Abstract
Background
Mutations in complement factor H (CFH) are associated with complement dysregulation and the development of an aggressive form of atypical hemolytic uremic syndrome (aHUS) that progresses to end-stage renal disease and in most patients has a high rate of recurrence following transplantation. Sequence analysis of CFH and its downstream complement factor H-related genes (CFHR1-5) reveals several macrohomologous blocks caused by large genomic duplications. This high degree of sequence identity renders this area susceptible to nonallelic homologous recombination (NAHR) events, resulting in large-scale deletions, duplications, and the generation of hybrid CFH genes.
Case-Diagnosis
Here, we report the finding of a novel CFHR1/CFH hybrid gene created by a de novo NAHR event in a 14-year-old girl with aHUS. The resulting fusion protein contains the first three short consensus repeats (SCRs) of CFHR1 and the terminal two SCRs of CFH.
Conclusions
This finding demonstrates a novel pathogenic mechanism for the development of aHUS. Additionally, since standard Sanger sequencing is unable to detect such rearrangements, all aHUS patients should receive comprehensive genetic screening that includes analysis of copy number variation in order to identify patients with poor clinical prognoses.
Keywords: Atypical hemolytic uremic syndrome, alternative pathway of complement, nonallelic homologous recombination, factor H hybrid genes
Introduction
Hemolytic uremic syndrome is a thrombotic microangiopathy (TMA) characterized by microangiopathic hemolytic anemia, thrombocytopenia, and acute renal failure. While the typical form is a diarrheal illness related to infection with Shiga-toxin producing bacteria, the rare, atypical form (aHUS) is associated with genetic mutations in the alternative complement system. In aHUS, sporadic and inherited genetic abnormalities affecting both alternative pathway (AP) regulators and activators are found in approximately 60% of patients.[1]
Complement factor H (CFH), the major regulator of the AP, is the most frequently mutated gene in aHUS.[2] Phenotypic-genotypic correlations have shown that patients with aHUS-causing CFH mutations have a poor clinical prognosis, with 60-70% of these patients progressing to end stage renal disease (ESRD) and 70-90% losing their allograft following transplantation.[3] While the majority of mutations in CFH occur in the final two short consensus repeats (SCRs 19 and 20), nonallelic homologous recombination (NAHR) events can create hybrid CFH genes that lead to complement dysregulation and aHUS pathology.[4]
CFH and its related genes (CFHR1-5) are located within the Regulators of Complement Activation (RCA) cluster on chromosome 1 (1q32). Sequence analysis shows that these genes share a high degree of sequence identity resulting from large-scale genomic duplications.[5] These homologous repeat regions render this area susceptible to complex NAHR between CFH and the other CFH related genes, resulting in several different copy number variations (CNVs).
The majority of NAHR events in this region occur between two homologous blocks, B and B′, most commonly leading to the deletion of CFHR3-CFHR1, a recognized CNV associated with the development of CFH autoantibodies.[6] A slightly different rearrangement involving these blocks results in the formation of hybrid CFH/CFHR1 genes, two of which, the Venables deletion and the Maga-Meyer deletion, have been described in patients with aHUS.[7, 8]
Given the high degree of homology within the RCA cluster, we routinely utilize multiplex ligation-dependent probe amplification (MLPA) to screen for NAHR in aHUS patients. Herein, we report the finding of a novel CFHR1/CFH hybrid gene occurring de novo in a patient with aHUS. This hybrid gene is the product of a NAHR event that results in an additional copy of CFHR3 and the formation of a CFHR1/CFH fusion protein that predisposes to the development of aHUS.
Case Report
Our patient, described in a report on the successful preemptive use of eculizumab for renal transplantation in aHUS, initially presented at 8 years of age with hypertension, severe anemia (hemoglobin 5.5 g/dL [55 g/L]), and renal failure (creatinine [Cr] 13.7 mg/dL [1211.1 μmol/L], blood urea nitrogen [BUN] 196 mg/dL [70 mmol/L]).[9] No cause of renal dysfunction was determined, and the patient was started on hemodialysis and subsequently received a living-related kidney transplant from her maternal aunt. Immediately following transplantation, the patient did well; however, 25 months post-transplantation she developed hypertension, acute kidney injury and anemia, and rapidly progressed to ESRD (BUN 109 mg/dL [38.9 mmol/L], Cr 8.5 mg/dL [751.4 μmol/L], hemoglobin 4.5 g/dL [45 g/L], platelets 144 ×103/μL [144 ×109/L]). Complement component assessment showed a slight decrease in C3 (81 mg/dL [81 g/L]; normal 90-180 mg/dL [90-180 g/L]) and a normal C4 (23 mg/dL [23 g/L]; normal 16-47 mg/dL [16-47 g/L]). Allograft biopsy was consistent with severe TMA, confirming the diagnosis of aHUS.
After 15 months of hemodialysis, the patient underwent renal transplantation from a living non-related donor and received eculizumab preemptively. One week following transplantation, the serum Cr dropped from 11.7 to 1.5 mg/dL (5012.3 to 132.6 μmol/L) and continued to decline to near normal over the following months. The patient is currently 26 months post-transplant and continues to receive bi-weekly doses of eculizumab. Her CH50 remains suppressed. C3 and C4 levels are within normal limits, and she has no signs of disease recurrence.
Genetic
Analysis
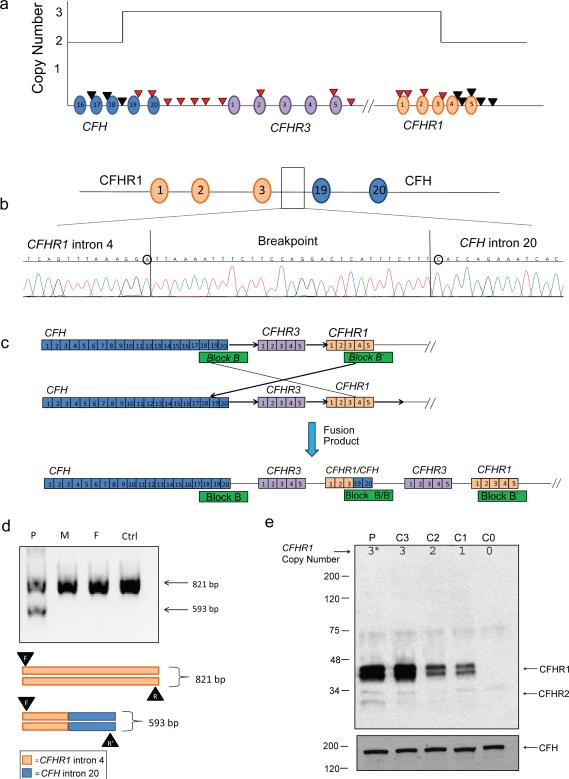
Genomic DNA was extracted from blood, and mutation screening was completed on complement genes associated with aHUS (CFH, CFI, CD46, CFB, C3, THBD, and CFHR5); [2] no disease-causing variants were identified. Next, CNVs across the CFH-CFHR5 region were assayed by MLPA, revealing a large heterozygous duplication containing exon 21 (SCR 19) of CFH through exon 4 (SCR 3) of CFHR1 (Figure 1a). MLPA reaction was performed as described previously.[10] MLPA probe sequences for the CFH-CFHR1 region are included in Supplementary Table S1.
Figure 1. A novel hybrid CFHR1/CFH gene.
a) MLPA determination of copy number over the CFH-CFHR region identified a duplication beginning with CFH exon 21 (CFH SCR 19) and ending after CFHR1 exon 4 (CFHR1 SCR 3). MLPA probes are represented by arrows and SCRs by ovals. Red arrows indicate duplicated probes. The breakpoint sequence was localized to a 29 bp sequence between intron 4 of CFHR1 and intron 20 of CFH. SNP differences (circled) between CFH and CFHR1 defined the exact location of the breakpoint. SCRs are represented by ovals. b) The breakpoint sequence was localized to a 29 bp sequence between intron 4 of CFHR1 and intron 20 of CFH. SNP differences (circled) between CFH and CFHR1 defined the exact location of the breakpoint. c) The novel hybrid CFHR1/CFH gene originates from NAHR between homologous blocks B and B′. The resulting fusion protein comprises SCRs 1-3 of CFHR1 and SCRs 19-20 of CFH. The direction of the recombination is indicated by black arrows. d) Diagnostic PCR utilizing a common forward primer (F) in CFHR1 intron 4, a reverse primer (R) in CFHR1 intron 4, and a reverse primer in CFH intron 20 (R′) in the same reaction resulted in both a 593-bp breakpoint and a 821-bp wild-type (WT) product in our patient. The 593-bp breakpoint fragment was not seen in the mother (M), father (F), or in a control patient (Ctrl). e) Western blot using a polyclonal rabbit anti-CFHR1 antibody against sera from the patient (P) and controls (C0-C3) with known genetic copy number variations in CFHR1 (C0 = zero copies of CFHR1, C1 = one copy, C2 = two copies, and C3 = three copies). The presence of an additional CFHR1/CFH fusion protein (*) accounts for the increased band density seen in the patient. Differential glycosylation of CFHR1 SCRs 2 and 3 accounts for the two different band sizes at 37 and 43 kDa. CFH (~150 kDa) was used as a loading control.
Breakpoint mapping was performed by DNA amplification across CFHR1 intron 4 (homologous to CFH intron 20) using long range PCR, subcloning, and bidirectional sequencing.[8, 10] Analysis of sequence data placed the breakpoint in CFHR1 intron 4, 2,104 bp downstream of exon 4 (Figure 1b), implying that a NAHR event between homologous blocks B and B′ had created a novel CFHR1/CFH hybrid gene (Figure 1c).
Diagnostic PCR of the patient's nuclear family to amplify both the breakpoint (593 bp) and the corresponding wild-type intron 4 of CFHR1 (821 bp), yielded a 593 bp product solely in our patient, indicating that the NAHR was a de novo event (Figure 1d).
Complement Functional Assays and CFH Autoantibody Screening
An alternative pathway functional assay (APFA), a hemolytic assay, and CFH autoantibodies were measured as described.[11] APFA was extremely low at 0.3%, consistent with excessive complement activation and consumption of complement proteins. The hemolytic assay was normal, a false negative result secondary to depletion of complement components. Excessive consumption of complement components in vitro can result in decreased membrane attack complex formation and decreased RBC lysis. CFH autoantibodies were absent.
Western Blot
Patient and control sera with varying copy numbers of CFHR1 were run on SDS-PAGE 10% gels under reducing conditions and blotted with a polyclonal rabbit anti-CFHR1 antibody (1:500) (in house). Anti-CFH (1:1000) (ab8842; Abcam, Cambridge, MA) was used as a loading control. Our patient showed a relative density similar to that of a control known to have 3 copies of CFHR1, suggesting that the increase density is caused by the expression of the novel fusion protein which contains SCRs 1-3 of CFHR1.
Discussion
Here we identify a de novo NAHR duplication event that results in an additional copy of CFHR3 and the formation of a novel CFHR1/CFH hybrid gene that predisposes to the development of aHUS. To our knowledge, this report is now the second to describe a de novo NAHR event in a patient with aHUS.
Sequence analysis of CFHR1 shows that the two C-terminal SCRs (4-5) are 98.5% identical to those of CFH. As expected from this degree of sequence similarity, the C-termini of CFH and CFHR1 have overlapping function. Like CFH, CFHR1, is capable of binding to C3b, C3d, heparin and cell surfaces, though it does so with lesser affinity.[12] Unlike CFH, however, CFHR1 does not contain the domains necessary for cofactor and decay acceleration activities (CFH SCRs 1-4) and so has no ability to regulate the C3 convertase. CFHR1's ability to bind to cell surfaces allows it to compete with CFH for surface binding, thus contributing to complement dysregulation through the displacement of CFH. This is exemplified by the finding that baseline C3b degradation can be reduced by increasing concentrations of CFHR1 and enhanced by increasing concentrations of CFH.[12]
The presence of a CFHR1/CFH fusion protein capable of binding to cell surfaces means that the fusion protein will actively compete with CFH for surface C3b binding in a fashion similar to wild-type CFHR1. However, because the fusion protein contains the last two C-terminal SCRs of CFH, it is a stronger competitive binder than wild-type CFHR1. This increased avidity of the fusion protein leads to decreased local CFH regulatory activity, the consequence of which is complement dysregulation and aHUS pathology (Supplementary Figure S1).
The additional copy number of CFHR3 also contributes to aHUS pathology. CFHR3 is a weak modulator of complement regulation, serving to enhance CFH cofactor activity. However, CFHR3 also binds to surface bound C3b, suggesting an antagonistic effect at cell surfaces.[13] In a mechanism similar to the CFHR1/CFH fusion protein, additional CFHR3 contributes to local dysregulation through CFH displacement.
Based on phenotypic-genotypic reports, kidney transplantation alone in patients with hybrid CFH/CFHR1 genes is not recommended due to the high risk of disease recurrence. However, the use of eculizumab prophylactically reduces TMA following transplantation.[9, 14] In our patient, renal transplantation with eculizumab has thus far been successful in protecting against aHUS, with no signs of recurrent disease 26 months post-transplantation. Other patients with hybrid CFH genes have also had successful renal transplants with concomitant eculizumab.[15] The recent success of eculizumab in renal transplant patients, however, is dampened by the economic burden of such an expensive drug if life-long use is required, emphasizing the need to identify patients who are most likely to benefit from adjuvant eculizumab therapy.
In summary, we present a novel CFHR1/CFH hybrid gene that occurred de novo in an aHUS patient. The inability to detect such rearrangements with standard Sanger sequencing, underscores the importance of comprehensive genetic testing. Since the CFH-CFHR genomic region is predisposed to NAHR events, the testing process must include routine screening for CNVs. The presence of such variations may skew the interpretation of novel variants or variants of unknown significance. We recommend that all aHUS patients be screened for CNVs. Additionally, all patients previously found to be negative for complement mutations who rapidly progress to ESRD should have additional CNV screening.
Supplementary Material
Figure S1: Competitive surface binding may contribute to local complement dysregulationa proposed mechanism. The C-terminal CFH SCRs of the CFHR1/CFH fusion protein allow it to compete with CFH for surface binding to glycosaminoglycans (GAGs) and C3b on host cell surfaces. Less surface bound CFH contributes to alternative pathway dysregulation. CFH SCRs 1-4 contain the cofactor and decay acceleration activity domains and are absent in the fusion protein. Homology in the C-terminal SCRs of CFHR1 allow it compete for surface binding as well, though it does so with lesser affinity.
Acknowledgments
Support:
This study was supported by grants from the Doris Duke Clinical Research Foundation (SJE), the University of Iowa Institute for Clinical and Translational Science (SJE), the Foundation for Children with Atypical HUS (RJHS), and NIH grant DK074409 (RJHS).
Footnotes
Financial disclosure:
The authors declare that they have no relevant financial interests.
References
- 1.Loirat C, Fremeaux-Bacchi V. Atypical hemolytic uremic syndrome. Orphanet J Rare Dis. 2011;6:60. doi: 10.1186/1750-1172-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maga TK, Nishimura CJ, Weaver AE, Frees KL, Smith RJH. Mutations in alternative pathway complement proteins in American patients with atypical hemolytic uremic syndrome. Hum Mutat. 2010;31:E1445–E1460. doi: 10.1002/humu.21256. [DOI] [PubMed] [Google Scholar]
- 3.Sellier-Leclerc A, Fremeaux-Bacchi V, Dragon-Durey M, Macher M-A, Niaudet P, Guest G, Boudailliez B, Bouissou F, Deschenes G, Gie S, Tsimaratos M, Fischbach M, Morin D, Nivet H, Alberti C, Loirat C. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2007;18:2392–2400. doi: 10.1681/ASN.2006080811. [DOI] [PubMed] [Google Scholar]
- 4.Caprioli J, Noris M, Brioschi S, Pianetti G, Castelletti F, Bettinaglio P, Mele C, Bresin E, Cassis L, Gamba S, Porrati F, Bucchioni S, Monteferrante G, Fang CJ, Liszewski MK, Kavanagh D, Atkinson JP, Remuzzi G. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006;108:1267–1279. doi: 10.1182/blood-2005-10-007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Józsi M, Zipfel PF. Factor H family proteins and human diseases. Trends Immunol. 2008;29:380–387. doi: 10.1016/j.it.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Józsi M, Licht C, Strobel S, Zipfel SL, Richter H, Heinen S, Zipfel PF, Skerka C. Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood. 2008;111:1512–1514. doi: 10.1182/blood-2007-09-109876. [DOI] [PubMed] [Google Scholar]
- 7.Venables JP, Strain L, Routledge D, Bourn D, Powell HM, Warwicker P, Diaz-Torres ML, Sampson A, Mead P, Webb M, Pirson Y, Jackson MS, Hughes A, Wood KM, Goodship JA, Goodship THJ. Atypical haemolytic uraemic syndrome associated with a hybrid complement gene. PLoS Med. 2006;3:e431. doi: 10.1371/journal.pmed.0030431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maga TK, Meyer NC, Belsha C, Nishimura CJ, Zhang Y, Smith RJH. A novel deletion in the RCA gene cluster causes atypical hemolytic uremic syndrome. Nephrol Dial Transplant. 2011;26:739–741. doi: 10.1093/ndt/gfq658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nester C, Stewart Z, Myers D, Jetton J, Nair R, Reed A, Thomas C, Smith R, Brophy P. Pre-emptive eculizumab and plasmapheresis for renal transplant in atypical hemolytic uremic syndrome. Clin J Am Soc Nephro. 2011;6:1488–1494. doi: 10.2215/CJN.10181110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 2002;30:e57. doi: 10.1093/nar/gnf056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sethi S, Sukov WR, Zhang Y, Fervenza FC, Lager DJ, Miller DV, Cornell LD, Krishnan SGS, Smith RJH. Dense deposit disease associated with monoclonal gammopathy of undetermined significance. Am J Kidney Dis. 2010;56:977–982. doi: 10.1053/j.ajkd.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinen S, Hartmann A, Lauer N, Wiehl U, Dahse H-M, Schirmer S, Gropp K, Enghardt T, Wallich R, Hälbich S, Mihlan M, Schlötzer-Schrehardt U, Zipfel PF, Skerka C. Factor H–related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood. 2009;114:2439–2447. doi: 10.1182/blood-2009-02-205641. [DOI] [PubMed] [Google Scholar]
- 13.Hellwage J, Jokiranta TS, Koistinen V, Vaarala O, Meri S, Zipfel PF. Functional properties of complement factor H-related proteins FHR-3 and FHR-4: binding to the C3d region of C3b and differential regulation by heparin. FEBS Lett. 1999;462:345–352. doi: 10.1016/s0014-5793(99)01554-9. [DOI] [PubMed] [Google Scholar]
- 14.Chatelet V, Frémeaux-Bacchi V, Lobbedez T, Ficheux M, Hurault de Ligny B. Safety and long-term efficacy of eculizumab in a renal transplant patient with recurrent atypical hemolytic–uremic syndrome. Am J Transplant. 2009;9:2644–2645. doi: 10.1111/j.1600-6143.2009.02817.x. [DOI] [PubMed] [Google Scholar]
- 15.Krid S, Roumenina LT, Beury D, Charbit M, Boyer O, Frémeaux-Bacchi V, Niaudet P. Renal transplantation under prophylactic eculizumab in atypical hemolytic uremic syndrome with CFH/CFHR1 hybrid protein. Am J Transplant. 2012;12:1938–1944. doi: 10.1111/j.1600-6143.2012.04051.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Competitive surface binding may contribute to local complement dysregulationa proposed mechanism. The C-terminal CFH SCRs of the CFHR1/CFH fusion protein allow it to compete with CFH for surface binding to glycosaminoglycans (GAGs) and C3b on host cell surfaces. Less surface bound CFH contributes to alternative pathway dysregulation. CFH SCRs 1-4 contain the cofactor and decay acceleration activity domains and are absent in the fusion protein. Homology in the C-terminal SCRs of CFHR1 allow it compete for surface binding as well, though it does so with lesser affinity.