceptions of control as mediators and moderators. Development and Psychopathology. 2001;13:913–940. [PubMed] [Google Scholar]
- Bremner JD. Neuroimaging of childhood trauma. Semin Clin Neuropsychiatry. 2002;7(2):104–112. doi: 10.1053/scnp.2002.31787. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Long-term effects of childhood abuse on brain and neurobiology. Child & Adolescent Psychiatric Clinics of North America. 2003;12(2):271–292. doi: 10.1016/s1056-4993(02)00098-6. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8(4):445–461. doi: 10.31887/DCNS.2006.8.4/jbremner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent L, Koban T, Ramirez S. Abnormal, abusive and stress-related behaviors in baboon mothers. Biological Psychiatry. 2002;52:1047–1056. doi: 10.1016/s0006-3223(02)01540-8. [DOI] [PubMed] [Google Scholar]
- Buss KA, Davidson RJ, Kalin NH, Goldsmith HH. Context-specific freezing and associated physiological reactivity as a dysregulated fear response. Developmental Psychology. 2004;40:583–594. doi: 10.1037/0012-1649.40.4.583. [DOI] [PubMed] [Google Scholar]
- Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biological psychiatry. 2009;65(3):227–234. doi: 10.1016/j.biopsych.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch F. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001;13:783–780. [PubMed] [Google Scholar]
- Cohen MM, Jing D, Yang RR, Tottenham N, Lee FS, Casey B. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proceedings of the National Academy of Sciences. 2013;110(45):18274–18278. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Brown J, Smailes E. Child abuse and neglect and the development of mental disorders in the general population. Development and Psychopathology. 2001;13:981–999. [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Casey B, Dahl RE, Birmaher B, Williamson DE, Thomas KM, Axelson DA, Frustaci K, Boring AM, Hall J, Ryan ND. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2000;48(1):51–57. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- De Brito SA, Viding E, Sebastian CL, Kelly PA, Mechelli A, Maris H, McCrory EJ. Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. Journal of Child Psychology and Psychiatry. 2013;54(1):105–112. doi: 10.1111/j.1469-7610.2012.02597.x. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Lochman JE, Harnish JD, Bates JE, Pettit GS. Reactive and proactive aggression in school children and psychiatrically impaired chronically assaultative youths. Journal of Abnormal Psychology. 1997;106:37–51. doi: 10.1037//0021-843x.106.1.37. [DOI] [PubMed] [Google Scholar]
- Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, Blumberg HP. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Archives of Pediatrics & Adolescent Medicine. 2011;165(12):1069–1077. doi: 10.1001/archpediatrics.2011.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehardt CL, Bernstein IS. Patterns of affiliation among immature rhesus monkeys (Macaca mulatta) American Journal of Primatology. 1987;13:255–269. doi: 10.1002/ajp.1350130304. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Williamson DE, Ryan ND, Dahl RE. Positive and negative affect in depression: influence of sex and puberty. Annals of the NewYork Academy of Sciences. 2004;1021:341–347. doi: 10.1196/annals.1308.042. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu. Rev. Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology: Developmental Neuroscience. Wiley Press; New York: 2006. pp. 533–577. [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Rudolph KD, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD. Structural variations in prefrontal cortex mediate the relationship between early childhood stress and spatial working memory. The Journal of Neuroscience. 2012;32(23):7917–7925. doi: 10.1523/JNEUROSCI.0307-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J Neurosci. 2010;30(22):7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, Shirtcliff EA, Pollak SD, Davidson RJ. Behavior Problems After Early Life Stress: Contributions of the Hippocampus and Amygdala. Biological Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.04.020. Advance online publication. DOI: http://dx.doi.org/10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BR, McCormack KM, Grand AP, Zhang X, Maestripieri D, Hu X, Sanchez MM. Brain white matter microstructure alterations in adolescent rhesus monkeys exposed to early life stress: associations with high cortisol during infancy. Biology of mood & anxiety disorders. 2013;3(1):21. doi: 10.1186/2045-5380-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski AP, Douglas-Palumberi H, Jackowski M, Win L, Schultz RT, Staib LW, Krystal JH, Kaufman J. Corpus callosum in maltreated children with posttraumatic stress disorder: a diffusion tensor imaging study. Psychiatry Research: Neuroimaging. 2008;162(3):256–261. doi: 10.1016/j.pscychresns.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Calogero AE, Gold PW, Chrousos GP. Effects of early parenting on growth and development in a small primate. Pediatric Research. 1996;39:999–1005. doi: 10.1203/00006450-199606000-00012. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: Environmental cues and neurochemical regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. Journal of Neuroscience. 2001;21:2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Turner JG. Effects of beta-carboline on fear-related behavioral and neurohormonal responses in infant rhesus monkeys. Biological Psychiatry. 1992;31:1008–1019. doi: 10.1016/0006-3223(92)90094-g. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biological Psychiatry. 2007;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus monkeys. Ontogeny and context-dependent selective expression. Child Development. 1991;62:1175–1183. [PubMed] [Google Scholar]
- Keiley MK, Howe TR, Dodge KA, Bates JE, Pettit GS. The timing of child physical maltreatment: A cross-domain growth analysis of impact of adolescent externalizing and internalizing problems. Development and Psychopathology. 2001;13:891–912. [PMC free article] [PubMed] [Google Scholar]
- Landers MS, Sullivan RM. The Development and Neurobiology of Infant Attachment and Fear. Dev Neurosci. 2012;34(2-3):101–114. doi: 10.1159/000336732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Kazama AM, Bachevalier J. Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9:147–163. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D. Maternal anxiety in rhesus macaques (Macaca mulatta). I. Measurement of anxiety and identification of anxiety eliciting situations. Ethology. 1993;95:19–31. [Google Scholar]
- Maestripieri D. Parenting styles of abusive mothers in group-living rhesus macaques. Animal Behavior. 1998;55:1–11. doi: 10.1006/anbe.1997.0578. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. The biology of human parenting: Insights from nonhuman primates. Neuroscience and Behavioral Reviews. 1999;23:411–422. doi: 10.1016/s0149-7634(98)00042-6. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Carroll KA. Risk factors for infant abuse and neglect in group-living rhesus monkeys. Psychological Science. 1998;9:143–145. [Google Scholar]
- Maestripieri D, Jovanovic T, Gouzoules H. Crying and infant abuse in rhesus macaques. Child Development. 2000;71:301–309. doi: 10.1111/1467-8624.00145. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Lindell SG, Higley JD, Newman TK, McCormack KM, Sanchez MM. Early maternal rejection affects the development of monoaminergic systems and adult abusive parenting in rhesus macaques. Behavioral Neuroscience. 2006;120:1017–1024. doi: 10.1037/0735-7044.120.5.1017. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Tomaszycki M, Carroll KA. Consistency and change in the behavior of rhesus macaque abusive mothers with successive infants. Developmental Psychobiology. 1999;34:29–35. [PubMed] [Google Scholar]
- Manly JT, Kim JE, Rogosch FA, Cicchetti D. Dimensions of child maltreatment and children’s adjustment: Contributions of developmental timing and subtype. Development and Psychopathology. 2001;13:759–782. [PubMed] [Google Scholar]
- McCormack KM, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Hormones and Behavior. 2009;55:538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack KM, Sanchez MM, Bardi M, Maestripieri D. Maternal care patterns and behavioral development of rhesus macaque abused infants in the first six months. Developmental Psychobiology. 2006;48:537–550. doi: 10.1002/dev.20157. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Brain on stress: How the social environment gets under the skin. Proceedings of the National Academy of Sciences. 2012;109(Supplement 2):17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen JC, Zima BT, Scott LD, Jr, Auslander WF, Munson MR, Ollie MT, Spitznagel EL. Prevalence of psychiatric disorders among older youths in the foster care system. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44(1):88–95. doi: 10.1097/01.chi.0000145806.24274.d2. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Murray EA, Malkova L, Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. European Journal of Neuroscience. 1999;11:4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- Nelson CA. Biological embedding of early life adversity. JAMA Pediatrics. 2013;167(12):1098–1100. doi: 10.1001/jamapediatrics.2013.3768. [DOI] [PubMed] [Google Scholar]
- Paul R, Henry L, Grieve SM, Guilmette TJ, Niaura R, Bryant R, Bruce S, Williams LM, Richards CC, Cohen RA, Gordon E. The relationship between early life stress and microstructural integrity of the corpus callosum in a non-clinical population. Neuropsychiatric disease and treatment. 2008;4(1):193. doi: 10.2147/ndt.s1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl) 2011;214(1):55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the Amygdala to Emotion Processing: From Animal Models to Human Behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Tolley-Schell SA. Selective attention to facial emotion in physically abused children. Journal of Abnormal Psychology. 2003;112:323–338. doi: 10.1037/0021-843x.112.3.323. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, Amaral DG. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Price JL, Russcher FT, Amaral DG. The limbic region: II. The Amygdaloid complex. In: Bjorklund A, Hokfelt T, Swanson L, editors. Handbook of Chemical Neuroanatomy. Elsevier; Amsterdam: 1987. [Google Scholar]
- Price JM, Glad K. Hostile attributional tendencies in maltreated children. Journal of Abnormal Child Psychology. 2003;31:329–343. doi: 10.1023/a:1023237731683. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16(6):723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Raper J, Wilson M, Sanchez M, Machado CJ, Bachevalier J. Pervasive alterations of emotional and neuroendocrine responses to an acute stressor after neonatal amygdala lesions in rhesus monkeys. Psychoneuroendocrinology. 2013;38(7):1021–1035. doi: 10.1016/j.psyneuen.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem KS, Logothetis NK. A combined MRI and histology atlas of the rhesus monkey brain in stereotaxic coordinates. Academic Press; 2012. [Google Scholar]
- Sanchez MM. The impact of early adverse care on HPA axis development: nonhuman primate models. Hormones & Behavior. 2006;50:623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13(3):419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, Winslow JT. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biological Psychiatry. 2005;57:373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Schino G, Perretta G, Taglioni AM, Monaco V, Troisi A. Primate displacement activities as an ethopharmacological model of anxiety. Anxiety. 1996;2:186–191. doi: 10.1002/(SICI)1522-7154(1996)2:4<186::AID-ANXI5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Shields A, Cicchetti D. Reactive aggression among maltreated children: The contributions of attention and emotion dysregulation. Journal of Clinical Child Psychology. 1998;27:381–395. doi: 10.1207/s15374424jccp2704_2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Behavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stouthamer-Loeber M, Loeber R, Homish DL, Wei E. Maltreatment of boys and the development of disruptive and delinquent behavior. Development and Psychopathology. 2001;13:941–955. [PubMed] [Google Scholar]
- Styner M, Knickmeyer R, Joshi S, Coe C, Short SJ, Gilmore J. Automatic brain segmentation in rhesus monkeys; Paper presented at the SPIE Medical Imaging; 2007. [Google Scholar]
- Suomi SJ. Mother-infant attachment, peer relationships, and the development of social networks in rhesus monkeys. Human Development. 2005;48:67–79. [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27(1-2):33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proceedings of the National Academy of Sciences. 2012;109(9):E563–E572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Millner A, Galvan A, Davidson MC, Eigsti I. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2010;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi A. Displacement activities as a behavioral measure of stress in nonhuman primates and human subjects. Stress:The International Journal on the Biology of Stress. 2002;5:47–54. doi: 10.1080/102538902900012378. [DOI] [PubMed] [Google Scholar]
- Troisi A, D’ Amato FR. Is monkey maternal abuse of offspring aggressive behavior? Aggressive Behavior. 1983;9:173–176. [Google Scholar]
- van Harmelen AL, van Tol MJ, van der Wee NJ, Veltman DJ, Aleman A, Spinhoven P, van Buchem MA, Zitman FG, Penninx BWJH, Elzinga BM. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biol Psychiatry. 2010;68(9):832–838. doi: 10.1016/j.biopsych.2010.06.011. [DOI] [PubMed] [Google Scholar]
- van Wagenen G, Catchpole HR. Physical growth of the rhesus monkey (Macaca mulatta) American Journal of Physical Anthropology. 1956;14:245–256. doi: 10.1002/ajpa.1330140219. [DOI] [PubMed] [Google Scholar]
- Williamson DE, Coleman K, Bacanu S, Devlin BJ, Rogers J, Ryan ND, Cameron JL. Heritability of fearful-anxious endophenotypes in infant rhesus macaques: A preliminary report. Biological Psychiatry. 2003;53:284–291. doi: 10.1016/s0006-3223(02)01601-3. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Walker ML, Gordon TP. Consequences of first pregnancy in rhesus monkeys. American Journal of Physical Anthropology. 1983;61:103–110. doi: 10.1002/ajpa.1330610111. [DOI] [PubMed] [Google Scholar]
- Young EA, Abelson JL, Curtis GC, Nesse RM. Childhood adversity and vulnerability to mood and anxiety disorders. Depression and Anxiety. 1997;5:66–72. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.