Species or populations may be categorized by sexual system. Here, we examine the frequency of sexual systems in Silene. We found that hermaphroditism is the most common sexual system, followed by dioecy, gynodioecy and gynodioecy-gynomonoecy (females, hermaphrodites and gynomonoecious plants) with similar frequency. These sexual systems are equally represented in the two phylogenetically supported subgenera Silene and Behenantha. We specifically studied the sexual systems of section Psammophilae (four species and 26 populations), and found that most populations are gynodioecious-gynomonoecious. Hermaphrodites are the most common sexual morph, and females tend to produce fewer flowers than other morphs.
Keywords: Behenantha, Caryophyllaceae, dioecy, gynodioecy, gynodioecy–gynomonoecy, hermaphroditism, Psammophilae, sexual polymorphism, sexual system, Silene
Abstract
Species and populations can be categorized by their sexual systems, depending on the spatial distribution of female and male reproductive structures within and among plants. Although a high diversity of sexual systems exists in Silene, their relative frequency at the genus and infrageneric level is unknown. Here, we carried out an extensive literature search for direct or indirect descriptions of sexual systems in Silene species. We found descriptions of sexual systems for 98 Silene species, where 63 and 35 correspond to the phylogenetically supported subgenera Silene and Behenantha, respectively. Hermaphroditism was the commonest sexual system (58.2 %), followed by dioecy (14.3 %), gynodioecy (13.3 %) and gynodioecy–gynomonoecy (i.e. hermaphroditic, female and gynomonoecious plants coexisting in the same population; 12.2 %). The presence of these sexual systems in both subgenera suggests their multiple origins. In 17 species, the description of sexual systems varied, and in most cases these differences corresponded to variations within or among populations. Interestingly, the poorly studied gynodioecy–gynomonoecy sexual system showed similar frequency to dioecy and gynodioecy in both subgenera. In addition, the incidence of gynodioecy–gynomonoecy was analysed in the species of section Psammophilae (Silene littorea, S. psammitis, S. adscendens and S. cambessedesii), in a survey of 26 populations across the distribution area of the species. The four species showed gynomonoecy–gynodioecy in most populations. Hermaphrodites were the most frequent morph, with a low number of females and gynomonoecious plants in all populations. The frequency of sexual morphs varied significantly among the studied populations but not among species. Female plants generally produced smaller numbers of flowers than hermaphroditic or gynomonoecious plants, and the percentages of female flowers per population were low. All these findings suggest that the gynodioecious–gynomonoecious sexual system in section Psammophilae is closer to hermaphroditism or gynomonoecy than gynodioecy.
Introduction
The study of the diversity and evolution of sexual systems in plants has been the focus of many scientists since early days. Species or populations may be categorized by sexual system, depending on the spatial distribution of male and female reproductive structures within and among plants (Bawa and Beach 1981). Although many different sexual systems may exist (Sakai and Weller 1999), most of the angiosperm species belong to one of the five main types: hermaphroditism (72 %), gynodioecy (7 %, female and hermaphroditic individuals), monoecy (5 %, individuals with female and male flowers), dioecy (5–6 %, female and male individuals) and gynomonoecy (3 %, individuals with female and hermaphroditic flowers) (Richards 1997; Renner 2014). Although only 5–6 % of total angiosperms are dioecious, dioecious species are present in 43 % of families, and from 871 to 5000 independent origins of dioecy have been proposed (Renner 2014). Therefore, the evolutionary pathways to dioecy have been the focus of interesting debate, specially the transition from hermaphroditism to dioecy, with gynodioecy or monoecy as intermediate steps (Charlesworth 1999). The association of gynodioecy or monoecy with dioecy at the family or genera level suggests that both are possible pathways to dioecy (Renner and Ricklefs 1995; Dufay et al. 2014; Renner 2014). Gynomonoecy occurs frequently in families such as Compositae or Chenopodiaceae (Yampolsky and Yampolsky 1922; Torices et al. 2011), and has been considered the main route to monoecy from hermaphroditism and vice versa (Torices et al. 2011).
The genus Silene (Caryophyllaceae) has been widely used to study the evolution of sexual systems and gender variation (Meagher 2007; Bernasconi et al. 2009; Charlesworth 2013; Weingartner and Delph 2014), and is one of the groups used for the phylogenetic approach (Desfeux et al. 1996; Rautenberg et al. 2010; Marais et al. 2011). Nonetheless, the complete phylogenetic relationship within this genus is not yet resolved (Rautenberg et al. 2010; Petri et al. 2013). What seems clear is the subdivision of Silene (sensu Oxelman and Lidén 1995) into two clades: subgenera Silene and Behenantha (Popp and Oxelman 2004, 2007; Rautenberg et al. 2010). The first of these phylogenetic studies found that dioecy appeared independently at least twice (in subsection Otites and section Melandrium; according to Oxelman et al. 2013), and that gynodioecy was the most probable ancestral condition for the genus (Desfeux et al. 1996). More recently, Marais et al. (2011) found that either gynodioecy or hermaphroditism could be the ancestral condition of Silene. In Otites and Melandrium, different types of sex-determining systems with a different date of origin are implicated (Käfer et al. 2013; Slancarova et al. 2013). In addition to dioecy, hermaphroditism and gynodioecy are common in Silene (Desfeux et al. 1996; Jürgens et al. 2002). However, monoecy is not present, suggesting the evolution of dioecy through the gynodioecy pathway.
Gynomonoecy, andromonoecy (individuals with male and hermaphroditic flowers) and trioecy (populations with hermaphroditic, male and female individuals) have also been reported for Silene, but are very rare (Desfeux et al. 1996; Jürgens et al. 2002; present study). However, in a non-negligible number of gynodioecious species, the existence of gynomonoecious individuals (i.e. plants with female and hermaphroditic flowers) in the populations is reported (e.g. Shykoff 1988; Talavera et al. 1996; Lafuma and Maurice 2006; Dufay et al. 2010). Species or populations containing hermaphroditic, female and gynomonoecious individuals must be considered as gynodioecious–gynomonoecious (Gd–Gm hereafter) (Desfeux et al. 1996). The frequency of gynomonoecious plants may be highly variable among populations and species; in some cases, this sexual morph is rare and in others it may be the most frequent (Charlesworth and Laporte 1998; Maurice 1999; Dufay et al. 2010; Casimiro-Soriguer et al. 2013). The genetic mechanism for sex determination of gynodioecy may be based on the interaction of cytoplasmic male sterility genes with nuclear restorers of male fertility (Bailey and Delph 2007), as found in S. vulgaris (Charlesworth and Laporte 1998). In some cases, the incomplete restoration of the cytoplasmic male sterility factors or heteroplasmy (the occurrence of different cytotypes within an individual) can cause partially male-sterile plants that are able to produce females and hermaphroditic flowers (i.e. gynomonoecious plants) (Koelewijn and Van Damme 1996; McCauley et al. 2005). Thus, although the genetic basis for gynomonoecious and female individuals in Gd–Gm species has been hypothesized in Silene species (Glaettli and Goudet 2006; Garraud et al. 2011), their incidence remains unclear.
Silene littorea is one of the most studied species with a Gd–Gm sexual system (Guitián and Medrano 2000; Vilas and García 2006; Vilas et al. 2006; Casimiro-Soriguer et al. 2013). In several populations from two contrasting sites in their distribution area, the frequency of hermaphrodites or gynomonoecious plants varied highly among populations, but female plants were always rare (Guitián and Medrano 2000; Casimiro-Soriguer et al. 2013). Analysis of functional gender showed that nearly all plants in the population transmit their genes via both pollen and ovules; thus, the Gd–Gm sexual system of S. littorea seems to be closer to hermaphroditism or gynomonoecy than gynodioecy (Casimiro-Soriguer et al. 2013). Interestingly, S. stockenii also shows a Gd–Gm sexual system with a very low frequency of female plants (Talavera et al. 1996). Both species belong to the section Psammophilae, composed of three other annual species (S. adscendens, S. cambessedesii and S. psammitis). Therefore, the question which arises from these findings is whether the Gd–Gm sexual system is widespread in the whole Psammophilae section. In addition, the reproductive output of the different morphs may vary in the Gd–Gm sexual system, which may be important to the stable maintenance of these morphs in the populations (Dufay et al. 2010). For instance, Shykoff et al. (2003) found that overall females produce more but smaller flowers, set more fruits and produce more and heavier seeds than hermaphrodites.
In this study, two different approaches were used to evaluate the occurrence of sexual systems, particularly gynodioecy–gynomonoecy, in Silene. For the general approach, we searched the literature extensively to locate any direct or indirect description of the sexual system of the species of Silene. This search allows us to know the frequency of sexual systems at the genus and infrageneric level as well as their variability within species. Accurate estimates of the frequency of the Gd–Gm sexual system may shed light on their possible evolution and stability in Silene, and also in other groups of angiosperms. For the specific approach, we have studied the sexual systems of a total 26 populations of the species of the section Psammophilae. Specifically, we seek to answer the following questions. (i) Is the Gd–Gm sexual system widespread throughout the distribution area of S. littorea and the other species of section Psammophilae? (ii) What is the frequency of the different sexual morphs and types of flowers in the populations? (iii) Are there differences in the number of flowers produced by each morph?
Methods
Study system
Silene littorea, S. cambessedesii, S. psammitis and S. stockenii are endemic to the Iberian Peninsula and Balearic Islands (Talavera 1979). Talavera (1979) included these taxa together with S. almolae, S. germana and S. pendula within the section Erectorefractae. We follow Greuter (1995) who proposed the section Psammophilae, previously considered a subsection of Erectorefractae (Talavera 1979). Oxelman et al. (2013) consider the species status of S. adscendens (previously considered a subspecies of S. littorea). All the species are spring-flowering annuals and grow in different types of soil: sandy substrates from the coast (S. cambessedesii, S. littorea), dolomites or slates (S. psammitis), calcareous sandstones (S. stockenii) or schists (S. adscendens) (Talavera 1979).
Analysis of the sexual system of section Psammophilae
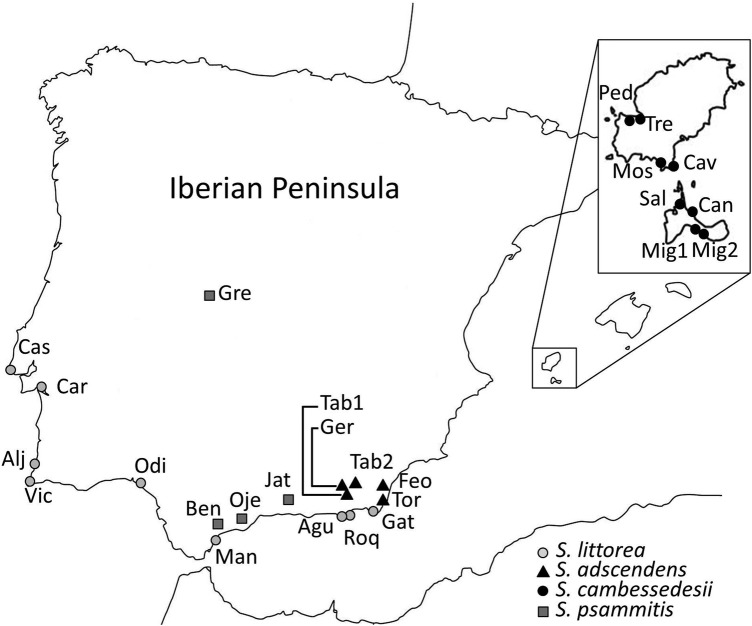
During the peak of the flowering period from 2010 to 2012, we visited 5 populations of S. adscendens, 8 of S. cambessedesii, 11 of S. littorea and 4 of S. psammitis (Fig. 1) [see Supporting Information]. We did not include S. stockenii because: (i) it is a critically endangered species with only a few populations (Bañares et al. 2004), (ii) Talavera et al. (1996) have already studied the sexual system of S. stockenii in the most important population and (iii) we visited some of the remnant populations, detecting high florivory levels and a small number of individuals. We performed single-day linear transects of 100 plants, with the exception of some very small populations [see Supporting Information]. We chose plants separated by at least 1 m to avoid microhabitat or clustering effects in sex expression (Klaas and Olson 2006). For each plant, we counted all the flowers in anthesis, and noted their sex (female or hermaphroditic). Withered flowers were also analysed when sex differentiation was possible. Plants bearing only female or hermaphroditic flowers were considered female or hermaphroditic individuals, respectively, whereas individuals with female and hermaphroditic flowers were considered gynomonoecious. In a previous study in S. littorea, Casimiro-Soriguer et al. (2013) found that the probability of recording female flowers in gynomonoecious plants was higher when the whole flowering period of a plant was studied than when estimates were based on a single census. Thus, our sampling methodology would underestimate the frequency of gynomonoecious plants in the population. In addition, plants with a large number of flowers would have a higher probability to be classified as gynomonoecious.
Figure 1.
Populations sampled from the different species of section Psammophilae: nine populations of S. littorea (grey dots), five populations of S. adscendens (black triangles), eight populations of S. cambessedesii (black dots) and four populations of S. psammitis (grey squares).
Literature search on Silene sexual systems
We performed a literature search in the SCOPUS and JSTOR databases including the terms: Silene, breeding system, sexual system, hermaphroditic, hermaphrodite, hermaphroditism, dioecious, dioecy, gynodioecious, gynodioecy, gynomonoecious, gynomonoecy, androdioecious, androdioecy, andromonoecious, andromonoecy, monoecious and monoecy. We also revised the description of the Silene species in main floras and revision studies or books previous to 1938 that contain information about plant sexuality, see Table 1 and [Supporting Information]. In addition, for those species with numerous sexual system descriptions or present in the Euro + Med database, specific individual searches were performed. We annotated the information about the sexual systems of the species in one of the following categories: hermaphrodite (H), dioecious (D), gynodioecious (Gd), gynomonoecious (Gm), androdioecious (Ad), andromonoecious (Am) or trioecious (T). In some cases, mixed sexual systems were found within a single population, for instance Am–Ad (male, hermaphroditic and andromonoecious plants), H–Gm (hermaphrodites and gynomonoecious plants) and gynodioecious–gynomonoecious (Gd–Gm, female, hermaphroditic and gynodioecious plants). When various studies described a species with different sexual systems, all of them were annotated with the respective reference; however, the principal or most frequent sexual system was used for calculating the frequency at the genus or subgenus level (see Jürgens et al. 2002 for similar criteria). The H–Gm category was assigned as H because in most cases gynomonoecious individuals are extremely rare and bear only a few female flowers (e.g. A. Jürgens, pers. comm.; Giménez-Benavides et al. 2007). We will follow the classification criteria of Oxelman et al. (2013) for Silene and the infrageneric level. The subspecies level was not considered.
Table 1.
Sexual systems in Silene. Sexual system description recognizes all the sexual systems described for the species in the literature. The sexual system assigned here is the principal or most frequent sexual system for the species according to our review. Species classification follows Oxelman et al. (2013). H, hermaphrodite; D, dioecious; Gd, gynodioecious; Gm, gynomonoecious; Ad, androdioecious; Am, andromonoecious and T, trioecious. Mixed sexual systems are denoted by a dash.
| Subgenus, section, species | Sexual system |
|
|---|---|---|
| Described in literature | Assigned | |
| Subgenus Behenantha (Otth) Endl. | ||
| Section Behenantha Otth | ||
| S. pendula L. | Gd1,2,51 | Gd |
| S. uniflora Roth | Gd3,4,5 | Gd |
| S. vulgaris (Moench) Garcke | Am–Ad6; Gd2; Gd–Gm6,7,8,9,10 | Gd–Gm |
| Section Conoimorpha Otth | ||
| S. conica L. | H2,7; H–Gm2 | H |
| S. conoidea L. | H1,2 | H |
| S. subconica Friv. | Gd2 | Gd |
| Section Dichotomae (Rohrb.) Chowdhuri | ||
| S. dichotoma Ehrh. | Gd2,6,7 | Gd |
| Section Elisanthe (Fenzl) Fenzl | ||
| S. noctiflora L. | H11;H–Gm2,7;Gm12,13;Gd–Gm1 | Gm |
| Section Erectorefractae Chowdhuri | ||
| S. germana Gay | H51 | H |
| Section Melandrium (Röhl.) Rabeler | ||
| S. astrachanicum (Pacz.) Takht. | D14 | D |
| S. diclinis (Lag.) M.Laínz | D1,8,14,15,16,17 | D |
| S. dioica (L.) Clairv. | Am6; D2,6,7,8,14,15 | D |
| S. integripetala Bory and Chaub. | H–Gm30 | H |
| Section Viscosae (Boiss.) C.L.Tang | ||
| S. viscosa (L.) Pers. | H2,7 | H |
| Others | ||
| S. acutifolia Link ex Rohrb. | H8,31 | H |
| S. elisabethae Jan | H6,7 | H |
| Subgenus Silene | ||
| Section Auriculatae (Boiss.) Schischkin | ||
| S. disticha Willd. | H2,8 | H |
| S. echinata Otth | H8; H–Gm2 | H |
| S. linicola C.C.Gmel. | H2,6,8 | H |
| S. schafta J.G.Gmel. ex Hohen. | Gd2 | Gd |
| S. spergulifolia (Willd.) M.Bieb. | H2 | H |
| S. vallesia L. | Gd2,7 | Gd |
| Section Silene | ||
| S. apetala Willd. | H1,2 | H |
| S. ciliata Pourr. | H–Gm32 | H |
| S. colorata Poir. | H8,33; H–Gm2 | H |
| S. gallica L. | H8; H–Gm2 | H |
| S. gracilis DC. | H51 | H |
| S. micropetala Lag. | H8; H–Gm2 | H |
| S. nicaeensis All. | H2,8,51 | H |
| S. nocturna L. | H1,2,8 | H |
| S. pseudoatocion Desf. | Gd–Gm2 | Gd–Gm |
| S. ramosissima Desf. | H8,51 | H |
| S. scabriflora Brot. | H51 | H |
| S. roemeri Friv. | Gd–Am15; H–Gd2 | Gd |
| S. saxifraga L. | Am–Gm–T6; Gm1; Gd–Gm2,7 | Gd–Gm |
| S. sendtneri Boiss. | D15 | D |
| S. senneni Pau | H45 | H |
| S. thessalonica Boiss. and Heldr. | H–Gm2 | H |
| S. viridiflora L. | H7; Gd–Gm2 | Gd–Gm |
| S. waldsteinii Griseb. | H–Gm2 | H |
| S. wolgensis (Hornem.) Otth | D36 | D |
| Section Spergulifoliae (Boiss.) Schischkin | ||
| S. brahuica Boiss. | Gd21 | Gd |
| Others | ||
| S. bupleuroides L. | H2,7 | H |
| S. heuffelii Soó | D14 | D |
| S. latifolia Poir. | Am6; D2,6,7,8,14,15 | D |
| S. marizii Samp. | D8 | D |
| Section Physolychnis (Bentham) Bocquet | ||
| S. caroliniana Walter | H18 | H |
| S. douglasii Hook. | H11,19 | H |
| S. gangotriana Pusalkar, D.K.Singh and Lakshmin | H20 | H |
| S. laxantha Majumdar | Gd20,21 | Gd |
| S. regia Sims | H22,23 | H |
| S. rotundifolia Nutt. | H23 | H |
| S. scouleri Hook. | H11 | H |
| S. stellata (L.) W.T. Aiton | H18,24 | H |
| S. tibetica Lidén and Oxelman | H–Am25 | Am |
| S. virginica L. | H18,26 | H |
| S. zawadzkii Herbich | H2 | H |
| Section Psammophilae (Talavera) Greuter | ||
| S. adscendens Lag. | Gd–Gm50 | Gd–Gm |
| S. cambessedesii Boiss. and Reut. | Gd–Gm50 | Gd–Gm |
| S. littorea Brot. | Gd–Gm2,27,28,50 | Gd–Gm |
| S. psammitis Link ex Spreng | Gd–Gm50 | Gd–Gm |
| S. stockenii Chater | Gd–Gm29,51 | Gd–Gm |
| Section Sedoideae Oxelman and Greuter | ||
| S. secundiflora Otth | H2,8 | H |
| S. sericea All. | Gd2 | Gd |
| S. succulenta Forssk. | H2 | H |
| Section Siphonomorpha Otth | ||
| S. acaulis (L.) Jacq. | D2,6,7,14; T6,7,15,34; Gd–Gm35 | D |
| S. andryalifolia Pomel | Gd2 | Gd |
| S. borysthenica (Gruner) Walters | D15,36 | D |
| S. colpophylla Wrigley | D37 | D |
| S. cyri Schischkin | D14 | D |
| S. fernandezii Jeanm. | H–Gm8 | H |
| S. flavescens Waldst. and Kit. | H–Gm2 | H |
| S. fruticosa L. | H2 | H |
| S. gazulensis Galán, Cortés, Orell. and Morales Alonso | H38 | H |
| S. gigantea L. | H–Gm39 | H |
| S. hayekiana Hand.-Mazz. and Janch. | Gd2 | Gd |
| S. hellmannii Claus | D14,15 | D |
| S. hifacensis Rouy | Gd23; Gd–Gm40 | Gd–Gm |
| S. italica (L.) Pers. | H7; Gd2; Gd–Gm1,41,42 | Gd–Gm |
| S. multicaulis Guss. | H2 | H |
| S. multiflora (Ehrh) Pers. | H7 | H |
| S. nocteolens Webb and Berthel. | H38 | H |
| S. nutans L. | Am–Ad6; Gd–Gm1,2,6,7,43 | Gd–Gm |
| S. otites (L.) Wibel | Ad6; D1,2,6,7,15,36 | D |
| S. paradoxa L. | H2,23 | H |
| S. parnassica Boiss. and Spruner | H2 | H |
| S. patula Desf. | H44 | H |
| S. capitellata Boiss. | H46 | H |
| S. chlorantha (Willd.) Ehrh | H2,7,47 | H |
| S. cretica L. | H2,7,8 | H |
| S. friwaldskyana Hampe. | H2 | H |
| S. hawaiiensis Sherff | H23,48 | H |
| S. inaperta L. | H1,2 | H |
| S. isaurica Contandr. and Quézel | Gd46 | Gd |
| S. kemoniana C. Brullo, Brullo, Giusso, Ilardi and Sciandr. | H49 | H |
| S. muscipula L. | H2 | H |
| S. portensis L. | H2,51 | H |
| S. struthioloides A.Gray | H23,48 | H |
1Desfeux et al. (1996), 2Jürgens et al. (2002), 3Baker and Dalby (1980), 4Pettersson (1997), 5Warren and James (2008), 6Knuth (1908), 7Meusel and Mühlberg (1979), 8Talavera (1990), 9Glaettli and Goudet (2006), 10Miyake and Olson (2009), 11Touzet and Delph (2009), 12Folke and Delph (1997), 13Davis and Delph (2005), 14Schischkin (1970), 15Chater and Walters (1964), 16Prentice (1976), 17Montesinos et al. (2006), 18Reynolds et al. (2009), 19Kephart et al. (1999), 20Pusalkar et al. (2004), 21Tropicos.org (2014), 22Dolan (1994), 23Moyle (2006), 24Castillo et al. (2013), 25Oxelman et al. (2001), 26Dudash and Fenster (2001), 27Guitián and Medrano (2000), 28Casimiro-Soriguer et al. (2013), 29Talavera et al. (1996), 30Oxelman (1995), 31Buide and Guitián (2002), 32Giménez-Benavides et al. (2007), 33Terrab et al. (2007), 34Alatalo and Molau (2001), 35Shykoff (1992), 36Lihua et al. (2001), 37Mrackova et al. (2008), 38Bañares et al. (2004), 39Ghazanfar (1989), 40Prentice et al. (2003), 41Maurice (1999), 42Lafuma and Maurice (2006), 43Dufay et al. (2010), 44Naciri et al. (2010), 45Martinell et al. (2010), 46Yildiz and Çirpici (2013), 47Lauterbach et al. (2011), 48Westerbergh and Saura (1994), 49Brullo et al. (2012), 50Casimiro-Soriguer et al. present study and 51E. Narbona, M. L. Buide and I. Casimiro-Soriguer, pers. observations.
Statistical analysis
To test for differences in the proportion of the different sexual morphs among species and populations, a generalized linear model (GLM) with a multinomial distribution and a probit link function was carried out. We considered the sexual morph of each individual (female, hermaphrodite or gynomonoecious) as the multinomial response variable; and species and population (nested within species) as fixed factors. Population was treated as a fixed factor rather than a random factor because we are interested in examining the differences in morph frequencies among our specific populations, and the same populations would be analysed in future studies (Bennington and Thayne 1994; Potvin 2001). Comparisons of the number of flowers between female plants and hermaphrodite or gynomonoecious plants were performed using GLMs with a log link function and a Poisson error distribution. The dependent variable was the number of flowers produced by each individual; and sexual morph, population (nested within species) and species were included as fixed factors. On the other hand, the frequency of each sexual system between the subgenus Silene and Behenantha was compared using χ2 tests for contingency tables (Quinn and Keough 2002). All the analysis were carried out in IBM® SPSS® Statistics v.22.
Results
Sexual system of the section Psammophilae
A total of 2478 individuals belonging to 26 populations were surveyed. In general, each studied taxa of section Psammophilae showed Gd and Gd–Gm populations, although Gd–Gm populations were the most frequent (Fig. 2). Silene littorea, S. adscendens and S. psammitis showed one Gd population each, whereas S. cambessedesii showed two Gd populations. The remaining populations were all Gd–Gm (Fig. 2).
Figure 2.
Frequency of hermaphroditic (grey), gynomonoecious (white) and female (black) individuals of species from section Psammophilae in each population. The number of individuals per sexual morph sampled in each population is shown elsewhere [see Supporting Information].
Overall, the most frequent morph of section Psammophilae was the hermaphrodite, with an 86.8 % of individuals included in this category, followed by the female and the gynomonoecious morphs (7.9 and 5.3 %, respectively). The proportion of hermaphroditic plants within populations ranged from 64.0 % (in S. littorea) to 99 % (in S. adscendens), whereas the proportion of female plants varied from 1.0 to 18.0 % (in S. littorea), and that of gynomonoecious plants ranged from zero (at least one population in each species) to 20 % (in S. psammitis) (Fig. 2). The frequency of sexual morphs per population varied significantly among the studied populations (Wald χ2 = 62.62, df = 22, P < 0.0001) but not among species (Wald χ2 = 4.59, df = 3, P = 0.21).
On the whole, 92.1 % of the 7001 flowers analysed were hermaphrodites, and 7.9 % were females. At the species level, S. psammitis showed the highest proportion of female flowers (16.1 %) in the population and S. adscendens the lowest (4.8 %) [see Supporting Information]. The predominance of hermaphrodite flowers was also found at the population level; the mean percentage of female flowers per population ranged from 1.3 to 18.7 % in S. littorea, from 0.8 to 10.2 % in S. adscendens, from 1.1 to 14.1 % in S. cambessedesii and from 7.1 to 29.4 % in S. psammitis [see Supporting Information]. On the other hand, the percentage of female flowers in gynomonoecious individuals was 34.8 ± 2.0 % (mean ± 1 SE) in S. littorea, 34.5 ± 5.2 % in S. adscendens, 31.4 ± 3.0 % in S. cambessedesii and 43.4 ± 3.1 % in S. psammitis [see Supporting Information].
The average number of flowers in female plants was generally smaller than in hermaphroditic or gynomonoecious plants [see Supporting Information]. The number of flowers per individual showed significant differences among sex morphs (Wald χ2 = 328.85, df = 2, P < 0.0001), populations (Wald χ2 = 2126.73, df = 22, P < 0.0001) and species (Wald χ2 = 571.88, df = 3, P < 0.0001).
Diversity and frequency of sexual systems in Silene
We found that 98 Silene species have been specifically studied or described in terms of the sexual system (Table 1). We have collected the data from 46 different species in addition to those formerly found by Desfeux et al. (1996) and Jürgens et al. (2002). The number of species described in the subgenus Silene (63 species) is nearly double that in subgenus Behenantha (35 species) (Table 1). The most frequent sexual system at the genus level is hermaphroditism (58.2 %), followed by dioecy (14.3 %), gynodioecy (13.3 %) and gynodioecy–gynomonoecy (12.2 %). Interestingly, all four sexual systems are present in both subgenera, with a statistically similar frequency (P > 0.43 for all the sexual systems, except for hermaphroditism, that showed marginally significant differences P = 0.09) (Fig. 3). In addition, one Gm and one Am species were found, and both belong to the subgenus Behenantha. The fact that 13 of our assigned H species (21.1 %) are described as H–Gm in the literature is worthy of mention.
Figure 3.
Proportion of sexual systems in subgenus Behenantha (black bars) and subgenus Silene (white bars). H, Hermaphroditism; D, dioecy; Gd–Gm, gynodioecy–gynomonoecy; Gd, gynodioecy; Gm, gynomonoecy; Am, andromonoecy.
There are some sections whose species present mainly the same sexual system. For instance, section Melandrium are all dioecious, section Psammophilae are all Gd–Gm and section Physolychnis are all hermaphroditic except one species (S. laxantha, Table 1). Other sections seem more variable. Thus, section Silene includes H, Gd and Gd–Gm species, and section Siphonomorpha includes all the sexual systems present in the subgenus; however, it can be said that both sections have the highest number of species with known sexual systems.
In the literature, we have found 17 species (17.3 %) whose description of the sexual system varies. For instance, S. noctiflora has been described as H, H–Gm, Gm or Gd–Gm, and S. acaulis as D, T or Gd–Gm. By contrast, in other species, the sexual system description is consistently confirmed by several studies (e.g. the dioecious S. diclinis or the hermaphroditic S. chloranta).
Discussion
Studies of sexual systems over an entire section of Silene are mostly focussed on those groups containing dioecious species (Marais et al. 2011; Slancarova et al. 2013). To the best of our knowledge, this is the first study that analyses the sexual system of a whole section in Silene composed of non-dioecious species, including multiple populations across their distribution area. All species of section Psammophilae should be considered Gd–Gm, despite their traditional description as hermaphrodites (Talavera 1990; Greuter 1995). The Gd–Gm sexual system has been described in other species of the Caryophyllaceae family (e.g. Dianthus sylvestris, Gypsophila repens, Stellaria longipes; Philipp 1980; Collin and Shykoff 2003; López-Villavicencio et al. 2005). However, their presence in other families seems scarce; only a few cases are known in the Plantaginaceae and Lamiaceae (Kheyr-Pour 1980; Koelewijn and Van Damme 1996; Widén and Widén 1999).
We have found that hermaphroditic plants are the most frequent morph in all populations and species, including other species in the section Psammophilae, S. stockenii (Talavera et al. 1996). Similar results have been found in other Gd–Gm species of Silene, such as S. italica and S. nutans (Maurice 1999; Dufay et al. 2010). We also have demonstrated that the proportion of gynomonoecious plants varies among populations. Other previously studied populations of S. littorea showed hermaphroditic individuals in a similar or smaller frequency than the gynomonoecious (Guitián and Medrano 2000; Casimiro-Soriguer et al. 2013). This fact may be explained by this high inter-population variability in the frequency of hermaphroditic plants, but variations due to the different sampling methodology cannot be excluded. At least in S. littorea it is possible that plants classified as hermaphrodites in a single-census day could produce a female flower throughout the flowering period (Casimiro-Soriguer et al. 2013).
Most Silene species reviewed here were hermaphroditic (ca. 60 %), but dioecious, gynodioecious and Gd–Gm species were also relatively frequent. Interestingly, this genus was considered predominantly gynodioecious by some authors (Matsunaga and Kawano 2001; Lengerova et al. 2003; Slancarova et al. 2013). Knuth (1908) reported the presence of androdioecy or andromonoecy in several species, but this has never been confirmed in further studies. More recently, Oxelman et al. (2001) described the presence of apparently functionally male flowers in the lateral positions of the dichasium in S. tibetica, but again no further studies have confirmed this finding. On the other hand, hermaphroditism, dioecy, gynodioecy and Gd–Gm are present in both subgenus Behenantha and Silene at similar frequencies. The presence of each sexual system in both phylogenetically supported subgenera suggests a repeated independent evolution of sexual systems in these Silene clades, as found in other groups (Renner and Won 2001; Soza et al. 2012). In fact, repeated evolution of dioecy is phylogenetically confirmed in Silene (Marais et al. 2011; Slancarova et al. (2013).
Our survey of sexual systems in Silene showed that although most species seem to be consistent in their sexual system, 17 % of the reported species were described with more than one sexual system. This variation may be caused by different authors assigning sexual systems [e.g. S. dioica and S. latifolia are dioecious, but have been considered andromonoecious by Knuth (1908)] or by authors' simplification due to the low frequency of some sexual morphs in populations. However, in most cases, these differences could correspond to variations within or among populations (e.g. S. acaulis, S. noctiflora, S. saxifraga and S. vulgaris; see references in Table 1). This variation may be related to the genetic basis of sex determination and/or ecological factors acting on sexual expression (Delph 2003; McCauley and Bailey 2009). For example, the sexually plastic S. acaulis shows dioecy, trioecy, gynomonoecy or gynodioecy across its distribution area (Maurice et al. 1999; Alatalo and Molau 2001; Delph and Carroll 2001) and a cytoplasmic determination of sex with nuclear male fertility restorer genes is suggested (Delph et al. 1999; Klaas and Olson 2006). In addition, the role of environmental factors in sex expression has also been demonstrated in different species with higher frequency of female plants in harsher or dryer environments (Delph 2003). For instance, a higher female frequency in low-quality sites was found in S. acaulis (Delph and Carroll 2001).
A question arising from the relative high frequency of gynodioecy–gynomonoecy in Silene, and particularly in section Psammophilae, is whether this sexual system is an evolutionarily stable strategy. Theoretical models suggest that gynodioecy can evolve into dioecy, but also can be stable (Charlesworth and Charlesworth 1978; Dufay et al. 2014). Less is known about the maintenance of gynomonoecy (De Jong et al. 2008; Mamut et al. 2014), and especially Gd–Gm (McCauley and Bailey 2009; Garraud et al. 2011). In an evolutionarily stable Gd–Gm sexual system, female and gynomonoecious individuals must compensate for their loss of male function at the individual and flower level, respectively (Lloyd 1984). In gynodioecy, the advantage of female plants over hermaphrodites can be through inbreeding avoidance, resource reallocation or sex difference interactions with herbivores (Ashman 2002; Dufay and Billard 2012). The degree of female advantage should have an impact on the frequency of females (Dufay et al. 2007). In that case, those species with low female advantage will have low or variable frequency of female plants (Dufay et al. 2014). We found a low frequency of female plants per population in all species of section Psammophilae, as well as in the Gd–Gm species S. italica and S. nutans (Maurice 1999; Dufay et al. 2010). In these species, female advantage over hermaphrodites due to reallocation of resources seems to be low (Lafuma and Maurice 2006; Dufay et al. 2010). For instance, in S. nutans there were no differences in seed mass, germination rate or offspring quality between females and hermaphrodites (Dufay et al. 2010). In S. stockenii, females produced similar fruit set and number of seeds to hermaphroditic or gynomonoecious plants (Talavera et al. 1996). Similarly, in S. littorea female plants set similar fruits than gynomonoecious or hermaphrodites plants (Guitián and Medrano 2000). We have found that the number of flowers in female plants was smaller than that in the other morphs in some of the populations analysed. Thus reproductive output of female plants in the section Psammophilae seems to be lower than those of gynomonoecious or hermaphrodites, but further studies are needed to assess the possible female advantage in these species. On the other hand, the avoidance of inbreeding depression by female plants of S. littorea could help to maintain this morph in the population, although in a low frequency (Vilas and García 2006).
With regard to reproductive compensation of gynomonoecious plants over hermaphrodites, three main hypotheses have been proposed: (i) two types of flowers may allow the reallocation of resources to male and female functions (Lloyd 1979), (ii) female flowers can partially avoid inbreeding depression by favouring outcrossing (Marshall and Abbott 1984; Mamut et al. 2014) and (iii) flowers can escape florivory since hermaphrodites are usually more often attacked (Ashman 2002; Bertin et al. 2010). The outcrossing–benefit hypothesis of gynomonoecy has been demonstrated in Eremurus anisopterus (Mamut et al. 2014) and in S. noctiflora (Davis and Delph 2005). In the former, perfect flowers promote seed quantity by increasing pollinator attraction, whereas in the latter perfect flowers provide reproductive assurance by autonomous selfing when pollinators are scarce. As Silene species are self-compatible, autogamous selfing is possible where there is an overlap between sexual phases in the protandrous hermaphroditic flowers (Davis and Delph 2005; M. L. Buide, unpubl. data). In three populations of S. littorea, around 20 % of seed set was due to autonomous selfing (Hidalgo-Triana 2010), with similar findings for S. stockenii (23 %; Talavera et al. 1996). Thus, in these species of section Psammophilae, perfect flowers in gynomonoecious plants could allow some levels of reproductive assurance, whereas female flowers could partially avoid inbreeding depression. On the other hand, environmental factors could also affect the production of female flowers in gynomonoecious plants, and consequently affect sex expression in species of section Psammophilae. In the gynomonoecious S. noctiflora, an increase of 6 °C in a greenhouse, increased the production of female flowers in gynomonoecious plants (Folke and Delph 1997).
Conclusions
To sum up, we have confirmed the high diversity of sexual systems in Silene, but we have also demonstrated that the most important sexual systems are similarly represented in both subgenera Silene and Behenantha. The Gd–Gm sexual system is found in a similar number of species as dioecy and gynodioecy. In addition, we have documented that most populations of species from section Psammophilae showed a Gd–Gm sexual system, but variations in sexual expression also exist. The low number of females and gynomonoecious plants, and the low percentage of female flowers at the population level, suggest that the Gd–Gm sexual system in section Psammophilae is closer to hermaphroditism or gynomonoecy than gynodioecy. Thus, our study generates an important question: Has the Gd–Gm sexual system any advantage over hermaphroditism and gynodioecy, or is it just a consequence of the genetic mechanism of gynodioecious sex determination? The main non-exclusive hypotheses proposed for the determination of the gynomonoecious morph are the effect of environmental factors, and the partial restoration of male fertility (Dufay et al. 2010 and references therein). However, to the best of our knowledge, explicit evolutionary models do not exist including the gynomonoecious plants and their role on evolutionary transitions (Garraud et al. 2011). Gd–Gm species of Silene, and especially those of the section Psammophilae, could be a good model system to study the maintenance of gynomonoecious individuals in Gd–Gm populations.
Sources of Funding
This work was supported with FEDER funds and grants by the Spanish Ministerio de Ciencia e Innovación through a Formación de Personal Investigador grant to I.C.-S. [BES-2010-031073] and the research projects CGL2009-08257 and CGL2012-37646.
Contributions by the Authors
E.N., M.L.B. and I.C.-S. conceived the idea and collected the field data. I.C.-S. performed the literature review. E.N. and I.C.-S. ran the statistics. I.C.-S. led the writing with assistance of the others.
Conflict of Interest Statement
None declared.
Supporting Information
The following additional information is available in the online version of this article –
File S1. Geographic coordinates and number of individuals analysed (N) in the populations of species from section Psammophilae.
File S2. Revised literature that was not cited in the manuscript because no information about the sexual system of species was found.
File S3. Percentage of female flowers (FF) in populations, average number of flowers per sexual morph and percentage of female flowers in gynomonoecious plants. Mean ± s.e. per species is highlighted in bold.
Acknowledgements
We thank D. Wolf and two anonymous reviewers for critically reading the manuscript and offering constructive comments, F. Casimiro-Soriguer, E. Sánchez Gullón and M. Luceño for providing helpful information about species locations, S. Carrión for field assistance and Anna Crandell for English proofreading. The Servei de Protecció d'Especies from the Conselleria Medi Ambient i Mobilitat granted permission for sampling S. cambessedesii. The Junta de Andalucía granted permission for sampling S. littorea in the Paraje Natural ‘Marismas del Odiel’ and S. adscendens in the Parque Natural ‘Cabo de Gata’.
Literature Cited
- Alatalo JM, Molau U. 2001. Pollen viability and limitation of seed production in a population of the circumpolar cushion plant, Silene acaulis (Caryophyllaceae). Nordic Journal of Botany 21:365–372. 10.1111/j.1756-1051.2001.tb00780.x [DOI] [Google Scholar]
- Ashman TL. 2002. The role of herbivores in the evolution of separate sexes from hermaphroditism. Ecology 83:1175–1184. 10.1890/0012-9658(2002)083[1175:TROHIT]2.0.CO;2 [DOI] [Google Scholar]
- Bailey MF, Delph LF. 2007. Sex-ratio evolution in nuclear-cytoplasmic gynodioecy when restoration is a threshold trait. Genetics 176:2465–2476. 10.1534/genetics.107.076554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AJM, Dalby DH. 1980. Morphological variation between some isolated populations of Silene maritima within the British Isles with particular reference to inland populations on metalliferous soils. New Phytologist 84:123–138. 10.1111/j.1469-8137.1980.tb00755.x [DOI] [Google Scholar]
- Bañares A, Blanca G, Güemes J, Moreno JC, Ortiz S. 2004. Atlas y libro rojo de la flora vascular amenazadas de España. Madrid: Dirección General de Conservación de la Naturaleza. [Google Scholar]
- Bawa KS, Beach JH. 1981. Evolution of sexual systems in flowering plants. Annals of the Missouri Botanical Garden 68:254–274. 10.2307/2398798 [DOI] [Google Scholar]
- Bennington CC, Thayne WV. 1994. Use and misuse of mixed model analysis of variance in ecological studies. Ecology 75:717–722. 10.2307/1941729 [DOI] [Google Scholar]
- Bernasconi G, Antonovics J, Biere A, Charlesworth D, Delph LF, Filatov D, Giraud T, Hood ME, Marais GAB, McCauley D, Pannell JR, Shykoff JA, Vyskot B, Wolfe LM, Widmer A. 2009. Silene as a model system in ecology and evolution. Heredity 103:5–14. 10.1038/hdy.2009.34 [DOI] [PubMed] [Google Scholar]
- Bertin RI, Connors DB, Kleinman HM. 2010. Differential herbivory on disk and ray flowers of gynomonoecious asters and goldenrods (Asteraceae). Biological Journal of the Linnean Society 101:544–552. 10.1111/j.1095-8312.2010.01508.x [DOI] [Google Scholar]
- Brullo C, Brullo S, Giusso del Galdo G, Ilardi V, Sciandrello S. 2012. A new species of Silene sect. Dipterosperma (Caryophyllaceae) from Sicily. Anales del Jardín Botánico de Madrid 69:209–216. 10.3989/ajbm.2327 [DOI] [Google Scholar]
- Buide ML, Guitián J. 2002. Breeding system in the dichogamous hermaphrodite Silene acutifolia (Caryophyllaceae). Annals of Botany 90:691–699. 10.1093/aob/mcf251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro-Soriguer I, Buide ML, Narbona E. 2013. The roles of female and hermaphroditic flowers in the gynodioecious-gynomonoecious Silene littorea: insights into the phenology of sex expression. Plant Biology 15:941–947. 10.1111/j.1438-8677.2012.00697.x [DOI] [PubMed] [Google Scholar]
- Castillo DM, Kula AAR, Fenster KAD, Fenster CB, Dudash MR. 2013. Specialist pollinating seed predator exhibits oviposition strategy consistent with optimal oviposition theory. Ecological Entomology 38:164–172. 10.1111/een.12003 [DOI] [Google Scholar]
- Charlesworth B, Charlesworth D. 1978. A model for the evolution of dioecy and gynodioecy. The American Naturalist 112:975–997. 10.1086/283342 [DOI] [Google Scholar]
- Charlesworth D. 1999. Theories of the evolution of dioecy. In: Geber MA, Dawson TE, Delph LF, eds. Gender and sexual dimorphism in flowering plants. Berlin: Springer, 33–60. [Google Scholar]
- Charlesworth D. 2013. Plant sex chromosome evolution. Journal of Experimental Botany 64:405–420. 10.1093/jxb/ers322 [DOI] [PubMed] [Google Scholar]
- Charlesworth D, Laporte V. 1998. The male-sterility polymorphism of Silene vulgaris: analysis of genetic data from two populations and comparison with Thymus vulgaris. Genetics 150:1267–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater AO, Walters SM. 1964. Silene L. In: Tutin TG, Heywood VH, Burges NA, Valentine DH, Walters SM, eds. Flora Europaea, Vol. 1 Cambridge: University Press, 158–181. [Google Scholar]
- Collin CL, Shykoff JA. 2003. Outcrossing rates in the gynomonoecious-gynodioecious species Dianthus sylvestris (Caryophyllaceae). American Journal of Botany 90:579–585. 10.3732/ajb.90.4.579 [DOI] [PubMed] [Google Scholar]
- Davis SL, Delph LF. 2005. Prior selfing and gynomonoecy in Silene noctiflora L. (Caryophyllaceae): opportunities for enhanced outcrossing and reproductive assurance. International Journal of Plant Sciences 166:475–480. [Google Scholar]
- De Jong TJ, Shmida A, Thuijsman F. 2008. Sex allocation in plants and the evolution of monoecy. Evolutionary Ecology Research 10:1087–1109. [Google Scholar]
- Delph LF. 2003. Sexual dimorphism in gender plasticity and its consequences for breeding system evolution. Evolution and Development 5:34–39. 10.1046/j.1525-142X.2003.03006.x [DOI] [PubMed] [Google Scholar]
- Delph LF, Carroll SB. 2001. Factors affecting relative seed fitness and female frequency in a gynodioecious species, Silene acaulis. Evolutionary Ecology Research 3:487–505. [Google Scholar]
- Delph LF, Bailey MF, Marr DL. 1999. Seed provisioning in gynodioecious Silene acaulis (Caryophyllaceae). American Journal of Botany 86:140–144. 10.2307/2656963 [DOI] [PubMed] [Google Scholar]
- Desfeux C, Maurice S, Henry JP, Lejeune B, Gouyon PH. 1996. Evolution of reproductive systems in the genus Silene. Proceedings of the Royal Society B: Biological Sciences 263:409–414. 10.1098/rspb.1996.0062 [DOI] [PubMed] [Google Scholar]
- Dolan RW. 1994. Patterns of isozyme variation in relation to population size, isolation, and phytogeographic history in royal catchfly (Silene regia; Caryophyllaceae). American Journal of Botany 81:965–972. 10.2307/2445289 [DOI] [Google Scholar]
- Dudash MR, Fenster CB. 2001. The role of breeding system and inbreeding depression in the maintenance of an outcrossing mating strategy in Silene virginica (Caryophyllaceae). American Journal of Botany 88:1953–1959. 10.2307/3558422 [DOI] [PubMed] [Google Scholar]
- Dufay M, Billard E. 2012. How much better are females? The occurrence of female advantage, its proximal causes and its variation within and among gynodioecious species. Annals of Botany 109:505–519. 10.1093/aob/mcr062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufay M, Touzet P, Maurice S, Cuguen J. 2007. Modelling the maintenance of male-fertile cytoplasm in a gynodioecious population. Heredity 99:349–356. 10.1038/sj.hdy.6801009 [DOI] [PubMed] [Google Scholar]
- Dufay M, Lahiani E, Brachi B. 2010. Gender variation and inbreeding depression in gynodioecious-gynomonoecious Silene nutans (Caryophyllaceae). International Journal of Plant Sciences 171:53–62. 10.1086/647916 [DOI] [Google Scholar]
- Dufay M, Champelovier P, Käfer J, Henry JP, Mousset S, Marais GAB. 2014. An angiosperm-wide analysis of the gynodioecy-dioecy pathway. Annals of Botany 114:539–548. 10.1093/aob/mcu134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folke SH, Delph LF. 1997. Environmental and physiological effects on pistillate flower production in Silene noctiflora L. (Caryophyllaceae). International Journal of Plant Sciences 158:501–509. 10.1086/297460 [DOI] [Google Scholar]
- Garraud C, Brachi B, Dufay M, Touzet P, Shykoff JA. 2011. Genetic determination of male sterility in gynodioecious Silene nutans. Heredity 106:757–764. 10.1038/hdy.2010.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazanfar SA. 1989. Hybridization studies in the genus Silene sectt. Siphonomorpha and Auriculatae (Caryophyllaceae). Plant Systematics and Evolution 168:143–148. 10.1007/BF00936094 [DOI] [Google Scholar]
- Giménez–Benavides L, Dötterl S, Jürgens A, Escudero A, Iriondo JM. 2007. Generalist diurnal pollination provides greater fitness in a plant with nocturnal pollination syndrome: assessing the effects of a Silene–Hadena interaction. Oikos 116:1461–1472. [Google Scholar]
- Glaettli M, Goudet J. 2006. Inbreeding effects on progeny sex ratio and gender variation in the gynodioecious Silene vulgaris (Caryophyllaceae). New Phytologist 172:763–773. 10.1111/j.1469-8137.2006.01866.x [DOI] [PubMed] [Google Scholar]
- Greuter W. 1995. Silene (Caryophyllaceae) in Greece: a subgeneric and sectional classification. Taxon 44:543–581. 10.2307/1223499 [DOI] [Google Scholar]
- Guitián P, Medrano M. 2000. Sex expression and fruit set in Silene littorea (Caryophyllaceae): variation among populations. Nordic Journal of Botany 20:467–473. 10.1111/j.1756-1051.2000.tb01589.x [DOI] [Google Scholar]
- Hidalgo-Triana N. 2010. Pollination system and pollen limitation in Sistema Silene littorea Brot. (Caryophyllaceae), an annual species of the Iberian coast. Master Thesis, Pablo de Olavide University, Spain. [Google Scholar]
- Jürgens A, Witt T, Gottsberger G. 2002. Pollen grain numbers, ovule numbers and pollen-ovule ratios in Caryophylloideae: correlation with breeding system, pollination, life form, style number, and sexual system. Sexual Plant Reproduction 14:279–289. 10.1007/s00497-001-0124-2 [DOI] [Google Scholar]
- Käfer J, Talianová M, Bigot T, Michu E, Guéguen L, Widmer A, Žlůvová J, Glémin S, Marais GAB. 2013. Patterns of molecular evolution in dioecious and non-dioecious Silene. Journal of Evolutionary Biology 26:335–346. 10.1111/jeb.12052 [DOI] [PubMed] [Google Scholar]
- Kephart SR, Brown E, Hall J. 1999. Inbreeding depression and partial selfing: evolutionary implications of mixed-mating in a coastal endemic, Silene douglasii var. oraria (Caryophyllaceae). Heredity 82:543–554. 10.1038/sj.hdy.6885250 [DOI] [PubMed] [Google Scholar]
- Kheyr-Pour A. 1980. Nucleo-cytoplasmic polymorphism for male sterility in Origanum vulgare L. Journal of Heredity 71:253–260. [Google Scholar]
- Klaas AL, Olson MS. 2006. Spatial distributions of cytoplasmic types and sex expression in Alaskan populations of Silene acaulis. International Journal of Plant Sciences 167:179–189. 10.1086/498965 [DOI] [Google Scholar]
- Knuth P. 1908. Handbook of flower pollination, Vol. II (English translation by JR Ainsworth Davis) Oxford: Clarendon Press. [Google Scholar]
- Koelewijn HP, Van Damme JMM. 1996. Gender variation, partial male sterility and labile sex expression in gynodioecious Plantago coronopus. New Phytologist 132:67–76. 10.1111/j.1469-8137.1996.tb04510.x [DOI] [PubMed] [Google Scholar]
- Lafuma L, Maurice S. 2006. Reproductive characters in a gynodioecious species, Silene italica (Caryophyllaceae), with attention to the gynomonoecious phenotype. Biological Journal of the Linnean Society 87:583–591. 10.1111/j.1095-8312.2006.00597.x [DOI] [Google Scholar]
- Lauterbach D, Ristow M, Gemeinholzer B. 2011. Genetic population structure, fitness variation and the importance of population history in remnant populations of the endangered plant Silene chlorantha (Willd.) Ehrh. (Caryophyllaceae). Plant Biology 13:667–677. 10.1111/j.1438-8677.2010.00418.x [DOI] [PubMed] [Google Scholar]
- Lengerova M, Moore RC, Grant SR, Vyskot B. 2003. The sex chromosomes of Silene latifolia revisited and revised. Genetics 165:935–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lihua Z, Zhengyi W, Lidén M, Oxelman B. 2001. Silene L. In: Zhengyi W, Raven PH, Deyuan H, eds. Flora of China, Vol. 6 Science Press (Beijing) & Missouri Botanical Garden (St. Louis), 66–100. http://flora.huh.harvard.edu/china/. [Google Scholar]
- Lloyd DG. 1979. Parental strategies of angiosperms. New Zealand Journal of Botany 17:595–606. 10.1080/0028825X.1979.10432573 [DOI] [Google Scholar]
- Lloyd DG. 1984. Gender allocations in outcrossing cosexual plants. In: Dirzo R, Sarukhane J, eds. Perspectives on plant population ecology. Sunderland: Sinauer, 277–300. [Google Scholar]
- López-Villavicencio M, Genton BJ, Porcher E, Shykoff JA. 2005. The role of pollination level on the reproduction of females and hermaphrodites in the gynodioecious plant Gypsophila repens (Caryophyllaceae). American Journal of Botany 92:1995–2002. 10.3732/ajb.92.12.1995 [DOI] [PubMed] [Google Scholar]
- Mamut J, Xiong YZ, Tan DY, Huang SQ. 2014. Pistillate flowers experience more pollen limitation and less geitonogamy than perfect flowers in a gynomonoecious herb. New Phytologist 201:670–677. 10.1111/nph.12525 [DOI] [PubMed] [Google Scholar]
- Marais GAB, Forrest A, Kamau E, Käfer J, Daubin V, Charlesworth D. 2011. Multiple nuclear gene phylogenetic analysis of the evolution of dioecy and sex chromosomes in the genus Silene. PLoS ONE 6:e21915 10.1371/journal.pone.0021915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall DF, Abbott RJ. 1984. Polymorphism for outcrossing frequency at the ray floret locus in Senecio vulgaris L. III. Causes. Heredity 53:145–149. 10.1038/hdy.1984.70 [DOI] [Google Scholar]
- Martinell MC, Dötterl S, Blanché C, Rovira A, Massó S, Bosch M. 2010. Nocturnal pollination of the endemic Silene sennenii (Caryophyllaceae): an endangered mutualism? Plant Ecology 211:203–218. 10.1007/s11258-010-9785-y [DOI] [Google Scholar]
- Matsunaga S, Kawano S. 2001. Sex determination by sex chromosomes in dioecious plants. Plant Biology 3:481–488. 10.1055/s-2001-17735 [DOI] [Google Scholar]
- Maurice S. 1999. Gynomonoecy in Silene italica (Caryophyllaceae): sexual phenotypes in natural populations. Plant Biology 1:346–350. 10.1111/j.1438-8677.1999.tb00262.x [DOI] [Google Scholar]
- Maurice S, Desfeux C, Mignot A, Henry JP. 1999. Is Silene acaulis (Caryophyllaceae) a trioecious species? Reproductive biology of two subspecies. Canadian Journal of Botany 76:478–485. [Google Scholar]
- McCauley DE, Bailey MF. 2009. Recent advances in the study of gynodioecy: the interface of theory and empiricism. Annals of Botany 104:611–620. 10.1093/aob/mcp141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley DE, Bailey MF, Sherman NA, Darnell MZ. 2005. Evidence for paternal transmission and heteroplasmy in the mitochondrial genome of Silene vulgaris, a gynodioecious plant. Heredity 95:50–58. 10.1038/sj.hdy.6800676 [DOI] [PubMed] [Google Scholar]
- Meagher TR. 2007. Linking the evolution of gender variation to floral development. Annals of Botany 100:165–176. 10.1093/aob/mcm035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meusel H, Mühlberg H. 1979. Unterfamilie Silenoideae (Lindl). In: Hegi G, ed. Illustrierte Flora von Mitteleuropa(Band III). Berlin: Verlag Paul Parey. [Google Scholar]
- Miyake K, Olson MS. 2009. Experimental evidence for frequency dependent self-fertilization in the gynodioecious plant, Silene vulgaris. Evolution 63:1644–1652. 10.1111/j.1558-5646.2009.00646.x [DOI] [PubMed] [Google Scholar]
- Montesinos D, García-Fayos P, Mateu I. 2006. Selective forces underlying seed dispersal in the endangered plant Silene diclinis. International Journal of Plant Sciences 167:103–110. 10.1086/497843 [DOI] [Google Scholar]
- Moyle LC. 2006. Correlates of genetic differentiation and isolation by distance in 17 congeneric Silene species. Molecular Ecology 15:1067–1081. 10.1111/j.1365-294X.2006.02840.x [DOI] [PubMed] [Google Scholar]
- Mrackova M, Nicolas M, Hobza R, Negrutiu I, Monéger F, Widmer A, Vyskot B, Janousek B. 2008. Independent origin of sex chromosomes in two species of the genus Silene. Genetics 179:1129–1133. 10.1534/genetics.107.085670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naciri Y, Cavat F, Jeanmonod D. 2010. Silene patula (Siphonomorpha, Caryophyllaceae) in North Africa: a test of colonisation routes using chloroplast markers. Molecular Phylogenetics and Evolution 54:922–932. [DOI] [PubMed] [Google Scholar]
- Oxelman B. 1995. A revision of the Silene sedoides-group (Caryophyllaceae). Willdenowia 25:143–169. [Google Scholar]
- Oxelman B, Lidén M. 1995. Generic boundaries in the tribe Sileneae (Caryophyllaceae) as inferred from nuclear rDNA sequences. Taxon 44:525–542. 10.2307/1223498 [DOI] [Google Scholar]
- Oxelman B, Lidén M, Turland NJ. 2001. Taxonomic and nomenclatural notes on Chinese Silene (Caryophyllaceae). Novon 11:322–324. 10.2307/3393038 [DOI] [Google Scholar]
- Oxelman B, Rautenberg A, Thollesson M, Larsson A, Frajman B, Eggens F, Petri A, Aydin Z, Töpel M, Brandtberg–Falkman A. 2013. Sileneae taxonomy and systematics. http://www.Sileneae.info (14 July 2014). [Google Scholar]
- Petri A, Pfeil BE, Oxelman B. 2013. Introgressive hybridization between anciently diverged lineages of Silene (Caryophyllaceae). PLoS ONE 8:e67729 10.1371/journal.pone.0067729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson MW. 1997. Solitary plants do as well as clumped ones in Silene uniflora (Caryophyllaceae). Ecography 20:375–382. 10.1111/j.1600-0587.1997.tb00382.x [DOI] [Google Scholar]
- Philipp M. 1980. Reproductive biology of Stellaria longipes Goldie as revealed by a cultivation experiment. New Phytologist 85:557–569. 10.1111/j.1469-8137.1980.tb00771.x [DOI] [Google Scholar]
- Popp M, Oxelman B. 2004. Evolution of a RNA polymerase gene family in Silene (Caryophyllaceae)–Incomplete concerted evolution and topological congruence among paralogues. Systematic Biology 53:914–932. 10.1080/10635150490888840 [DOI] [PubMed] [Google Scholar]
- Popp M, Oxelman B. 2007. Origin and evolution of North American polyploid Silene (Caryophyllaceae). American Journal of Botany 94:330–349. 10.3732/ajb.94.3.330 [DOI] [PubMed] [Google Scholar]
- Potvin C. 2001. ANOVA: experimental layout and analysis. In: Scheiner SM, Gurevitch J, eds. Design and analysis of ecological experiments, 2nd edn New York: Oxford University Press, 63–76. [Google Scholar]
- Prentice HC. 1976. A study in endimism: Silene diclinis. Biological Conservation 10:15–30. 10.1016/0006-3207(76)90021-5 [DOI] [Google Scholar]
- Prentice HC, Malm JU, Mateu-Andrés I, Segarra-Moragues JG. 2003. Allozyme and chloroplast DNA variation in island and mainland populations of the rare Spanish endemic, Silene hifacensis (Caryophyllaceae). Conservation Genetics 4:543–555. 10.1023/A:1025603328704 [DOI] [Google Scholar]
- Pusalkar PK, Singh DK, Lakshminarasimhan P. 2004. Silene gangotriana (Caryophyllaceae): a new species from Western Himalaya, India. Kew Bulletin 59:621–624. 10.2307/4110923 [DOI] [Google Scholar]
- Quinn GP, Keough MJ. 2002. Experimental design and data analysis for biologists. Cambridge: Cambridge University Press. [Google Scholar]
- Rautenberg A, Hathaway L, Oxelman B, Prentice HC. 2010. Geographic and phylogenetic patterns in Silene section Melandrium (Caryophyllaceae) as inferred from chloroplast and nuclear DNA sequences. Molecular Phylogenetics and Evolution 57:978–991. 10.1016/j.ympev.2010.08.003 [DOI] [PubMed] [Google Scholar]
- Renner SS. 2014. The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an updated online database. American Journal of Botany 101:1588–1596. 10.3732/ajb.1400196 [DOI] [PubMed] [Google Scholar]
- Renner SS, Ricklefs RE. 1995. Dioecy and its correlates in the flowering plants. American Journal of Botany 82:596–606. 10.2307/2445418 [DOI] [Google Scholar]
- Renner SS, Won HS. 2001. Repeated evolution of dioecy from monoecy in Siparunaceae (Laurales). Systematic Biology 50:700–712. 10.1080/106351501753328820 [DOI] [PubMed] [Google Scholar]
- Reynolds RJ, Westbrook MJ, Rohde AS, Cridland JM, Fenster CB, Dudash MR. 2009. Pollinator specialization and pollination syndromes of three related North American Silene. Ecology 90:2077–2087. 10.1890/08-1141.1 [DOI] [PubMed] [Google Scholar]
- Richards AJ. 1997. Plant breeding systems. London: Chapman and Hall. [Google Scholar]
- Sakai AK, Weller SG. 1999. Gender and sexual dimorphism in flowering plants: a review of terminology, biogeographic patterns, ecological correlates, and phylogenetic approaches. In: Geber MA, Dawson TE, Delph LF, eds. Gender and sexual dimorphism in flowering plants. Berlin: Springer, 1–31. [Google Scholar]
- Schischkin BK. 1970. Silene L. In: Komarov VL, Schischkin BK, eds. Flora of the USSR, Vol. 6 Jerusalem: Israel Program for Scientific Translations. [Google Scholar]
- Shykoff JA. 1988. Maintenance of gynodioecy in Silene acaulis (Caryophyllaceae): stage-specific fecundity and viability selection. American Journal of Botany 75:844–850. 10.2307/2444003 [DOI] [Google Scholar]
- Shykoff JA. 1992. Sex polymorphism in Silene acaulis (Caryophyllaceae) and the possible role of sexual selection in maintaining females. American Journal of Botany 79:138–143. 10.2307/2445100 [DOI] [Google Scholar]
- Shykoff JA, Kolokotronis SO, Collin CL, López-Villavicencio M. 2003. Effects of male sterility on reproductive traits in gynodioecious plants: a meta-analysis. Oecologia 135:1–9. 10.1007/s00442-002-1133-z [DOI] [PubMed] [Google Scholar]
- Slancarova V, Zdanska J, Janousek B, Talianova M, Zschach C, Zluvova J, Siroky J, Kovacova V, Blavet H, Danihelka J, Oxelman B, Widmer A, Vyskot B. 2013. Evolution of sex determination systems with heterogametic males and females in Silene. Evolution 67:3669–3677. 10.1111/evo.12223 [DOI] [PubMed] [Google Scholar]
- Soza VL, Brunet J, Liston A, Salles Smith P, Di Stilio VS. 2012. Phylogenetic insights into the correlates of dioecy in meadow-rues (Thalictrum, Ranunculaceae). Molecular Phylogenetics and Evolution 63:180–192. 10.1016/j.ympev.2012.01.009 [DOI] [PubMed] [Google Scholar]
- Talavera S. 1979. Revisión de la sect. Erectorefractae Chowdhuri del género Silene L. Lagascalia 8:135–164. [Google Scholar]
- Talavera S. 1990. Silene L. In: Castroviejo S, Aedo C, Laínz M, Muñoz Garmendia F, Nieto Feliner G, Paiva J, Benedí C, eds. Flora Iberica, Vol. 2 Madrid: Real Jardín Botánico. [Google Scholar]
- Talavera S, Arista M, Salgueiro FJ. 1996. Population size, pollination and breeding system of Silene stockenii Chater (Caryophyllaceae), an annual gynodioecious species of southern Spain. Botanica Acta 109:333–339. 10.1111/j.1438-8677.1996.tb00581.x [DOI] [Google Scholar]
- Terrab A, García-Castaño JL, Romero JM, Berjano R, de Vega C, Talavera S. 2007. Analysis of amino acids in nectar from Silene colorata Poiret (Caryophyllaceae). Botanical Journal of the Linnean Society 155:49–56. 10.1111/j.1095-8339.2007.00673.x [DOI] [Google Scholar]
- Torices R, Méndez M, Gómez JM. 2011. Where do monomorphic sexual systems fit in the evolution of dioecy? Insights from the largest family of angiosperms. New Phytologist 190:234–248. 10.1111/j.1469-8137.2010.03609.x [DOI] [PubMed] [Google Scholar]
- Touzet P, Delph LF. 2009. The effect of breeding system on polymorphism in mitochondrial genes of Silene. Genetics 181:631–644. 10.1534/genetics.108.092411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropicos.org. Missouri Botanical Garden. 2014. Flora of Pakistan. http://www.tropicos.org (November 2014).
- Vilas C, García C. 2006. The role of genetic mechanisms of sex determination in the survival of small populations of Silene littorea: a reintroduction experiment. Biological Conservation 129:124–133. 10.1016/j.biocon.2005.10.028 [DOI] [Google Scholar]
- Vilas C, San Miguel E, Amaro R, Garcia C. 2006. Relative contribution of inbreeding depression and eroded adaptive diversity to extinction risk in small populations of shore campion. Conservation Biology 20:229–238. 10.1111/j.1523-1739.2005.00275.x [DOI] [PubMed] [Google Scholar]
- Warren J, James P. 2008. Do flowers wave to attract pollinators? A case study with Silene maritima. Journal of Evolutionary Biology 21:1024–1029. 10.1111/j.1420-9101.2008.01543.x [DOI] [PubMed] [Google Scholar]
- Weingartner LA, Delph LF. 2014. Neo-sex chromosome inheritance across species in Silene hybrids. Journal of Evolutionary Biology 27:1491–1499. 10.1111/jeb.12371 [DOI] [PubMed] [Google Scholar]
- Westerbergh A, Saura A. 1994. Genetic differentiation in endemic Silene (Caryophyllaceae) on the Hawaiian Islands. American Journal of Botany 81:1487–1493. 10.2307/2445321 [DOI] [Google Scholar]
- Widén M, Widén B. 1999. Sex expression in the clonal gynodioecious herb Glechoma hederacea (Lamiaceae). Canadian Journal of Botany 77:1689–1698. 10.1139/cjb-77-12-1689 [DOI] [Google Scholar]
- Yampolsky C, Yampolsky H. 1922. Distribution of sex forms in the phanerogamic flora. Bibliotheca Genetica 3:1–62. [Google Scholar]
- Yildiz K, Çirpici AH. 2013. Taxonomic revision of Silene (Caryophyllaceae) sections Siphonomorpha, Lasiostemones, Sclerocalycinae, Chloranthae, Tataricae, and Otites in Turkey. Turkish Journal of Botany 37:191–218. 10.3906/bot-1301-4 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.